Introduction
Malignant pleural effusion (MPE) is a common and
devastating complication of various advanced malignancies, of which
lung cancer is the most common cause, accounting for one-third of
patients (1). The incidence of MPE
parallels that of lung cancer and its presence impedes effective
surgery and is predictive of poor prognosis, with a short median
survival after MPE diagnosis between 4 and 9 months (2). It has been well demonstrated that
CD4+ T lymphocytes frequently accumulate in MPE
secondary to direct pleural invasion from malignant tumors
(3,4). More recently, two subsets of
CD4+ T cell, regulatory T cells (Tregs) and T helper
IL-17-producing (Th17) cells, have attracted much attention in
MPE.
Previous evidence suggests that Tregs,
characteristically expressing Foxp3 (5), are essential for maintainance of
self-tolerance and exert a potential for inhibiting effector T
cells, by a cell contact-manner or by secreting inhibitory
cytokines, such as interleukin-10 (IL-10) and transforming growth
factor-β1 TGF-β (6). Importantly,
Tregs may function as an independent prognostic factor in several
types of cancers and are alternately harmful or beneficial to
patient survival (7). Similar to
Tregs, Th17 cells express retinoic acid-related orphan receptor γt
(RORγt) and release pro-inflammatory cytokines, and are involved in
the development of autoimmune and tumor diseases (8). Controversially, Th17 cells have been
found in both pro- and antitumorigenic processes (9,10).
Previous studies have demonstrated that increased Tregs are
recruited into the pleural space in MPE induced by chemokine C-C
motif ligand 22 (CCL22) (8),
whereas the number of Th17 cells are elevated in MPE and such an
elevation predicts improved survival of patients with MPE (11).
In the present study, we compared the frequency of
Tregs and Th17 cells, mRNA expression of relevant transcription
factors, their hallmark cytokines and related chemokines between
MPE and parapneumonic effusion (PPE), to investigate the role of
Treg/Th17 imbalance in MPE. A higher percentage of Tregs and a
lower percentage of Th17 cells were found in MPE in contrast to
these percentages in PPE, accompanied by increased levels of FoxP3
mRNA and concentrations of IL-10 and TGF-β. Moreover, an alteration
of CCL17 and CCL20 might be responsible for the Treg/Th17 imbalance
in MPE. Furthermore, an elevated Treg/Th17 ratio was predictive of
a poor prognosis of patients with MPE.
Materials and methods
Subjects
Twenty-six patients newly diagnosed with lung cancer
with MPE, and 12 patients newly diagnosed with community-acquired
pneumonia with PPE, who were admitted to the Department of
Respiratory Diseases at the First Affiliated Hospital of Zhejiang
University from May 2012 to October 2012 were enrolled in the
present study. Histologically, all of the patients were diagnosed
with adenocarcinoma. A diagnosis of MPE was established by
verification of malignant cells in the pleural fluid or/and on
closed pleural biopsy specimen. The patients were excluded if they
had received any invasive manipulations directed into the pleural
cavity or if they had a chest trauma within 3 months prior to
hospitalization. Up to the time of sample collection, none of the
patients had received any drug that could affect the immune
response within 3 months before enrollment. The study was approved
by the ethics committee of our institution, and informed consent
was obtained from all patients or their surrogates.
Sample collection and processing
The pleural fluid samples were collected within 24 h
of hospitalization in heparin-treated tubes using a standard
thoracentesis technique. Following centrifugation at 4,000 rpm for
10 min at 4°C, the cell-free supernatants were dispensed into
1.5-ml Eppendorf tubes and frozen at −80°C for the detection of
cytokines and chemokines. Then, the cell pellets were resuspended
in lysis buffer (BD Biosciences, San Jose, CA, USA) for removal of
the red blood cells, and were then analyzed by flow cytometry and
real-time PCR.
