Introduction
Glioblastoma multiforme (GBM) is the most aggressive
malignant brain tumor in humans with a survival time often less
than 1 year even following treatment (1–3). There
remains a need for animal models that can be used to assess the
efficacy of new and innovative treatments for experimental
neuro-oncology (4,5). The blood-brain barrier (BBB) composed
of tight junctions prevents the central nervous system (CNS) from
being infiltrated by neurotoxic substances or pharmaceuticals from
the peripheral circulatory system. Although the BBB of a
tumor-bearing animal is severely compromised (6), some difficulties still remain in
monitoring brain tumor progression or treatment response in
experimental and clinical approaches. For example, since the
introduction of 2-deoxy-2-[18F]fluoro-D-glucose
(18F-FDG)/PET, it has been widely used in clinical
settings for evaluation of several malignancies, such as lung,
colon, breast, head and neck and esophageal cancers (7). 18F-FDG is metabolized as
glucose but is stopped at the early step of metabolism, and can be
used to detect the proliferation of tumor cells. Hence radiolabeled
glucose analogs may serve as probes for molecular imaging of brain
tumors. However, 18F-FDG also accumulates in benign
lesions, and false-positive results may occur during infection,
inflammation and other therapy-related conditions (8).
3′-Deoxy-3′-[18F]fluorothymidine (18F-FLT), a
thymidine analog, often used in PET imaging for monitoring
non-invasive cancer treatment, can be phosphorylated by thymidine
kinase 1 (TK1) and trapped within tumor cells (9). It has been suggested that a
multitracer approach using a combination of [18F]FLT and
[18F]FDG may monitor early treatment response more
effectively following cancer therapy (10). Other radiolabeled pyrimidine
nucleoside derivatives, such as
2′-fluoro-2′-deoxy-β-D-arabinofuranosyl-5-iodouracil (FIAU) labeled
with radioiodine,
9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine
([18F]FHBG), and
9-[(1-[18F]fluoro-3-hydroxy-2-propoxy)methyl]guanine
([18F]FHPG), have been demonstrated to accumulate
selectively in HSV1-tk transfected cells, but significant
non-specific phosphorylation is still noted (11,12).
On the other hand,
5-[76Br]bromo-2′-fluoro-2′-deoxyuridine
([76Br]BFU) and
1-[2-deoxy-2-[18F]fluoro-β-arabinofuranosyl-5-bromouracil]
([18F]FBAU), both uracil analogs for DNA incorporation,
have been developed for the imaging of tumor proliferation with
superior specificity for the HSV1-tk reporter system
(13,14). However, one disadvantage of
[76Br] is that patients may be exposed to a higher
radiation dose due to its long half-life and high energy (16.2 h,
3.6 MeV). The shorter half-life and optimal energy of
[18F] (110 min, 0.64 MeV) makes [18F]-labeled
pharmaceuticals more convenient for clinical PET imaging. It has
been shown that [18F]-labeled proteins provide higher
availability and better imaging quality (15). The advantages of
[18F]-labeled FBAU and combined with PET imaging in
cellular proliferation, neuroscience and oncology have been
reported (16–18).
In our previous studies,
4-borono-2-[18F]-fluoro-L-phenylalanine-fructose
([18F]FBPA-Fr) has been shown to have higher
accumulation in tumors of F98 glioma-bearing rats, and can be used
as a probe to mimic BPA-Fr in the treatment of brain tumors by
boron neutron capture therapy (BNCT) (19). Furthermore, specific tumor
accumulation with higher tumor-to-normal brain ratio of
[18F]FBPA-Fr than that of [18F]FDG has also
been demonstrated (20).
Establishment of a brain tumor-bearing animal model
with a reporter gene-reporter probe (RGRP) system for
multimodalities of molecular imaging may provide a useful platform
for monitoring theranostics of tumor treatment (21–23),
particularly for aggressive brain tumors such as GBM. The purpose
of the present study was to investigate the potential application
of [18F]FBAU as a PET reporter probe for monitoring
tumor progression in an F98/tk-luc glioma-bearing rat
model.
Materials and methods
Establishment of the pC1-tk-IRES-luc
plasmid and the F98-tk-luc stable clone
The pC1-tk-IRES-luc plasmid was
constructed according to our previous study (24). F98 rat glioma tumor cells were
transfected with this plasmid using cationic polymer, jetPEI
(Polyplus Transfection, Strasbourg, France). F98 cells were
cultured in a 6-well plate (2×105 cells/well) after
transfection. In a 1.5-ml Eppendorf vial, 3 μg of DNA plasmid was
mixed with 6 μl jetPEI reagent in 100 μl of 150 mM NaCl followed by
gentle vortexing, and then briefly spun down. The mixtures were
incubated for 30 min at room temperature and were added to the
well. Transfection was performed at 37°C for 24 h in a humidified
incubator with 5% CO2. Stable clones were selected with
600 μg/ml of G418 (Calbiochem, Darmstadt, Germany).
