Introduction
Angiogenesis, the formation and recruitment of new
blood vessels, is known to play important roles in cancer growth
and progression, and is regulated by the production of several
angiogenic and anti-angiogenic factors (1,2). Among
angiogenic factors, vascular endothelial growth factor-A (VEGF-A)
and VEGF receptor-2 (VEGFR-2) signaling pathways stimulate the
secretion and activation of matrix metalloproteinases (MMPs) in
endothelial cells which result in cell proliferation, migration and
survival, and are widely appreciated as the therapeutic targets for
a variety of angiogenesis-related diseases. The central role of the
VEGF-A/VEGFR-2 in this process is evidenced by the development and
approval of pegaptanib and ranibizumab for age-related macular
degeneration as well as bevacizumab and sunitinib for cancer
treatment (3–5). However, current approved
anti-angiogenic drugs frequently lead to drug resistance and
recurrence of cancer growth and progression. Thus, further
understanding of the molecular mechanisms and targets of angiogenic
responses may help to develop potential therapeutic strategies for
the treatment of cancer as well as angiogenesis-related
diseases.
MMPs belong to a family of zinc-dependent
endopeptidases and are responsible for tissue remodeling by
selective proteolytic degradation, resulting in the promotion of
cancer growth and progression through alterations of cell adhesion,
migration, epithelial to mesenchymal transition, tumor angiogenesis
and release of growth factors (6–8). These
MMPs are regulated by their endogenous inhibitors, tissue
inhibitors of metalloproteinases (TIMPs) (9,10). In
addition to MMP-inhibitory activity, TIMPs have been reported to
regulate cell growth, migration and differentiation through the
MMP-independent mechanism (11–14).
Identification of molecular mechanisms and targets in regulating
expression and activities of MMPs and TIMPs within the tumor
microenvironment has become an attractive strategy for therapeutic
intervention in cancer growth and progression.
Broussonetia kazinoki (BK) Siebold (Moraceae)
has been used as a traditional medicine for the amelioration of
vision, inflammatory and infectious diseases as well as a material
for production of paper in Northeastern Asia. Previous
investigations have demonstrated that the extracts and bioactive
phytochemicals of BK have anti-diabetic, anti-hyperglycemic,
anti-inflammatory, anti-allergic and anticancer activities
(15–19). However, the effects and molecular
mechanisms of BK on angiogenesis remain unknown. In the present
study, we evaluated the effects and signaling pathways of BK on
proliferation, migration and tubular formation in human umbilical
vein endothelial cells.
Materials and methods
Cell culture conditions
Primary cultures of human umbilical vein endothelial
cells (HUVECs) were purchased from Lonza (Walkersville, MD, USA)
and used between passages 4 and 6 for all the experiments. The
cells were cultured in EGM-2® BulletKit media, according
to the manufacturer’s instructions (Lonza).
Reagents
The following pharmacological agents and antibodies
were purchased from commercial sources: vascular endothelial growth
factor-A (VEGF-A) and LY294002 (Merck Millipore, Billerica, MA,
USA); rapamycin (Sigma-Aldrich, St. Louis, MO, USA); PD98059,
anti-phospho-ERK (T202/Y204), anti-phospho-Akt (S473),
anti-phospho-p70S6K (T421/S424),
anti-phospho-p38MAPK (T180/Y182), anti-phospho-pRb
(S780) and anti-MMP-2 (Cell Signaling Technology, Beverly, MA,
USA); anti-VEGFR-2, anti-ERK, anti-Akt, anti-p70S6K,
anti-p38MAPK, anti-TIMP-2, anti-Cdk4, anti-Cdk2,
anti-cyclin D, anti-cyclin E, anti-actin antibodies and mouse and
rabbit IgG-horseradish peroxidase conjugates (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA).
Preparation of Broussonetia kazinoki (BK)
extract
The ethanol extract was prepared by mixing 100 g of
twigs of BK with 1 liter of 100% ethanol and stirring for 72 h. The
extract was then filtered through a filter paper (Advantec No. 1;
Toyo Roshi Kaisha, Ltd., Tokyo, Japan), and the filtrate was
concentrated using a rotary evaporator (Tokyo Rikakikai Co., Ltd.,
Tokyo, Japan) at 40°C under vacuum. The yield of the dried extract
was ~1.37%.
