Introduction
Osteosarcoma is the most common malignant bone tumor
that predominantly occurs in childhood or adolescence. Current
therapies include surgical tumor resection and multi-agent
chemotherapy. The introduction of (neo)-adjuvant chemotherapy has
increased the 5-year survival rate for localized disease to more
than 50% compared to surgery alone. However, patients with
metastases at the initial diagnosis or poor response to
chemotherapy have a worse prognosis (1,2), and
critical non-surgical treatments have not yet been established. The
development of new therapeutic drugs with fewer side-effects is
strongly expected. Recently, various biological markers in
osteosarcoma have been reported and several therapeutic drugs
targeting these markers have been established, such as
17-N-allylamino-17-demethoxygeldanamycin (17-AAG) (Hsp90 inhibitor)
(3), trastuzumab (anti-Her2
antibody) (4), cixutumumab
(IMC-A12) (anti-IGF-1R antibody) (5) and temsirolimus (mTOR inhibitor)
(6). Yet, their clinical effects
were found to be limited at the clinical investigation stage.
Ubiquilin (UBQLN), a member of the ubiquitin-like
protein family (ubiquilins), delivers ubiquitinated proteins to the
proteasome for degradation and maintains protein homeostasis.
Recent evidence has shown that mutations of UBQLN1 or UBQLN2, as
scaffold proteins that allow abnormally modified proteins to pass
from the endoplasmic reticulum (ER) to the proteasome, are closely
associated with the pathogenesis of neurodegenerative disorders
such as Alzheimer’s disease and amyotrophic lateral sclerosis (ALS)
(7,8). Mutations of the UBQLN2 gene suppress
ubiquitin-mediated proteasomal degradation, leading to the
accumulation of inclusions composed of misfolded proteins linked to
the onset of ALS (8). Moreover,
higher UBQLN mRNA levels are associated with shorter survival of
lung cancer patients and UBQLN is an important factor in tumor
progression (9).
In the present study, we aimed to clarify the roles
of UBQLN2 in osteosarcoma by in vitro and in vivo
experiments. UBQLN2 overexpression was found to be a poor
prognostic factor for metastasis-free survival. In addition, UBQLN2
silencing through siRNA transfection effectively induced cellular
apoptosis and growth suppression of osteosarcoma cells both in
vitro and in vivo under hypoxic conditions. We found
that osteosarcoma progression was enhanced by UBQLN2 through
hypoxic stress tolerance.
Materials and methods
Cell culture
The human osteosarcoma cell line MG63 was obtained
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). The rat osteosarcoma cell line COS1NR was established from a
chemically induced osteosarcoma in a Fischer 344 rat by 4-hydroxy
quinolone 1-oxide in our laboratory (10,11).
MG63 and COS1NR cells were maintained in Dulbecco’s minimum
essential medium (Nacalai Tesque, Kyoto, Japan) with 10% fetal
bovine serum (Nichirei, Tokyo, Japan) and 50 U/ml
penicillin/streptomycin (Nacalai Tesque) under 5% CO2 at
37°C.
For the hypoxia experiments, the cells were cultured
and treated in Forma™ Series II 3130 Water-Jacketed CO2
Incubators (Thermo Fisher Scientific, Rockford, IL, USA) under 1%
O2, 5% CO2 and 94% N2.
Antibodies and chemicals
Antibodies against phosphorylated (p)-p38 and
p-c-Jun NH2-terminal kinase (JNK) were supplied by Cell
Signaling (Boston, MA, USA). An antibody against UBQLN2 was
obtained from Abnova Corporation (Taipei, Taiwan). Antibodies
against p38, JNK and actin were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). An antibody against
hypoxia-inducible factor (HIF)-1α was obtained from Novus
Biologicals (Littleton, MA, USA). An antibody against Ki-67 was
obtained from Thermo Fisher Scientific. An antibody against
vascular endothelial growth factor (VEGF) was obtained from Bioss
Antibodies (Woburn, MA, USA). The JNK inhibitor SP600125 and p38
inhibitor SB203580 were obtained from Calbiochem (San Diego, CA,
USA).
Preparation of cell lysates and western
blot analysis
MG63 cells were washed with phosphate-buffered
saline (PBS) and suspended in lysis buffer (40 mmol/l HEPES pH 7.4,
10% glycerol, 1% Triton X-100, 0.5% NP-40, 150 mmol/l NaCl, 50
mmol/l NaF, 20 mmol/l h-glycerophosphate, 1 mmol/l EDTA, 1 mmol/l
EGTA, 1 mmol/l phenylmethylsulfonyl fluoride and 0.1 mmol/l
vanadate) containing a protease inhibitor mixture (aprotinin,
leupeptin and pepstatin). Cell lysates were cleared by
centrifugation at 15,000 rpm for 30 min. Protein concentrations
were determined using the bicinchoninic acid (BCA) protein assay
reagent (Thermo Fisher Scientific). MG63 cell lysates were resolved
in SDS-polyacrylamide gels (Wako, Osaka, Japan) and transferred
onto polyvinylidene difluoride membranes (Millipore, Temecula, CA,
USA), followed by blocking with 5% skimmed milk at room temperature
for 1 h. The membranes were then incubated with the individual
primary antibodies described above overnight at 4°C, followed by
incubation with horseradish peroxidase-conjugated anti-mouse or
anti-rabbit IgG (Santa Cruz Biotechnology Inc.). We detected the
resulting peroxidase activity on X-ray film (Amersham Hyperfilm MP;
GE Healthcare, Amersham, UK) using an enhanced chemiluminescence
detection system (Western Lightning; Perkin Elmer, Waltham, MA,
USA).