Luciferase (luc) gene expression assayed
with bioluminescent imaging (BLI)
For the luc activity assay, the cell lysate
was prepared by performing a freeze-thaw cycle in reporter lysis
buffer (Promega, Madison, WI, USA). The bioluminescence was
measured with a luminometer (Wallac 1420 Multilabel Counter; Life
Sciences, Pittsburgh, PA, USA). The protein concentration of the
cell lysate in each well was measured with a Bio-Rad protein assay
kit (Bio-Rad Laboratories, Hercules, CA, USA) (24). F98 cells transfected with
pC1-tk-IRES-luc vectors were cultured in a 6-cm diameter
dish for 14 days, and 30 colonies were selected. Each colony was
cultured in a 3-cm diameter dish for cell proliferation. Cells were
treated with 200 μl lysis buffer, and were frozen at −80°C for 1 h.
After thawing and centrifugation (1,200 rpm, Kubota Laboratory
Centrifuge, model 5100; Kubota Corp., Tokyo, Japan), the
supernatant was transferred to a black 96-well plate filled with
D-luciferin. The bioluminescence was quantified as photons/sec/mg.
For BLI imaging in vivo, male Fischer 344 rats and NOD/SCID
mice were anaesthetized with 1–3% isofluorane, and then an i.p.
injection with 300 μl D-luciferin (150 mg/kg in PBS) as the
substrate. The IVIS 50 Imaging System (Xenogen, Alameda, CA, USA)
was used for image acquisition. The time duration was 15 min for
rats and 2 min for mice, followed by quantification with the Living
Image software (Xenogen).
HSV1-tk gene expression assay
F98/tk-luc cells were cultured in 96-well
plates (3,000 cells/200 μl/well) for 24 h, and then treated with
0–200 mM ganciclovir (GCV; F. Hoffmann-La Roche Ltd., Basel,
Switzerland) for 120 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) colorimetric assay was used to
determine cell survival (24).
Radiosynthesis of [131I]FIAU
and [18F]FBAU
[131I]FIAU was prepared according to our
previous study (25).
[18F]FBAU was synthesized according to a study reported
by Alauddin et al (26). The
purification of [18F]FBAU was followed according to our
previous study (27).
[131I]FIAU cellular uptake
assay
[131I]FIAU (3.7×104 Bq/μl)was
added to each well (1×106 cells/3 ml/well in 6-well
culture plates), followed by incubation at 37°C for 15 and 30 min,
1, 2 and 4 h, respectively. Triplicates were performed at each time
point.
Cell culture, orthotropic F98/tk-luc
glioma-bearing rat model and subcutaneous mouse tumor
inoculation
F98 glioma cells (a kind gift from Dr Rolf F. Barth,
The Ohio State University, USA) were transfected with the
pC1-tk-IRES-luc plasmid (24), and renamed as F98/tk-luc
glioma cells. These cell lines were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (containing 10% FBS, 100 U/ml penicillin and
100 μg/ml streptomycin) at 37°C in a humidified atmosphere of 5%
CO2. Male Fischer 344 rats (12–14 weeks of age, 250–280
g) were anesthetized intraperitoneally (i.p.) with a mixture of 110
mg/kg ketamine and 9 mg/kg xylazine. F98/tk-luc glioma cells
(1×105) in 10 μl Mg2+/Ca2+-free
Hanks’ balanced salt solution (19)
were then slowly (15–20 sec) injected into the left brain region of
the rats. The syringe was held still for 2 min after injection
before withdrawing, and the injection hole was sealed with bone
wax. Finally, the wound was flushed with iodinated alcohol and
sutured with a sterilized steel clip. Male NOD/SCID mice were
inoculated with 5×105 F98/tk-luc cells in 200 μl
serum-free medium subcutaneously in the right flank. The tumor
volume was measured with a digital caliper.
Tumor volume assayed by MR imaging
3T MR scanning was performed within 24 h post BLI
imaging. A 40-mm diameter field-of-view (FOV) orbital surface coil
was used as the receiver (28).
Each rat was anesthetized with 3–4 ml 10% chloride hydrate. To
obtain accurate position of the brain slice, the coronal and
transverse plane scout images were scanned first. 2–4 coronal
T1-weighted slices with a 1.5-mm thickness would cover the tumor.
The T1-weighted images were acquired with a 256×256 matrix, FOV=89
mm, repetition time=435 msec and echo time=12 msec. For
contrast-enhanced images, each animal received 0.2 mmol/kg contrast
medium i.v. 5 min prior to T1-weighted sequence imaging.
T2-weighted images were acquired with a 256×256 matrix, FOV=89 mm,
repetition time=5,000 msec and echo time=104 msec. The DICOM images
were collected, and the tumor boundary visualized in each slice was
defined as the region-of-interest (ROI) using ImageJ (National
Institutes of Health, Bethesda, MD, USA) for calculation. The sum
of the ROI areas was processed by slice thickness to obtain the
tumor volume.
Pathology examination
After examination, the rats were sacrificed with
CO2. The brains were removed and fixed with 4%
paraformaldehyde. The sections (5-μm) obtained from the
paraffin-embedded samples were stained with hematoxylin and eosin
(H&E).