Cell viability and proliferation
assay
HUVECs, plated on 6-well plates (1×105
cells/well; BD Biosciences, Bedford, MA, USA), were serum-starved
for 14 h to synchronize cells in the G1/G0
phase of the cell cycle, and pretreated with BK (0.1–10 μg/ml) for
30 min in the presence or absence of PD98059 (25 μM), LY294002 (10
μM) or rapamycin (50 nM) as indicated, and then incubated with
VEGF-A (10 ng/ml) for 24 h. Following culture for 24 h, cell
viability was determined by a Muse™ cell analyzer using a cell
count and viability assay kit (Merck Millipore), and the cell
proliferation was quantified as previously described (20–22).
The results from triplicate determinations (mean ± standard
deviation) are presented as the number of cells per culture.
Western blot analysis
HUVECs in 100-mm dishes (1×106
cells/dish; BD Biosciences) were serum-starved for 14 h in
endothelial cell basal medium (EBM-2; Lonza) and replaced with
fresh media, followed by treatments for different time-points, as
indicated in the figure legends, at 37°C. The cells were rinsed
twice with ice-cold phosphate-buffered saline (PBS) and lysed by
incubation in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10% glycerol,
1% Triton X-100, 1 mM EDTA, 100 μg/ml
4-(2-aminoethyl)benzenesulfonyl fluoride, 10 μg/ml aprotinin, 1
μg/ml pepstatin A, 0.5 μg/ml leupeptin, 80 mM β-glycerophosphate,
25 mM sodium fluoride and 1 mM sodium orthovanadate for 30 min at
4°C. Cell lysates were clarified at 12,500 × g for 20 min at 4°C,
and the supernatants were subjected to western blot analysis as
previously described (23,24). All the western blots were performed
in triplicate and representative gels were shown.
Migration assay
Cell migration was quantified in the in vitro
wound-healing assay as previously described (25,26).
Cells were plated on 48-well plates (4×104 cells/well),
grown to confluence, and a single wound was created in the center
of the cell monolayer by the gentle removal of the attached cells
with a sterile plastic pipette tip. Following serum starvation with
EBM-2 for 2 h, the cells were pretreated with BK (0.1–10 μg/ml) for
30 min, followed by VEGF-A (10 ng/ml) stimulation for 16 h. The
cells were fixed with methanol, and then stained with 0.04% Giemsa
solution (Sigma-Aldrich). The migration of the cells into the wound
was observed using images captured at the indicated
time-points.
Tube formation assays
Matrigel® basement membrane matrix (10.4
mg/ml; BD Biosciences) was thawed overnight at 4°C, and each well
of pre-chilled 24-well plates was coated with 200 μl
Matrigel® and then incubated at 37°C for 30 min.
Following serum starvation with EBM-2 for 2 h, the cells
(4×104 cells/ml) were added to
Matrigel®-coated plates and pretreated with BK (0.1–10
μg/ml) for 30 min, followed by VEGF-A (10 ng/ml) for 6 h. Tube
formation was observed with an Olympus CKX41 inverted microscope
(CAchN 10/0.25php objective) and ToupTek Toupview software (version
x86, 3.5.563; Hangzhou ToupTek Photonics Co., Ltd., Zhejiang,
China).
Zymogram analysis
Activities of MMPs were measured by zymography
(27,28). Aliquots of conditioned medium
collected from HUVECs treated with BK (10 μg/ml) and VEGF-A (10
ng/ml) for 16 h were diluted in sample buffer, and applied to 8%
polyacrylamide gels containing 1 mg/ml gelatin (Sigma-Aldrich) as a
substrate. After electrophoresis, the gels were incubated in 2.5%
Triton X-100 for 1 h to remove SDS and allow the re-naturalization
of MMPs, and then incubated in developing buffer containing 50 mM
Tris-HCl (pH 7.5), 10 mM CaCl2, and 150 mM NaCl for 16 h
at 37°C. The gels were stained with 0.5% Coomassie brilliant blue
R-250 in 30% methanol-10% acetic acid for 3 h and followed by
destaining with 30% methanol-10% acetic acid. Gelatinolytic
activities were detected as unstained bands against the background
of the Coomassie blue-stained gelatin.
