Introduction
Antigen-specific response and tolerance to tumors of
the immune system are regulated by multiple networks of stimulatory
and inhibitory signals. Delivery of inhibitory signals to T cells
mediated by cytotoxic T-lymphocyte antigen 4 (CTLA-4) may mediate
the development of tumor antigen-specific T-cell tolerance. CTLA-4
is expressed on the cell surface of activated T cells and is
critical to restrict cell cycle progression and inhibit the
production of interleukin (IL)-2 (1). CTLA-4 presents a degree of sequence
homology with the T-cell costimulatory molecule CD28 and binds with
higher avidity and affinity than CD28 to its ligands, B7-1 and B7-2
(2). Consequently, CTLA-4 promotes
the termination of immune responses by preventing continuous T-cell
costimulation and activation (2).
CD4+ and CD8+ T cells not expressing CTLA-4
exhibit an activated phenotype and increased proliferation
potential both in vitro and in vivo (3,4).
CTLA-4-deficient mice develop a CD28-dependent expansion of
autoreactive T cells in lymph nodes, spleen and several peripheral
organs, which leads to death within 4 weeks after birth due to
diffuse lymphoproliferative disease (5).
Due to the central role of CTLA-4 in the inhibition
of T-cell activation, targeting of this molecule holds great
promise for several clinical applications. Clinical trials
conducted with various anti-CTLA-4 antibodies (α-CTLA-4)
demonstrated that selective inhibition of CTLA-4 enhances the
endogenous antitumor immune response. The fully human antibodies
tremelimumab and ipilimumab have been studied extensively in
melanoma and were found to act by blocking the interaction of
CTLA-4 with B7 ligands to enhance T-cell proliferation and
activation (6–8). In a phase III study, tremelimumab
administration did not improve overall survival when compared with
dacarbazine chemotherapy (6).
Conversely, ipilimumab administration improved survival in
comparison with patients with melanoma previously treated with a
peptide vaccine (7). Furthermore, a
phase III study demonstrated that ipilimumab and dacarbazine
combination therapy is more effective than dacarbazine treatment
alone (8). Subsequently, ipilimumab
was approved in 2011 for the treatment of unresectable or
metastatic melanoma by regulatory agencies in the US and the
European Union.
CTLA-4 inhibition combined with multiple therapeutic
interventions in murine tumor models has been explored (9). Synergistic effects were demonstrated
in combination with chemotherapy (10), radiation (11,12),
cryoablation (13) and surgery
(14). These studies indicate that
CTLA-4 inhibition can be an effective therapeutic strategy to
extend and elicit the immune response in cancer-bearing hosts.
Preclinical studies have demonstrated that CTLA-4 suppression is
effective against tumors in combination with other immunotherapies
such as cancer vaccines (15–18),
cytosine-phosphate-guanine oligodeoxynucleotide (CpG-ODN) adjuvants
(18,19) and antibodies (20,21).
However, it is not clear whether CTLA-4 inhibition and adoptive
T-cell transfer combination therapy is effective against
tumors.
The state of differentiation of T cells is crucial
to the success of adoptive T-cell therapy, and less-differentiated
T cells are ideal due to their in vivo persistence, high
proliferative potential, receptiveness to homeostatic and
costimulatory signals, and their ability to target secondary
lymphoid tissues and secrete IL-2 (22,23).
CTLA-4 suppression has the potential to enhance the activation of
less-differentiated transferred T cells in vivo. In the
present study, we evaluated whether a combination of CTLA-4
inhibition and transfer of adoptive T cells at different stages of
differentiation exhibit synergistic antitumor effects in a murine
colon cancer model.
Materials and methods
Mice and cell line
All experiments were performed according to the
protocols approved by the Animal Care Committee of Kyoto
Prefectural University of Medicine. BALB/c male mice were purchased
from Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan), fed a
standard laboratory diet, and were provided with water ad
libitum under standard laboratory conditions. Mice between 7
and 8 weeks of age were used for the subsequent experiments.