Biodistribution of [18F]FBAU
in the F98/tk-luc glioma-bearing rats
Biodistribution of [18F]FBAU was
determined on the 13th day post tumor implantation as previously
described (19). Each rat was
anesthetized with 3–4 ml of 10% chloride hydrate. The
F98/tk-luc glioma-bearing rats were injected with 15 MBq/0.2
ml [18F]FBAU via lateral tail veins. At 0.5, 1, 1.5 and
2 h post injection, the rats (n=5 per group) were sacrificed with
CO2. Left and right brains, tumors, intestine, spleen,
heart, blood, lung, liver, stomach, pancreas, kidneys, bone and
muscles were removed, and the radioactivity was measured in a
well-type multichannel analyzer (Raytest, Straubenhardt, Germany)
(27). The uptake of
[18F]FBAU in tissues or organs was expressed in counts
per second (cps) corrected with the decay, and was normalized as
the percentage injected dose per gram of tissue (%ID/g).
PET scanning
The PET images of F98/tk-luc glioma-bearing
rats i.v. injected with 50 MBq/0.1 ml [18F]FBAU were
acquired up to 160 min using the PET/CT system (GE Discovery LS
PET-CT; GE Healthcare, Cleveland, OH, USA) on day 13 post tumor
implantation. The imaging parameters were set as follows: 2-D brain
model, FOV=15.52 cm, spatial resolution=5.46 mm, 128×128 matrix
size. The reconstruction algorithm using ordered subset expectation
maximization (OSEM) was similar to that of a previous study
(19). The images were acquired
with axial, coronal and sagittal planes, respectively. Each rat was
anesthetized with 3–4 ml 10% chloride hydrate and i.v. injected
with 0.1 ml of 50 MBq [18F]FBAU. Data acquisition by PET
scanning was initiated at the first minute after drug injection.
Dynamic coronal and sagittal images were acquired using 10 60-sec
frames and 10 2-min frames, followed by 10-min frames up to 2 h.
Standardized uptake values (SUVs) were calculated and normalized to
body weight (BW) (29). %SUV was
calculated using the following formula: %SUV = [(left brain SUV -
right brain SUV)/right brain SUV] × 100%.
Ex vivo autoradiography
The ex vivo autoradiography was performed
according to a previous report (20). Fischer 344 rats were anesthetized
with 1–3% isofluorane. At 30, 60, 90 and 120 min post i.v.
injection of [18F]FBAU (15–20 MBq/0.7 ml), the rats were
sacrificed with CO2. The brains were removed and
embedded with OCT on a cryostat holder (diameter 2.7 cm), and were
frozen immediately at −80°C. Coronal sections (20-μm thick) were
performed using a cryomicrotome (Leica CM3050; Leica Microsystems,
Bensheim, Germany). The imaging plates were assayed with an FLA5000
reader (Fuji Photo Film Co., Ltd., Tokyo, Japan) with the following
settings: PMT=800 V, resolution=50 μm, gradation=16 bits, dynamic
range=L5 and sensitivity=5,000 to acquire the phosphor images. ROIs
were circled along the tumor contour of the F98/tk-luc
glioma, and ROIs of equal size in the normal brain region at
corresponding brain position were used as the reference. The
intensity of photo-stimulated luminescence (PSL) of the tumor and
normal brain tissue was measured with Image Gauge (version 4.0,
Science Lab 2001; Fuji Photo Film).
Statistical analysis
The Studen’t t-test was used to analyze the
significant difference between the control and drug-treated rats.
All data are shown as the means ± standard error (SE). Differences
between the means were considered significant at P≤0.05. The
correlation between tumor volume and BLI photon flux was performed
by Pearson correlation.
Results
Selection of stable clones and luc gene
expression assay
The pC1-tk-IRES-luc vectors as shown
in Fig. 1A were transfected into
the F98 cell line, which was renamed F98/tk-luc. The colony
exhibiting luc expression of >1,500 counts per sec per mg
(cps/mg) was selected for cell expansion as shown in Fig. 1B (colony no. 11). The
bioluminescence of luc gene expression in the
F98/tk-luc cells was 3.24 photons/sec/cell (Fig. 1C).
Expression of thymidine kinase (tk)
F98/tk-luc cells were treated with various
concentrations of ganciclovir (GCV). The surviving fraction (SF) of
F98/tk-luc cells was 18% at 15 μM and remained ~100% up to
200 μM for parental F98 cells after GCV treatment (Fig. 2A). In addition, the uptake of
[131I]FIAU in the F98/tk-luc cells was increased
with time, but was not found in the parental F98 cells (Fig. 2B).
