Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies and the second leading cause of cancer-related
mortality in China (1). Progression
of multimodality therapy has improved the outcome for HCC patients,
but it has not yet achieved satisfactory curative effect.
Therefore, it is important to elucidate the precise molecular
mechanisms of HCC development and to develop new therapeutic
targets (2).
MicroRNAs (miRNAs) are a large class of
evolutionarily conserved non-coding RNAs 18–25 nucleotides in
length that negatively regulate genes involved in many fundamental
cell processes including development, differentiation,
proliferation, survival and death (3). Recent studies have shown that miRNAs
play key roles in the initiation and progression of cancer
(4). Deregulation of miRNAs has
been reported in various types of human cancers including lymphoma,
colorectal and breast cancer, glioblastoma, lung cancer, papillary
thyroid carcinoma and HCC, suggesting it is a hallmark of cancer
(5). Specific miRNAs have been
shown to regulate known oncogenes or tumor suppressor genes or
function as so called onco-miRs or tumor suppressor-miRs by
directly targeting other genes involved in cell differentiation,
proliferation, invasion, apoptosis and angiogenesis in various
types of cancer (4).
miRNAs have been reported to play a critical role in
the hepatocarcinogenesis with dysfunction of targeting genes
(6). Several miRNAs have been found
to be aberrantly expressed in HCC and most of them are related to
the malignant behavior of the tumors (7). Recently, microRNA-218 (miR-218) was
recognized as a tumor suppressor and its downregulation was found
in human cancer, including cervical, gastric, lung, colon, prostate
and bladder cancer (8–16). miR-218 suppresses cell
proliferation, inhibits cell cycle progression and induces
apoptosis in colon cancer by downregulating B lymphoma Mo-MLV
insertion region 1 homolog (BMI-1)(13). However, the status, clinical
significance and function of miR-218 in HCC remain poorly
understood.
In the present study, we demonstrated that reduced
miR-218 expression is correlated with poor clinicopathological
parameters in HCC. miR-218 is an independent prognostic factor for
predicting both the overall and the disease-free 5-year survival of
HCC patients. miR-218 functions as a tumor suppressor in HCC by
inhibiting cell proliferation and inducing apoptosis in
vitro and in vivo. Furthermore, miR-218 negatively
regulates BMI-1 abundance in HCC cells and it is inversely
correlated with BMI-1 mRNA in HCC tissues. Our results suggest that
miR-218 may inhibit BMI-1 expression, thereby inhibiting HCC growth
and, hence, tumor progression.
Materials and methods
Clinical samples and cell lines
This study included a total of 60 HCC patients,
including 49 males and 11 females (range, 36–73 years; median 51
years), who underwent curative liver resection at the Department of
Hepatobiliary Surgery, The First Affiliated Hospital Xi’an Jiaotong
University from March 2006 to November 2008, with a median
follow-up time of 31.5 months. None of the patients received
chemotherapy, radiotherapy or radiofrequency ablation before
operation. The clinicopathological data are shown in Table I. HCC tissues and matched normal
tumor-adjacent tissues (>2 cm distance of the surgical margin)
were collected and used after obtaining informed consent. The Xi’an
Jiaotong University Ethics Committee approved all protocols
according to the 1975 Helsinki Declaration.
 | Table IClinical significance of miR-218
expression in HCC (n=60). |
Table I
Clinical significance of miR-218
expression in HCC (n=60).
| Clinicopathological
characteristics | R-value | P-value |
|---|
| Age (years) |
| <50 | 0.035 | 0.809 |
| ≥50 | | |
| Gender |
| Male | 0.108 | 0.501 |
| Female | | |
| HBV |
| Absent | 0.183 | 0.204 |
| Present | | |
| Serum AFP level
(ng/ml) |
| <400 | −0.159 | 0.221 |
| ≥400 | | |
| Tumor size (cm) |
| <5 | −0.429 | 0.029a |
| ≥5 | | |
| No. of tumor
nodules |
| 1 | −0.191 | 0.471 |
| ≥2 | | |
| Cirrhosis |
| Absent | −0.203 | 0.352 |
| Present | | |
| Venous
infiltration |
| Absent | −0.198 | 0.205 |
| Present | | |
| Edmondson-Steiner
grading |
| I+II | −0.514 | 0.008a |
| III+IV | | |
| TNM tumor stage |
| I+II | −0.571 | 0.002a |
| III+IV | | |
The human immortalized normal hepatocyte cell line
(LO2) and five HCC cell lines (HepG2, Hep3B, SMMC-7721, Bel-7402
and Huh7) were obtained from the Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in complete Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal bovine serum (FBS) (both from Gibco, USA) with
100 U /ml penicillin and 100 μg/ml streptomycin (Sigma, USA) and
cultured in a humidified 5% CO2 incubator at 37°C.
Real-time quantitative reverse
transcription-PCR (qRT-PCR)
The PCR amplification for the quantification of the
miR-218 and RNU6B was performed using TaqMan miRNA Reverse
Transcription kit and TaqMan Human MiRNA Assay kit (both from
Applied Biosystems, USA). The relative expression of miR-218 was
shown as fold difference relative to RNU6B.