The colon-26 murine colon adenocarcinoma cell line
was used. Cells were cultured in monolayer with RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), l-glutamine and
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2.
Reagents
For in vivo CTLA-4 inhibition, purified
hamster anti-mouse CD152 (CTLA-4; clone UC10-4F10, #BE0032)
immunoglobulin (Ig)G or hamster IgG control antibodies (#BE0091)
were purchased from Bio X Cell (West Lebanon, NH, USA).
Preparation of therapeutic
CD62Lhigh and CD62Llow T cells
The effects of CTLA-4 inhibition combined with
transfer of adoptive T cells at different stages of differentiation
were determined. To this end, CD62Lhigh and
CD62Llow T cells were prepared for less-differentiated
(naïve phenotype) and differentiated (effector phenotype) adoptive
transfer therapy, respectively. T cells were obtained from spleens
harvested from 7-week-old male BALB/c mice sacrificed by cervical
dislocation. Splenocytes were mechanically dissociated and strained
through a 40-μm nylon mesh to produce a single-cell
suspension. CD3+ T cells were separated by AutoMACS Pro
using the Pan T isolation kit (both from Miltenyi Biotec, Tokyo,
Japan) and seeded on 12-well plates (Thermo Fisher Scientific K.K.,
Yokohama, Japan) previously coated with 5 μg/ml of mCD3
antibody (R&D Systems, Rockville, MD, USA), and 5 μg/ml
RetroNectin®. Cells were cultured in GT-T503 medium
(Takara Bio, Inc., Otsu, Japan) containing 10% FBS,
penicillin/streptomycin, nonessential amino acids, sodium pyruvate
and 2-ME for 3 days (1.5×106 cells/2.5 ml/well).
Subsequently, cells were transferred into a T225 flask (BD Falcon,
Franklin Lakes, NJ, USA) and cultured with GT-503 containing 100
U/ml recombinant mouse IL-2 and 10 ng/ml recombinant mouse IL-7
(both from R&D Systems). Seven days after harvesting,
CD62Lhigh and CD62Llow populations were
sorted with a MACS CD62L+ selection column (Miltenyi
Biotec).
Adoptive cell transfer and α-CTLA-4
administration
Male BALB/c mice at 7–8 weeks of age were injected
s.c. with 1×106 colon-26 cells. Mice (n=9 for all
groups) were treated with i.v. adoptive T-cell transfer
(5×107 cells) 6 and 13 days after tumor challenge.
α-CTLA-4 or control IgG [100 μg in phosphate-buffered saline
(PBS)] was delivered intraperitoneally 5, 8, 10, 12 and 15 days
after tumor challenge. The percentage of CD62L+ cells in
the transferred population was confirmed by flow cytometry. Tumor
growth was monitored twice a week, and tumor volume was expressed
as (a × b2)/2, where a is the largest and b is the
smallest diameter of the tumor. Mice were sacrificed by cervical
dislocation 17 days after tumor inoculation.
Flow cytometry
The phenotype of the lymphocytes in the transferred
cells and in the draining lymph nodes was analyzed by flow
cytometry. For lymph node analysis, the tissue was mechanically
dissociated and strained through a 40-μm nylon mesh to
produce a single-cell suspension.
Cells were stained with fluorescein isothiocyanate
(FITC)-, phycoerythrin (PE)-, phycoerythrin-Texas Red (ECD)-, or
phycoerythrin-cyanin (PC5)-conjugated monoclonal antibodies
specific for CD3, CD4, CD8, CD62L (Beckman Coulter, Marseille,
France), forkhead box P (Foxp)-3, and interferon (IFN)-γ (both from
eBioscience, San Diego, CA, USA). Five hours before cell
harvesting, brefeldin A (BD Biosciences, San Jose, CA, USA) was
added for intracellular blocking of IFN-γ. A single aliquot was
thawed, and mononuclear cells were stained with
fluorescence-conjugated antibodies and analyzed with a FACSCalibur
flow cytometer (BD Biosciences). Data acquisition and analysis were
conducted with the CellQuest software version 6.0 for Mac OS 10 (BD
Biosciences).
