Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, accounting for 1.37 million deaths annually
(1–3). Of all lung cancer cases, ~85–90% are
non-small cell lung cancers (NSCLC). Lung cancer is a multistep
process in which the activation of oncogenes and inactivation of
tumor-suppressor genes play a critical role in the process of
malignant transformation. Smoking and occupational asbestos
exposure also interact with other genetic susceptibility factors to
synergistically enhance lung cancer risk (4–7).
Cigarette smoking (both active and passive inhalation) is the
leading cause of multiple tumor types, and there is a broad
consensus that cigarette smoking increases the risk of lung cancer
in the general population (8–12). Due
to the special physiological function, there is no malignancy more
closely linked with smoking than lung cancer. In China, the
incidence and mortality rates of lung cancer have increased rapidly
(4), which may be attributed to the
dramatic increase in the cigarette smoking rate during the past 2
decades; a peak in lung cancer incidence is still expected. Among
adult Chinese, ~2/3 individuals are smokers, representing 1/3 of
all smokers worldwide.
Despite recent advances in early diagnosis/screening
and development of novel treatment strategies, the overall survival
rate of lung cancer patients remains low (2). The identification of reliable new
biomarkers and better understanding of the tumorgenesis of NSCLC,
as well as the development of more efficient therapeutic targets
will improve the prognosis of NSCLC patients.
The p53 gene, is the most frequently mutated
tumor suppressor in all cancers. Structural alteration has been
found in more than 50% of human tumors. Wild-type p53 protein
remains difficult to detect by IHC, because of a very short
half-life; however, the mutated type of p53, which is encoded by
the p53 gene with missense mutations has (but not always) a
prolonged half-life, and is easily detected by IHC (12). p21 (also known as
p21WAF1/CIP1) is a member of the Cip/Kip family of
cyclin-dependent kinase inhibitors, and the expression of p21 is
tightly regulated at the transcriptional and post-transcriptional
levels (13). p21 protein can bind
to and inhibit the activity of cyclin-CDK2 or cyclin-CDK4
complexes, which could participate as a regulator of cell cycle
progression at the G1 phase. Previous studies have shown that p21
is regulated by both p53-dependent and p53-independent mechanisms,
but the p53-dependent p21 pathway plays a critical role in cell
cycle arrest and prevents cellular DNA synthesis in response to DNA
damage (14,15). p21 can interact with proliferating
cell nuclear antigen (PCNA), a DNA polymerase accessory factor, and
act as a regulatory role in S phase DNA replication and DNA damage
repair (16). On the other hand,
p21 was reported to be specifically cleaved by CASP3-like caspase,
which leads to activation of CDK2, and then activates downstream
caspase leading to programmed cell death (9).
To our knowledge, there are few studies that have
focused on the relationship between both p53/p21 expression and
cigarette smoking and lung cancer. We hypothesized that both
p53/p21 expression in combination with smoking history may have a
close relationship with lung cancer progression, as well as
prognosis. In the present study, we analyzed the expression of p53
and p21 in tumor and adjacent non-cancerous tissues obtained from
50 NSCLC patients by performing western blot analysis. We further
investigated the association between p53/p21 expression as well as
smoking with NSCLC parameters by conducting IHC in tissue
microarrays (TMAs), including the survival status of NSCLC
patients.
Materials and methods
Reagents and antibodies
Protease inhibitor cocktail tablet was obtained from
Roche Applied Science (Indianapolis, IN, USA). Polyclonal anti-p21
Waf1/Cip1 was obtained from Proteintech (Wuhan, China); monoclonal
anti-p53, anti-actin and horseradish peroxidase-conjugated
secondary antibodies were all from Abmart (Shanghai, China). Pierce
BCA protein assay kit and ECL reagent were purchased from Thermo
Scientific (Waltham, MA, USA). Biotinylated goat anti-rabbit IgG
and the streptavidin-biotin complex were purchased from Boster
(Wuhan, China)
Patient specimens and tissue
microarrays
A total of 50 paired human non-small lung cancer and
adjacent non-cancerous tissues were obtained from the Department of
Cardiothoracic Surgery, The First Affiliated Hospital of Wenzhou
Medical University, Wenzhou, China, from March 2010 to November
2013. The tissues were frozen immediately in liquid nitrogen after
surgery and stored at −80°C for subsequent extraction of RNA and
tissue lysate preparation. NSCLC TMAs were constructed with
established routine methods by experienced pathologists (R.W. and
K.H.). TMAs contained a total of 150 formalin-fixed,
paraffin-embedded tissue samples from NSCLC patients collected
between 2005 and 2011. None of the patients included in the present
study received chemotherapy or/and radiation therapy prior to
surgery. Written informed consent for experimental use of the
specimens was obtained from all patients, and the study was
approved by the Board and Ethics Committee of Wenzhou Medical
University, China. All of the patients were clinically and
pathologically confirmed to have NSCLC. The tumor tissues were
classified according to the TNM system guidelines of the American
Joint Committee on Cancer (AJCC)/Union Internationale Contre Cancer
(UICC), and the histologic classification of the tumors was based
on the World Health Organization criteria (WHO).