Flow cytometry of Th17 cells and
Tregs
To analyze the perecentage of Th17 cells,
IL-17-producing CD4+ cells were detected. MNCs
(2×106) from pleural effusion were stimulated with 50
ng/ml PMA (BioVision, Mountain View, CA, USA) and 500 ng/ml
ionomycin (Enzo Life Sciences, Farmingdale, NY, USA) in the
presence of GolgiPlug (BD Biosciences) for 4 h, after which the
cells were stained for FITC-labeled antihuman CD4, then fixed and
permeabilized with IC fixation/permeabilization buffer
(eBioscience, San Diego, CA, USA), washed and intracellularly
stained with PE-labeled antihuman IL-17.
To detect Tregs, a human regulatory T-cell staining
kit (eBioscience) was used according to the manufacturer’s
protocol. MNCs were surfacely stained with FITC-labeled anti-human
CD4 and PE-labeled anti-human CD25 for 30 min in the dark at 4°C,
then washed and incubated with 1 ml Foxp3 fixation/permeabilization
buffer (eBioscience) for 60 min at 4°C in the dark. The cells were
washed with 1 ml 1× permeabilization buffer twice and
intracellularly stained using APC-labeled anti-human FoxP3 or an
isotype control (PE-labeled rat IgG; BD Biosciences) for 30 min
away from light at 4°C. Flow cytometry acquisition was performed
using an FACSCalibur (BD Biosciences), and data were analyzed using
CellQuest software (BD Biosciences).
Real-time PCR of RORγt and Foxp3
levels
Total RNA was extracted from pleural MNCs using the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse
transcription based on the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction was performed
using the SYBR-Green PCR Mix (Takara, Dalian, China). Cycling
reactions were performed using an ABI 7500 Sequence Detection
System. The following primer pairs were used: RORγt: forward,
5′-TGAGAAGGACAGGGAGCCAA-3′ and reverse,
5′-CCACAGATTTTGCAAGGGATCA-3′; FoxP3: forward,
5′-GAGAAGCTGAGTGCCATGCA-3′ and reverse, 5′-AGAG CCCTTGTCGGATGAT-3′;
GAPDH: forward, 5′-GGTCTC CTCTGACTTCAACA-3′ and reverse,
5′-GTGAGGGTCTCT CTCTTCCT-3′. The PCR thermal cycle was 95°C for 30
sec, 40 cycles of 95°C for 5 sec, 60°C for 34 sec. GAPDH was
utilized as a housekeeping gene for normalization, and a deionized
water sample was used as a negative control. All reactions were
carried out in triplicate per sample.
ELISA measurement of cytokines and
chemokines
The levels of cytokines, including IL-6, IL-10,
TGF-β1 and IL-17 (eBioscience), as well as chemokines CCL17 and
CCL20 (R&D Systems, Minneapolis, MN, USA) in pleural fluids
were measured by ELISA kits according to the manufacturer’s
protocols. All samples were detected in duplicate.
Statistical analysis
Values are expressed as mean ± SEM. Differences
between values were determined using the non-paired Student’s
t-test. Correlations between values were evaluated by Spearman’s
rank correlation coefficients. Survival was assessed by the
Kaplan-Meier method and compared by the log-rank test. Analysis was
performed with the SPSS Statistical Software (version 21.0; SPSS,
Inc., Chicago, IL, USA), and P<0.05 was considered to indicate a
statistically significant result.
Results
Treg/Th17 imbalance exists in MPE
In the present study, flow cytometry was used to
investigate the balance of Tregs and Th17 cells, by detecting the
percentages of CD4+IL-17+ and
CD4+CD25+FoxP3+ cells in MPE and
PPE. We observed that patients with MPE had a higher percentage of
Tregs (Fig. A and C) (MPE vs. PPE: 3.86±0.50 vs. 1.09±0.31%,
P=0.0004) but a lower level of Th17 cells (MPE vs. PPE: 1.18±0.16
vs. 2.52±0.40%, P=0.0022) than patients with PPE (Fig. 1B and D). Moreover, the ratio of
Tregs/Th17 cells was obviously higher in MPE than PPE (MPE vs. PPE:
3.89±0.61 vs. 0.39±0.09, P=0.0003) (Fig. 1E). Notably, Tregs had a negative
correlation with Th17 cells in the pleural effusion (r=−0.5032,
P=0.023) (Fig. 1F).