Small interfering RNA (siRNA)
transfection for UBQLN2
For transfections, 8×104 MG63 cells/well
were seeded in 6-well dishes and transfected with 165 nmol/l of
siRNA against UBQLN2 (Qiagen, Venlo, The Netherlands).
Transfections were carried out using Lipofectamine RNAiMAX (Life
Technologies, Foster City, CA, USA) in accordance with the
manufacturer’s protocol. The UBQLN2 siRNA sequence was designed
after selection of appropriate DNA target sequences and was as
follows: 5′-TCCCATAAAGAGACCCTAATA-3′. The UBQLN2 siRNA sequence for
the rat experiments was also designed after selection of
appropriate DNA target sequences and was as follows:
5′-AACCATCGCGGCCATGTCAAA-3′.
Preparation of total RNA and RT-PCR
Total RNA was extracted using an RNeasy Mini kit
(Qiagen). Template cDNA was synthesized from 1 μg of total RNA
using a PrimeScript RT reagent kit (Perfect Real-Time), and RT-PCR
was performed using SYBR Premix Ex Taq II (Tli RNaseH Plus)
(both from Takara, Shiga, Japan). The PCR conditions were 95°C for
30 sec followed by 55–63°C for 30 sec, for a total of 35–45 cycles.
The amount of actin mRNA (sense, 5′-ATGGGTCAGAAGGATTCCTATGT-3′ and
antisense, 5′-GAAGGTCTCAAACATGATCTGGG-3′) was used to standardize
the quantity of UBQLN2 mRNA (sense, 5′-GCTGAATGAACTGCTGGTTGGG-3′
and antisense, 5′-CATAGGACCCACTGGCCCTG-3′).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labelling (TUNEL) assay
DNA cleavage, a characteristic of apoptosis, was
detected using the TUNEL assay (Apop Tag Plus Peroxidase In
Situ Apoptosis Detection Kit; Millipore). After siRNA
transfection, the cells were washed with PBS and fixed with
CytoRich Red (Becton-Dickinson, Franklin Lakes, NJ, USA) at room
temperature for 30 min. The fixed cells were washed with distilled
water, deposited on a slide, further fixed in 95% ethanol and
stained with the above kit. At least 600 cells from three different
fields were examined in each experiment, and cell death was
expressed as the percentage of TUNEL-positive cells.
Formalin-fixed and paraffin-embedded 5-μm-thick
sections of all rat tumor samples were deparaffinized in xylene,
dehydrated in a graded ethanol series and subsequently rinsed with
distilled water. Antigen retrieval was performed with proteinase K
(Dako, Glostrup, Denmark). Identification of apoptotic cells by
TUNEL staining was performed using the same kit. The apoptotic
index (per microscopic field at ×400 magnification) was calculated
as follows: Number of apoptotic cells × 100/total number of
cells.
Cell proliferation assay
Cells were stimulated with various reagents for a
specified period, followed by addition of MTS reagent,
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy
phenyl)-2-(4-sulphonyl)-2H-tetrazolium, inner salt (Promega, Tokyo,
Japan). After a 2-h incubation period, the optical absorbance at
490 nm was measured using a microplate reader (Multiskan FC; Thermo
Fisher Scientific). Cell viability was expressed as the mean ratio
± SD of the absorbance values after control RNA and UBQLN2 siRNA
transfections. All experiments were performed in triplicate.
In vivo UBQLN2 treatment in syngeneic
rats
The animal experiments were approved by The
Institutional Animal Care and Use Committee at Nara Medical
University. COS1NR cells (5×106) in 100 μl of PBS were
inoculated into subcutaneous tissue in the back of 7-week-old male
Fischer 344 rats. When each tumor diameter reached 2 mm, we started
injections of 20 μmol/l of control RNA or 20 μmol/l of UBQLN2 siRNA
with atelocollagen (AteloGene; Koken Co., Ltd., Tokyo, Japan)
mixture into groups of 5 rats every week for a total of three
times. The growth rate of each tumor was evaluated twice a week.
The sizes of the tumors were calculated by the formula for volume
(V): V = 0.2618 × L × W × (L+W) (12). The rats were euthanized at 5 weeks
after the first injection, and the subcutaneous tumors were
excised, weighed, and fixed in 10% formalin for histological,
immunohistochemical and TUNEL assay assessments.