Protein extraction from tissue samples
and immunoblotting
The tissue samples were retrieved at −80°C, washed 3
times with ice-cold PBS and homogenized using a homogenizer
(Kinematica AG, Luzern, Switzerland) in 1.5 ml tissue RIPA lysis
buffer [50 mMTris-HCl (pH 7.4), 1.0% Triton X-100, 1% sodium
deoxycholate, 0.1% SDS, 150 mM NaCl], containing protease inhibitor
cocktail tablet, 1 mM NaF and 1 mM Na3VO4.
Tissue homogenates were centrifuged at 13,000 rpm for 25 min at
4°C, and the supernatants were collected in clean microcentrifuge
tubes on ice.
The protein concentrations of the tissue homogenates
were determined using the Pierce BCA protein assay kit. Proteins
were resolved by SDS-PAGE and transferred onto nitrocellulose
membranes. Blots were incubated with the appropriate primary
antibodies overnight at 4°C and horseradish peroxidase-conjugated
secondary antibodies for 1 h followed by detection with the
enhanced chemiluminescence (ECL) detection system according to the
manufacturer’s instructions. The optical density was quantified
using the National Institutes of Health ImageJ software.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as
described previously (17,18). Briefly, sections were deparaffinized
in xylene and then gradually hydrated using a graded alcohol series
followed by blocking endogenous peroxidase activity by 0.5%
H2O2 in methanol for 60 min at room
temperature. Nonspecific binding was blocked by 5% BSA. Following
antigen retrieval, the sections were incubated overnight at 4°C
with rabbit polyclonal anti-p21 (dilution 1:100) and mouse
monoclonal anti-p53 (dilution 1:100). After washing with ice-cold
PBS, the TMA was incubated with biotinylated secondary antibody for
30 min, followed by further incubation with the
streptavidin-horseradish peroxidase for 20 min at room temperature.
Finally, 3,3′-diaminobenzidine (DAB) was applied to visualize the
signals of the TMA staining and lightly counterstained with Mayer
hematoxylin. For the negative control staining, PBS was used to
replace the primary antibody and no staining was observed. A brown
particle in the tissue was considered as positive labeling. The
staining was evaluated independently by two pathologists (K.H. and
R.W.) without knowledge of the clinicopathological data and p53/p21
expression. An average value of two independent scores was
presented in the present study.
An immunoreactivity scoring system was applied as
described elsewhere (19,20). The percent of p53/p21-positive cells
was scored according to four grades (percentage scores): <10%
(1), 10–50% (2), 51–80% (3) and >80% (4). The intensity of
staining was divided into four grades (intensity scores): no
staining (0), weakly stained (1), moderately stained (2) and
strongly stained (3). The overall p21 and p53 immunostaining score
was calculated using the percentage score × intensity score.
Overall scores 0–6 were defined as low p21 or p53 expression, and
scores >6 were high p21 or p53 expression.
Statistical analysis
The optical density of the immunoblotting signals
was quantified by National Institutes of Health ImageJ software.
All statistical analyses were performed using SPSS 15.0 software
(Chicago, IL, USA). The Student’s t-test was used to analyze the
relationship between the p21/p53 protein expression levels in
NSCLC. The χ2 test was performed to evaluate the
significance of the IHC results for the association between p53/p21
expression in IHC and clinicopathological parameters. The overall
survival was calculated according to Kaplan-Meier method and
compared with the log-rank test. Univariate and multivariate
analyses were based on Cox regression model. This model was used to
identify which independent factors jointly had significant effects
on survival. Differences were considered statistically significant
at P<0.05.
Results
Overexpression of p21 and p53 protein in
NSCLC tumor tissues
To investigate the role of p53 and p21 in NSCLC
progression, we examined the p21 and p53 protein expression in 50
paired tumor tissues and adjacent non-cancerous tissues (2 cm away
from the cancer tissues) by western blotting. Our results showed
that p53 and p21 protein expression levels were significantly
higher in the NSCLC tumor tissues than these levels in the matched
adjacent non-cancerous tissues. Representative immunoblotting
images are shown in Fig. 1A.
Additionally, the quantitative results of p53 and p21 expression
are shown in Fig. 1B (n=50;
P<0.001 and P<0.05, respectively).