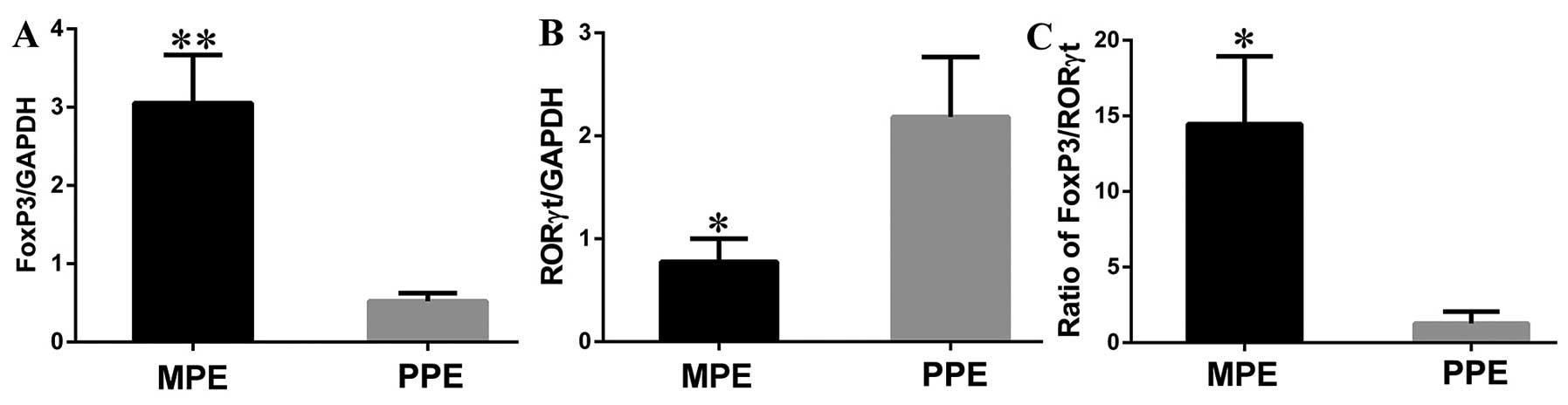
Meanwhile, both FoxP3 and RORγt were determined by
real-time PCR in MPE and PPE. We found that Foxp3 mRNA expression
level from cells in the pleural effusion was significantly
increased in patients with MPE compared to patients with PPE (MPE
vs. PPE: 3.05±0.62 vs. 0.52±0.11, P=0.0012) (Fig. 2A). In contrast, RORγt mRNA
expression presented an opposite result, which was markedly lower
in MPE than the level in PPE (MPE vs. PPE: 0.78±0.23 vs. 2.19±0.58,
P=0.0253) (Fig. 2B). The ratio of
Foxp3/RORγt was significantly higher in MPE as compared with that
in PPE (MPE vs. PPE: 14.45±0.28 vs. 0.11±0.05, P=0.008) (Fig. 2C).
Moreover, concentrations of Treg/Th17-related
cytokines, IL-10, IL-6, TGF-β1 and IL-17, were detected by ELISA.
The levels of IL-10 and TGF-β1 exhibited increasing trends from MPE
to PPE (MPE vs. PPE: IL-10, 166.3±39.53 vs. 40.38±10.92 pg/ml,
P=0.0307; TGF-β1, 10,720±1,274 vs. 17,47±293.2 pg/ml, P<0.0001)
(Fig. 3A and B). In contrast, the
levels of IL-6 and IL-17 exhibited a relative decreasing trend (MPE
vs. PPE: IL-6, 2,490±257.7 vs. 4427±215.6 pg/ml, P<0.0001;
IL-17, 2.542±0.1408 vs. 4.272±0.5413 pg/ml, P=0.0148) (Fig. 3C and D).