Tissue samples and
immunohistochemistry
After obtaining approval from our Institutional
Review Board (authorization no. 575), we searched the surgical
pathology database of Nara Medical University from 1984 to 2012,
and identified 34 cases of human high-grade osteosarcomas and 6
cases of low-grade osteosarcomas. As benign counterparts, 11
specimens of reactive bone (including fracture callus, reactive new
bone formation and osteomyelitis) were also retrieved. The
diagnosis of osteosarcoma was made according to the latest edition
of the World Health Organization classification (13). The clinicopathological data of the
high-grade osteosarcoma cases are summarized in Table I. The surgical staging was based on
the 7th edition of the American Joint Committee on Cancer (AJCC)
Staging Manual (14). Clinical
details and follow-up information were obtained by reviewing the
medical charts. All primary tumors were treated either surgically
or with carbon ion radiotherapy, accompanied by neoadjuvant and
adjuvant chemotherapy. The median follow-up period after surgery
was 6 years (range, 1–16 years). Metastasis-free survival was
defined as the interval from diagnosis to discovery of a metastasis
or the last follow-up examination.
 | Table IClinicopathological characteristics of
the high-grade osteosarcoma cases. |
Table I
Clinicopathological characteristics of
the high-grade osteosarcoma cases.
| Characteristics | Data |
|---|
| Age (years) |
| <40 | 30 |
| ≥40 | 4 |
| Median | 20.1 |
| Range | 7–51 |
| Gender |
| Male | 24 |
| Female | 10 |
| Site |
| Femur | 13 |
| Tibia or fibula | 12 |
| Others | 9 |
| Surgical stage |
| IA | 0 |
| IB | 0 |
| IIA | 7 |
| IIB | 24 |
| III | 0 |
| IVA | 3 |
| IVB | 0 |
| Surgery |
| Amputation | 12 |
| Limb salvage | 18 |
| Total | 34 |
Immunohistochemical staining for UBQLN2 was
performed in all cases. All of the osteosarcoma samples were
obtained by open biopsy. Tumor tissues from each case were fixed in
10% neutral-buffered formalin and embedded in paraffin. Specimens
were cut at 4-μm intervals and mounted for immunohistochemical
analyses and histopathological analysis by conventional hematoxylin
and eosin (H&E) staining. The H&E-stained sections served
as a guide for the immunohistochemical analyses.
Immunohistochemistry was performed using a Histofine Simple Stain
kit (Nichirei) according to the manufacturer’s instructions. After
deparaffinization in xylene and sequential hydration in 100 and 95%
ethanol, the sections were heated at 120°C for 10 min in 10 mM