Statistical analysis
Statistical analysis was performed using the
Student’s t-test, and was based on at least three different
experiments. The results were considered to be statistically
significant when P<0.05.
Results
BK suppresses VEGF-A-stimulated
endothelial cell proliferation by modulating the expression of cell
cycle-related proteins
We first examined the ability of BK to regulate
proliferation in HUVECs. BK treatment suppressed VEGF-A-stimulated
cell proliferation in a dose-dependent manner (Fig. 1A) and did not alter cell viability
(Fig. 1B), suggesting that BK
inhibition of cell proliferation is not mediated by the induction
of apoptosis or cytotoxicity. Based on these findings, we examined
the regulatory effect of BK on cell cycle progression by analyzing
the changes of cyclin-dependent kinases (Cdks) and cyclins. Cell
cycle progression requires activation of Cdks through formation
with cyclins and subsequent phosphorylation of retinoblastoma
protein (pRb) (29). As shown in
Fig. 1C, BK treatment markedly
suppressed the expression of Cdks and cyclin D, resulting in pRb
hypophosphorylation in VEGF-A-treated HUVECs. These findings
indicate that BK downregulates the expression of cell cycle-related
proteins, resulting in inhibition of cell cycle progression and
proliferation.
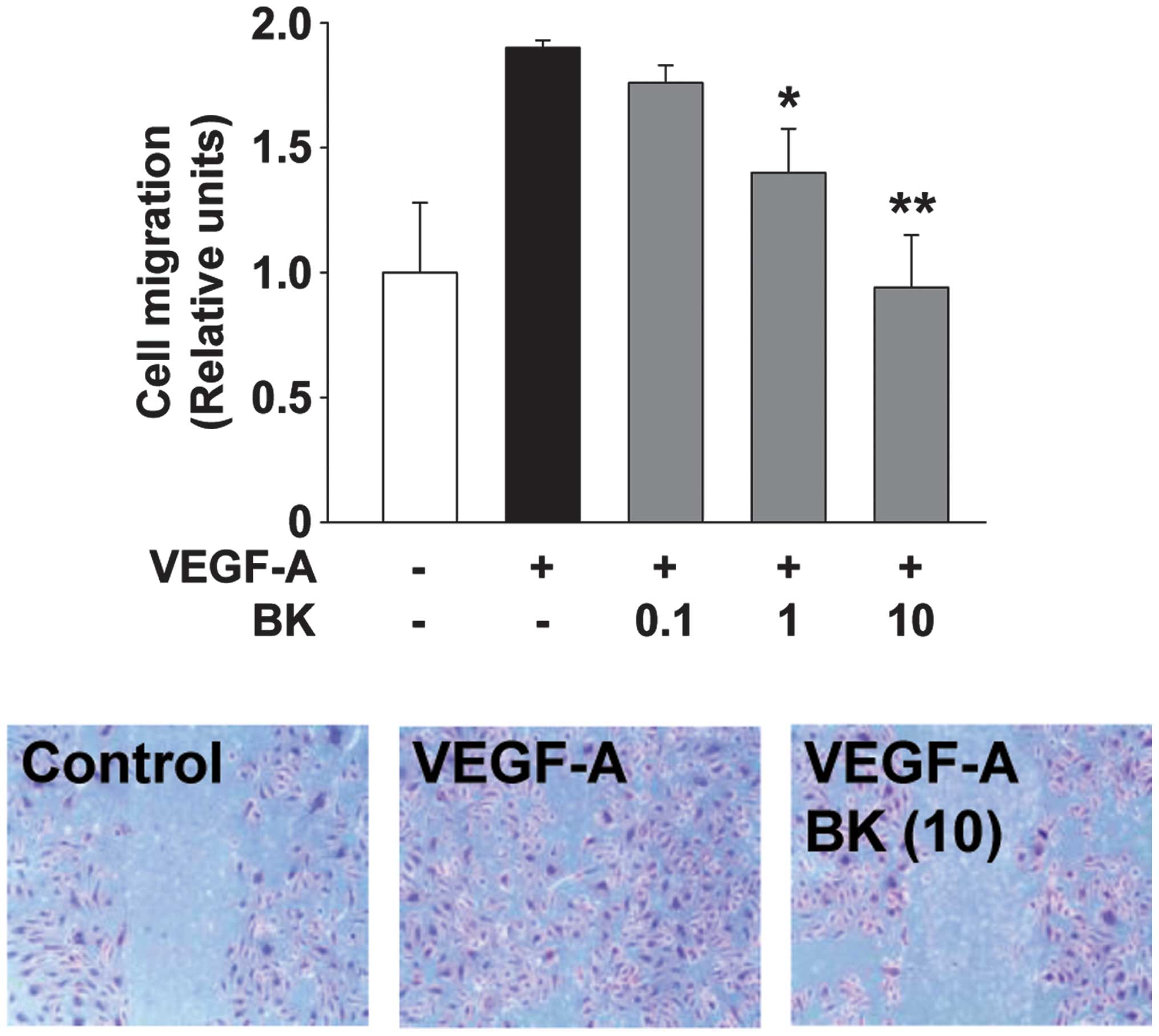
BK inhibits VEGF-A-stimulated endothelial
cell migration and capillary structure formation
Endothelial cell migration and tubular formation are
coordinately controlled by the interactions from ECM molecules to
the intracellular and intercellular components of cells, and play
important roles in angiogenic responses associated with cancer
growth and progression (2). BK
treatment dose-dependently inhibited VEGF-A-stimulated cell
migration and tubular formation in HUVECs (Figs. 2 and 3). Collectively, these findings suggested
that the bioactive components from the ethanolic extracts of BK act
on multiple targets and mechanisms involved in cell proliferation,
migration and tubular formation, resulting in the regulation of
angiogenic responses in vitro.
BK suppresses VEGFR-2 and MMP-2
expression in VEGF-A-treated HUVECs
The expression and activation of VEGFR-2 and MMP-2
have been reported to promote angiogenic responses including
endothelial cell proliferation, migration, invasion and tubular
formation (1,2,30). To
understand the molecular mechanism underlying the regulatory