Alteration of CCL17 and CCL20 may be
responsible for the Treg/Th17 imbalance in MPE
Previous studies demonstrated that CCL17 induce the
migration of Tregs (12), while
CCL20 exerts a potent chemoattractant activity for Th17 cells
(8). We measured CCL17 and CCL20 by
ELISA and showed that a higher level of CCL17 (MPE vs. PPE:
341.10±88.22 vs. 119.20±19.80 pg/ml, P=0.0427) and a lower level of
CCL20 (MPE vs. PPE: 26.51±5.32 vs. 125.90±30.89 pg/ml, P=0.0039)
were noted in MPE in contrast to these levels in PPE (Fig. 4A and B). Importantly, there was a
positive correlation between the level of CCL17 and the ratio of
Tregs/Th17 cells (r=0.7975, P<0.0001) (Fig. 4C), but a negative correlation
between the level of CCL20 and the ratio of Tregs/Th17 cells
(r=−0.6097, P<0.0001) (Fig.
4D).
Higher Treg/Th17 ratio predicts the poor
survival of patients with MPE
The characteristics of patients with MPE and the
measurement of pleural effusion were listed in Table I. To test whether a Treg/Th17
imbalance impacted the prognosis of patients with MPE, we analyzed
the correlation of overall survival time with the Treg/Th17 ratio
in 26 patients with MPE. Subjects were divided into two equal
groups according to the ratio of Tregs/Th17 cells. The low group
included all those with a Treg/Th17 ratio <3.54% (n=13) and the
high group included those with a Treg/Th17 ratio >3.54% (n=13).
As shown in Fig. 5, there was a
significant correlation between the pleural Treg/Th17 ratio and
survival (P=0.0002). Patients with a higher Treg/Th17 ratio had a
significantly shorter overall survival (median, 3.4 months) than
patients with a lower Treg/Th17 ratio (median, 6.7 months).
Patients with MPE in the higher Treg/Th17 ratio group experienced a
9-fold higher death hazard as compared with those in the lower
Treg/Th17 ratio group (95% confidence interval, 3.12–26.26). The
multivariate Cox proportional hazards analysis revealed that the
Treg/Th17 ratio was an independent prognostic factor for survival
(hazard ratio = 0.094; P<0.001) (Table II). However, the levels of lactate
dehydrogenase (LDH) and carcinoembryonic antigen (CEA) were not
independent prognostic factors for survival (P>0.05). Thus, we
considered that an increase in the Treg/Th17 ratio was related to
high risk for death and for poor survival in patients with MPE.
 | Table ICharacteristics of the patients with
MPE. |
Table I
Characteristics of the patients with
MPE.
| Characteristics | N | Mean ± SEM | Median (range) |
|---|
| Gender |
| Male | 14 | | |
| Female | 12 | | |
| Age (years) | 26 | 63.77±2.53 | 66 (42–83) |
| Protein (g/l) | 26 | 42.10±1.71 | 40.88
(31.77–70.20) |
| Lactate dehydrogenase
(LDH, U/l) | 26 | 494.4±75.9 | 388.5
(129–1596) |
| Carcinoembryonic
antigen (CEA, ng/ml) | 26 | 345.1±78.1 | 214.7
(1.4–1388) |
| Total cell count,
×109/l | 26 | 1.51±0.14 | 1.41
(0.72–3.62) |
| Lymphocytes, % | 26 | 48.85±5.01 | 45.00 (10–90) |
| Tregs/Th17
cells | 26 | 3.89±0.61 | 3.54
(1.00–9.28) |
 | Table IIUnivariate and multivariate analyses
of the correlation between factors of the patients with MPE and
survival. |
Table II
Univariate and multivariate analyses
of the correlation between factors of the patients with MPE and
survival.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variables | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Gender | 0.612 | | | |
| Age (years) | 0.405 | | | |
| Protein | 0.863 | | | |
| Total cell
count | 0.671 | | | |
| Lymphocytes | 0.119 | | | |
| LDH | 0.042 | 1.975 | 0.764–5.106 | 0.160 |
| CEA | 0.022 | 0.717 | 0.315–1.633 | 0.428 |
| Treg/Th17 | 0.0003 | 0.094 | 0.026–0.348 | <0.0001 |
Discussion
In recent years, the balance of Th17 and Treg cells,
and the regulatory mechanisms between T cell subsets in the
pathogenesis of pleural effusion have attracted more and more
attention. The present study focused on the comparison of function
of these two new subsets between MPE and PPE and showed that lung
cancer patients with MPE exhibited a marked elevation in the
percentage of Tregs, IL-10, TGF-β1 and Foxp3 mRNA levels, and a
marked decline in the percentage of Th17 cells, IL-6, IL-17 and
RORγt mRNA levels in contrast to those with PPE. The imbalance of
the Treg/Th17 ratio may provide a mechanistic insight into
immunomodulation involved in MPE.