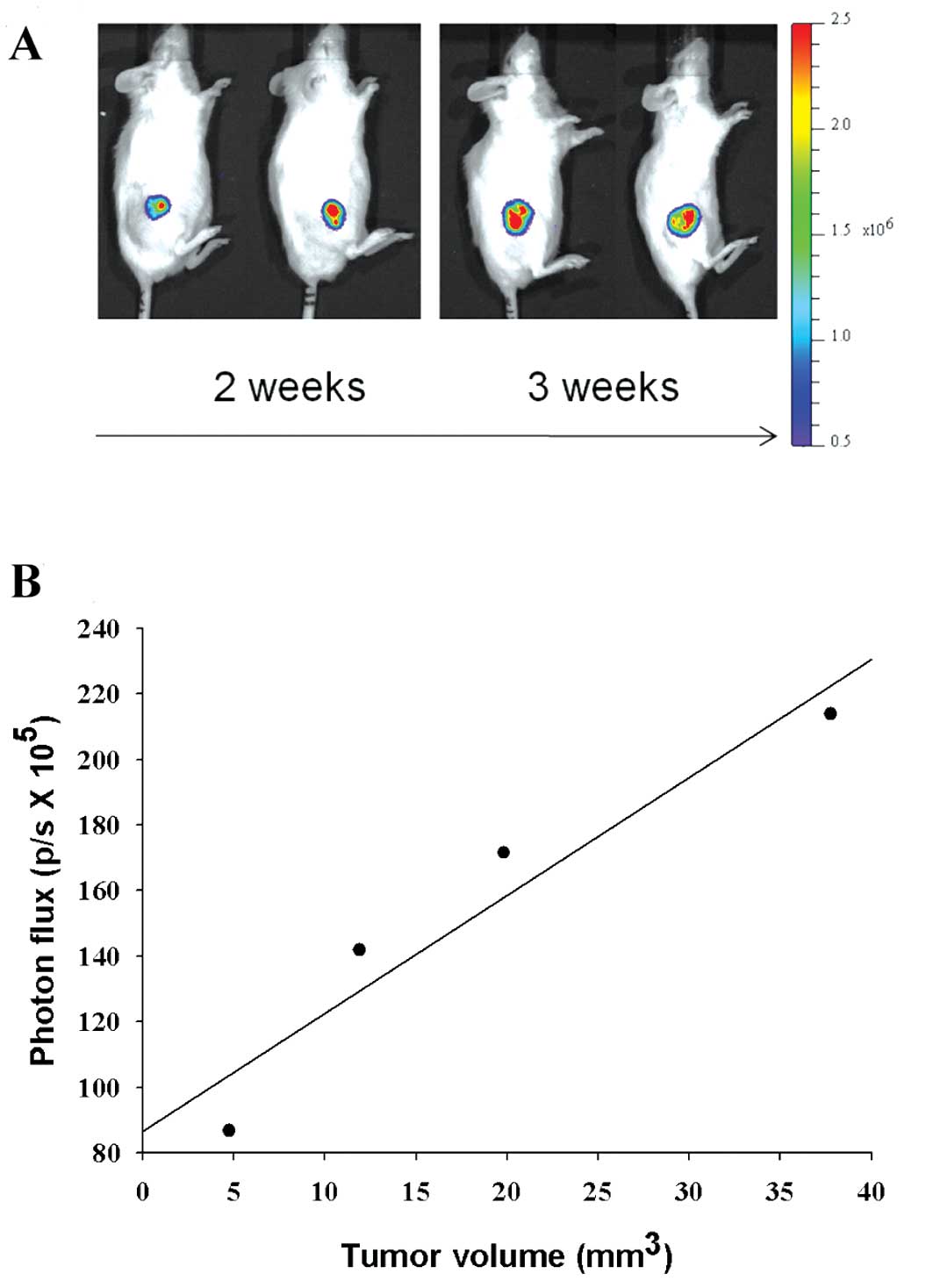
Tumor growth monitored using
bioluminescent imaging (BLI) in F98/tk-luc tumor-bearing mice
F98/tk-luc cells were inoculated
subcutaneously into the right flank of NOD/SCID mice. Two weeks
later, tumor growth was monitored with BLI as shown in Fig. 3A. The quantification of
bioluminescence (photons/sec × 105) vs. tumor volume
(mm3 × 10) from representative mice is plotted in
Fig. 3B with linear regression
(r2=0.90).
Bioluminescent imaging (BLI), MR imaging
and histopathology
The BLI of F98/tk-luc glioma-bearing rats was
obtained using the IVIS50 optical imaging system (Xenogen Corp.) on
day 13 post inoculation of F98-tk-luc glioma cells into the
left brain. The total photon flux rates of the tumors were
5.50×105-4.14×106 as shown in Fig. 4A (images were from representative
rats). MR images of the representative rat brains were obtained
from a 3T MR scanner including T1-, T2-weighted and
contrast-enhanced images (Gd 0.2 mmol/kg) on day 14 (Fig. 4B).
In the present study, the tumor volume and its
extent were not able to be demonstrated well by T1-weighted images
but were greatly enhanced post contrast injection, which showed a
clear boundary between the tumor and normal brain tissue. On the
other hand, the tumor location could be shown by T2-weighted images
whereas the boundary between the tumor and normal tissue was still
indistinguishable. Fischer 344 rats after MR scanning were
sacrificed, and the brain tissues were removed and preserved in 4%
paraformaldehyde. H&E staining was subsequently performed. The
results are shown in Fig. 4C, in
which the tumor location and tumor volume were the same as those of
the MR images shown in Fig. 4B.