effects of BK on endothelial cell proliferation, migration and
tubular formation, we analyzed the changes in the expression of
VEGFR-2, MMP-2 and TIMP-2, an endogenous inhibitor of MMPs, in
VEGF-A-treated HUVECs. BK treatment markedly suppressed the
VEGF-A-induced expression of VEGFR-2 and MMP-2 (Fig. 4A). As shown in Fig. 4B, BK treatment also inhibited
VEGF-A-induced activation of MMP-2 in conditioned medium of HUVECs.
By contrast, the levels of TIMP-2 in HUVECs were not altered by
VEGF-A or BK treatment (Fig. 4A).
Taken together, these findings indicates that the inhibitory
effects of BK on endothelial cell proliferation, migration and
tubular formation may be mediated at least in part through the
suppression of VEGFR-2 and MMP-2 expression.
In vitro anti-angiogenic activities of BK
are mediated through the inhibition of mitogenic signaling pathways
and downregulation of VEGFR-2 and MMP-2
To investigate the molecular mechanisms by which BK
regulates endothelial cell responses, we examined the changes in
the activation of signaling pathways including extracellular
signal-regulated kinase (ERK), p38 mitogen-activated protein kinase
(p38MAPK), phosphatidylinositol 3-kinase (PI3-K)/Akt and
mammalian target of rapamycin (mTOR)/p70S6K, which play
pivotal roles in cell fate (31).
As shown in Fig. 5A, VEGF-A
stimulation markedly increased the phosphorylation/activation of
ERK, Akt and p70S6K, but not that of p38MAPK,
when compared with unstimulated controls. By contrast, BK treatment
significantly inhibited the VEGF-A-stimulated phosphorylation of
ERK, Akt, and p70S6K in HUVECs. Pretreatment of cells
with PD98059 (an inhibitor of the ERK pathway), LY294002 (an
inhibitor of the PI3-K/Akt pathway) or rapamycin (an inhibitor of
the mTOR/p70S6K pathway) suppressed the expression of
VEGFR-2 and MMP-2 in response to VEGF-A stimulation, suggesting
that BK may contain pharmacologically effective components similar
to these inhibitors, and share the roles and mechanisms of action
in regulating endothelial cell proliferation, migration and tubular
formation (Fig. 5B). Taken
together, these findings demonstrated that the suppression of
endothelial cell proliferation, migration and tubular formation by
BK ethanol extracts might be mediated through the inactivation of
VEGFR-2 downstream signaling pathways and subsequent downregulation
of VEGFR-2 and MMP-2.