Treg cells have been broadly considered as a
critical subset of T cells which execute their suppressive function
against T cell-mediated immune response and play an important role
in peripheral tolerance, autoimmunity and tumor immunity (13). Studies have confirmed the
accumulation of Tregs in tumor tissues and peripheral blood of
tumor-bearing animals and patients with cancer, and such an
increase in Tregs predict a grim prognosis in cancer (14,15).
Thus, Treg cells have the potential to prevent the host from
launching an immune response to tumor antigens (16), subsequently facilitating tumor
progression. In MPE, overrepresentation of Tregs also exist
(17), which is consistent with our
findings that the tumor microenvironment may induce more Tregs to
gather in the pleural cavity compared with inflammation.
Since the identification of Th17 cells in 2005
(18), their contributions to tumor
immunity have been extensively explored. However, these studies
have yielded controversial results. The protumor function mediated
by Th17 and IL-17 has been proven both in animal tumor models and
in patients with cancer (19,20).
Th17 cells characteristically produce IL-17 which acts as an
angiogenic factor that accelerates tumor growth and metastasis
through neovascularization (21).
Meanwhile, Th17 cells induce the secretion of inflammatory
cytokines, such as IL-8 and TNF-α, that attract neutrophil
recruitment and destroy the niche of immunity (22,23).
However, the role of Th17 in tumors is hardly conclusive. Adequate
evidence has proven that Th17 may contribute to protective tumor
immunity via stimulating the production of Th1-type chemokines
CXCL9 and CXCL10 to recruit effector cells to tumor tissues
(10). Th17 cells can also
facilitate dendritic cell (DC) accumulation and elicit activation
of tumor-specific CD8+ T cells, exerting antitumor
immunity (24). More recently, Ye
et al (11) showed that the
number of Th17 cells was significantly increased in MPE, which
predicted prolonged survival, implying a beneficial role for Th17
cells in human cancer.
It has been well demonstrated that the developmental
processes of Tregs and Th17 cells are reciprocally interactive
(25). The deviated balance of
TGF-β and IL-6 might control the switch of Tregs or Th17 cells via
antagonistic competition of Foxp3 and RORγt (26). In this process, TGF-β induces both
Foxp3 and RORγt expression, but exclusively converts naïve T cells
into Treg cells (27), while IL-6
can abrogate the inhibition of RORγt by Foxp3 and initiate the
differentiation of Th17 cells (28). In MPE, we found that the numbers of
Tregs and Th17 cells were inversely correlated, and both the ratios
of Tregs/Th17 cells and Foxp3/RORγt were markedly lower in MPE than
in PPE, suggesting a close interplay between Tregs and Th17 cells
in tumor development, and that the Treg/Th17 ratio might be a
valuable index in the differentiation of malignant from benign
pleural effusion. Furthermore, we investigated the levels of
cytokines associated with Tregs and Th17 cells in pleural effusion.
In the present study, higher levels of TGF-β1 and IL-10, but lower
levels of IL-6 and IL-17 were in MPE in contrast to that in PPE.
TGF-β and IL-10, serving as negative regulators, might promote
tumor progression through inducing immunosuppression and assisting
in evasion from tumor immune surveillance (29,30).
Chemokines with chemoattracting and activating
properties play pivotal roles in tumor immunity. CCL17 in the tumor
microenvironment is related to Treg cells in lung carcinomas,
gastric carcinomas and ovarian carcinomas (12,31,32).
Such tumor-infiltrating Treg cells trigger impaired tumor-specific
immune responses and unfavorable prognosis (33). CCL20 has been shown to serve as an
immunotherapeutic mediator which can cause accumulation in DCs and
CD8+ cells into the tumors and suppress tumor growth
(33). Ye and the coworkers
(11) demonstrated that CCL20 in
MPE might contribute to chemoattract Th17 cells into the pleural
space. Our results suggest that CCL17, rather than CCL20, exerted a
dominant effect on Treg cell infiltration in MPE.
