Introduction
Gastric cancer (GC) causes 12% of all cancer-related
deaths each year (1,2). The interaction of multiple factors
contributes to the development of gastric carcinogenesis, including
regulatory changes in oncogenes and tumor suppressor genes
(3,4). The regulation of oncogenes and tumor
suppressor genes occurs not only at the transcriptional level, but
also at the post-transcriptional level.
MicroRNAs (miRNAs, miRs) are a new class of small
(~22 nucleotide) noncoding RNAs that negatively regulate
protein-coding gene expression by targeting mRNA degradation or
translation inhibition (5–7). Profiling studies revealed the
contribution of aberrant miRNA expression to GC initiation and
progression by perturbing the function of target genes (8,9).
Previous studies had shown that miR-182 is poorly expressed in
human breast cancer stem cells, human and mouse normal mammary stem
cells, and embryonic carcinoma (10). miR-182 could deregulate RGS17 by
targeting its 3′UTR to ultimately suppress the occurrence of lung
cancer (11). Although miR-182 is
downregulated and can suppress cell growth and target
cAMP-responsive element-binding protein 1 in human gastric cancer
(12), the various mechanisms of
miR-182 inhibiting proliferation of gastric cancer cells are still
emerging.
The roles of ANUBL1, also known as ZFAND4, are
scarely reported in cancer. Firstly, we demonstrated that ANUBL1
expression was specifically upregulated in gastric cancer and it
was positively associated with grades of gastric cancer. Moreover,
it promoted proliferation of gastric cancer SGC-7901 cells.
Secondly, we analyzed the mechanism of ANUBL1 upregulation in
gastric cancer. We predicted that miR-182 could target 3′UTR of
ANUBL1 mRNA by bioinformatic methods and the following studies
confirmed the prediction that miR-182 suppressed expression of
ANUBL1 through targeting its 3′UTR. Moreover, we found that ANUBL1
could downregulate anti-proliferation-associated miRNA (miR-148b,
miR-375 and miR-182) expression in SGC-7901 cells. Finally, we
demonstrated that introduction of ANUBL1 cDNA lacking predicted
sites of 3′-UTR abrogates miR-182 cellular function (unpublished
data).
In this study, we not only showed that ANUBL1 as an
oncogene was upregulated and could promote proliferation of
SGC-7901 cells, but also demonstrated that its overexpression led
to a strong decrease of miR-182 expression and expression of
ANUBL1 was in turn directly downregulated by miR-182,
thereby establishing a negative feedback loop between miR-182 and
ANUBL1. The elucidation of the mechanisms of miR-182 targeting
ANUBL1 in gastric cancer helps us to further understand the
mechanism of gastric cancer initiation and progression.
Material and methods
Human tissue samples
One hundred and fifty pairs of human gastric tissue
samples were obtained from patients who underwent surgical
resection at the Division of General Surgery, Shanghai Jiao Tong
University Affiliated Sixth People’s Hospital between 2009 and 2014
and were diagnosed with gastric cancer based on histopathological
evaluation (32, WHO grade I; 34, WHO grade I; 40, WHO grade III;
and 37, WHO grade IV). The mean age was 55 years (range: 30–74).
The matched non-tumor adjacent tissue was obtained from a segment
of the resected specimens that was the farthest from the tumor
(>5 cm). The samples were snap-frozen in liquid nitrogen and
stored at −80°C. No local or systemic treatment was conducted in
these patients before the operation. The use of human tissue
samples followed internationally recognized guidelines as well as
local and national regulations. Research carried out on humans
followed international and national regulations. Medical ethics
committees of Shanghai Jiao Tong University Affiliated Sixth
People’s Hospital approved the experiments undertaken. Informed
consent was obtained from each individual.
Immunohistochemistry
Immunohistochemisty was performed using standard
techniques. Antigen retrieval was performed by autoclaving.
Incubation with 10% normal goat serum in phosphate-buffered saline
was performed for 15 min to eliminate non-specific staining.
Incubation with anti-ANUBL1 antibody (Abcam, Cambridge, MA, USA)
was carried out. Finally, sections were lightly counterstained with
10% Mayer hematoxylin, dehydrated, mounted, and observed. Staining
was evaluated by a pathologist and an investigator blinded to
diagnosis. Sections were classified as - (negative), + (focal and
weak immunoreactivity), ++ (diffuse and weak or focal and intense
immunoreactivity), +++ (diffuse and intense immunoreactivity). The
data were analyzed by SPSS 11.5 statistical package. Expression of
ANUBL1 was evaluated according to the ratio of positive cells per
specimen and staining intensity (13). The comparison of the expression
rates was by Chi-square test.