BMI-1 sense primers, 5′-GTGCTTTGTGGAGGGTACTT CAT-3′
and antisense, 5′-TTGGACATCACAAATAGGACAA TACTT-3′. Total RNA was
isolated from HCC tissues and cells using TRIzol®
reagent (Invitrogen, USA) according to the manufacturer’s protocol.
The first strand cDNA was synthesized using the RevertAid™ First
Strand cDNA synthesis kit (Fermentas, USA). cDNA (2 μl) obtained
from each sample was amplified and quantified by real-time PCR
using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus; Takara,
Japan). The human GAPDH gene served as an internal control gene to
ensure that an equal amount of mRNA was analyzed from each
sample.
miRNA transfection
Cells were seeded in a 24-well plate at the
concentration of 1×105/well and divided into two groups
(miR-control and miR-218 group). Cells were transfected with
pre-miR-218 or pre-miR control at 30 nmol/l using Lipofectamine
2000 (Invitrogen, USA) according to the manufacturer’s
guidelines.
Cell proliferation and apoptosis
assay
For the proliferation assay, HCC cells were seeded
into 96-well plates at 5,000 cells/well for 24 h and assessed using
a Cell Proliferation ELISA, BrdU (chemiluminescent) (Roche, USA),
as described in our previous study (17). An Annexin V-FLUOS Staining kit
(Roche) was used to analyze the level of apoptosis, as previously
described (2).
Western blotting
The following primary antibodies were used in the
immunoblotting assays: BMI-1 (D20B7, #6964; Cell Signaling
Technology, USA) (1:1,000) and GAPDH (G8140; US Biological, USA)
(1:5,000). Horseradish peroxidase-conjugated goat anti-mouse or
anti-rabbit secondary antibodies (Bio-Rad, USA) were used at a
1:1,000–1:5,000 dilution and detected using a western blotting
luminol reagent (sc-2048; Santa Cruz, USA), as described in our
previous study (2).
In vivo experiments
Female BALB/c nude mice 4–6 weeks old (Centre of
Laboratory Animals, The Medical College of Xi’an Jiaotong
University, Xi’an, China) were used to establish a nude mouse
xenograft model. Mice were housed in sterilized cages (2
animals/cage) at a constant temperature and humidity and fed a
regular autoclaved chow diet with water ad libitum (2). SMMC-7721 cells (5×106) were
inoculated subcutaneously into the flank of each nude mouse. At day
5 after implantation, miR-control or miR-218 was injected into the
tumor every 3 days, respectively (n=6 mice each group). miRNA (1.2
nmol) was mixed with 10 μl Lipofectamine 2000 and incubated for 15
min, then injections were made in a final volume of 100 μl in
McCoy’s 5A medium (Sigma-Aldrich, USA) (13). The tumor volume for each mouse was
determined by measuring two of its dimensions and then calculated
as Tumor volume = length × width × width/2. After 3 weeks, the mice
were sacrificed by cervical dislocation under anesthesia with ether
and the xenograft tumor tissue was explanted for routine
pathological examination. The amount of apoptosis in the isolated
tumor tissues was detected using a TUNEL assay kit (4810–30-K;
R&D Systems, USA) according to the manufacturer’s guidelines.
All animal protocols were approved by the Institutional Animal Care
and Use Committee of Xi’an Jiaotong University.
Immunohistochemical staining
Immunohistochemistry was performed on
paraformaldehyde-fixed paraffin sections. Ki-67 (D2H10, #9027; Cell
Signaling Technology) (1:400) antibodies were used in
immunohistochemistry with the streptavidin peroxidase-conjugated
(SP-IHC) method. Immunohistochemistry was performed as previously