The total photon fluxes of F98-tk-luc tumors
obtained from BLI vs. tumor volumes calculated by 3T MR
contrast-enhanced images are tabulated in Table I and plotted in Fig. 4D. The Pearson correlation
coefficient (r) is 0.95.
 | Table IThe tumor volumes assayed by 3T MR
and photon flux rates of regions-of-interest (ROI) obtained from
BLI of the rat brains were compared. |
Table I
The tumor volumes assayed by 3T MR
and photon flux rates of regions-of-interest (ROI) obtained from
BLI of the rat brains were compared.
| Rat ID | Tumor volume
(mm3) assayed by MR | Total photon flux
rate (photons/sec, × 105) obtained from BLI |
|---|
| 1 | 1.93 | 4.38 |
| 2 | 5.07 | 4.56 |
| 3 | 8.62 | 9.78 |
| 4 | 8.94 | 5.51 |
| 5 | 52.77 | 41.40 |
| 6 | 126.90 | 41.00 |
| 7 | 213.0 | 58.90 |
Biodistribution of 18F-FBAU in
the F98/tk-luc tumor-bearing rats
The accumulation of [18F]FBAU in tissues
of the F98/tk-luc tumor-bearing Fischer 344 rats (n=5) was
measured at different time points after administration of 15
MBq/0.2 ml. The highest uptake of [18F]FBAU was found in
kidneys, suggesting that the elimination route of
[18F]FBAU is via renal clearance. The uptake of
[18F]FBAU in the left and right normal brain tissues was
very low in the range of 0.03–0.09 %ID/g (Fig. 5A and Table II). Notably, the uptake of
[18F]FBAU in the F98/tk-luc glioma tumors reached
up to 0.51±0.06 at 90 min post injection (Fig. 5B). The uptake of
[18F]FBAU in the tumors was significantly higher than
those of normal brain tissues (P<0.01). In addition, the uptake
of [18F]FBAU in tumors in the 60 and 90 min
post-injection groups was significantly higher than that of the 30
min post-injection group (P<0.05).
 | Figure 5Biodistribution of
[18F]FBAU in F98/tk-luc tumor-bearing rats (n=5).
(A) Specific radioactivities (%ID/g) in the left and right brain,
intestine, spleen, heart, kidney, lung, liver, stomach, pancreas,
bone, muscle, blood and tumor at various time points are shown. (B)
The ratios calculated from %ID/g of [18F]FBAU in
F98/tk-luc glioma-to-normal right brain at 30, 60, 90 and
120 min post injection of [18F]FBAU were 9.16, 14.24,
5.70 and 13.70, respectively. The %ID/g of [18F]FBAU in
the F98/tk-luc glioma was significantly higher than that of
the right brain (**P<0.005). In addition, %ID/g
values for the 60 and 90 min groups were also significantly higher
than that of the 30 min post injection group
(#P<0.05). |
 | Table IIBiodistribution of
[18F]FBAU in various tissues/organs of the
F98/tk-luc glioma-bearing Fischer 344 rats after intravenous
injection (n=5; mean ± SE). |
Table II
Biodistribution of
[18F]FBAU in various tissues/organs of the
F98/tk-luc glioma-bearing Fischer 344 rats after intravenous
injection (n=5; mean ± SE).
| Uptake (%ID/g) |
|---|
|
|
|---|
| Tissues/organs | 30 min | 60 min | 90 min | 120 min |
|---|
| Left brain | 0.048±0.004 | 0.047±0.008 | 0.094±0.023 | 0.081±0.028 |
| Right brain | 0.030±0.001 | 0.029±0.004 | 0.090±0.019 | 0.030±0.005 |
| Tumor | 0.275±0.034 | 0.413±0.023 | 0.513±0.066 | 0.411±0.061 |
| Large
intestine | 0.212±0.051 | 0.325±0.060 | 0.826±0.282 | 0.311±0.040 |
| Small
intestine | 0.231±0.039 | 0.368±0.175 | 0.759±0.248 | 0.362±0.071 |
| Spleen | 0.315±0.025 | 0.311±0.030 | 0.650±0.158 | 0.936±0.375 |
| Heart | 0.296±0.054 | 0.252±0.044 | 0.648±0.212 | 0.176±0.059 |
| Blood | 0.296±0.032 | 0.207±0.027 | 0.699±0.217 | 0.178±0.058 |
| Lung | 0.203±0.030 | 0.146±0.012 | 0.562±0.158 | 0.172±0.066 |
| Liver | 0.248±0.031 | 0.189±0.033 | 0.476±0.240 | 0.191±0.053 |
| Stomach | 0.120±0.017 | 0.186±0.028 | 0.448±0.127 | 0.136±0.031 |
| Pancreas | 0.179±0.027 | 0.117±0.026 | 0.227±0.112 | 0.120±0.046 |
| Kidney | 2.151±0.544 | 1.822±0.480 | 4.183±1.604 | 1.868±0.550 |
| Bone | 0.170±0.026 | 0.159±0.046 | 0.657±0.160 | 0.479±0.116 |
| Muscle | 0.165±0.019 | 0.161±0.026 | 0.484±0.143 | 0.195±0.031 |
| Tumor/right
brain | 9.16 | 14.24 | 5.70 | 13.70 |
PET images of 18F-FBAU in the
F98/tk-luc tumor-bearing rat model
The coronal and sagittal PET images of the
F98/tk-luc glioma-bearing rats were imaged with a clinical
PET. The result showed that accumulation of [18F]FBAU
was noted in F98/tk-luc glioma in the brain (arrows in
Fig. 6A). The
[18F]FBAU/PET images at 30–90 min post injection showed
significantly higher %SUV values than that acquired at the initial
time point (P<0.005; Fig.