Cell culture, plasmids and
transfection
Human gastric cancer SGC-7901 cells were obtained
from MD Anderson Cancer Center (Houston, TX, USA). SGC-7901 human
gastric cancer cells were maintained in RPMI-1640 (Gibco, Grand
Island, NY, USA) with 10% heat-inactivated fetal bovine serum
(Gibco), 100 IU/ml penicillin, and 0.1 mg/ml streptomycin, in a
humidified 5% atmosphere of CO2 at 37°C.
ANUBL1-expressing plasmids/empty vector (pcDNA3.1) and
sh-ANUBL1/scramble were purchased from the National RNAi Core
Facility in Academic Sinica, Taipei, Taiwan. The ANUBL1-expressing
plasmids/empty vector (pcDNA3.1) and sh-ANUBL1/scramble used for
each transfection is 10 nM, unless otherwise specified. Pre-miR-182
and control-miR were purchased from Ambion (Austin, TX, USA). For
transfection experiments, the cells were cultured in serum-free
medium without antibiotics at 60% confluence for 24 h, and then
transfected with transfection reagent (Lipofectamine 2000,
Invitrogen, Carlsbad, CA, USA) according to manufacturer’s
instructions. After incubation for 6 h, the medium was removed and
replaced with normal culture medium for 48 h.
Colony formation
For colony formation assay, cells were transfected
with ANUBL1 expressing plasmids or sh-ANUBL1 plasmids for 24 h, and
then seeded in a 6-well plate. FBS (0.2 ml) was added per well on
day 5. After 9–10 days of incubation, plates were washed with PBS
and stained with 0.1% of crystal violet. Colonies with over 50
cells were manually counted. Plating efficiency was calculated by
dividing the number of colonies formed in the treated group by that
in control.
Cell-cycle analysis
Cells (8.0×105) were seeded into a 100-mm
culture plate and allowed to attach overnight. The cells were
transfected with plasmids for 24 h, washed twice with NaCl/Pi, and
then centrifuged at 200 × g at room temperature. The pellet was
resuspended in 1 ml cold NaCl/Pi and fixed in 70% ethanol for at
least 12 h at 4°C. The fixed cells were incubated with 100 μl
DNase-free RNaseA (200 μg/ml) for 30 min at 37°C, and then 1 mg/ml
propidium iodide was added. The stained cells were analyzed using a
fluorescence-activated cell sorter (BD Accuri C6; BD Biosciences,
Ann Arbor, MI, USA). The percentages of cells in the G1, S and G2/M
phases of the cell cycle were determined using CellQuest Pro
software (FlowJo, Ashland, OR, USA).
MTT assay
The effect of the cell proliferation was assessed by
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium (MTT; Sigma,
St. Louis, MO, USA) assay. SGC-7901 cells were transfected with
ANUBL1 or pcDNA3.1 (mock), sh-ANUBL1 or scramble (mock). These
cells were incubated in a humidified atmosphere with 5%
CO2. Then, MTT solution (4.14 mg/ml) was added to each
well and the optical density was measured after incubation at 37°C
for 4 h (14). Absorbance was
directly proportional to the number of survival cells.
Bromodeoxyuridine labeling and
immunofluorescence
Cells grown on coverslips (Fisher, Pittsburgh, PA)
were incubated with bromodeoxyuridine (BrdUrd) for 1 h and stained
with anti-BrdUrd antibody (Upstate Biotechnology, Temecula, CA,
USA) according to the manufacturer’s instruction. Images were
acquired under a laser scanning microscope (Axioskop 2 plus, Carl
Zeiss Co. Ltd., Jena, Germany).
Reverse transcription-polymerase chain
reaction and real-time for ANUBL1
Total RNA was isolated from cells or tissues using
Trizol reagent (cat no. 11647229001, Invitrogen). cDNA was
synthesized from 1 μg of total RNA in a 20 μl reverse transcription
(RT) system followed by PCR amplification in a 50 μl PCR system
performed using an RT-PCR kit (cat no. A3500, Promega, Madison, WI,
USA). Housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as RNA loading control. The PCR primer sequences
are as follows: GAPDH: Forward - ATTCAACGGCACAGT CAAGG, Reverse -
GCAGAAGGGGCGGAGATGA; PCNA: Forward - CTGTAGCGGCGTTGTTGC, Reverse -
TCG TTGATGAGGTCCTTG; Ki67: Forward - CAACTATCCT CGTCTGTCC, Reverse
- GGTCCCTAAAGATGTGCT; p21: Forward - CCCGTGAGCGATGGAACT, Reverse -
CGA GGCACAAGGGTACAAGA; p53: Forward - CCTCCTCA GCATCTTATCCG,
Reverse - CACAAACACGCACCTC AAA; Rb: Forward - AAGGTTTCAGGGTATCAG,
Reverse - GTGGGTCTGTATGTTGTG; E2F1: Forward - AAGAAC CGCTGTTGTCCCG,
Reverse - TCGAGGCCGAAGTGG TAGTC; CDK2: Forward -
AGAAACAAGTTGACGGGAG, Reverse - GAAGAGGAATGCCAGTGAG; CDK4: Forward -
CAGTTCGTGAGGTGGCTTTA, Reverse - GGGGTGCC TTGTCCAGATA; CDK6: Forward
- TGCCCACTGAAA CCATAAAGG, Reverse - ATCCACAGCGTGACGACCA; The PCR
cycles were conducted according to manufacturer’s instruction and
the PCR products were analyzed by agarose gel electrophoresis. Gels
were photographed and densities of the bands were determined with a
computerized image analysis system (Alpha Innotech, San Leandro,
CA, USA). The area of each band was calculated as the integrated
density value (IDV). Real-time PCR for ANUBL1 was done with a Power
SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA)
according to the manufacturer’s protocol. The primer sequences for
ANUBL1: Forward - CCGAGACAGATA ACGAGGAT-3′, Reverse -
GAGGTCTTTCTGCTAGAC-3′.