Association of p53 and p21 expression
with clinicopathological parameters of NSCLC
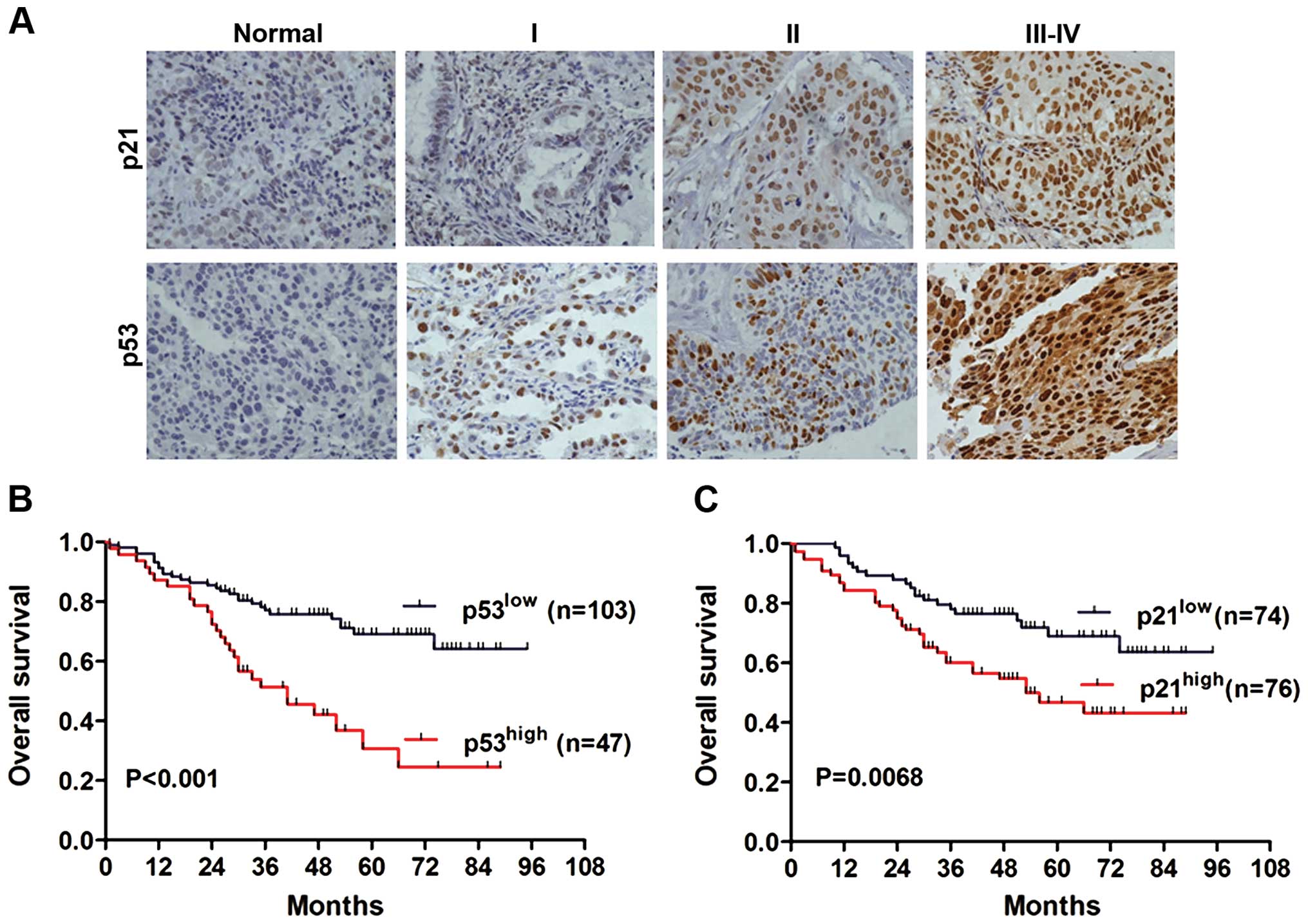
To further confirm the expression pattern of p53 and
p21 in NSCLC tissues, IHC staining analysis was conducted in TMA
containing 150 archived paraffin-embedded NSCLC tissues. The
representative IHC images of p53 and p21 in non-cancerous and
cancer tissues of different TNM stage are shown in Fig. 2A. We found that p21 and p53 protein
levels were predominantly localized in the nucleus. According to
the scoring system, we divided the 150 NSCLC cases into two groups:
high-expression and low-expression. In total, 50.67% (76 of 150)
showed high p21 expression within the nucleus (Table I), whereas high p53 expression was
detected in 31.3% (47 of 150) of the NSCLC cases (Table II). As summarized in Table I, p21 expression was significantly
associated with gender (P=0.004), smoking history (P=0.006), T
stage (P=0.014) and TNM stage (P=0.001), but not with alcohol
history, age, tumor grade and LN metastases. We next analyzed the
relationship between p53 expression and clinicopathological
characteristics. As documented in Table II, our results showed that p53
expression was significantly associated with gender (P=0.013), age
(P=0.026), smoking history (P=0.021), tumor grade (P=0.012), TNM
stage (P=0.023) and LN metastases (P=0.014). However, no
significant relationship was found between p53 expression and
variables such as alcohol history and T stage (P=0.853 and P=0.502,
respectively).
 | Table ICorrelation between p21 expression and
various clinicopathological factors of the NSCLC patients. |
Table I
Correlation between p21 expression and
various clinicopathological factors of the NSCLC patients.