sodium citrate buffer (pH 6.0). The sections were cooled down,
incubated in 3% H2O2 solution for 5 min to
block endogenous peroxidase activity, rinsed in PBS for 5 min, and
incubated with the primary antibody against UBQLN2 (1:500 dilution)
for 1 h at room temperature. After three washes with PBS, the
sections were incubated with secondary antibodies for 30 min at
room temperature. Diaminobenzene was used as the chromogen, with
hematoxylin as a nuclear counterstain. The stained sections were
dehydrated, cleared and mounted. The intensity of
immunohistochemical staining was evaluated at ×400 magnification
with a microscope (BX51; Olympus, Tokyo, Japan). The levels of
immunostaining were graded by scoring the percentages of positivity
into two groups: negative (<60%) and positive (≥60%).
Statistical analysis
Data were statistically analyzed using the Student’s
t-test, with the Mann-Whiney U test used for non-parametric
analyses. Metastasis-free survival was estimated by the
Kaplan-Meier method. The log-rank test was used to evaluate the
differences between survival curves. All analyses were performed
with IBM SPSS version 20.0 (IBM Co., Armonk, NY, USA). Values of
P<0.05 were considered to indicate a statistically significant
result.
Results
Expression of UBQLN2 in human
osteosarcoma
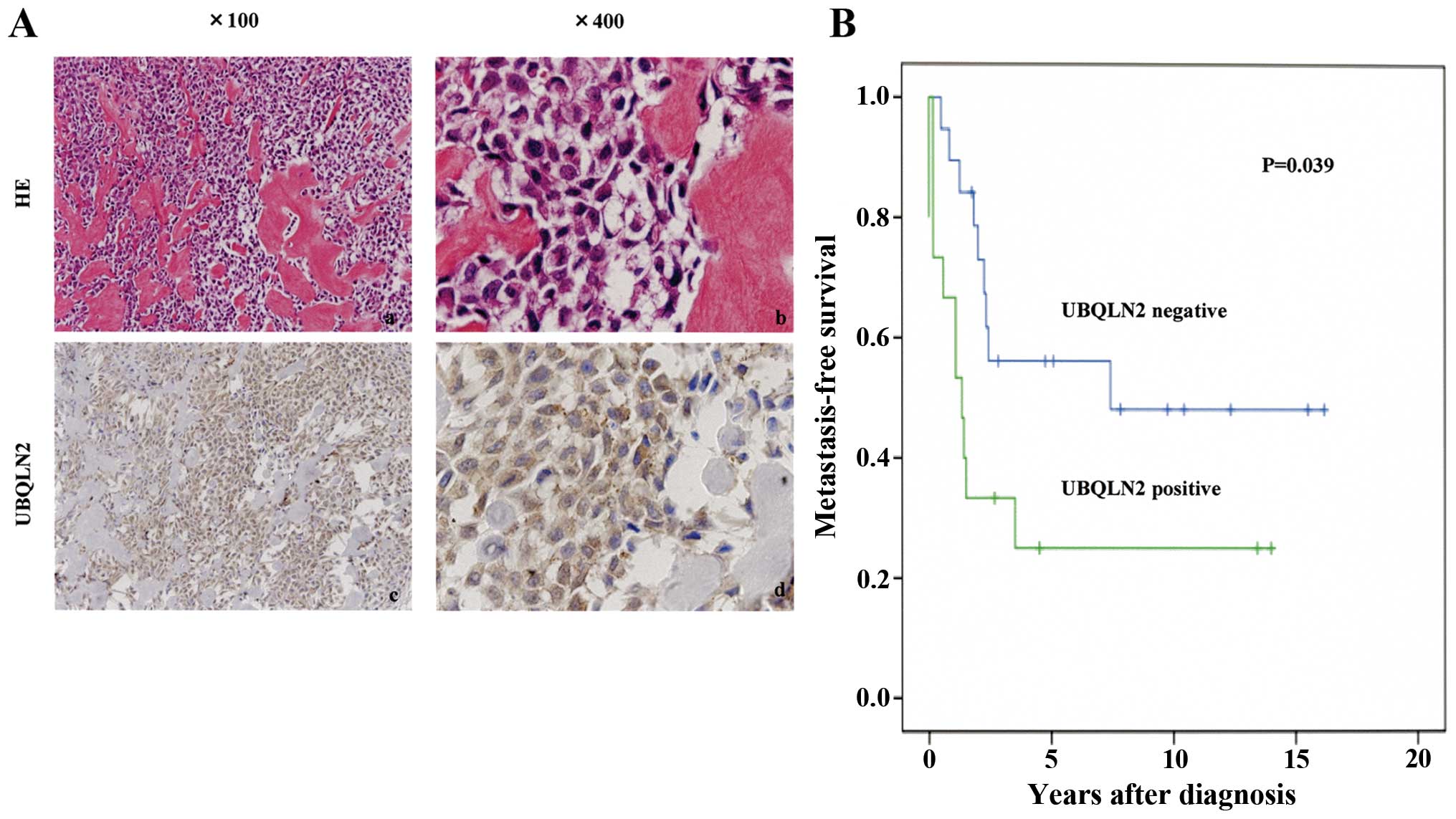
UBQLN2 expression was positive in 44% (15/34) of the
high-grade osteosarcomas (Fig. 1A)
and 33% (2/6) of the low-grade osteosarcomas in contrast to no
expression in reactive bone (0/11). Positivity for UBQLN2 (>60%
UBQLN2-positive tumor cells) was significantly associated with
unfavorable metastasis-free survival of the osteosarcoma patients
as well as AJCC surgical stage (IIB, III and IVA/B) in the
univariate prognostic analyses (P<0.05; Fig. 1B, Table
II).
 | Table IIUnivariate analysis for
metastasis-free survival. |
Table II
Univariate analysis for
metastasis-free survival.
| Variable | No. of
patients | 5-year metastasis-
free survival (%) | P-value |
|---|
| Age (years) | | | 0.49 |
| <40 | 30 | 41.6 | |
| ≥40 | 4 | 50.0 | |
| Gender | | | 0.62 |
| Male | 24 | 40.7 | |
| Female | 10 | 46.7 | |
| Site |
| Extremity | 30 | 41.7 | 0.85 |
| Trunk | 4 | 50.0 | |
| AJCC staging | | | 0.007 |
| IA.B, IIA | 7 | 100.0 | |
| IIB, III,
IVA.B | 27 | 26.6 | |
| UBQLN2 | | | 0.039 |
| Negative | 19 | 56.1 | |
| Positive | 15 | 25.0 | |
Silencing of UBQLN2 induces apoptosis in
human osteosarcoma cells under hypoxic conditions
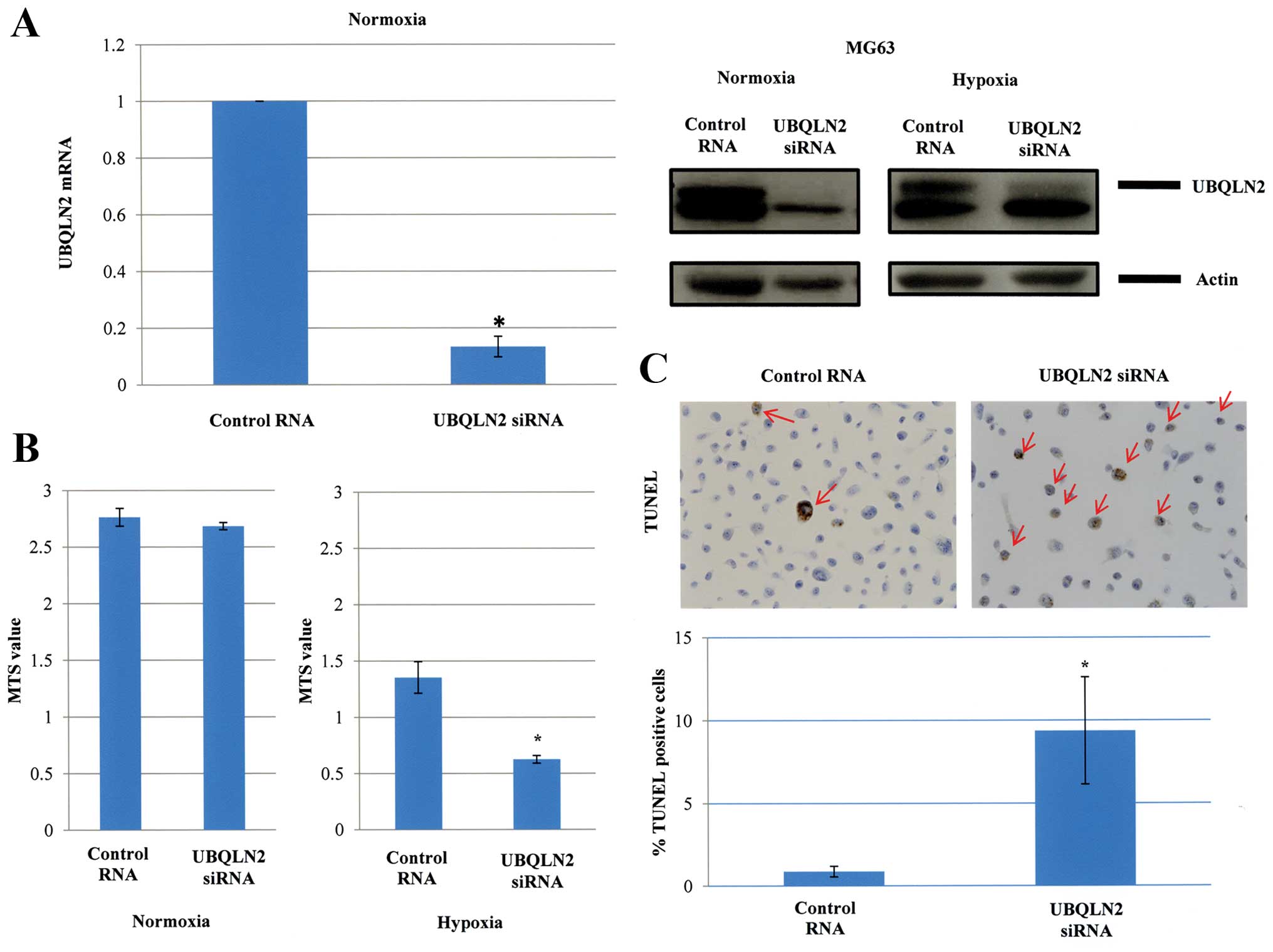