Western blot analysis
Protein extracts from the cells or tissue samples
were separated by SDS-PAGE, transferred to PVDF membrane (15). After incubation with rabbit
anti-ANUBL1, anti-Ki67, anti-PCNA, anti-p21, anti-p53, anti-RB,
anti-CDK2, anti-CDK4, anti-CDK6 (all from Abcam) and anti-β-actin
antibodies overnight at 4°C, IRDyeTM-800 conjugated
anti-rabbit secondary antibodies (Li-COR, Biosciences, Lincoln, NE,
USA) were used for 30 min at room temperature. The specific
proteins were visualized by Odyssey™ Infrared Imaging System (Gene
Company, Lincoln, NE, USA).
Immunofluorescence analyses
For immunofluorescence analyses, cells were plated
on glass coverslips in six-well plates and transfected with 30 nM
pre-miR-182 or control miR. At 36 h after transfection, coverslips
were stained with the mentioned anti-ANUBL1 antibodies. Alexa Fluor
488 goat anti-mouse IgG antibody or goat anti-rabbit IgG antibody
was used as secondary antibody (Invitrogen). Coverslips were
counterstained with DAPI (Invitrogen-Molecular Probes, Eugene,
Oregon, USA) for visualization of the nuclei. Microscopic analysis
was performed with a confocal laser-scanning microscope (Leica
Microsystems, Bensheim, Germany). Fluorescence intensities were
measured in a few viewing areas for 200–300 cells per coverslip and
analyzed using ImageJ 1.37v software (http://rsb.info.nih.gov/ij/index.html).
miRNA microarray
Total RNA from cultured cells, with efficient
recovery of small RNAs, was isolated using the mirVana miRNA
Isolation kit (cat no. AM1561, Ambion). cRNA for each sample was
synthesized by using 3′IVT Express kit according to the
manufacturer’s protocols. The purified cRNA was fragmented by
incubation in fragmentation buffer (provided in the 3′IVT express
kit) at 95°C for 35 min and chilled on ice. The fragmented labeled
cRNA was applied to MicroRNA 2.0 Array and hybridized in Genechip
hybridization oven 640, at 45°C for 18 h. After washing and
staining in Genechip fluidics station 450, the arrays were scanned
by using Genechip scanner 3000 (all from Affymetrix, Santa Clara,
CA, USA). The gene expressions levels of samples were normalized
and compared by using Partek GS 6.5 (Partek, Inc, St. Louis, MO,
USA). Average-linkage hierarchical clustering of the data was
applied by using the Cluster and the results were obtained by using
TreeView developed by Michael Eisen (Stanford University, CA, USA;
http://rana.lbl.gov).
Real-time PCR for microRNAs
Detection of the mature form of miRNAs was performed
using the mirVana qRT-PCR miRNA Detection kit (cat no. AM1558,
Ambion), according to the manufacturer’s instructions. The U6 small
nuclear RNA was used as an internal control.
Methods of bioinformatics
The analysis of potential microRNA target sites
using 4 commonly used prediction algorithms - miRanda (http://www.microrna.org/), TargetScan (http://www.targetscan.org), miRDB (http://mirdb.org/miRDB/) and PicTar (http://pictar.bio.nyu.edu).
Luciferase reporter assay
The 3′UTR of ANUBL1 was donated by Yao Xu (Harvard
University, USA) Briefly, it was amplified using cDNA from SGC-7901
cells, cloned into pRL-TK (Promega), checked for orientation,
sequenced and named Luc-ANUBL1-WT. Site-directed mutagenesis of the
miR-182 target-site in the ANUBL1-3′-UTR was carried out using Quik
change mutagenesis kit (cat no. 200519, Stratagene, Heidelberg,
Germany), with Luc-ANUBL1-WT as a template. For reporter assays,
SGC-7901 cells were transiently transfected with wt or mutant
reporter plasmid and pre-miR-182 (as indicated in Fig. 4) using Lipofectamine 2000
(Invitrogen). Reporter assays were performed 36 h post-transfection
using the Dual-luciferase assay-system (Promega), normalized for
transfection efficiency by cotransfected Renilla-luciferase.