| | p21 protein
expression | |
|---|
| |
| |
|---|
| Variables | Total (n=150) | Low (n=74) | High (n=76) | P-value |
|---|
| Gender | | | | 0.004 |
| Male | 100 | 41 | 59 | |
| Female | 50 | 33 | 17 | |
| Alcohol
history | | | | 0.640 |
| Yes | 40 | 21 | 19 | |
| No | 110 | 53 | 57 | |
| Age (years) | | | | 0.634 |
| <61 | 68 | 35 | 33 | |
| ≥61 | 82 | 39 | 43 | |
| Smoking
history | | | | 0.006 |
| Yes | 78 | 30 | 48 | |
| No | 72 | 44 | 28 | |
| Grade | | | | 0.302 |
| G1 | 16 | 8 | 8 | |
| G2 | 91 | 49 | 42 | |
| G3 | 43 | 17 | 26 | |
| T stage | | | | 0.014 |
| T1 | 11 | 10 | 1 | |
| T2 | 126 | 59 | 67 | |
| T3–T4 | 13 | 5 | 8 | |
| LN metastasis
(N) | | | | 0.090 |
| N0 | 89 | 49 | 40 | |
| N≥1 | 61 | 25 | 36 | |
| TNM stage | | | | 0.001 |
| I | 65 | 43 | 22 | |
| II | 40 | 13 | 27 | |
| III–IV | 45 | 18 | 27 | |
 | Table IICorrelation between p53 expression
and various clinicopathological factors of the NSCLC patients. |
Table II
Correlation between p53 expression
and various clinicopathological factors of the NSCLC patients.
| | p53 protein
expression | |
|---|
| |
| |
|---|
| Variables | No. (n=150) | Low (n=103) | High (n=47) | P-value |
|---|
| Gender | | | | 0.013 |
| Male | 100 | 62 | 38 | |
| Female | 50 | 41 | 9 | |
| Alcohol
history | | | | 0.853 |
| Yes | 40 | 27 | 13 | |
| No | 110 | 76 | 34 | |
| Age (years) | | | | 0.026 |
| <61 | 68 | 53 | 15 | |
| ≥61 | 82 | 50 | 32 | |
| Smoking
history | | | | 0.021 |
| Yes | 78 | 47 | 31 | |
| No | 72 | 56 | 16 | |
| Grade | | | | 0.012 |
| G1 | 16 | 13 | 3 | |
| G2 | 91 | 68 | 23 | |
| G3 | 43 | 22 | 21 | |
| T stage | | | | 0.502 |
| T1–T2 | 137 | 93 | 44 | |
| T3–T4 | 13 | 10 | 3 | |
| LN metastasis
(N) | | | | 0.014 |
| N0 | 89 | 68 | 21 | |
| N≥1 | 61 | 35 | 26 | |
| TNM stage | | | | 0.023 |
| I–II | 105 | 78 | 27 | |
| III–IV | 45 | 25 | 20 | |
Survival analysis
To understand the prognostic value of p53/p21 for
NSCLC, Kaplan-Meier analysis with a log-rank test was performed to
evaluate the association between p53/p21 expression and overall
survival. A total of 150 NSCLC patients who had adequate follow-up
data were used for survival analysis. The log-rank test results
showed that patients with high p53 expression were associated with
poor overall survival when compared to those with low p53
expression (Fig. 2B, P<0.001).
Similarly, the high p21 expression group also showed decreased
overall survival compared with the low p21 expression group
(Fig. 2C, P=0.0068). Moreover, we
proceeded to analyze the relationship between NSCLC patient
survival status with combinations of p53/p21 and found a strikingly
reverse correlation with overall survival (Fig. 3A, P=0.0002). Particularly, in the
low p53 expression group (p53low), the high p21
expression patients had a lower survival rates compared to the low
p21 expression group. While in the group of patients with high p53
expression (p53high), the overall survival was
independent of p21 expression. Collectively, these results suggest
that the aberrant overexpression of p53 and p21 may potentially
contribute to NSCLC carcinogenesis and tumor progression.
Cigarette smoking may play a marked role
in NSCLC progression
As known, cigarette smoking plays critical roles in
lung cancer carcinogenesis. We first analyzed the role of cigarette
smoking in NSCLC patient overall survival. Consistently, NSCLC
patients who had a smoking history exhibited obviously poor overall
survival than those without a smoking history (Fig. 3B, P=0.0379). As our statistical
results showed (Fig. 3C), p53 and
p21 expression were both significantly positively associated with
smoking history. Thus, we proposed that cigarette smoking together
with upregulation of p53 and p21 may contribute to NSCLC
carcinogenesis. To validate this hypothesis, we quantified the
corresponding integrated optical density (IOD) value of IHC images
by Image-Pro Plus 6.0. We divided the p53/p21 expression into two
groups based on smoking status. Interestingly, we found a notable
increase in p53 expression in the smoking (+) group compared with
that in the smoking (−) group (Fig.
3C, P=0.0137). Similarly, p21 protein expression was also
significantly higher in the smoking (+) group (Fig. 3C, P=0.0423). Moreover, we examined
the effect of the combination of smoking and p53 on overall
survival of NSCLC patients. A marked negative correlation was
observed in overall survival (Fig.
3D, P<0.001). In detail, the low p53 expression
(p53low)/smoking (+) group exhibited worse outcomes
compared with the low p53 expression (p53low)/smoking
(−) group; whereas no statistically significant difference was
found in the high p53 expression (p53high) group,
whether or not the patients had a smoking history. We further
analyzed the overall survival with the combination of smoking and
p21. A negative correlation was found by Kaplan-Meier analysis
(Fig. 3E, P=0.0171). Finally, a
significant decrease in survival rates was found in the smoking (+)
and high p21 expression group (p21high). Taken together,
our results indicate that cigarette smoking may play a critical
role in promoting NSCLC progression via modulation of p53 and p21
protein expression.