reported (18).
Statistical analysis
All data are presented as the means ± SEM. The SPSS
statistical package for Windows version 13 (SPSS, USA) was used for
the multi-variant Cox regression analysis. A two-tailed Student’s
t-test, a Spearman’s rank correlation coefficient test, a
Kaplan-Meier plot, a log-rank test or an ANOVA was used to evaluate
statistical significance using GraphPad Prism 5 software (GraphPad
Software, Inc., USA). p<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical significance of reduced miR-218
expression in HCC specimens
Previous studies reported that miR-218 expression is
impaired in various types of human cancers (8–16). To
determine the status of miR-218 and its clinical significance in
HCC, we tested miR-218 expression by qRT-PCR in a retrospective
cohort of 60 pairs of HCC and matched normal tumor-adjacent tissues
from HCC patients who received liver resection. In these cases, we
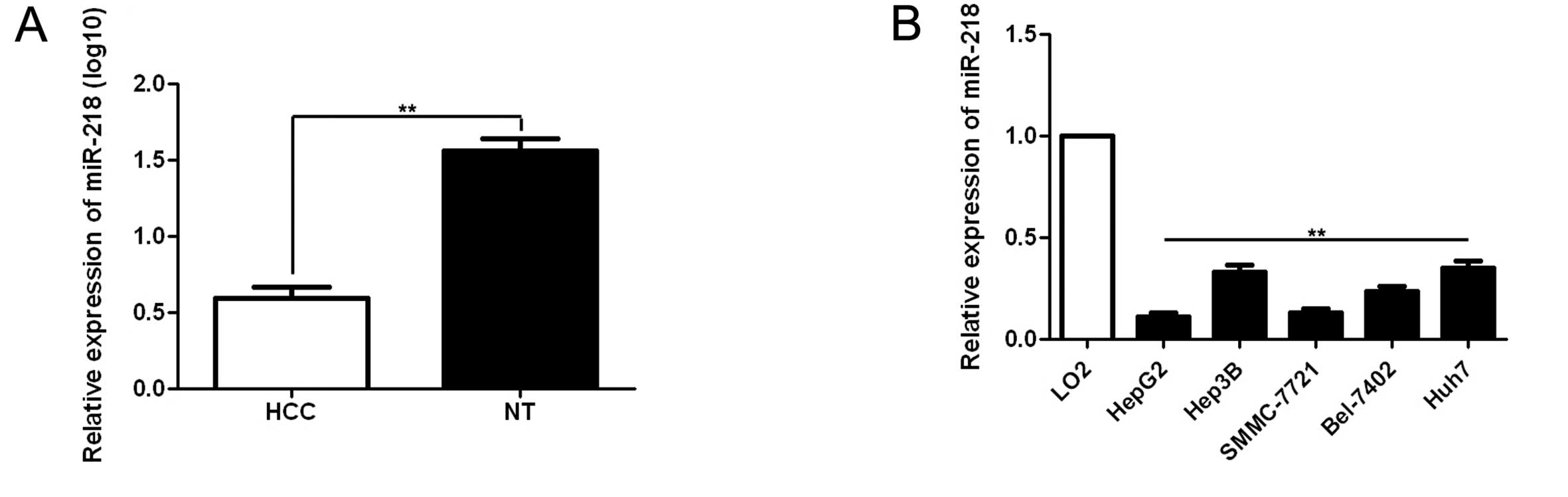
found that miR-218 expression in HCC was significantly lower than
that in matched non-cancerous tissues (the mean of log10 was 0.59
in the tumors and 1.56 in the matched non-tumor tissues, p<0.01,
Fig. 1A). Of these 60 paired
samples, 75.00% (45/60) of the HCC tissues showed lower miR-218
expression as compared with matched normal tumor-adjacent tissues.
Furthermore, we detected miR-218 expression in normal hepatocyte
cell line (LO2) and five HCC cell lines (Hep3B, HepG2, SMMC-7721,
Huh7 and Bel-7402). Consistent with the tissue samples, miR-218
expression was downregulated in all HCC cell lines as compared with
that in the normal hepatocyte cell line (p<0.01, Fig. 1B). As shown in Table I, clinical significance analysis
using a Spearman’s rank correlation coefficient test indicated that
the miR-218 expression in HCC tissues was significantly associated
with a large tumor size (r=-0.429, p=0.029), high Edmondson-Steiner
grading (r=-0.514, p=0.008) and advanced TNM tumor stage (r=-0.571,
p=0.002).
Reduced miR-218 expression correlates
with a poorer 5-year survival for HCC patients
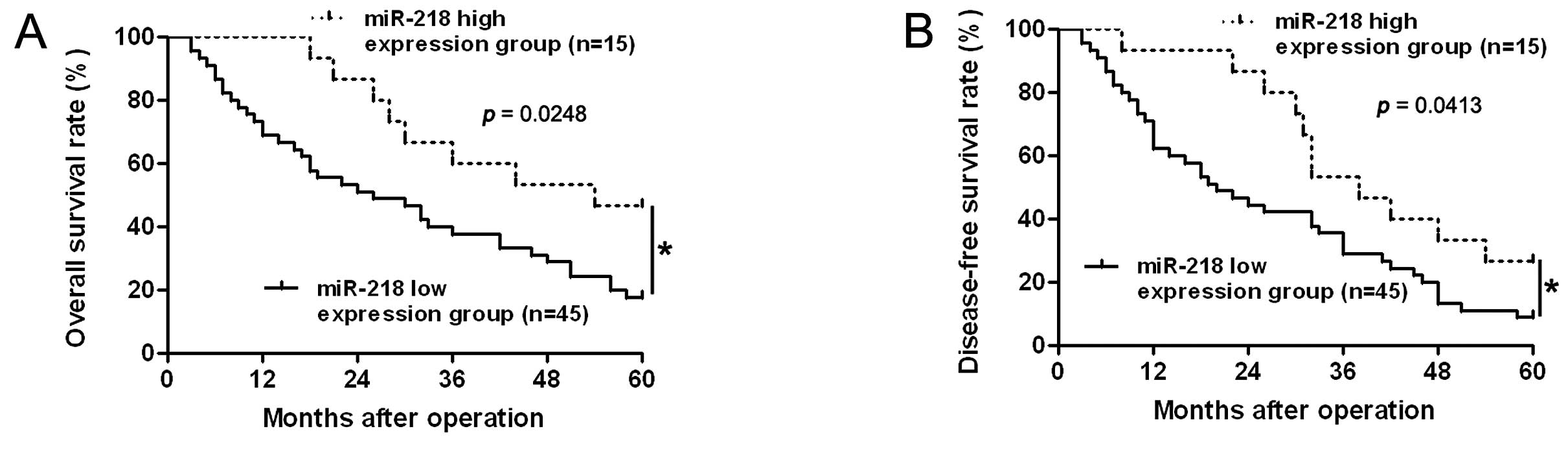
To determine the prognostic significance of miR-218
in HCC patients, quantification of miR-218 was performed to confirm
the correlation between miR-218 expression and 5-year patient
survival. We constructed Kaplan-Meier survival curves using the
overall 5-year patient survival date to analyze cases with high and
low miR-218 expression. Our data suggested overall survival in the
miR-218 high expression group was 46.67%, compared with 17.78% in
the low expression group. According to the overall survival curve,
patients in the miR-218 low expression group (n=45) had a
significantly poorer prognosis than those in the miR-218 high
expression group (n=15; log-rank=5.037; p=0.0248, Fig. 2A). The median disease-free survival
times in the miR-218 high and low expression subgroups of HCC
patients were 38.0 and 20.0 months, respectively. Kaplan-Meier
analysis also revealed that miR-218 loss was associated with a
shorter disease-free survival time (log-rank=4.163; p=0.0413,
Fig. 2B). These data indicate that
miR-218 may act as a potential biomarker for predicting prognosis
in HCC. Furthermore, multivariate Cox regression analysis indicated
that miR-218 expression was an independent factor for predicting
both 5-year overall and disease-free survival in HCC patients
(p=0.003 and 0.011, respectively, Table II).