Results
Aberrant ANUBL1 expression in gastric
cancer
To assess the expression of ANUBL1 in gastric
cancer, real-time PCR and western blot analysis were conducted in 7
pairs of gastric cancer tissues and matched adjacent normal tissue
samples. The expression of ANUBL1 was consistently higher in the
gastric cancer tissues than in normal tissues (Fig. 1A). These data support the notion
that ANUBL1 may act as an oncogene in gastric cancer.
To show the general importance of ANUBL1 in
pathogenesis of gastric cancer, we applied immunohistochemistry
analyses to detect ANUBL1 expression in human gastric cancer
specimens. Staining was evaluated by a pathologist and an
investigator blinded to diagnosis. Sections of gastric cancer of
every grade were divided into two groups (−/+ and ++/+++) (Fig. 1B). The expression rates of the
ANUBL1 protein in ++/+++ group in gastric cancer of grades I, II,
III and IV were 12.5, 29.4, 45.0 and 70.3%, respectively (Fig. 1B). Statistical analysis showed that
ANUBL1 expression level was higher in high-grade gastric cancer
tissues than in low-grade ones, grades I vs. IV (p<0.001), grade
II vs. grade IV (p<0.001), and grade I vs. grade III
(p<0.001).
In order to further confirm that high expression of
ANUBL1 was associated with high-grade human gastric cancer,
quantitative image analysis was performed to analyze ANUBL1 protein
expression in these tissue sections and we found that ANUBL1
expression was indeed associated with grades of gastric cancer
(Fig. 1C). These data implied that
ANUBL1 as an oncogene was upregulated in gastric cancer tissues and
associated with progression of gastric cancer.
ANUBL1 promotes proliferation of gastric
cancer SGC-7901 cells
To investigate whether ANUBL1 contributes to
proliferation of gastric cancer cells, firstly using western blot,
we tested whether ANUBL1 expressing plasmids could stably express
ANUBL1 protein in SGC-7901 cells. The results showed that ANUBL1
protein could be significantly increased by ANUBL1 expressing
plasmids in the cells (Fig.
2A).
 | Figure 2ANUBL1 promotes proliferation in
gastric cancer SGC-7901 cells. (A) Western blot for ANUBL1 in
SGC-7901 cells. SGC-7901 cells were transfected with ANUBL1
expressing plasmids or empty vector (mock). β-actin was a loading
control (n=3). (B) Colony formation assay for SGC-7901 cells
transfected with ANUBL1 expressing plasmids or empty vector (mock).
Colonies with over 50 cells were counted. Representative
micrographs (left) and quantification of colonies (right) after
transfection with ANUBL1 expressing plasmids or empty vector (mock)
(n=3). (C) MTT assay for SGC-7901 cells. SGC-7901 cells were
transfected with ANUBL1 expressing plasmids as indicated and then
cell viability was measured at the indicated time points by MTT
assay (n=3). (D) Cell cycle analysis for SGC-7901 cells transfected
with ANUBL1 or empty vector (mock). Histograms of DNA contents
obtained by FACS analysis are shown. The percentages of each cell
cycle stages are shown in the inset of the histograms (n=3). (E)
Brdu incorporation assay for SGC-7901 cells. Representative
micrographs (left) and quantification (right) of BrdU
incorporating-cells after transfection with ANUBL1 expressing
plasmids or empty vector (mock) (n=3). (F) RT-PCR for PCNA, Rb,
p21, p53, Ki67, CDK2, CDK4, CDK6 and E2F1 in SGC-7901 cells
infected with ANUBL1 or empty vector (mock). GAPDH was a loading
control (n=3). (G) Western blot for PCNA, Rb, p21, p53, Ki67, CDK2,
CDK4 and CDK6 in SGC-7901 cells transfected with ANUBL1 or empty
vector (mock). β-actin was a loading control (n=3). |
In order to identify the effect of ANUBL1 on colony
formation, we performed colony formation assay. The results showed
that over-expression of ANUBL1 significantly increased colony
formation rate of SGC-7901 cells after transfection (Fig. 2B).
In addition, we also performed MTT assay to detect
proliferation of SGC-7901 cells transfected with ANUBL1 expressing
plasmids. The results showed that ANUBL1 promoted proliferation in
SGC-7901 cells after 24 h of transfection and the promotion was
dose- and time-dependent (Fig. 2C).