Assessment of NSCLC prognostic
factors
To determine the factors which affect the overall
survival of NSCLC patients, univariate and multivariate analyses
using a Cox regression hazards model were conducted to evaluate the
impact of p53/p21 expression and clinicopathological factors on
survival status in 150 NSCLC patients. As the univariate analysis
results show in Table III, p21
and p53 expression levels (P=0.001 and P<0.001, respectively),
smoking history (P=0.041), tumor grade (P=0.004), T stage
(P<0.001), lymph node (LN) metastasis (P=0.041) and TNM stage
(P<0.001), but not patient age (P=0.065), gender (P=0.081) and
alcohol history (P=0.772) were significant prognostic factors for
overall survival of NSCLC. Next, as summarized in Table IV, our data showed that p53
expression (P=0.005) was an independent prognostic factor for
NSCLC, but not p21 (P=0.123).
 | Table IIIUnivariate analysis to identify
factors influencing the overall survival of NSCLC patientsa. |
Table III
Univariate analysis to identify
factors influencing the overall survival of NSCLC patientsa.
| Univariate
analysis |
|---|
|
|
|---|
| Variables | RR | 95% CI | P-value |
|---|
| p21 | 2.618 | 1.495–4.586 | 0.001 |
| p53 | 2.809 | 1.661–4.752 | <0.001 |
| Age | 1.029 | 0.988–1.061 | 0.065 |
| Gender | 1.694 | 0.938–3.061 | 0.081 |
| Smoking | 1.745 | 1.022–2.979 | 0.041 |
| Alcohol | 0.912 | 0.490–1.699 | 0.772 |
| Grade | 1.971 | 1.241–3.131 | 0.004 |
| T stage | 7.009 | 3.781–12.933 | <0.001 |
| LN metastasis | 1.719 | 1.020–2.896 | 0.042 |
| Clinical stage | 2.061 | 1.492–2.847 | <0.001 |
 | Table IVMultivariate analysis to identify
factors influencing the overall survival of NSCLC patientsa. |
Table IV
Multivariate analysis to identify
factors influencing the overall survival of NSCLC patientsa.
| p21 Multivariate
analysis | p53 Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Expression
level | 1.603 | 0.880–2.919 | 0.123 | 2.305 | 1.294–4.105 | 0.005 |
| Age | 1.026 | 0.991–1.062 | 0.148 | 1.024 | 0.989–1.059 | 0.181 |
| Gender | 1.239 | 0.481–3.194 | 0.657 | 1.293 | 0.500–3.344 | 0.597 |
| Smoking | 1.204 | 0.504–2.874 | 0.676 | 1.157 | 0.490–2.731 | 0.740 |
| Alcohol | 1.017 | 0.498–2.080 | 0.962 | 1.072 | 0.531–2.166 | 0.846 |
| Grade | 1.026 | 0.598–1.761 | 0.927 | 0.903 | 0.527–1.545 | 0.709 |
| T stage | 3.969 | 1.860–8.470 | <0.001 | 4.516 | 2.088–9.771 | <0.001 |
| LN metastasis | 0.432 | 0.178–1.051 | 0.064 | 0.033 | 0.133–0.835 | 0.019 |
| Clinical stage | 2.479 | 1.385–4.438 | 0.002 | 2.738 | 1.526–4.911 | 0.001 |
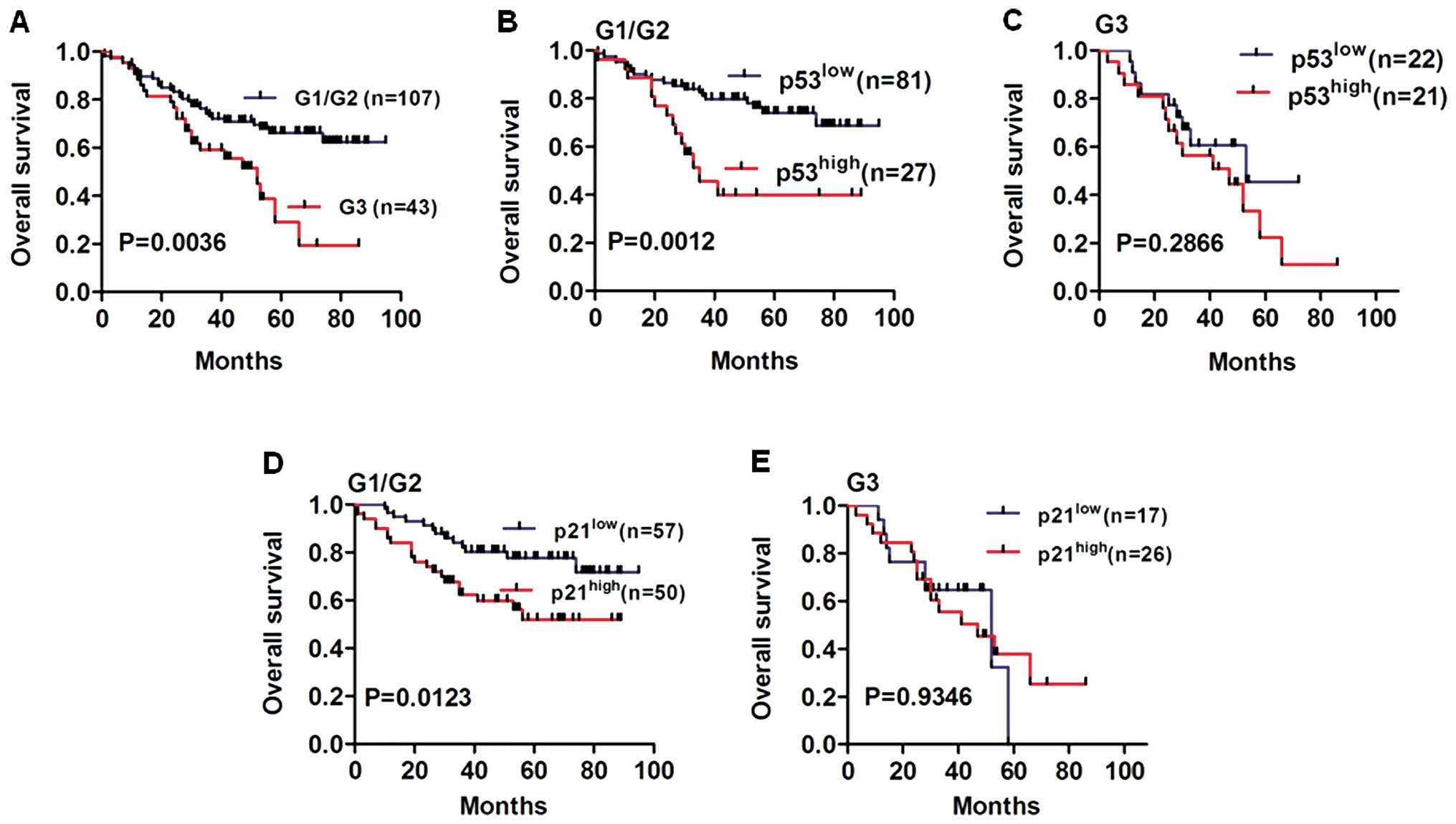
We performed Kaplan-Meier analysis to determine the
effects of p53/p21 expression on overall survival of NSCLC patients
in various tumor grades, and histological grade was a potential
prognostic factor in NSCLC patients (Fig. 4A, P=0.0036). We further proceeded to