Additionally, we evaluated the relationship between
the ratio of Tregs/Th17 cells and clinical pathological parameters
in MPE. Herein, we noted that a higher Treg/Th17 ratio predicted
worse survival, implying that the balance of Treg/Th17 might be a
significant prognostic factor for tumor progression.
In conclusion, our data showed that the ratio of
Tregs/Th17 cells was increased in MPE in contrast to that in PPE,
which is valuable for the differential diagnosis of pleural
effusion. Alteration of CCL17 and CCL20 might be responsible for
the Treg/Th17 imbalance in MPE. The Treg/Th17 imbalance might be
involved in the development of MPE and a higher Treg/Th17 ratio
predicted the poor prognosis of patients with MPE.
Acknowledgements
The present study was supported by grants from the
Major Project of the Science Technology Department of Zhejiang
Province, China (no. 2012C13022-2), the Project of Health and
Family Planning Commission of Zhejiang Province, China (no.
2013KYB105), and the Key Personnel of Zhejiang Medicine and Health
Platform (no. 2012RCA025).
References
|
1
|
Roberts ME, Neville E, Berrisford RG,
Antunes G and Ali NJ: BTS Pleural Disease Guideline Group:
Management of a malignant pleural effusion: British Thoracic
Society Pleural Disease Guideline 2010. Thorax. 65:ii32–ii40. 2010.
View Article : Google Scholar
|
|
2
|
Bielsa S, Martín-Juan J, Porcel JM and
Rodríguez-Panadero F: Diagnostic and prognostic implications of
pleural adhesions in malignant effusions. J Thorac Oncol.
3:1251–1256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marazioti A, Blackwell TS and Stathopoulos
GT: The lymphatic system in malignant pleural effusion. Drain or
immune switch? Am J Respir Crit Care Med. 189:626–627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aguiar LM, Antonangelo L, Vargas FS,
Zerbini MC, Sales MM, Uip DE and Saldiva PH: Malignant and
tuberculous pleural effusions: immunophenotypic cellular
characterization. Clinics (Sao Paulo). 63:637–644. 2008. View Article : Google Scholar
|
|
5
|
Muller YD, Seebach JD, Bühler LH, Pascual
M and Golshayan D: Transplantation tolerance: clinical potential of
regulatory T cells. Self Nonself. 2:26–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gregori S, Bacchetta R, Passerini L,
Levings MK and Roncarolo MG: Isolation, expansion, and
characterization of human natural and adaptive regulatory cells.
Methods Mol Biol. 380:83–106. 2007. View Article : Google Scholar
|
|
7
|
Wilke CM, Wu K, Zhao E, Wang G and Zou W:
Prognostic significance of regulatory T cells in tumor. Int J
Cancer. 127:748–758. 2010.PubMed/NCBI
|
|
8
|
Qin XJ, Shi HZ, Deng JM, Liang QL, Jiang J
and Ye ZJ: CCL22 recruits CD4-positive CD25-positive regulatory T
cells into malignant pleural effusion. Clin Cancer Res.
15:2231–2237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bettelli E, Oukka M and Kuchroo VK:
TH-17 cells in the circle of immunity and autoimmunity.
Nat Immunol. 8:345–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kryczek I, Banerjee M, Cheng P, et al:
Phenotype, distribution, generation, and functional and clinical
relevance of Th17 cells in the human tumor environments. Blood.
114:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye ZJ, Zhou Q, Gu YY, et al: Generation
and differentiation of IL-17-producing CD4+ T cells in
malignant pleural effusion. J Immunol. 185:6348–6354. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizukami Y, Kono K, Kawaguchi Y, Akaike H,
Kamimura K, Sugai H and Fujii H: CCL17 and CCL22 chemokines within
tumor microenvironment are related to accumulation of
Foxp3+ regulatory T cells in gastric cancer. Int J
Cancer. 122:2286–2293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin X, Chen M, Liu Y, Guo Z, He X, Brand D
and Zheng SG: Advances in distinguishing natural from induced
Foxp3+ regulatory T cells. Int J Clin Exp Pathol.