To further show the effects of ANUBL1 on proliferation, we
performed cell cycle analysis to analyze its effects on the cell
cycle. The results showed higher S phase fractions in SGC-7901
cells transfected with ANUBL1 than in SGC-7901 cells transfected
with empty vector (Fig. 2D). To
further identify that DNA synthesis promotion contributed to the
higher S phase fractions in SGC-7901 cells transfected with ANUBL1,
we performed Brdu incorporation assay to detect DNA synthesis in
the cells. The results confirmed that ANUBL1 significantly promoted
DNA synthesis in the cells and representative micrographs (left)
and quantification (right) of BrdU incorporating-cells after
transfection with ANUBL1 or empty vector (mock) are shown in
Fig. 2E. In the following studies,
we performed RT-PCR to identify whether mRNA of the proliferation
markers were also affected by ANUBL1 in the cells. The results of
RT-PCR showed that expression of PCNA, Ki67, CDK2, CDK4, CDK6, E2F1
was upregulated, and expression of p21 and p53 was downregulated by
ANUBL1 in the cells (Fig. 2F). In
addition, we performed western blot to further confirm that ANUBL1
could regulate these markers. The results of western blot
demonstrated that PCNA, Ki67, CDK2, CDK4 and CDK6 expression were
upregulated and p21 and p53 expression were downregulated by ANUBL1
(Fig. 2G). Due to lack of the E2F1
antibody, we did not analyze its protein expression in the cells.
These data implied that ANUBL1 was upregulated and could promote
proliferation in SGC-7901 cells.
Silencing ANUBL1 inhibits proliferation
in gastric cancer SGC-7901 cells
We demonstrated that ANUBL1 overexpression promoted
proliferation in SGC-7901 cells. To provide further evidence that
ANUBL1 were involved in proliferation of SGC-7901 cells, we studied
the effects of the sh-ANUBL1, an inhibitor of ANUBL1. After stable
transfection, ANUBL1 expression was detected by western blotting.
The results showed that exogenous sh-ANUBL1 significantly
downregulated ANUBL1 expression in SGC-7901 cells (Fig. 3A). Colony formation of SGC-7901
cells were tested by colony formation assay. Silencing ANUBL1
significantly suppressed colony formation rate of SGC-7901 cells
(Fig. 3B). Moreover, we also
performed MTT assay to detect proliferation of SGC-7901 cells with
sh-ANUBL1 and scramble transfection. The results showed that
sh-ANUBL1 inhibited proliferation in SGC-7901 cells, compared with
scramble-transfected groups (Fig.
3C). To further show the effects of silencing ANUBL1 on
proliferation, we performed cell cycle analysis to analyze the
effect of sh-ANUBL1 on the cell cycle. The results showed that
lower S phase fractions in SGC-7901 cells transfected with
sh-ANUBL1 than in SGC-7901 cells transfected with scramble
(Fig. 3D). To identify that DNA
synthesis inhibition contributed to lower S phase fractions in
SGC-7901 cells transfected with sh-ANUBL1, we performed Brdu
incorporation assay to detect DNA synthesis in the cells. The
results confirmed that sh-ANUBL1 significantly suppressed DNA
synthesis in the cells and representative micrographs and
quantification of BrdU incorporating-cells after transfection with
sh-ANUBL1 or scramble are shown in Fig.
3E. All the results confirmed that ANBUL1, as an oncogene
promoted proliferation in SGC-7901 cells.
ANUBL1 is a target of miR-182 in gastric
cancer SGC-7901 cells
Having demonstrated that ANUBL1 expression is
specifically upregulated and is associated with progression of the
disease and it promotes proliferation in SGC-7901 cells, we next
studied the mechanisms promoting ANUBL1 expression in the disease.
MicroRNAs (miRNAs) are a new class of small (~22 nucleotide)
noncoding RNAs negatively regulating protein-coding gene expression
by targeting mRNA degradation or translation inhibition (16). Downregulation of specific miRNA can
contribute to oncogene overexpression (12). Thus we reasoned ANUBL1 was
upregulated by defect of a specific miRNA.
As further confirmation, on the one hand, we
employed four commonly used prediction algorithms - miRanda
(http://www.microrna.org/), TargetScan (http://www.targetscan.org), Pic Tar (http://pictar.mdc-berlin.de/) and miRDB (http://mirdb.org/miRDB/index.html) to analyze
3′UTR of ANUBL1. All the four algorithms predicted that miR-182
could target 3′UTR of ANUBL1 (Fig.
4A) and the predicted target is shown in Fig. 4B.
To study biology function of miR-182, we tested
whether miR-182 expression could be increased by pre-miR-182 in
SGC-7901 cells. Real-time PCR was performed to detect miR-182
expression in the cells transfected with pre-miR-182 and the
results showed that pre-miR-182 could significantly upregulate
miR-182 expression (Fig. 4C).
In order to identify that ANUBL1 could be
downregulated by miR-182, we performed immunofluorescence analyses
and western blot to study whether miR-182 could affect ANUBL1
protein expression. Immunofluorescence analyses showed that ANUBL1
was significantly downregulated in SGC-7901 cells transfected with
pre-miR-182 (Fig. 4D). Consistent
with immunofluorescence analyses, the results of western blot
showed that ANUBL1 protein was significantly downregulated by
miR-182 in SGC-7901 cells (Fig.