Human osteosarcoma MG63 cells expressing UBQLN2 were
used in the present study. These cells expressed both UBQLN2 mRNA
and protein, which were strongly reduced under both normoxia and
hypoxia (1% O2) after UBQLN2 siRNA transfection
(Fig. 2A). MG63 cells were cultured
for 48 h under normoxia after UBQLN2 gene silencing. However, the
cell growth was not significantly affected. In contrast, the cell
survival was strongly suppressed by UBQLN2 gene silencing under
hypoxic conditions (1% O2) (Fig. 2B). As shown in Fig. 2C, the percentage of apoptotic cells
was significantly increased in response to UBQLN2 gene silencing as
assessed by TUNEL assays (0.9% for control RNA transfection vs.
9.4% for UBQLN2 siRNA transfection; P=0.04).
JNK and p38 activation contributes to
apoptosis induced by UBQLN2 downregulation under hypoxia
Since stress kinases, including JNK and p38, are
known to play essential roles in the apoptosis induced by various
extrinsic stimuli in cancer cells (15), we examined whether mitogen-activated
protein kinase (MAPK) activation contributed to the cytotoxicity
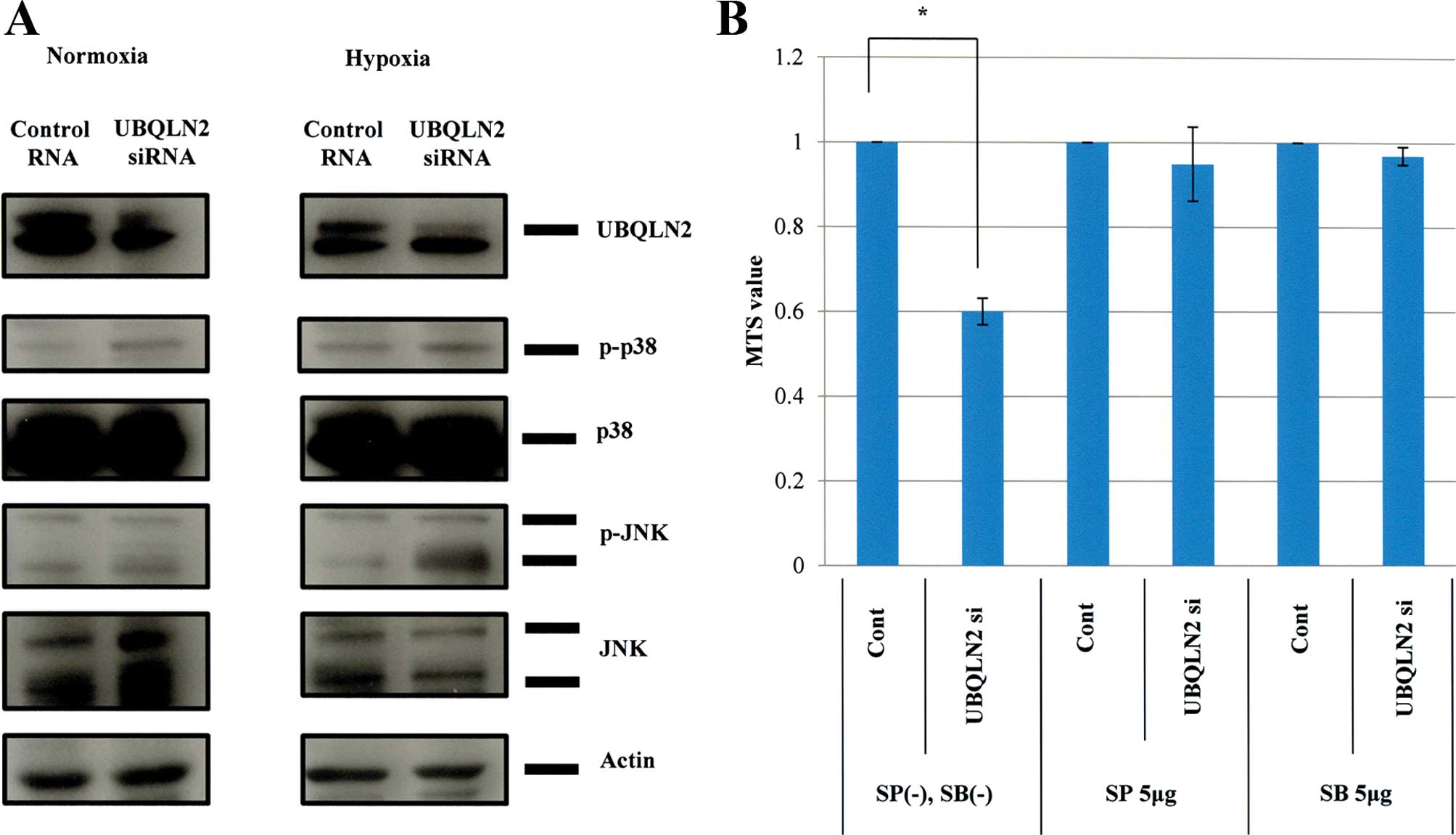
induced by UBQLN2 knockdown under hypoxic conditions. Western blot
analyses revealed that both p38 and JNK were activated in MG63
cells under hypoxic conditions following UBQLN2 siRNA transfection,
in contrast to only p38 activation under normoxic conditions
(Fig. 3A). Treatment with p38 or
JNK inhibitors cancelled the cytotoxic effects of UBQLN2 knockdown
under hypoxic conditions in the MG63 cells (Fig. 3B). TUNEL assays produced similar
findings (data not shown). Thus, activation of both p38 and JNK
appeared to be required for apoptosis induction by UBQLN2 gene
silencing. We examined whether the master of hypoxia, HIF-1α, was
inhibited by silencing of UBQLN2. The results showed that HIF-1α
was not inhibited by silencing of UBQLN2 (data not shown). These
findings showed that activation of the stress kinases JNK and p38
was essential for the apoptosis induced by silencing of UBQLN2
under hypoxia, and was independent of HIF-1α stabilization.
Growth of osteosarcoma is suppressed by
UBQLN2 siRNA transfection in vivo
The experimental protocol for this part of the
present study is illustrated in Fig.
4A. Briefly, COS1NR cells were inoculated into subcutaneous
tissue in the back of Fischer 344 rats. When each tumor diameter
reached 2 mm (at 4–6 weeks after inoculation), injection of either
20 μmol/l of control RNA or 20 μmol/l of UBQLN2 siRNA with
atelocollagen mixture was initiated every week for a total of three