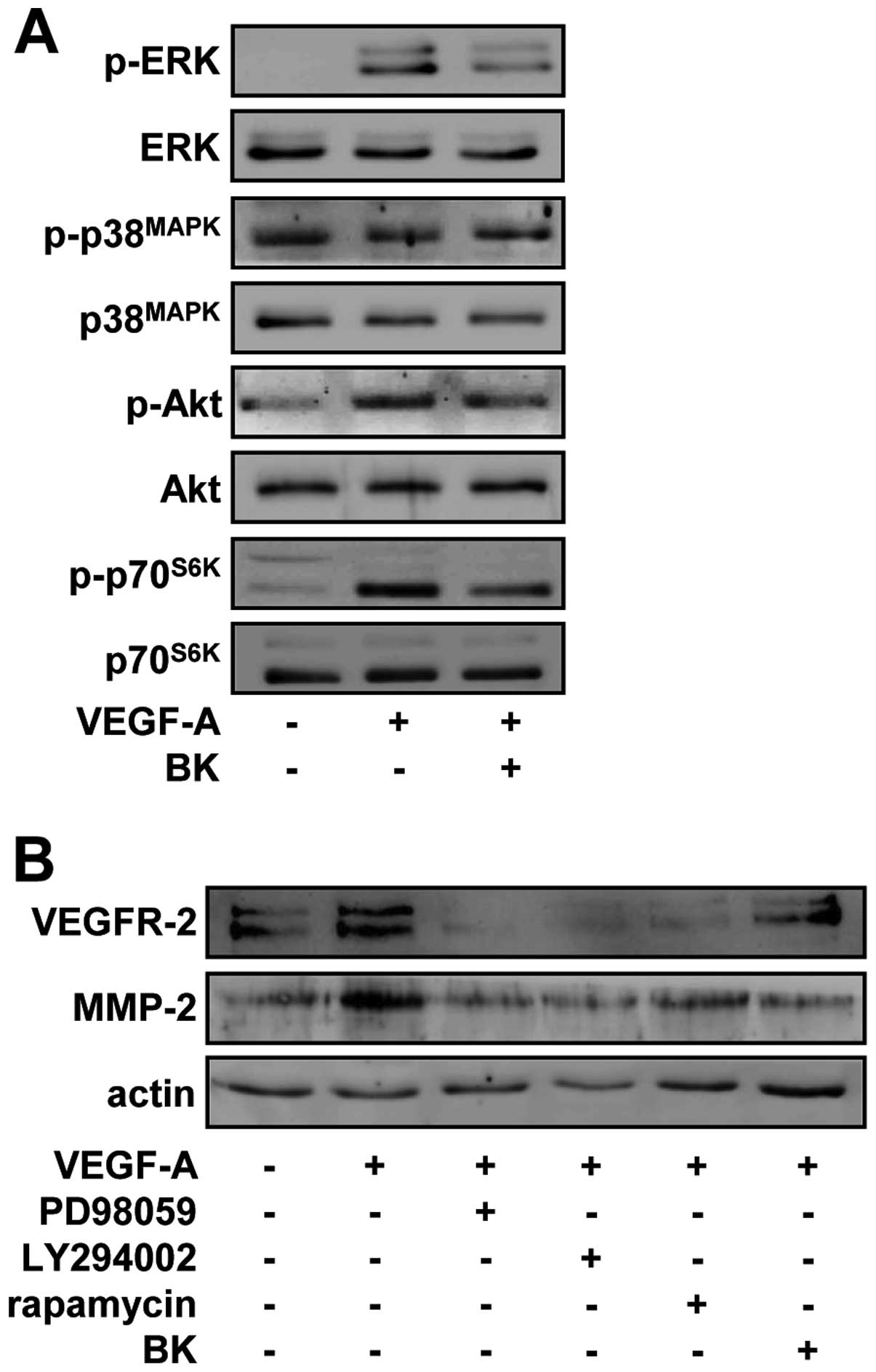 | Figure 5BK suppresses VEGFR-2 and MMP-2
expression through the inhibition of ERK, Akt and p70S6K
activity in VEGF-A-treated HUVECs. (A) Quiescent cells were
pretreated with BK (10 μg/ml) for 30 min, followed by VEGF-A (10
ng/ml) stimulation for 15 min. Western blotting was performed on
cell lysates using anti-phospho-ERK, anti-ERK,
anti-phospho-p38MAPK, anti-p38MAPK,
anti-phospho-Akt, anti-Akt, anti-phsopho-p70S6K or
anti-p70S6K antibodies. (B) Cells were pretreated with
PD98059 (25 μM), LY294002 (10 μM), rapamycin (50 nM) or BK (10
μg/ml) for 30 min, and then stimulated with VEGF-A (10 ng/ml) for
24 h. Western blotting was performed on cell lysates using
anti-VEGFR-2, anti-MMP-2 or anti-actin antibodies. Results shown
are representative of three independent experiments. |
Discussion
Broussonetia kazinoki (BK) has been used as a
traditional medicine for improving vision, as well as in
inflammatory and infectious diseases. These applications are well
supported by previous investigations that BK possesses bioactive
phytochemicals such as isoprenylated and prenylated flavans to
reduce inflammation (15,17). In addition to anti-inflammatory
activity, BK has been reported to have cytotoxicity against several
different cell lines including liver, cervical, oral, colon, lung
and gastric cancer (18,19,32).
However, no effects and molecular mechanisms of BK on angiogenesis
have been reported thus far.
VEGF-A/VEGFR-2 signaling pathways play pivotal roles
in the formation and stability of blood vessels associated with
cancer growth and progression (1,3,14,30,33).
These angiogenic responses induced by VEGF-A/VEGFR-2 signaling
pathways include the secretion and activation of MMPs, resulting in
the degradation of the extracellular matrix and remodeling of the
tumor microenvironment. Thus, selective inhibition of
VEGF-A/VEGFR-2 activation or downstream signaling pathways is
appreciated as a potent strategy, compared to conventional
chemotherapy.
To the best of our knowledge, in the present study,
we have shown for the first time that the ethanol extract of BK
inhibits VEGF-A-stimulated proliferation, migration and tubular