4E). We also performed real-time PCR to detect ANUBL1 mRNA in
the cells transfected with pre-miR-182. The result showed that
miR-182 indeed downregulated ANUBL1 mRNA expression in SGC-7901
cells (Fig. 4F).
To further demonstrate the direct regulation of
ANUBL1 by miR-182, we used luciferase reporters with the targeting
sequences of wild-type (ANUBL1-WT-luc) and mutated ANUBL1 3′UTRs
(ANUBL1-MUT-luc) (Fig. 4G). To
detect whether miR-182 targets 3′UTR of ANUBL1, luciferase assay
was performed. Our results showed that miR-182 inhibited
ANUBL1-WT-luc plasmids, but not ANUBL1-MUT-luc plasmids (Fig. 4H). All the results confirmed that
miR-182 negatively regulates protein-coding gene ANUBL1 expression
by targeting its 3′UTR.
ANUBL1 regulates proliferation-associated
microRNA expression
Oncogenes or tumor suppressor genes can play
important roles through regulating miRNAs in gastric cancer
(17–19). Having demonstrated that ANUBL1, as
an oncogene, promoted proliferation of SGC-7901 cells, we reasoned
that ANUBL1 also could regulate proliferation-associated microRNA
expression. In an attempt to identify that ANUBL1 contributed to
aberrant regulation of miRNA expression in SGC-7901 cells, we
performed miRNA profiling in SGC-7901 cells transfected with ANUBL1
expressing plasmids or empty vector. RNAs isolated from the cells
were hybridized to a custom miRNA microarray platform. Following
three hybridization cycles, quantification, and normalization, a
dozen miRNAs, were changed in the cells transfected with ANUBL1
expressing plasmids. We were interested in miR-148b, miR-375 and
miR-182, because they could suppress proliferation of gastric
cancer cells (12,20,21)
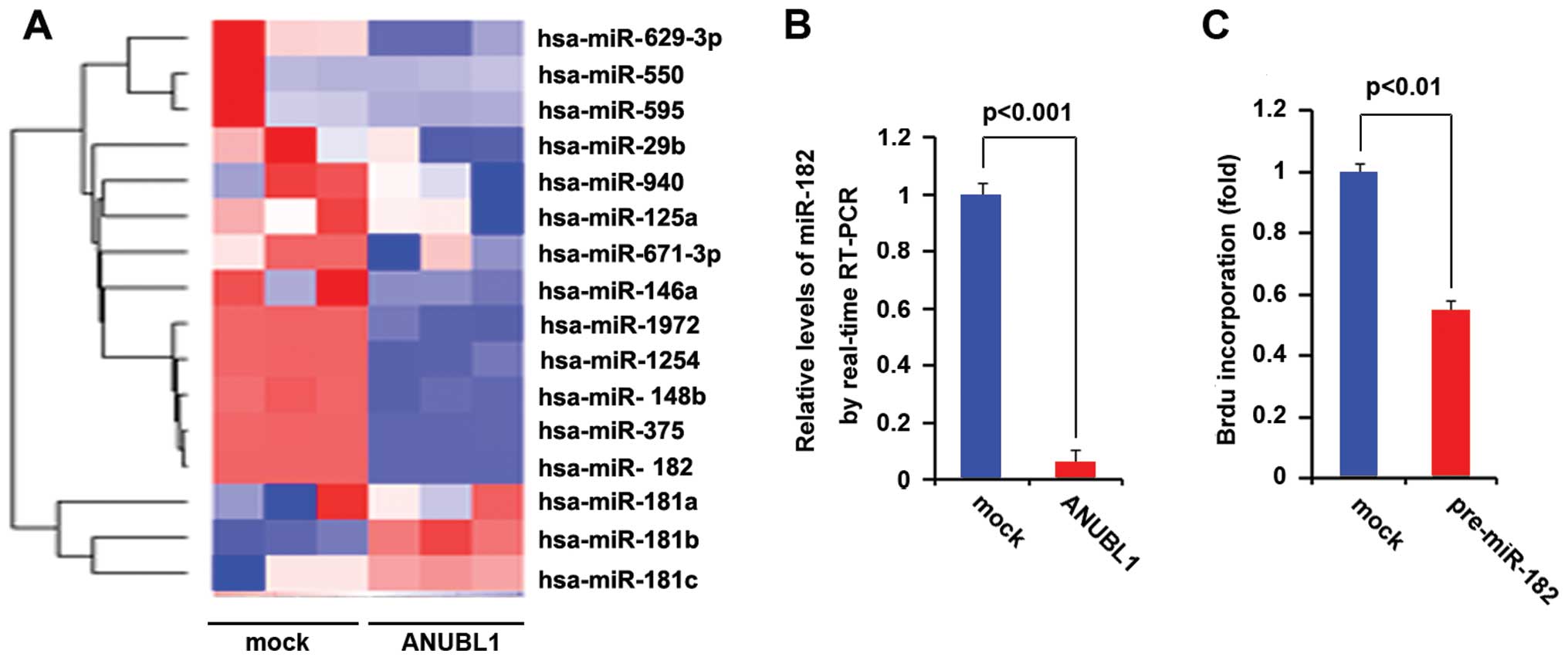
and they were downregulated in the cells transfected with ANUBL1
expressing plasmids (Fig. 5A). We
also performed real-time PCR to identify the miR-182 expression in
the cells transfected with ANUBL1 expressing plasmids, and the
results showed that ANUBL1 significantly inhibited the expression
of miR-182 (Fig. 5B). We performed
Brdu incorporation assay in SGC-7901 cells transfected with
pre-miR-182. Consistent with previous studies (12), the results showed that miR-182 could
inhibit DNA synthesis in SGC-7901 cells (Fig. 5C).