6B).
Ex vivo autoradiography
Biodistribution of [18F]FBAU in the rat
brain was also examined by ex vivo autoradiography. The
result showed that the uptake of [18F]FBAU in the
F98/tk-luc gliomas reached the maximum value at 90 min post
injection (Fig. 7A). Autoradiogram
of [18F]FBAU in the F98/tk-luc gliomas showed a
similar result as that of the biodistribution study with the
highest uptake at 90 min post injection. The quantification of the
autoradiogram is depicted in Fig.
7B. The uptake of [18F]FBAU assayed by BLI in units
of photo-stimulated luminescence (PSL)/mm2 in the
F98/tk-luc gliomas was significantly higher at each time
point from 30 to 120 min compared to that of the contralateral
normal brain tissue (Fig. 7B).
Discussion
[124I]FIAU has been reported to be the
first PET probe used in the HSV1-tk reporter gene expression
system (11,30). Although FIAU is preferentially
phosphorylated by HSV1-thymidine kinase (TK) compared to mammalian
TK, a certain amount of FIAU is accumulated in cells which do not
express HSV1-TK resulting in non-specific signals (31). It has been reported that no
difference in biodistribution exists between [14C]FIAU
and [76Br]FBAU; the uptake of the latter, however, is
constantly higher than that of [14C]FIAU in RG2-TK rat
glioma cells. Furthermore, the uptake of [76Br]FBAU is
not affected significantly by endogenous mammalian thymidine kinase
(14).
2′-Deoxy-2′-[18F]fluoro-5-ethyl-1-β-D-arabinofuranosyluracil
([18F]FEAU), another pyrimidine nucleoside analog, has
been shown to have higher selectivity and affinity for
HSV-tk in subcutaneous RG2-TK+ tumors and
C6-tk cells compared to other radiotracers (32,33),
but thorough evaluation by other modalities is lacking. In the
present study, we demonstrated the tumor-specific radiotracing
activity of [18F]FBAU in an F98/tk-luc
glioma-bearing animal model using HSV1-tk reporter
gene/[18F]FBAU reporter probe system combined with
multimodalities of molecular imaging with PET, BLI, MRI and ex
vivo autoradiography. [18F]FBAU showed better
differentiation with higher uptake in the tumors compared to the
contralateral normal brain in the F98/tk-luc glioma-bearing
animal model between 30 to 120 min post injection (Figs. 5B and 6B). Both PET imaging and ex vivo
autoradiography also showed the persistent accumulation of
[18F]FBAU in the F98/tk-luc glioma-bearing animal
model from 90 to 120 min post injection (Figs. 6A and 7B), which was ~5–14 times higher than the
other radiotracers, such as [76Br]FBAU, at 120 min post
injection as reported in other studies (14). The results suggest that
[18F]FBAU is a suitable reporter probe for the imaging
of HSV1-tk expression.
For the purpose of tracing tumor growth
progression, molecular imaging techniques other than traditional
caliper measurement have been developed. A statistical correlation
was developed to correlate the relationship between anatomical size
and bioluminescent intensity collected from ROI of the tumors in a
brain-tumor bearing animal model (23). Notably, the brain tumor volume
assayed by bioluminescent imaging (BLI) has been reported to poorly
correlate with images obtained from MRI (34,35).
Nevertheless, metastatic breast cancer in a rat model monitored
using MRI at 3 tesla was found to correlate well with that by BLI
(36). Although
[18F]FDG/PET imaging has been used for diagnosis of
tumors as well as the malfunction of the brain, the uptake of
[18F]FDG remains high in normal brain tissues, causing
difficulties in differentiating normal brain from malignancy
(20,37). In order to monitor the progression
of brain tumor growth in the glioma-bearing animal model, we found
a good correlation for the tumor volume acquired from MRI vs. that
obtained from BLI in the F98/tk-luc glioma-bearing rat model
(Fig. 4). The SUV of
[18F]FBAU in F98/tk-luc glioma was 20% higher
than that of contralateral normal brain tissue (Fig. 6). Hence [18F]FBAU may
provide another advantage in brain tumor diagnosis and disease
monitoring. As a result, this approach may have potential for using
[18F]FBAU as a molecular probe combined with a reporter
gene system, such as HSV1-tk, to monitor tumor growth
progression in preclinical or clinical applications.