 | Table IIMultivariate Cox regression analysis
of 5-year overall and disease-free survival of 60 HCC patients. |
Table II
Multivariate Cox regression analysis
of 5-year overall and disease-free survival of 60 HCC patients.
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 1.365 | 0.559–3.333 | 0.494 | 0.978 | 0.465–2.057 | 0.954 |
| Gender | 0.673 | 0.309–1.466 | 0.319 | 0.916 | 0.442–1.896 | 0.813 |
| HBV | 2.027 | 0.789–5.205 | 0.142 | 2.219 | 0.937–5.258 | 0.070 |
| No. of tumor
nodules | 0.766 | 0.368–1.595 | 0.476 | 0.686 | 0.343–1.370 | 0.285 |
| Tumor size | 1.655 | 0.731–3.745 | 0.227 | 1.468 | 0.691–3.120 | 0.318 |
| Venous
infiltration | 1.558 | 0.738–3.291 | 0.245 | 1.828 | 0.903–3.700 | 0.094 |
| Serum AFP
level | 0.951 | 0.446–2.025 | 0.896 | 1.019 | 0.514–2.020 | 0.956 |
| Cirrhosis | 1.634 | 0.834–3.204 | 0.468 | 1.561 | 0.859–2.838 | 0.144 |
| Edmondson-Steiner
grading | 0.636 | 0.280–1.443 | 0.279 | 0.600 | 0.295–1.218 | 0.157 |
| TNM tumor
stage | 0.208 | 0.074–0.585 | 0.003a | 0.297 | 0.124–0.710 | 0.006a |
| miR-218 expression
in tumor | 3.475 | 1.515–7.972 | 0.003a | 2.547 | 1.240–5.232 | 0.011a |
miR-218 inhibits proliferation and
promotes apoptosis in HCC cells
Previous studies demonstrated that miR-218 acts as a