formation in HUVECs. These anti-angiogenic activities of BK were
found to be mediated through the inhibition of VEGF-A-stimulated
phosphorylation/activation of ERK, Akt and p70S6K, the
downstream targets in VEGFR-2 signaling pathways, and
downregulation of VEGFR-2 and MMP-2 as evidenced by using
pharmacological inhibitors such as PD98059, LY294002 and
rapamycin.
In conclusion, our findings provide important
insights into the roles and pharmacological efficacy of BK in
regulation of angiogenesis. However, further investigation on the
development of BK for the treatment of a variety of diseases
associated with angiogenesis should be conducted.
Acknowledgements
The present study was carried out with the support
of ‘Forest Science and Technology Projects’ (Project no.
S121313L070100) provided by Korea Forest Service.
References
|
1
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Prac Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng EWM, Shima DT, Calias P, Cunningham ET,
Guyer DR and Adamis AP: Pegaptanib, a targeted anti-VEGF aptamer
for ocular vascular disease. Nat Rev Drug Discov. 5:123–132. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown DM and Regillo CD: Anti-VEGF agents
in the treatment of neovascular age-related macular degeneration:
applying clinical trial results to the treatment of everyday
patients. Am J Ophthalmol. 144:627–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: a moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): an ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: metalloproteinase-independent
biological activities. Sci Signal. 1:re62008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo DW, Li H, Guedez L, et al: TIMP-2
mediated inhibition of angiogenesis: an MMP-independent mechanism.
Cell. 114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi JH, Ebrahem Q, Moore N, et al: A novel
function for tissue inhibitor of metalloproteinases-3 (TIMP3):
inhibition of angiogenesis by blockage of VEGF binding to VEGF
receptor-2. Nat Med. 9:407–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung KK, Liu XW, Chirco R, Fridman R and
Kim HRC: Identification of CD63 as a tissue inhibitor of
metalloproteinase-1 interacting cell surface protein. EMBO J.