times. The rats were euthanized at 5 weeks after the first
injection, and evaluated for the volume and weight of their tumors.
UBQLN2 downregulation was found to inhibit tumor growth.
Significant differences in the tumor volume (P=0.01) and tumor
weight (P=0.01) at euthanasia were observed between the groups
treated with control RNA and UBQLN2 siRNA (Fig. 4A and B). TUNEL assays revealed a
significant increase in the number of cells undergoing apoptosis
after UBQLN2 knockdown (3.7% for UBQLN2 siRNA vs. 0% for control
RNA; P=0.009; Fig. 4C). There were
no differences in Ki-67, HIF-1α or VEGF expression between control
RNA and UBQLN2 siRNA injections (data not shown).
Discussion
In the present study, we demonstrated for the first
time that UBQLN2 protects osteosarcoma cells against hypoxic
stress-induced cytotoxicity, independently from HIF-1α-mediated
signaling. Hypoxia is known to activate two opposite cytotoxic and
cytoprotective pathways. The representative cytotoxicity is
apoptosis via hypoxia-induced mitochondrial membrane permeability,
leading to release of cytochrome c into the cytoplasm and
apoptosome formation in which caspase-9 is activated. Stress
kinases, particularly MAPKs such as JNK, have been shown to play
important roles in hypoxia-induced apoptosis (16). On the other hand, the cytoprotective
effect of hypoxia is mainly mediated by HIF-1α, which allows tumor
cells to adapt to the hypoxic microenvironment and to acquire
invasive and metastatic biological properties (17). Our results showed that silencing of
UBQLN2 induced apoptosis in human osteosarcoma MG63 cells under
hypoxic conditions without any change in HIF-1α expression in
vitro, and that osteosarcoma growth was suppressed and
apoptosis was induced by UBQLN2 siRNA transfection while HIF-1α
expression remained unchanged in vivo. Therefore, UBQLN2
enhanced osteosarcoma progression by inhibiting the apoptotic
pathway, yet not the HIF-1α-mediated pathway. Immunohistochemical
and prognostic analyses for UBQLN2 performed in human high-grade
osteosarcomas demonstrated unfavorable metastasis-free survival
with UBQLN2 overexpression. While no or reduced HIF-1α expression
in primary osteosarcoma was found in this study (data not shown),
HIF-1α is overexpressed in metastatic osteosarcoma rather than in
the primary tumors (18). Frequent
expression of two HIF-1α downstream molecules, VEGF and
platelet-derived growth factor (PDGF), is correlated with inferior
event free-survival in osteosarcoma (19,20).