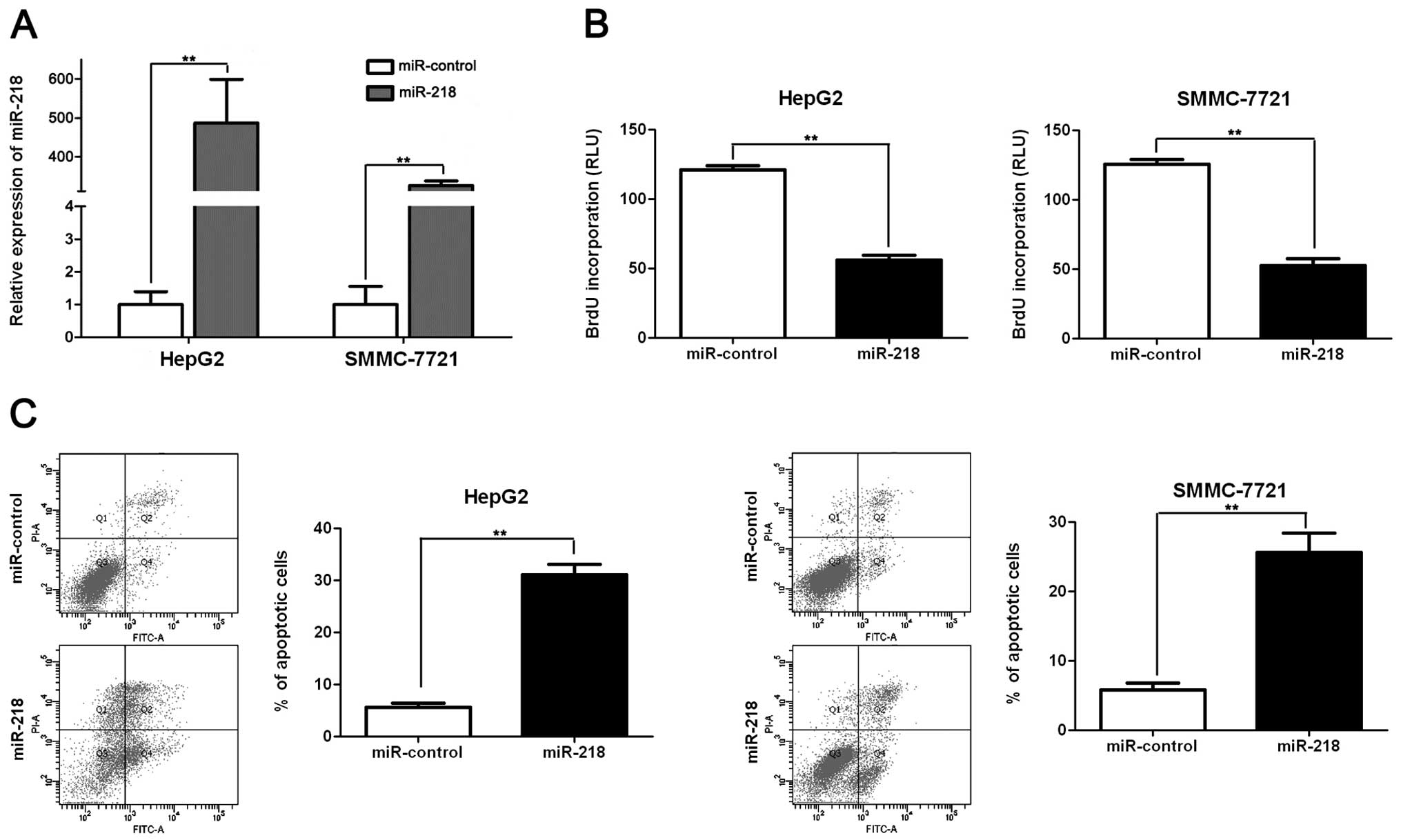
tumor suppressor by inducing apoptosis and growth arrest (13). To identify the role of miR-218 in
HCC, we restored miR-218 expression in two HCC cell lines, HepG2
and SMMC-7721. As assessed by qRT-PCR, the miR-218 level was raised
by ectopically expressing pre-miR-218 in both cell lines
(p<0.01, respectively, Fig. 3A).
BrdU assays were performed to test the effect of altering miR-218
levels on tumor cell proliferation. We found that miR-218
overexpression led to a significant reduction of cell proliferation
in both HepG2 and SMMC-7721 cells (p<0.01, respectively,
Fig. 3B). Furthermore, as
determined by flow cytometry, the percentage of apoptotic HepG2 and
SMMC-7721 cells was significantly elevated after miR-218
overexpression (p<0.01, respectively, Fig. 3C). Thus, miR-218 may exert an
anti-HCC effect by promoting both apoptosis and growth arrest.
miR-218 inhibits tumor growth in
mice
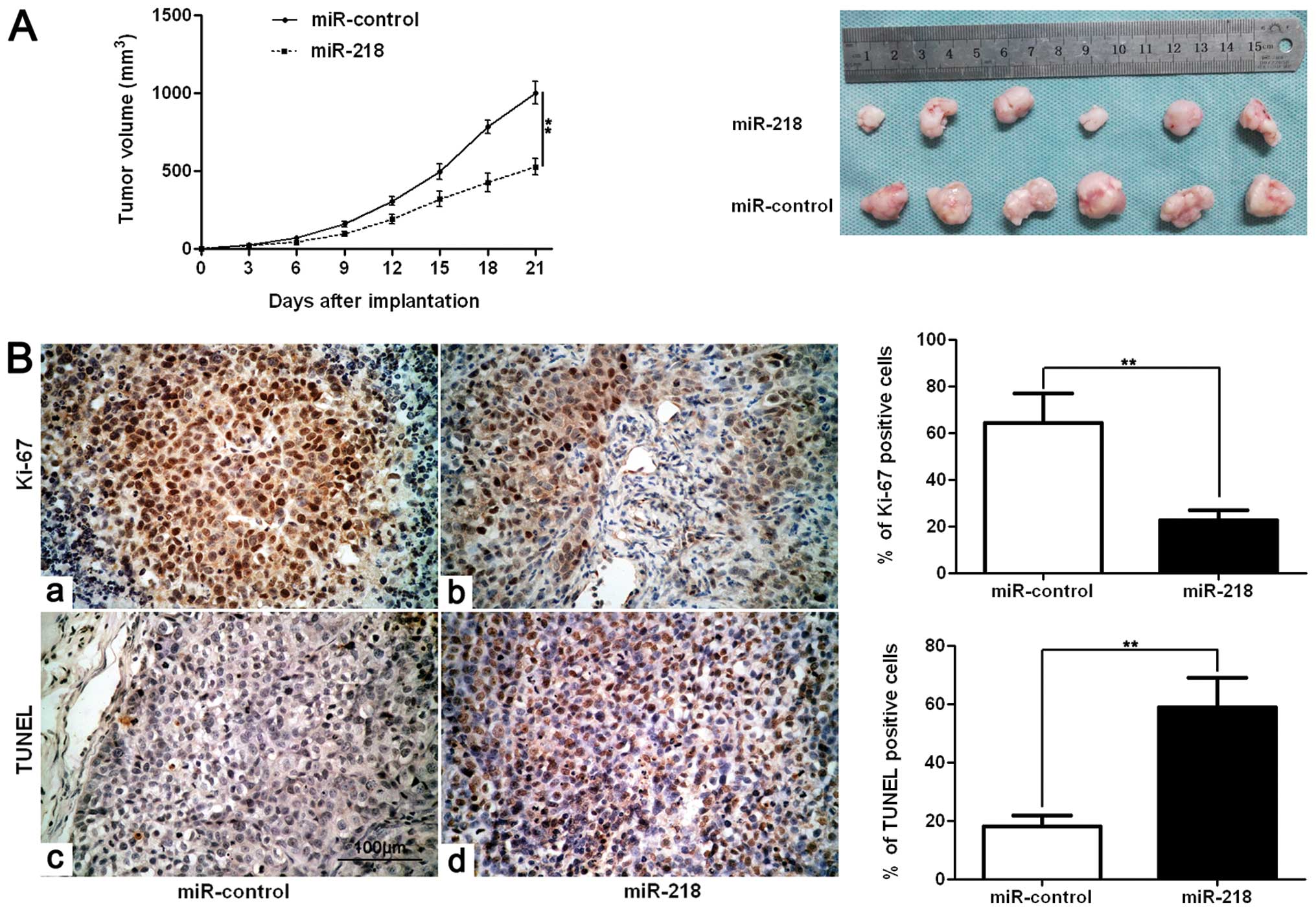
We next sought to determine whether miR-218 affects
tumor growth using an SMMC-7721 subcutaneous tumor model. Mice were
treated with miR-218 or miR-control by multi-center intratumoral
injection. Tumor growth curves revealed that miR-218 slowed down
tumor growth in mice (p<0.01, Fig.