In conclusion, the uptake of [18F]FBAU
and SUV of [18F]FBAU in brain tumors of an
F98/tk-luc glioma-bearing rat model was significantly higher
than that of normal brain tissues determined by biodistribution and
PET, respectively. Both the volume and progression of tumors
determined with MRI and BLI were well correlated in the
F98/tk-luc glioma-bearing rat model using
[18F]FBAU as a reporter probe. Radiolabeled FBAU
combined with multimodalities of molecular imaging may also provide
potential benefits for the evaluation of therapeutic efficacy of
newly developed drugs against other brain diseases.
Acknowledgements
The present study was supported by grant
NSC92-2745-P-010-002 from the National Science Council, Taipei,
Taiwan. We thank the staff of the Department of Radiopharmaceutical
Production, Buddhist Tzu Chi General Hospital, Hualien, Taiwan for
providing PET/CT imaging and advice for the synthesis of
[18F]FBAU. We also thank the Taiwan Mouse Clinic which
is funded by the National Research Program for Biopharmaceuticals
(NRPB) at the National Science Council (NSC) of Taiwan for
technical support with the bioluminescent imaging experiment.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sathornsumetee S and Rich JN: Designer
therapies for glioblastoma multiforme. Ann NY Acad Sci.
1142:108–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar
|
|
4
|
Huang FY, Lee TW, Kao CH, et al: Imaging,
autoradiography, and biodistribution of 188Re-labeled
PEGylated nanoliposome in orthotopic glioma bearing rat model.
Cancer Biother Radiopharm. 26:717–725. 2011.PubMed/NCBI
|
|
5
|
Miyata S, Kawabata S, Hiramatsu R, et al:
Computed tomography imaging of transferrin targeting liposomes
encapsulating both boron and iodine contrast agents by
convection-enhanced delivery to F98 rat glioma for boron neutron
capture therapy. Neurosurgery. 68:1380–1387. 2011.
|
|
6
|
Wolburg H, Wolburg-Buchholz K, Kraus J, et
al: Localization of claudin-3 in tight junctions of the blood-brain
barrier is selectively lost during experimental autoimmune
encephalomyelitis and human glioblastoma multiforme. Acta
Neuropathol. 105:586–592. 2003.
|
|
7
|
Valk PE, Townsend DW and Maisey MN:
Positron Emission Tomography: Basic Science and Clinical Practice.
Springer-Verlag Publishing; New York, NY: 2003
|
|
8
|
Strauss LG: Fluorine-18 deoxyglucose and
false-positive results: a major problem in the diagnostics of
oncological patients. Eur J Nucl Med. 23:1409–1415. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong XB, Zhu QY, Vidal PM, et al:
Comparisons of anti-human immunodeficiency virus activities,
cellular transport, and plasma and intracellular pharmacokinetics
of 3′-fluoro-3′-deoxythymidine and 3′-azido-3′-deoxythymidine.
Antimicrob Agents Chemother. 36:808–818. 1992.PubMed/NCBI
|
|
10
|
Jensen MM, Erichsen KD, Johnbeck CB, et
al: [18F]FLT and [18F]FDG PET for
non-invasive treatment monitoring of the nicotinamide
phosphoribosyltransferase inhibitor APO866 in human xenografts.
PLoS One. 8:e534102013.
|
|
11
|
Tjuvajev JG, Stockhammer G, Desai R, et
al: Imaging the expression of transfected genes in vivo.
Cancer Res. 55:6126–6132. 1995.PubMed/NCBI
|
|
12
|
Tjuvajev JG, Finn R, Watanabe K, et al:
Noninvasive imaging of herpes virus thymidine kinase gene transfer
and expression: a potential method for monitoring clinical gene
therapy. Cancer Res. 56:4087–4095. 1996.PubMed/NCBI
|
|
13
|
Borbath I, Gregoire V, Bergstrom M, Laryea
D, Langstrom B and Pauwels S: Use of
5-[76Br]bromo-2′-fluoro-2′-deoxyuridine as a ligand for
tumour proliferation: validation in an animal tumour model. Eur J
Nucl Med Mol Imaging. 29:19–27. 2002.
|
|
14
|
Cho SY, Ravasi L, Szajek LP, et al:
Evaluation of 76Br-FBAU as a PET reporter probe for
HSV1-tk gene expression imaging using mouse models of human glioma.
J Nucl Med. 46:1923–1930. 2005.
|
|
15
|
Kilbourn MR, Dence CS, Welch MJ and
Mathias CJ: Fluorine-18 labeling of proteins. J Nucl Med.
28:462–470. 1987.PubMed/NCBI
|
|
16
|
Gambhir SS, Barrio JR, Herschman HR and
Phelps ME: Assays for noninvasive imaging of reporter gene
expression. Nucl Med Biol. 26:481–490. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobs AH, Li H, Winkeler A, et al:
PET-based molecular imaging in neuroscience. Eur J Nucl Med Mol
Imaging. 30:1051–1065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan MH, Huang SC, Liao YP, et al: FLT-PET
imaging of radiation responses in murine tumors. Mol Imaging Biol.