6:116–123. 2013.
|
|
14
|
Larmonier N, Marron M, Zeng Y, et al:
Tumor-derived CD4+CD25+ regulatory T cell
suppression of dendritic cell function involves TGF-beta and IL-10.
Cancer Immunol Immunother. 56:48–59. 2007. View Article : Google Scholar
|
|
15
|
Badoual C, Hans S, Rodriguez J, et al:
Prognostic value of tumor-infiltrating CD4+ T-cell
subpopulations in head and neck cancers. Clin Cancer Res.
12:465–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kosmaczewska A, Ciszak L, Potoczek S and
Frydecka I: The significance of Treg cells in defective tumor
immunity. Arch Immunol Ther Exp (Warsz). 56:181–191. 2008.
View Article : Google Scholar
|
|
17
|
Ibrahim L, Salah M, Rahman AA, Zeidan A
and Ragb M: Crucial role of CD4+CD25+
FOXP3+ T regulatory cell, interferon-γ and
interleukin-16 in malignant and tuberculous pleural effusions.
Immunol Invest. 42:122–136. 2013. View Article : Google Scholar
|
|
18
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage
distinct from the T helper type 1 and 2 lineages. Nat Immunol.
6:1123–1132. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prabhala RH, Pelluru D, Fulciniti M, et
al: Elevated IL-17 produced by TH17 cells promotes myeloma cell
growth and inhibits immune function in multiple myeloma. Blood.
115:5385–5392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tosolini M, Kirilovsky A, Mlecnik B, et
al: Clinical impact of different classes of infiltrating T
cytotoxic and helper cells (Th1, th2, treg, th17) in patients with
colorectal cancer. Cancer Res. 71:1263–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi W, Huang X and Wang J: Correlation
between Th17 cells and tumor microenvironment. Cell Immunol.
285:18–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iida T, Iwahashi M, Katsuda M, et al:
Tumor-infiltrating CD4+ Th17 cells produce IL-17 in
tumor microenvironment and promote tumor progression in human
gastric cancer. Oncol Rep. 25:1271–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu FM, Li QL, Gao Q, et al: IL-17 induces
AKT-dependent IL-6/ JAK2/STAT3 activation and tumor progression in
hepatocellular carcinoma. Mol Cancer. 10:1502011. View Article : Google Scholar
|
|
24
|
Martin-Orozco N, Muranski P, et al: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ayyoub M, Deknuydt F, Raimbaud I, Dousset
C, Leveque L, Bioley G and Valmori D: Human memory
FOXP3+ Tregs secrete IL-17 ex vivo and constitutively
express the TH17 lineage- specific transcription factor
RORγt. Proc Natl Acad Sci USA. 106:8635–8640. 2009. View Article : Google Scholar
|
|
26
|
Weaver CT and Hatton RD: Interplay between
the TH17 and TReg cell lineages: a (co-) evolutionary perspective.
Nat Rev Immunol. 9:883–889. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou L, Lopes JE, Chong MM, et al:
TGF-beta-induced Foxp3 inhibits TH17 cell
differentiation by antagonizing RORγt function. Nature.
453:236–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura A and Kishimoto T: IL-6: regulator
of Treg/Th17 balance. Eur J Immunol. 40:1830–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith AL, Robin TP and Ford HL: Molecular
pathways: targeting the TGF-β pathway for cancer therapy. Clin
Cancer Res. 18:4514–4521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamidullah, Changkija B and Konwar R: Role
of interleukin-10 in breast cancer. Breast Cancer Res Treat.
133:11–21. 2012. View Article : Google Scholar
|
|
31
|
Olkhanud PB, Baatar D, Bodogai M, et al:
Breast cancer lung metastasis requires expression of chemokine
receptor CCR4 and regulatory T cells. Cancer Res. 69:5996–6004.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franciszkiewicz K, Boissonnas A, Boutet M,
Combadière C and Mami-Chouaib F: Role of chemokines and chemokine
receptors in shaping the effector phase of the antitumor immune
response. Cancer Res. 72:6325–6332. 2012. View Article : Google Scholar : PubMed/NCBI
|