4A). Furthermore, we performed immunohistochemistry for Ki-67
and TUNEL assays in the xenografted tissues. Consistent with our
in vitro data, miR-218 inhibited proliferation and induced
apoptosis in vivo (p<0.01, respectively, Fig. 4B).
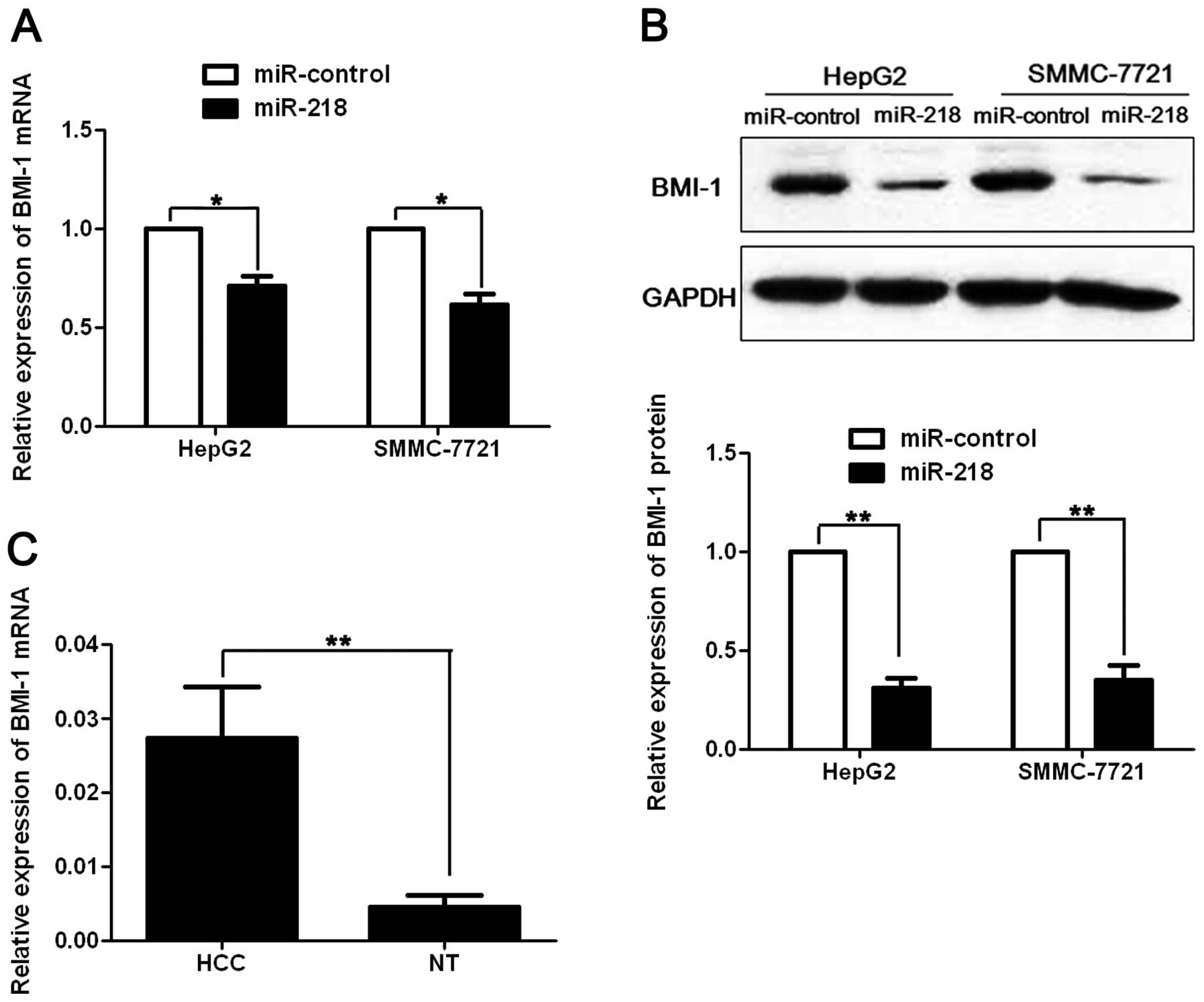
miR-218 regulates BMI-1 abundance in HCC
cells
Previous studies reported that BMI-1 was a potential
oncogene in human cancers and it was suppressed by miR-218 via
binding to its 3′UTR (13,19). To investigate whether BMI-1 is
involved in miR-218-induced apoptosis and growth arrest in HCC,
pre-miR-218 or pre-miR-control was transfected into HepG2 and
SMMC-7721 cells. As assessed by qRT-PCR and immunoblotting, miR-218
resulted in significant decrease of BMI-1 mRNA (p<0.05,
respectively, Fig. 5A) and protein
(p<0.01, respectively, Fig. 5B)
level in both HepG2 and SMMC-7721 cells. Furthermore, we compared
the expression of the BMI-1 mRNA between HCC and matched normal
tumor-adjacent tissues. Our data indicated that BMI-1 mRNA level
was significantly increased in HCC by ~6-fold compared with the
non-cancerous tissues (p<0.01, Fig.