analyze p53/p21 expression in well differentiated (G1), moderately
differentiated (G2) and poorly differentiated (G3) tumor tissues.
Notably, we found an obviously lower survival rate in the
p53high group compared to that in the p53low
group for G1/G2 NSCLC patients (Fig.
4B, P=0.0012). Similarly, we found that overall survival of the
p21high group was worse than that of the
p21low group for G1/G2 NSCLC patients (Fig. 4D, P=0.0123). However, no
statistically significant difference was found in the overall
survival of G3 NSCLC patients according to p53 or p21 expression
levels (Fig. 4C and E, P=0.2866 and
P=0.9346, respectively).
Next, we analyzed overall survival in different
TNM-stage NSCLC patients (Fig. 5A,
P<0.001). Notably, the NSCLC patients with high-expression of
p53 showed worse outcomes in both stages I/II (Fig. 5B, P=0.0021) compared to the the
stage III subgroup (Fig. 5C,
P=0.0422). Moreover, the p21high group had lower overall
survival rates than the p21low group in I/II stage NSCLC
patients (Fig. 5D, P=0.0263). In
patients diagnosed with III stage, no significant difference was
found for the p21high group and p21low group
(Fig. 5E, P=0.2261).
Finally, we examined the relationship between
p53/p21 expression and LN metastases (Fig. 6A, P=0.0386). Our results showed that
the overall survival of NSCLC patients with high p53 expression was
worse than patients with low p53 expression in both N0 (Fig. 6B, P=0.0034) and N≥1 stage groups
(Fig. 6C, P=0.0152). In addition,
the overall survival of NSCLC patients with high p21 expression was
much worse than patients with low p21 expression in the N0 stage
group (Fig. 6D, P=0.0084). However,
no statistically significant difference in overall survival was
observed in the N≥1 stage group regardless of p21 expression
(Fig. 6E, P=0.4258). Taken
together, these findings indicate that p53/p21 expression may play
a potential role in NSCLC progression and correlate with the
outcome of NSCLC patients.
Discussion
p53 is a well-known tumor suppressor encoded by the
p53 gene located on chromosome 17p13, which is composed of
393 amino acids and is a member of a highly conserved family
containing at least another two genes, p63 and p73. p53 is closely
related to many human cancers and is the most frequently mutated
tumor-suppressor gene in human cancers. The mutation or loss of the
p53 gene can be identified in more than 50% of all human
cancers (21,22), which results not only in the loss of
tumor-suppressor function, but also leads to the gain of novel
cancer-related functions that contribute to tumorigenesis. Under
normal conditions, p53 protein levels in cells are maintained at
very low levels due to extremely short half-life, and p53 is
largely inactive, unless the cells are activated by signals from
DNA damage and a number of other cellular stress factors, including
hypoxia and nucleotide deprivation (15,23).