Immunohistochemistry
Seventeen days after subcutaneous transplantation of
colon-26 cells, tumors were harvested, fixed in formalin and
analyzed by immunohistochemistry. For immunostaining, 4-μm
sections were cut, deparaffinized and subjected to heat-induced
epitope retrieval before incubation with the antibodies. Sections
were immersed in sodium citrate buffer at pH 7.0 and heated in a
high-pressure cooker, treated with 3% H2O2 in
methanol for 15 min, and blocked with Dako Protein Block Serum-Free
solution for 30 min. Two consecutive sections were then incubated
for 2–3 h at room temperature with a rabbit anti-CD3 antibody
(ab5690; Abcam, Cambridge, MA, USA) and a rabbit anti-Foxp3
antibody (14-5773-82; eBioscience) at a dilution of 1:100 and
1:300, respectively. After incubation with anti-rabbit MAX-PO
secondary antibody (Nichirei Bioscience, Tokyo, Japan), color
development was performed using a DAB substrate kit (Nichirei
Bioscience).
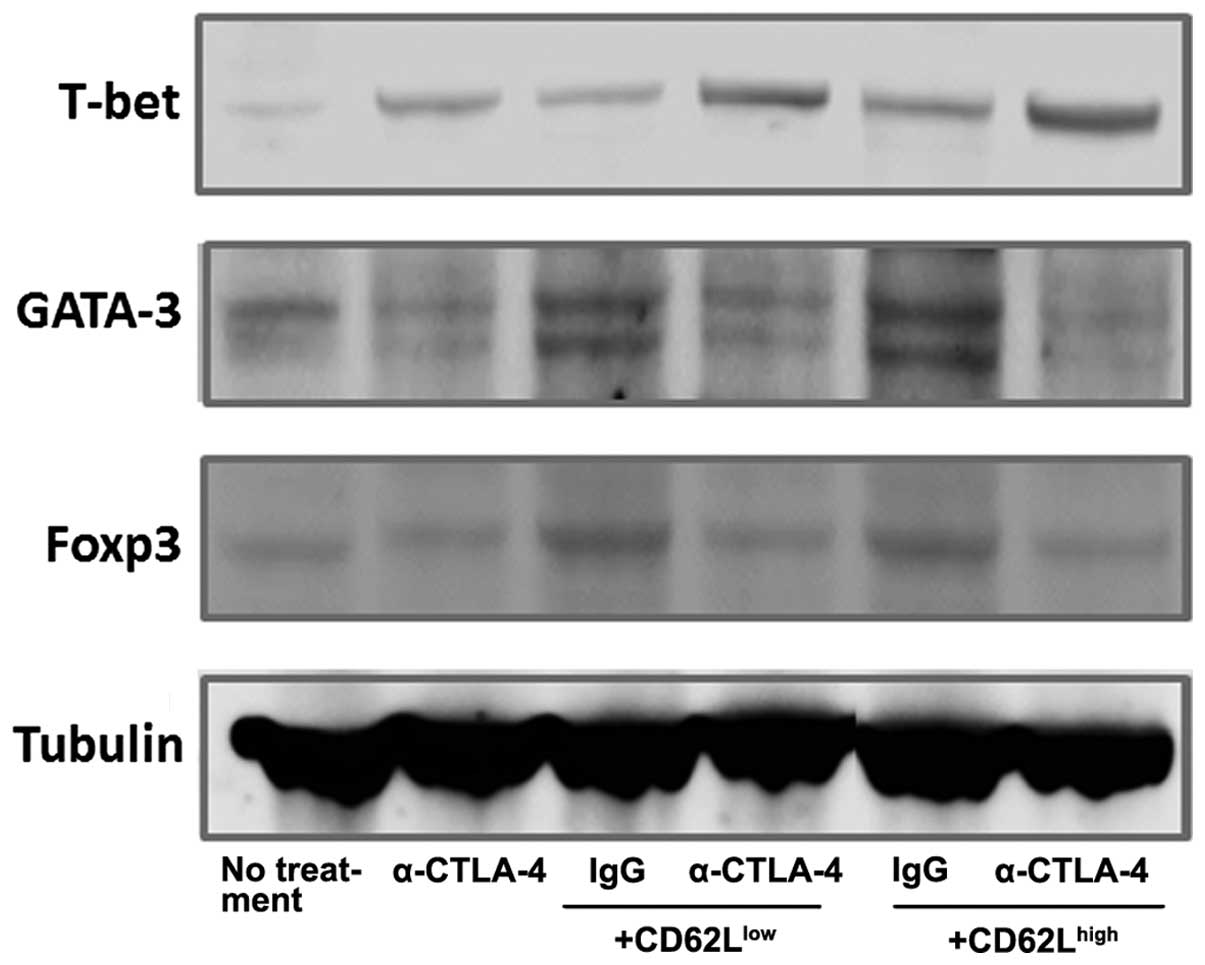
Detection of T-bet, GATA-3 and Foxp3
expression by western blot analysis
Subcutaneous tumors were harvested and frozen
immediately. Total cell protein was extracted by thawing on ice and
homogenizing at 4°C in a solution of 50 mmol/l Tris-HCl, pH 7.6,
300 mmol/l NaCl, 0.5% Triton X-100, 10 μg/ml aprotinin, 10
μg/ml leupeptin, 1 mmol/l phenylmethylsulfonyl fluoride, 1.8
mg/ml iodoacetamide, 50 mmol/l NaF and 1 mM DTT. Equal quantities
of protein (25 μg) were added to lysis buffer containing
protease inhibitors and boiled at 70°C for 10 min. The proteins
were separated by 10% NuPAGE® Novex Bis-Tris Gel and
electroblotted to nitrocellulose membranes (iBlot®
Transfer Stack) (both from Thermo Fisher Scientific, Hampton, NH,
USA). Membranes were incubated in blocking buffer (AE-1475; ATTO