10:325–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HE, Liao AH, Deng WP, et al:
Evaluation of
4-borono-2-18F-fluoro-L-phenylalanine-fructose as a
probe for boron neutron capture therapy in a glioma-bearing rat
model. J Nucl Med. 45:302–308. 2004.
|
|
20
|
Wang HE, Wu SY, Chang CW, et al:
Evaluation of F-18-labeled amino acid derivatives and
[18F]FDG as PET probes in a brain tumor-bearing animal
model. Nucl Med Biol. 32:367–375. 2005.
|
|
21
|
Massoud TF and Gambhir SS: Molecular
imaging in living subjects: seeing fundamental biological processes
in a new light. Genes Dev. 17:545–580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De A, Lewis XZ and Gambhir SS: Noninvasive
imaging of lentiviral-mediated reporter gene expression in living
mice. Mol Ther. 7:681–691. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bryant MJ, Chuah TL, Luff J, Lavin MF and
Walker DG: A novel rat model for glioblastoma multiforme using a
bioluminescent F98 cell line. J Clin Neurosci. 15:545–551. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang YL, Wang HE, Liu RS, Pang F and
Hwang JJ: Monitoring of tumor growth and metastasis potential in
MDA-MB-435s/tk-luc human breast cancer xenografts. Nucl Instrum
Meth A. 571:155–159. 2007. View Article : Google Scholar
|
|
25
|
Deng WP, Yang WK, Lai WF, et al:
Non-invasive in vivo imaging with radiolabelled FIAU for monitoring
cancer gene therapy using herpes simplex virus type 1 thymidine
kinase and ganciclovir. Eur J Nucl Med Mol Imaging. 31:99–109.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alauddin MM, Shahinian A, Park R, Tohme M,
Fissekis JD and Conti PS: A general synthesis of
2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-D-arabinofuranosyluracil
and its 5-substituted nucleosides. J Labelled Compds Radiopharm.
46:285–289. 2003.
|
|
27
|
Kao CH, Xie HL, Liao CH, Chen WM and Kao
PF: [18F]FBAU 3′,5′-dibenzoate, a lipophilic prodrug,
enhances brain uptake of the cell proliferation tracer
[18F]FBAU. Nucl Med Biol. 35:635–643. 2008.
|
|
28
|
Engelhorn T, Eyupoglu IY, Schwarz MA, et
al: In vivo micro-CT imaging of rat brain glioma: a comparison with
3T MRI and histology. Neurosci Lett. 458:28–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin C, Itti E, Haioun C, et al: Early
18F-FDG PET for prediction of prognosis in patients with
diffuse large B-cell lymphoma: SUV-based assessment versus visual
analysis. J Nucl Med. 48:1626–1632. 2007.
|
|
30
|
Tjuvajev JG, Avril N, Oku T, et al:
Imaging herpes virus thymidine kinase gene transfer and expression
by positron emission tomography. Cancer Res. 58:4333–4341.
1998.PubMed/NCBI
|
|
31
|
Fu DX, Foss CA, Nimmagadda S, Ambinder RF
and Pomper MG: Imaging virus-associated cancer. Curr Pharm Des.
14:3048–3065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyagawa T, Gogiberidze G, Serganova I, et
al: Imaging of HSV-tk reporter gene expression: comparison
between [18F]FEAU, [18F]FFEAU, and other
imaging probes. J Nucl Med. 49:637–648. 2008.
|
|
33
|
Buursma AR, Rutgers V, Hospers GA, Mulder
NH, Vaalburg W and de Vries EF: 18F-FEAU as a
radiotracer for herpes simplex virus thymidine kinase gene
expression: in-vitro comparison with other PET tracers. Nucl Med
Commun. 27:25–30. 2006. View Article : Google Scholar
|
|
34
|
Rehemtulla A, Stegman LD, Cardozo SJ, et
al: Rapid and quantitative assessment of cancer treatment response
using in vivo bioluminescence imaging. Neoplasia. 2:491–495. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jost SC, Collins L, Travers S,
Piwnica-Worms D and Garbow JR: Measuring brain tumor growth:
combined bioluminescence imaging-magnetic resonance imaging
strategy. Mol Imaging. 8:245–253. 2009.PubMed/NCBI
|
|
36
|
Song HT, Jordan EK, Lewis BK, et al: Rat
model of metastatic breast cancer monitored by MRI at 3 tesla and
bioluminescence imaging with histological correlation. J Transl
Med. 7:882009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Spaeth N, Wyss MT, Pahnke J, et al: Uptake
of 18F-fluorocholine,
18F-fluoro-ethyl-L-tyrosine and
18F-fluoro-2-deoxyglucose in F98 gliomas in the rat. Eur
J Nucl Med Mol Imaging. 33:673–682. 2006.
|