In response to DNA damage and cellular stresses, the p53 protein
level is upregulated, which leads to cell cycle arrest, DNA repair
or apoptosis. Hence, p53 plays a critical role in the inhibition of
cancer cell malignancy. In contrast to wild-type p53, the mutant
protein is stably expressed, which leads to the immunohistochemical
staining detection of mutant p53 with high levels of prognostic
significance.
p21 protein mediates p53-dependent G1 growth arrest
and acts as a tumor suppressor. However, it has been reported that
p21 is upregulated in a variety of human cancers including breast,
cervical, prostate and esophageal carcinoma (23). The upregulation of p21 is associated
with tumor grade, clinical stage, invasiveness and aggressiveness.
Moreover, p21 overexpression may also predict the poor prognosis of
human cancers. Thus, the role of p21 as a tumor suppressor is still
controversial and the association with tumor progression remains to
be further understood.
In the present study, we performed western blotting
using frozen tissues which were snap-frozen in liquid nitrogen
immediately after surgical resection and stored at −80°C. Our data
showed that both p53 and p21 protein levels were upregulated in
tumor tissues. Similar results were also reported by Zhang et
al (17) for hepatocellular
carcinomas.
Additionally, we examined the p53 and p21 protein
expression pattern with IHC analysis, and our results confirmed
that the tumor tissues of NSCLC patients had strong p53 and p21
protein staining, whereas there was no or weak staining in the
matched adjacent non-cancerous tissues. These results revealed that
overexpression of p53 and p21 in NSCLC patient tissues plays an
important role in the progression of NSCLC. The upregulation of p53
was significantly associated with gender, age, smoking history,
tumor grade, T stage and TNM stage of NSCLC patients. In addition,
upregulation of p21 was associated with gender, smoking history, T
stage and TNM stage; however, there was no association between p21
expression and age or tumor grade of the NSCLC patients.
Kaplan-Meier survival analysis showed that high
expression levels of both p53 and p21 were associated with poor
overall survival of patients with NSCLC. In cases with low p53
expression, the high expression of p21 promoted tumor progression
and led to poor survival. However, once p53 was mutated and with
stable high expression at the protein level, p21 protein had no
more effects on overall survival.
Among the 150 NSCLC patients included in this study,
we found that cigarette smoking led to poor overall survival for
NSCLC patients compared with never-smokers, which is consistent
with most previous reports on other cancers (24–26).
While the p53 protein level is low, cigarette smoking is
significantly associated with overall survival; however, when p53
is overexpressed, cigarette smoking is no longer associated with
overall survival of NSCLC patients, since overexpression of p53
dominates the effects on tumor progression. We further found that
cigarette smoking did not exhibit a significant effect on overall
survival while p21 protein expression was low. Contrary to p53, our
data showed that cigarette smoking was significantly associated
with overall survival for NSCLC patients with high p21 protein
expression levels. This could be attributed to the difference in
the p53 protein expression level in the p21 high expression group
(Table V). The ratio of
p53high/p53low was low (0.40) in the group of
p21high/smoking (−) (n=28), whereas the
p53high/p53low ratio was high (1.29) in the
group of p21high/smoking (+) (n=48). These results
suggest that the significant difference in overall survival for p21
high expression NSCLC patients with or without smoking history was
due to the difference in p53 protein expression level.
 | Table VRatio of
p53high/p53low in the p21 expression and
smoking combination groups. |
Table V
Ratio of
p53high/p53low in the p21 expression and
smoking combination groups.
| | p53 expression | |
|---|
| |
| |
|---|
| Group | | Low (n=103) | High (n=47) |
p53high/p53low |
|---|
| p21low
smoking (−) | n=44 | 36 | 8 | 0.22 |
| p21low
smoking (+) | n=30 | 26 | 4 | 0.15 |
| p21high
smoking (−) | n=28 | 20 | 8 | 0.40 |
| p21high
smoking (+) | n=48 | 21 | 27 | 1.29 |
Our results showed that tumor grade, TNM stage and
LN metastasis were all significantly associated with prognosis and
could serve as an independent outcome biomarker for NSCLC patients.
In patients with low tumor grade (G1/G2), both high p53 and p21
expression led to poor overall survival. On the contrary, neither
p53 or p21 expression had an impact on overall survival in the high
tumor grade (G3) NSCLC patients. This indicated that the p53 or p21
protein expression level had no further effects on tumor
progression in NSCLC patients with high tumor grade. The expression
of p53 protein was significantly associated with TNM stage and LN
stage. In addition, high expression of p21 was significantly
associated with the overall survival of NSCLC patients in TNM stage
I–II or LN stage N0; however, there was no difference for stage
III–IV patients. These results further suggest that p21 protein
expression had less effect on tumor progression in NSCLC patients
who were in late TNM or N stage. Moreover, the univariate analysis
showed that overexpression of both p53 and p21 was associated with
survival status of NSCLC patients. However, the multivariate
analysis showed that p53 but not p21 could be an independent
prognostic factor for NSCLC, which indicates that p53 plays a more
decisive role in the progression of NSCLC.
In conclusion, our findings suggest that cigarette
smoking and p53/p21 overexpression are associated with the poor
prognosis of NSCLC patients. Although the incidence of NSCLC is a
multifactor process, p53/p21 plays an important role in cell cycle
regulation. Better understanding of this cell cycle regulatory
system involved in NSCLC development is necessary for understanding
tumor biological behavior, seeking reliable diagnostic markers and
providing new therapeutic strategies. The combination of p53/p21
expression and cigarette smoking history could be a useful clinical
biomarker for tumor progression and prognosis of NSCLC
patients.