Corporation, Tokyo, Japan) for 20 min, followed by primary
antibodies (20 h) raised against mouse T-bet (1:500 dilution,
sc21003), GATA-3 (1:500 dilution, sc9009) (both from Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), Foxp3 (1:500 dilution,
320002; BioLegend, Inc., San Diego, CA, USA), tubulin (1:500
dilution, T9026; Sigma-Aldrich, St. Louis, MO, USA) in
Tris-buffered saline containing 0.1% Tween-20 (TBS-T).
Subsequently, membranes were incubated with secondary anti-mouse or
rabbit antibodies (GE Healthcare, Tokyo, Japan) in TBS-T (diluted
1:10,000) for 50 min at room temperature. Immunocomplexes were
detected using a commercial kit (ECL Plus; GE Healthcare
Bio-Sciences K.K., Tokyo, Japan) according to the manufacturer’s
recommendations.
Statistics
The results are presented as mean ± SEM. Statistical
significance of differences between means was analyzed by one-way
ANOVA, followed by Tukey’s multiple comparison test, and P<0.05
indicates a statistically significant difference. All analyses were
performed using the GraphPad Prism 4 program (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Phenotype of transferred cells
CD62Lhigh and CD62Llow cells
from mouse spleen were cultured, and before cell separation, double
CD62L+ and CD3+ T cells contributed to 55% of
the total cell population. After separation, the fractions of
double CD62L+ and CD3+ T cells among total
CD62Lhigh T cells from the first and second adoptive
transfer were 92.16 and 96.78%, respectively. In contrast, the
fraction of double-positive T cells among total CD62Llow
T cells was 26.63% in the first and 36.71% in the second adoptive
transfer. Subsequently, we considered T-cell separation between
CD62Lhigh and CD62Llow successful, and we
used these cells for further analysis.