With regard to malignant tumor effects related to UBQLN, it
stabilizes BCL2L10/BCLb, one of the six anti-apoptotic members of
the BCL2 family, inhibits apoptosis, and contributes to the
survival of human lung cancer cells (9). Thus, the function of UBQLN in cancer
cells is the same as that in osteosarcoma, in that it inhibits
hypoxia-induced apoptosis in tumor cells.
UBQLN, a member of the ubiquitin-like protein
family, is characterized by the presence of N-terminal
ubiquitin-like and C-terminal ubiquitin-associated domains, and
delivers ubiquitinated proteins to the proteasome for degradation.
In accordance with this function, the ubiquitin-like domain of
UBQLN binds to subunits of the proteasome, and its
ubiquitin-associated domain binds to polyubiquitin chains that
typically accelerate protein degradation mediated by the proteasome
(21). Moreover, recent studies
have shown that UBQLN regulates endoplasmic reticulum
(ER)-associated protein degradation (ERAD), by which misfolded
proteins are translocated from the ER to the cytoplasm for
proteasomal degradation (22,23).
In addition, UBQLN was shown to be linked to macroautophagy, an
alternative degradation pathway by which cellular cargos are
sequestered in double-membrane structures called autophagosomes and
subsequently fuse with lysosomes harboring the acid hydrolases
involved in protein degradation (24,25).
UBQLN maintains protein homeostasis and aids cell survival through
the degradation of misfolded or damaged proteins, which accumulate
upon stimulation with various stresses, including hypoxia and/or
starvation. Our finding that UBQLN2 inhibits hypoxic stress-induced
apoptosis in osteosarcoma cells can also be deduced from the above
reports. Mutations of the UBQLN2 gene suppress ubiquitin-mediated
proteasomal degradation, leading to the accumulation of inclusions
composed of misfolded proteins linked to the onset of ALS (8). Chronically reduced vascular perfusion
by aging or other factors can produce chronic or episodic deficits
in oxygen (hypoxia). This hypoxic stress may induce neuronal cell
death and lead to the occurrence of ALS (26). Thus, UBQLN2 inhibits hypoxic
stress-induced apoptosis in neuronal cells and prevents the
occurrence of ALS. It is of great interest that a number of common
signals involved in cell survival actually function in both
neuronal and osteosarcoma cells.
In the present study, JNK and p38 were activated in
MG63 cells under hypoxia following UBQLN2 siRNA transfection, while
p38, but not JNK was activated under normoxia. In addition, either
the JNK or the p38 inhibitor was able to significantly suppress the
apoptosis induction. Thus, activation of both JNK and p38 is
required for induction of apoptosis in these cells. Our results
indicate that UBQLN2 may inhibit JNK activation and apoptosis in
response to hypoxic stress, thereby successfully enhancing the
malignant potential of osteosarcoma cells. JNK plays a critical
role in both death receptor- and mitochondrial-mediated apoptotic
pathways by upregulating pro-apoptotic genes via transactivation of
specific transcription factors or by modulating the phosphorylation
of pro-apoptotic and anti-apoptotic proteins in mitochondria
(27). Similar to JNK, p38 seems to
sensitize cells to apoptosis via upregulation of pro-apoptotic
proteins and downregulation of survival pathways (28). Our results were thus in line with
these previous reports. We are currently investigating how the
expression and/or activity of upstream kinases that do not activate
p38, but do activate JNK, are modified by UBQLN2 gene silencing in
osteosarcoma cells under hypoxic conditions.
Solid tumors including osteosarcoma are well-known
to be exposed to hypoxia due to an inadequate oxygen supply,
particularly when the tumors outgrow their blood supply (29,30).
Coagulative tumor necrosis, which is caused by an inadequate blood
supply and hypoxic conditions, is closely related to advancement of
malignant tumors and poor response to chemotherapy, including cases
of osteosarcoma (31). Since UBQLN2
protected osteosarcoma cells against hypoxia-induced apoptosis, a
drug that inhibits UBQLN2 could lead osteosarcoma cells under
hypoxia toward apoptosis. Thus, we can provide new methods for
treating osteosarcoma that kill osteosarcoma cells under normoxia
using conventional anticancer agents and surgery or kill these
cells under hypoxia showing high invasion, metastatic potential and
resistance to chemotherapy using UBQLN2 inhibitors. From the
finding that UBQLN2 downregulation under normoxia did not inhibit
the growth of osteosarcoma cells, UBQLN2 inhibitors will not affect
normal tissues under normoxia and if used as drugs would confer few
side-effects.
In summary, UBQLN2 is essential for resistance to
hypoxic stress and acquisition of high malignant potential in
osteosarcoma, and silencing of UBQLN2 enhances hypoxia-induced
apoptosis in vitro and in vivo. In addition, UBQLN2
overexpression is significantly associated with unfavorable
metastasis-free survival. UBQLN2 could represent a new molecular
target and a useful clinicopathological marker in osteosarcoma.