5C). Pearson’s correlation analysis showed that the expression
of BMI-1 was inversely correlated with miR-218 in HCC tissues
(r=-0.572, p=0.003). Taken together, these data indicate that BMI-1
may function as a downstream factor in miR-218-induced apoptosis
and growth arrest in HCC.
Discussion
HCC is the most common primary tumor in liver and
the third most frequent malignant tumor due to the high incidence
of HBV infection in China. Several studies reported that miRNAs
regulate carcinogenesis-related gene expression, suggesting a new
mechanism involved in HCC initiation and development. We primarily
detected miR-218 expression in 60 samples of paired HCC and normal
tumor-adjacent tissues using qRT-PCR; our data indicated that
miR-218 level in the cancer tissues was significantly lower than
that in the non-cancerous tissues. Furthermore, miR-218 was
expressed at significantly lower levels in HCC patients with large
tumor size, high Edmondson-Steiner grading and advanced TNM tumor
stage. These results are consistent with the status and clinical
significance of miR-218 in other types of human cancer including
colorectal and pancreatic cancer, and glioma (13,20,21).
Notably, our data showed that reduced miR-218 expression conferred
a significantly poorer 5-year patient survival for HCC patients.
Multivariate Cox regression analysis indicated that miR-218 was an
independent factor for predicting both overall 5-year survival and
disease-free survival in HCC patients. Collectively, these results
show that the status of miR-218 is critical for prognosis
determination in HCC patients.
Functional studies demonstrated that miR-218
suppresses proliferation, inhibits cell cycle progression and
induces apoptosis in colorectal cancer and glioma (20,21).
Furthermore, miR-218 suppresses cell migration and invasion in
gastric cancer, cervical squamous cell carcinoma, lung cancer and
thyroid cancer and glioma (11,21).
In the present study, we showed that restoring miR-218 expression
led to reduced cell proliferation and elevated apoptotic HCC cells.
Ectopically expressing miR-218 conferred an inhibitory effect on
tumor growth in a nude mouse xenograft model. Furthermore,
immunostaining of Ki-67 and TUNEL assays indicated miR-218 may
suppress tumor growth by inducing apoptosis and growth arrest in
vivo.
B lymphoma Mo-MLV insertion region 1 homolog
(BMI-1), a member of the polycomb group (PcG), functions as a
transcriptional repressor and presents with high expression in many
tumors including HCC, indicating a poor prognosis (22,23).
BMI-1 has been shown to be an oncogene that regulates cell
proliferation and transformation (19). BMI-1 is also critical for the
self-renewal of stem cells and cancer initiation (24,25).
Several recent studies reported that miR-218 inhibited tumor
progression by targeting the polycomb group gene Bmi-1 (13,21).
miR-218 could directly bind to 3′-UTR of Bmi-1 and subsequently
suppress BMI-1 protein expression (13,21).
We investigated the regulatory effect of miR-218 on BMI-1 in HCC
and our data showed that ectopically expressing miR-218 resulted in
evident reduction of both BMI-1 mRNA and protein level in two
different HCC cell lines. We found that BMI-1 mRNA was
significantly higher in HCC tissues than in matched normal
tumor-adjacent tissues, which has been reported in previous studies
(26). Furthermore, Pearson’s
correlation analysis indicated that miR-218 expression was
negatively correlated with BMI-1 mRNA expression in HCC tissues.
Thus, BMI-1 may be a downstream target of miR-218 in HCC.
In conclusion, we demonstrated that miR-218
expression is impaired in HCC and reduced levels of miR-218 are
associated with poor clinicopathological characteristics. HCC
patients with low expression of miR-218 exhibit a poor 5-year
survival. miR-218 is an independent factor for predicting poor
prognosis in HCC patients. miR-218 acts as an HCC tumor suppressor
by inhibiting cell proliferation and promoting apoptosis in
vitro and in vivo. BMI-1 may be a target for miR-218 and
its abundance is inversely regulated by miR-218 in HCC cells.
Collectively, we hypothesize that loss of miR-218 function
contributes to hepatocarcinogenesis, in part through the
accumulation of BMI-1. We identified miR-218 as a potential
therapeutic target for HCC.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (nos. 81272645 and
81071897).