CTLA-4 inhibition enhances the
therapeutic potential of adoptive T-cell transfer
To determine whether CTLA-4 inhibition enhances the
antitumor effects of adoptive cell transfer, 1×106
colon-26 cells were injected subcutaneously, followed by
intravenous injection of CD62Lhigh or
CD62Llow T cells with or without administration of
α-CTLA-4. Tumor growth was monitored twice a week. The body weight
of the mice was not affected by the procedure and did not change
over time (data not shown). Administration of CD62Lhigh
T cells exhibited a tendency toward higher antitumor activity than
administration of CD62Llow T cells (Fig. 1). α-CTLA-4 monotherapy displayed
significant antitumor activity. Administration of α-CTLA-4 combined
with CD62Llow or CD62Lhigh cell
administration enhanced the antitumor activity to a greater extent
than did administration of CD62Llow or
CD62Lhigh in combination with IgG injection
(P<0.001). CTLA-4 suppression combined with administration of
CD62Lhigh T cells exhibited a tendency toward a higher
efficacy against tumors than injection of CD62Llow T
cells, although the effect was not statistically significant.
Adoptive T-cell transfer and CTLA-4
inhibition modifies the population of lymphocytes within the spleen
and draining lymph nodes
We assessed the phenotype of lymphocytes in the
spleen and draining lymph nodes of tumor-bearing mice. In the
spleen of mice subjected to adoptive T-cell transfer, the frequency
of CD4+ lymphocytes was decreased whereas that of
CD8-positive cells was increased (Fig.
2). The CD4/CD8 ratio was significantly lower in mice subjected
to adoptive cell transfer than this ratio in the controls (no
treatment) or mice injected with α-CTLA-4. Monotherapy with
α-CTLA-4 did not affect the frequency of CD4- and CD8-positive
cells or the CD4/CD8 ratio in the spleen. The frequency of Tregs