25:3934–3942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SH, Cho YR, Kim HJ, et al: Antagonism
of VEGF-A-induced increase in vascular permeability by an integrin
α3β1-Shp-1-cAMP/PKA pathway. Blood. 120:4892–4902. 2012.PubMed/NCBI
|
|
15
|
Ryu JH, Ahn H and Jin Lee H: Inhibition of
nitric oxide production on LPS-activated macrophages by kazinol B
from Broussonetia kazinoki. Fitoterapia. 74:350–354. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha JY, Kim YT, Kim HS and Cho YS:
Antihyperglycemic effect of stem bark powder from paper mulberry
(Broussonetia kazinoki Sieb.) in type 2 diabetic Otsuka
Long-Evans Tokushima fatty rats. J Med Food. 11:499–505. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JK, Ha H, Lee HY, et al: Inhibitory
effects of heartwood extracts of Broussonetia kazinoki Sieb
on the development of atopic dermatitis in NC/Nga mice. Biosci
Biotechnol Biochem. 74:1802–1806. 2010.PubMed/NCBI
|
|
18
|
Ko HH, Yen MH, Wu RR, Won SJ and Lin CN:
Cytotoxic isoprenylated flavans of Broussonetia kazinoki. J
Nat Prod. 62:164–166. 1998. View Article : Google Scholar
|
|
19
|
Zhang PC, Wang S, Wu Y, Chen RY and Yu DQ:
Five new diprenylated flavonols from the leaves of Broussonetia
kazinoki. J Nat Prod. 64:1206–1209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ, Cho YR, Kim SH and Seo DW:
TIMP-2-derived 18-mer peptide inhibits endothelial cell
proliferation and migration through cAMP/PKA-dependent mechanism.
Cancer Lett. 343:210–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo DW, Cho YR, Kim W and Eom SH:
Phytochemical linarin enriched in the flower of Chrysanthemum
indicum inhibits proliferation of A549 human alveolar basal
epithelial cells through suppression of the Akt-dependent signaling
pathway. J Med Food. 16:1086–1094. 2013.PubMed/NCBI
|
|
22
|
Cho YR, Kim JK, Kim J, Oh J and Seo DW:
Ligularia fischeri regulates lung cancer cell proliferation
and migration through down-regulation of epidermal growth factor
receptor and integrin β1 expression. Genes Genom. 35:741–746. 2013.
View Article : Google Scholar
|
|
23
|
Seo DW, Kim SH, Eom SH, et al: TIMP-2
disrupts FGF-2-induced downstream signaling pathways. Microvasc
Res. 76:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo DW, Li H, Qu CK, et al: Shp-1 mediates
the antiproliferative activity of tissue inhibitor of
metalloproteinase-2 in human microvascular endothelial cells. J
Biol Chem. 281:3711–3721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho YR, Kim S, Ko H, Kim MD, Choi S and
Seo DW: Sepiapterin inhibits cell proliferation and migration of
ovarian cancer cells via down-regulation of
p70S6K-dependent VEGFR-2 expression. Oncol Rep.
26:861–867. 2011.PubMed/NCBI
|
|
26
|
Yoon HJ, Cho YR, Joo JH and Seo DW:
Knockdown of integrin α3β1 expression induces proliferation and
migration of non-small cell lung cancer cells. Oncol Rep.
29:662–668. 2013.
|
|
27
|
Cho YR, Choi S and Seo DW: The in
vitro antitumor activity of Siegesbeckia glabrescens
against ovarian cancer through suppression of receptor tyrosine
kinase expression and the signaling pathways. Oncol Rep.
30:221–226. 2013.
|
|
28
|
Lee HN, Joo JH, Oh JS, Choi SW and Seo DW:
Regulatory effects of Siegesbeckia glabrescens on non-small
cell lung cancer cell proliferation and invasion. Am J Chin Med.
42:453–463. 2014.
|
|
29
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei BL, Chen YC and Hsu HY: Kazinol Q from
Broussonetia kazinoki enhances cell death induced by Cu(ll)
through increased reactive oxygen species. Molecules. 16:3212–3221.
2011.PubMed/NCBI
|
|
33
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|