Introduction of ANUBL1 cDNA lacking
predicted sites of 3′-UTR abrogates miR-182 cellular function
Since miR-182 directly degraded ANUBL1 through
targeting its 3′UTR, we reasoned that ectopic expression of ANUBL1
by transfection of the cDNA that did not contain the predicted
target of 3′UTR (in this study, the ANUBL1 expression plasmids did
not contain the predicted position in its 3′UTR) escapes the
regulation of miR-182 and thus attenuate or decrease the function
of miR-182. To this end, we transfected ANUBL1 expressing plasmids
or empty vector (pcDNA3.1) into control miR or pre-miR-182
treated-SGC-7901 cells. Immunoblotting analysis revealed that
transfection of ANUBL1 expressing plasmids attenuated the effect of
miR-182 on ANUBL1 protein (Fig.
6A).
Since overexpression of miR-182 in SGC-7901 cells
inhibited proliferation, in order to identify whether ANUBL1 could
abrogate or attentuate the roles of miR-182 on proliferation,
control miR or pre-miR-182-treated SGC-7901 cells were transfected
with either ANUBL1 expressing plasmids or empty vector (pcDNA3.1),
we performed MTT and Brdu incorporation assay and found that
pre-miR-182-treated SGC-7901 cells displayed ~40% decrease in
proliferation compared to control miR treated cells (Fig. 6B) and DNA synthesis (Fig. 6C). Restoration of ANUBL1 sufficed to
reverse the loss of proliferation (Fig.
6B) and DNA synthesis (Fig. 6C)
observed in pre-miR-182-treated cells. Thus, we concluded that
miR-182 promoted proliferation via downregulating ANUBL1
expression.
Discussion
The interaction of multiple factors contributes to
the development of gastric carcinogenesis, including regulatory
changes in oncogenes and tumor suppressor genes (3,4). The
alterations of these genes are diverse. For example, the oncogenes
K-ras and c-met are significantly amplified and overexpressed in
gastric cancer (22). Abnormal
expression of CD44v is closely related to the invasiveness and
metastasis of gastric cancer (23).
As tumor suppressor genes, p53, APC and DCC show absent expression
or lower expression levels in gastric cancer (24–26).
More genes are still emerging as oncogenes or tumor suppressor
genes in gastric cancer. In this study, we demonstrated that ANUBL1
expression was specifically upregulated in gastric cancer and it
was positively associated with progression of gastric cancer.
Moreover, as an oncogene, it can promote proliferation of gastric
cancer SGC-7901 cells.
We also exploited the mechanism promoting ANUBL1
expression. We found that ANUBL1 is a target of miR-182. Because
miR-182 is downregulated and can suppress cell growth in human
gastric cancer (12), we reasoned
that miR-182 inhibited proliferation by downregulating ANUBL1
expression. The fact that introduction of ANUBL1 cDNA lacking
predicted sites in 3′-UTR abrogated miR-182 cellular function
further confirmed the conclusion in the cells. Our results indicate
that upregulation of ANUBL1 was associated with miR-182 low
expression in gastric cancer. The elucidation of the mechanisms of
miR-182 targeting ANUBL1 in gastric cancer helps us to further
understand the mechanism of gastric cancer initiation and
progression.
miR-182-mediated ANUBL1 regulation in gastric cancer
demonstrated in this study has potential basic and clinical
implications. On one hand, ANUBL1 could be a powerful oncogene by
promoting proliferation and regulating relevant tumor suppressor
genes in gastric cancer and pharmacological inhibition of ANUBL1
may represent a promising therapeutic strategy. On the other hand,
miR-182 is a tumor suppressor gene and overexpression of ANUBL1
could further downregulate its expression. Further exploration of
the role of ANUBL1 in cancer is required to confirm its clinical
significance.