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: state
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bagatell R, Gore L, Egorin MJ, Ho R,
Heller G, et al: Phase I pharmacokinetic and pharmacodynamic study
of 17-N-allyl-amino-17-demethoxygeldanamycin in pediatric patients
with recurrent or refractory solid tumors: a pediatric oncology
experimental therapeutics investigators consortium study. Clin
Cancer Res. 13:1783–1788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ebb D, Meyers P, Grier H, et al: Phase II
trial of trastuzumab in combination with cytotoxic chemotherapy for
treatment of metastatic osteosarcoma with human epidermal growth
factor receptor 2 overexpression: a report from the Children’s
Oncology Group. J Clin Oncol. 30:2545–2551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigel B, Malempati S, Reid JM, et al:
Phase 2 trial of cixutumumab in children, adolescents, and young
adults with refractory solid tumors: a report from the Children’s
Oncology Group. Pediatr Blood Cancer. 61:452–456. 2014. View Article : Google Scholar
|
|
6
|
Moroney J, Fu S, Moulder S, et al: Phase I
study of the antiangiogenic antibody bevacizumab and the
mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with
liposomal doxorubicin: tolerance and biological activity. Clin
Cancer Res. 18:5796–5805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertram L, Hiltunen M, Parkinson M, et al:
Family-based association between Alzheimer’s disease and variants
in UBQLN1. N Engl J Med. 352:884–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng HX, Chen W, Hong ST, et al: Mutations
in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and
ALS/dementia. Nature. 477:211–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beverly LJ, Lockwood WW, Shah PP,
Erdjument-Bromage H and Varmus H: Ubiquitination, localization, and
stability of an anti-apoptotic BCL2-like protein, BCL2L10/BCLb, are
regulated by Ubiquilin1. Proc Natl Acad Sci USA. 109:E119–E126.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Honoki K, Mori T, Tsutsumi M, et al:
Heterogeneous pattern of gene expression in cloned cell lines
established from a rat transplantable osteosarcoma lung metastatic
nodule. Cancer Lett. 127:221–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honoki K, Tsutsumi M, Miyauchi Y, et al:
Increased expression of nucleoside diphosphate kinase/nm23 and
c-Ha-ras mRNA is associated with spontaneous lung metastasis in
rat-transplantable osteosarcomas. Cancer Res. 53:5038–5042.
1993.PubMed/NCBI
|
|
12
|
Luu HH, Kang Q, Park JK, et al: An
orthotopic model of human osteosarcoma growth and spontaneous
pulmonary metastasis. Clin Exp Metastasis. 22:319–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: Osteogenic tumours. WHO Classification of Tumours of
Soft Tissue and Bone. IARC; Lyon: pp. 275–296. 2013
|
|
14
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
The American Joint Committee on Cancer; 2010
|
|
15
|
Shimada K, Nakamura M, Ishida E and
Konishi N: Molecular roles of MAP kinases and FADD phosphorylation
in prostate cancer. Histol Histopathol. 21:415–422. 2006.PubMed/NCBI
|
|
16
|
Kunz M and Ibrahim SM: Molecular responses
to hypoxia in tumor cells. Mol Cancer. 2:232003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semenza GL: Hypoxia-inducible factors:
mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizobuchi H, García-Castellano JM, Philip
S, Healey JH and Gorlick R: Hypoxia markers in human osteosarcoma:
an exploratory study. Clin Orthop Relat Res. 466:2052–2059. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubo T, Piperdi S, Rosenblum J, et al:
Platelet-derived growth factor receptor as a prognostic marker and
a therapeutic target for imatinib mesylate therapy in osteosarcoma.
Cancer. 112:2119–2129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S and
Chen X: VEGF and EMMPRIN expression correlates with survival of
patients with osteosarcoma. Surg Oncol. 20:13–19. 2011. View Article : Google Scholar
|
|
21
|
Ko HS, Uehara T, Tsuruma K and Nomura Y:
Ubiquilin interacts with ubiquitylated proteins and proteasome
through its ubiquitin-associated and ubiquitin-like domains. FEBS
Lett. 566:110–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim TY, Kim E, Yoon SK and Yoon JB: Herp
enhances ER-associated protein degradation by recruiting
ubiquilins. Biochem Biophys Res Commun. 369:741–746. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim PJ, Danner R, Liang J, et al:
Ubiquilin and p97/VCP bind erasin, forming a complex involved in
ERAD. J Cell Biol. 187:201–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
N’Diaye EN, Kajihara KK, Hsieh I, et al:
PLIC proteins or ubiquilins regulate autophagy-dependent cell
survival during nutrient starvation. EMBO Rep. 10:173–179. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rothenberg C, Srinivasan D, Mah L, et al:
Ubiquilin functions in autophagy and is degraded by
chaperone-mediated autophagy. Hum Mol Genet. 19:3219–3232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skene JP and Cleveland DW: Hypoxia and Lou
Gehrig. Nat Genet. 28:107–108. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porras A, Zuluaga S, Black E, Valladares
A, Alvarez AM, Ambrosino C, Benito M and Nebreda AR: p38α
mitogen-activated protein kinase sensitizes cells to apoptosis
induced by different stimuli. Mol Biol Cell. 15:922–933. 2004.
View Article : Google Scholar :
|
|
29
|
Hockel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
30
|
Vaupel P and Harrison L: Tumor hypoxia:
causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9(Suppl 5): S4–S9. 2004. View Article : Google Scholar
|
|
31
|
Björnsson J, Inwards CY, Wold LE, Sim FH
and Taylor WF: Prognostic significance of spontaneous tumour
necrosis in osteosarcoma. Virchows Archiv A Pathol Anat.
423:195–199. 1993. View Article : Google Scholar
|