Acknowledgements
This study was partially supported by the National
Basic Research Program of China (no. 2013CB531702), the National
Natural Science Foundation of China (nos. 31070710 and 31171345),
Zhejiang Qianjiang Talent Project B Grant (no. 2010R10045) and the
Scientific Research Foundation for the Returned Overseas Chinese
Scholar, State Education Ministry to B.L., Natural Science
Foundation of Zhejiang Province (no. Y2110097) and Wenzhou Science
and Technology Bureau grant (no. H20100064) to Y.L.
References
|
1
|
An Q, Liu Y, Gao Y, et al: Deletion of
tumor suppressor genes in Chinese non-small cell lung cancer.
Cancer Lett. 184:189–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Liu X, Lin XJ, Wang CP, Yan KK, Zhao LY,
An WX and Liu XD: Association between smoking and p53 mutation in
lung cancer: a meta-analysis. Clin Oncol (R Coll Radiol). 26:18–24.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsou JA, Hagen JA, Carpenter CL and
Laird-Offringa IA: DNA methylation analysis: a powerful new tool
for lung cancer diagnosis. Oncogene. 21:5450–5461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Christiani DC, Wiencke JK, et al:
Mutations in the p53 gene in lung cancer are associated with
cigarette smoking and asbestos exposure. Cancer Epidemiol
Biomarkers Prev. 4:543–548. 1995.PubMed/NCBI
|
|
7
|
Hwang SJ, Cheng LS, Lozano G, Amos CI, Gu
X and Strong LC: Lung cancer risk in germline p53 mutation
carriers: association between an inherited cancer predisposition,
cigarette smoking, and cancer risk. Hum Genet. 113:238–243. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H and Cai B: The impact of tobacco
on lung health in China. Respirology. 8:17–21. 2003. View Article : Google Scholar
|
|
9
|
Fujino M, Dosaka-Akita H, Harada M,
Hiroumi H, Kinoshita I, Akie K and Kawakami Y: Prognostic
significance of p53 and ras p21 expression in nonsmall cell lung
cancer. Cancer. 76:2457–2463. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furrukh M: Tobacco smoking and lung
cancer: perception-changing facts. Sultan Qaboos Univ Med J.
13:345–358. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pfeifer GP, Denissenko MF, Olivier M,
Tretyakova N, Hecht SS and Hainaut P: Tobacco smoke carcinogens,
DNA damage and p53 mutations in smoking-associated cancers.
Oncogene. 21:7435–7451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levine AJ, Momand J and Finlay CA: The p53
tumour suppressor gene. Nature. 351:453–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi C, Wang Q, Wang L, et al: MiR-663, a
microRNA targeting p21(WAF1/CIP1), promotes the proliferation and
tumorigenesis of nasopharyngeal carcinoma. Oncogene. 31:4421–4433.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giono LE and Manfredi JJ: The p53 tumor
suppressor participates in multiple cell cycle checkpoints. J Cell
Physiol. 209:13–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang MF, Zhang ZY, Fu J, Yang YF and Yun
JP: Correlation between expression of p53, p21/WAF1, and MDM2
proteins and their prognostic significance in primary
hepatocellular carcinoma. J Transl Med. 7:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi H, Miyamoto H, Ito T, Kameda Y,
Nakamura N, Kubota Y and Kitamura H: Analysis of p21Waf1/Cip1
expression in normal, premalignant, and malignant cells during the
development of human lung adenocarcinoma. Am J Pathol. 151:461–470.
1997.PubMed/NCBI
|
|
19
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German).
|
|
20
|
Cheng AN, Jiang SS, Fan CC, et al:
Increased Cdc7 expression is a marker of oral squamous cell
carcinoma and overexpression of Cdc7 contributes to the resistance
to DNA-damaging agents. Cancer Lett. 337:218–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hainaut P and Hollstein M: p53 and human
cancer: the first ten thousand mutations. Adv Cancer Res.
77:81–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hollstein M, Moeckel G, Hergenhahn M, et
al: On the origins of tumor mutations in cancer genes: insights
from the p53 gene. Mutat Res. 405:145–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lakin N and Jackson S: Regulation of p53
in response to DNA damage. Oncogene. 18:7644–7655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taghavi N, Biramijamal F, Sotoudeh M,
Moaven O, Khademi H, Abbaszadegan MR and Malekzadeh R: Association
of p53/p21 expression with cigarette smoking and prognosis in
esophageal squamous cell carcinoma patients. World J Gastroenterol.
16:4958–4967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cruz I, Snijders PJ, Van Houten V, Vosjan
M, Van der Waal I and Meijer CJ: Specific p53 immunostaining
patterns are associated with smoking habits in patients with oral
squamous cell carcinomas. J Clin Pathol. 55:834–840. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizobuchi S, Furihata M, Sonobe H, et al:
Association between p53 immunostaining and cigarette smoking in
squamous cell carcinoma of the esophagus. Jpn J Clin Oncol.
30:423–428. 2000. View Article : Google Scholar : PubMed/NCBI
|