References
|
1
|
Tu K, Zheng X, Zan X, Han S, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with the
expression of c-Myc, cyclin E and p53 in human hepatocellular
carcinoma. Hepatol Res. 42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tu K, Zheng X, Zhou Z, et al: Recombinant
human adenovirus-p53 injection induced apoptosis in hepatocellular
carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls
c-Myc and cyclin E. PLoS One. 8:e685742013. View Article : Google Scholar
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia Z, Wang K, Wang G, Zhang A and Pu P:
MiR-30a-5p antisense oligonucleotide suppresses glioma cell growth
by targeting SEPT7. PLoS One. 8:e550082013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baer C, Claus R and Plass C: Genome-wide
epigenetic regulation of miRNAs in cancer. Cancer Res. 73:473–477.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong CM, Kai AK, Tsang FH and Ng IO:
Regulation of hepatocarcinogenesis by microRNAs. Front Biosci.
5:49–60. 2013.PubMed/NCBI
|
|
7
|
Gramantieri L, Fornari F, Callegari E, et
al: MicroRNA involvement in hepatocellular carcinoma. J Cell Mol
Med. 12:2189–2204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davidson MR, Larsen JE, Yang IA, et al:
MicroRNA-218 is deleted and down-regulated in lung squamous cell
carcinoma. PLoS One. 5:e125602010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiyomaru T, Enokida H, Kawakami K, et al:
Functional role of LASP1 in cell viability and its regulation by
microRNAs in bladder cancer. Urol Oncol. 30:434–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song L, Huang Q, Chen K, et al: miR-218
inhibits the invasive ability of glioma cells by direct
downregulation of IKK-β. Biochem Biophys Res Commun. 402:135–140.
2010.PubMed/NCBI
|
|
11
|
Tie J, Pan Y, Zhao L, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Ping Z and Ning H: MiR-218 impairs
tumor growth and increases chemo-sensitivity to cisplatin in
cervical cancer. Int J Mol Sci. 13:16053–16064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He X, Dong Y, Wu CW, et al: MicroRNA-218
inhibits cell cycle progression and promotes apoptosis in colon
cancer by downregulating BMI1 polycomb ring finger oncogene. Mol
Med. 18:1491–1498. 2012.PubMed/NCBI
|
|
14
|
Leite KR, Sousa-Canavez JM, Reis ST, et
al: Change in expression of miR-let7c, miR-100, and miR-218 from
high grade localized prostate cancer to metastasis. Urol Oncol.
29:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uesugi A, Kozaki K, Tsuruta T, et al: The
tumor suppressive microRNA miR-218 targets the mTOR
component Rictor and inhibits AKT phosphorylation in oral
cancer. Cancer Res. 71:5765–5778. 2011.PubMed/NCBI
|
|
16
|
Li BS, Zhao YL, Guo G, et al: Plasma
microRNAs, miR-223, miR-21 and miR-218, as novel potential
biomarkers for gastric cancer detection. PLoS One. 7:e416292012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X, Gai X, Ding F, Lu Z, Tu K, Yao Y
and Liu Q: Histone acetyltransferase PCAF up-regulated cell
apoptosis in hepatocellular carcinoma via acetylating histone H4
and inactivating AKT signaling. Mol Cancer. 12:962013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu K, Zheng X, Yin G, Zan X, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with
expression of SREBP-1 in a mouse model of NAFLD. Mol Med Rep.
6:525–530. 2012.PubMed/NCBI
|
|
19
|
Kang MK, Kim RH, Kim SJ, et al: Elevated
Bmi-1 expression is associated with dysplastic cell transformation
during oral carcinogenesis and is required for cancer cell
replication and survival. Br J Cancer. 96:126–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Z, Xu Y, Du J, Tan J and Jiao H:
Expression of microRNA-218 in human pancreatic ductal
adenocarcinoma and its correlation with tumor progression and
patient survival. J Surg Oncol. 109:89–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu Y, Gao X, Li G, et al: MicroRNA-218
inhibits glioma invasion, migration, proliferation, and cancer
stem-like cell self-renewal by targeting the polycomb group gene
Bmi1. Cancer Res. 73:6046–6055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong YQ, Liu B, Zheng HY, He YJ, Gu J, Li
F and Li Y: Overexpression of BMI-1 is associated with poor
prognosis in cervical cancer. Asia Pac J Clin Oncol. 8:e55–e62.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin T, Wei H, Leng Z, et al: Bmi-1
promotes the chemoresistance, invasion and tumorigenesis of
pancreatic cancer cells. Chemotherapy. 57:488–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schuringa JJ and Vellenga E: Role of the
polycomb group gene BMI1 in normal and leukemic hematopoietic stem
and progenitor cells. Curr Opin Hematol. 17:294–299. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Douglas D, Hsu JH, Hung L, et al: BMI-1
promotes ewing sarcoma tumorigenicity independent of CDKN2A
repression. Cancer Res. 68:6507–6515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Yang Z, Song W, et al:
Overexpression of Bmi-1 contributes to the invasion and metastasis
of hepatocellular carcinoma by increasing the expression of matrix
metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth
factor via the PTEN/ PI3K/Akt pathway. Int J Oncol. 43:793–802.
2013.PubMed/NCBI
|