Acknowledgments
This study was supported by the Technology
Development Foundation of Pudong District (PKJ2013-Y67), the
Experimental Animal Special Purpose Foundation of Science and
Technology Commission of Shanghai Municipality (13140902901). We
thank Professor Zhen Zhan from Nanjing University of Chinese
Medicine for constructive inputs and critical review of the
manuscript.
References
|
1
|
Parkin DM, Bray F, Ferlay J, et al: Global
cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khushalani N: Cancer of the esophagus and
stomach. Mayo Clin Proc. 83:712–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JM, Sohn HY, Yoon SY, et al:
Identification of gastric cancer-related genes using a cDNA
microarray containing novel expressed sequence tags expressed in
gastric cancer cells. Clin Cancer Res. 11:473–482. 2005.PubMed/NCBI
|
|
4
|
Chen X, Leung SY, Yuen ST, et al:
Variation in gene expression patterns in human gastric cancers. Mol
Biol Cell. 14:3208–3215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasquinelli AE, Reinhart BJ, Slack F, et
al: Conservation of the sequence and temporal expression of let-7
heterochronic regulatory RNA. Nature. 408:86–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reinhart BJ, Slack FJ, Basson M, et al:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Zhang Y, Zhang Y, et al: Survival
prediction of gastric cancer by a seven-microRNA signature. Gut.
59:579–585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueda T, Volinia S, Okumura H, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: a microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar
|
|
10
|
Shimono Y, Zabala M, Cho RW, et al:
Downregulation of miRNA-200c links breast cancer stem cells with
normal stem cells. Cell. 138:592–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Fang R, Li C, et al: Hsa-miR-182
suppresses lung tumorigenesis through down regulation of RGS17
expression in vitro. Biochem Biophys Res Commun. 396:501–507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong WQ, Bai R, Liu T, et al: MicroRNA-182
targets cAMP-responsive element-binding protein 1 and suppresses
cell growth in human gastric adenocarcinoma. FEBS J. 279:1252–1260.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bacus SS, Zelnick CR, Plowman G, et al:
Expression of the erbB-2 family of growth factor receptors and
their ligands in breast cancers. Implication for tumor biology and
clinical behavior. Am J Clin Pathol. 102(Suppl 1): S13–S24.
1994.PubMed/NCBI
|
|
14
|
Liao XH, Lu DL, Wang N, et al: Estrogen
receptor α mediates proliferation of breast cancer MCF-7 cells via
a p21/PCNA/ E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar
|
|
15
|
Luo XG, Zou JN, Wang SZ, et al: Novobiocin
decreases SMYD3 expression and inhibits the migration of MDA-MB-231
human breast cancer cells. IUBMB Life. 62:194–199. 2010. View Article : Google Scholar
|
|
16
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Zhan W, Wang Z, et al: Inhibition of
PRL-3 gene expression in gastric cancer cell line SGC7901 via
micoRNA suppressed reduces peritoneal metastasis. Biochem Biophys
Res Commun. 348:229–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao C, Zhan Z, Liu W, et al: Reduced
microRNA-218 expression is associated with high nuclear factor
kappa B activation in gastric cancer. Cancer. 116:41–49. 2010.
|
|
19
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song YX, Yue ZY, Wang ZN, et al:
MicroRNA-148b is frequently down-regulated in gastric cancer and
acts as a tumor suppressor by inhibiting cell proliferation. Mol
Cancer. 10:12011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding L, Xu Y, Zhang W, et al: miR-375
frequently downregulated in gastric cancer inhibits cell
proliferation by targeting JAK2. Cell Res. 20:784–793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaji M, Yonemura Y, Harada S, et al:
Participation of c-met in the progression of human gastric cancers:
anti-c-met oligonucleotides inhibit proliferation or invasiveness
of gastric cancer cells. Cancer Gene Ther. 3:393–404.
1996.PubMed/NCBI
|
|
23
|
Chen GY and Wang DR: The expression and
clinical significance of CD44v in human gastric cancers. World J
Gastroenterol. 6:125–127. 2000.
|
|
24
|
Tamura G, Kihana T, Nomura K, et al:
Detection of frequent p53 gene mutations in primary gastric cancer
by cell sorting and polymerase chain reaction single-strand
conformation polymorphism analysis. Cancer Res. 51:3056–3058.
1991.PubMed/NCBI
|
|
25
|
Horii A, Nakatsuru S, Miyoshi Y, et al:
The APC gene, responsible for familial adenomatous polyposis, is
mutated in human gastric cancer. Cancer Res. 52:3231–3233.
1992.PubMed/NCBI
|
|
26
|
Uchino S, Tsuda H, Noguchi M, et al:
Frequent loss of heterozygosity at the DCC locus in gastric cancer.
Cancer Res. 52:3099–3102. 1992.PubMed/NCBI
|