(CD4+ and Foxp3+) in the spleen was higher in
mice treated with α-CTLA-4 than the frequency in the controls. The
frequency of IFN-γ-producing cells in CD4+ lymphocytes
was higher in mice subjected to α-CTLA-4 and adoptive cell transfer
combination therapy when compared with the frequency in the control
mice. The frequency of IFN-γ-producing cells among CD8+
lymphocytes was higher in the context of α-CDLA-4 and
CD62Llow adoptive transfer combination therapy, and in
mice subjected to CD62Lhigh cell transfer irrespective
of CTLA-4 suppression, than under control conditions. The frequency
in the context of α-CTLA-4 monotherapy or CD62Llow
adoptive transfer was higher than under basal conditions, yet the
effect reached statistical significance.
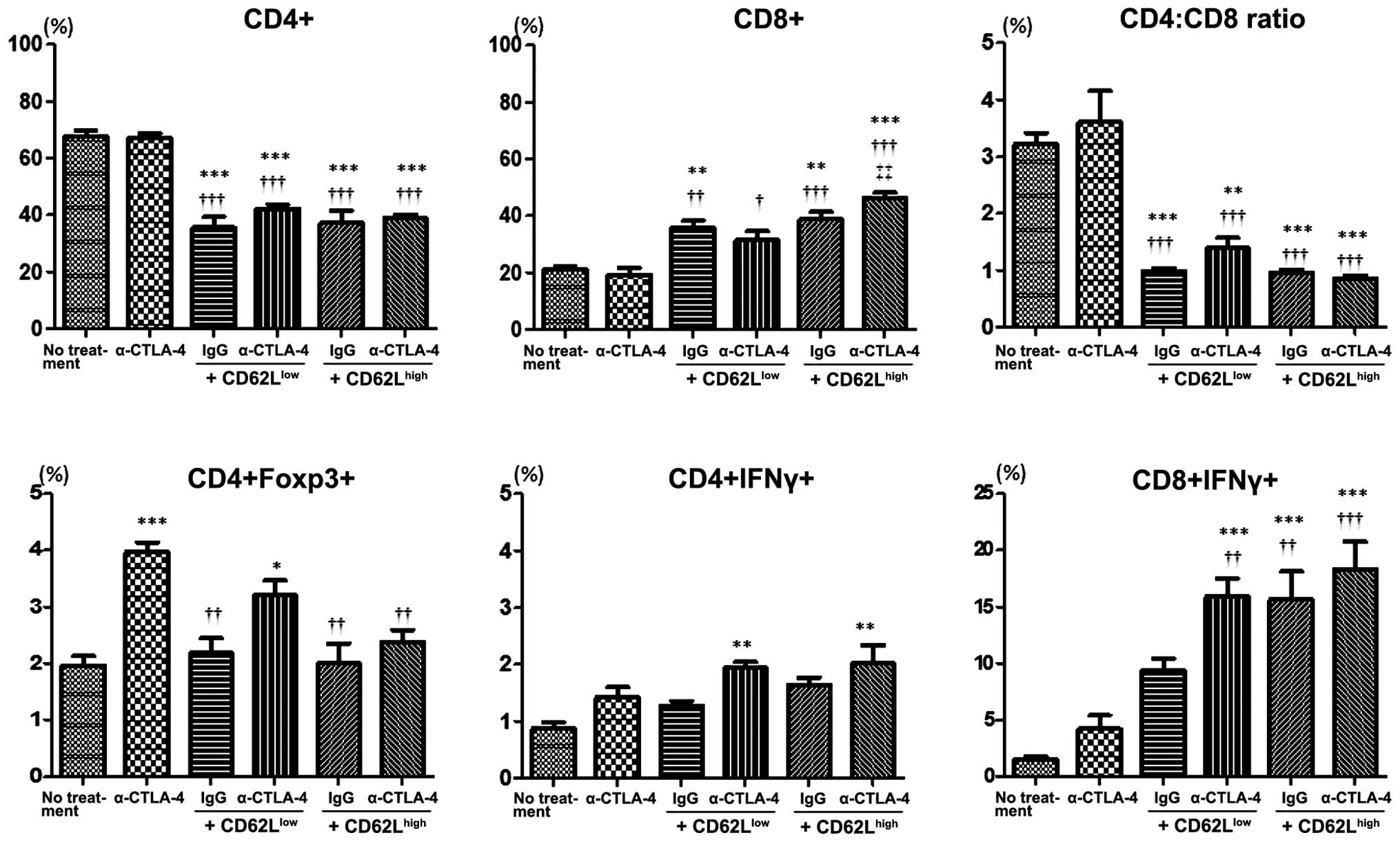 | Figure 2Flow cytometric analysis of
lymphocytes from the spleen of control mice (no treatment), mice
treated with anti-CTLA-4 Ab and mice treated with adoptive cell
transfer (CD62Llow or CD62Lhigh subsets)
combined with control IgG or anti-CTLA-4 Ab at day 17 after tumor
challenge. The percentage of CD4+, CD8+,
CD4+Foxp3+, CD4+IFNγ+
and CD8+IFNγ+ T cells was analyzed using flow
cytometry (n=3 mice in each group). Error bars represent means ±
SEM. *P<0.05, **P<0.01, ***P<0.001
vs. no treatment. †P<0.05, ††P<0.01,
†††P<0.001 vs. anti-CTLA-4 Ab. ‡‡P<0.01
vs. CD62Llow + anti-CTLA-4 Ab. CTLA-4, cytotoxic
T-lymphocyte-associated antigen 4; IgG, immunoglobulin G. |
Similar trends were observed in draining lymph nodes
(Fig. 3). The frequency of
CD4+ T cells was decreased in mice administered
CD62Lhigh T cells combined with control IgG or α-CTLA-4
in comparison to control mice or mice subjected to α-CTLA-4
monotherapy. The frequency of CD8+ T cells was
significantly higher in the context of adoptive CD62Llow
or CD62Lhigh transfer than under control conditions, and
this effect was more pronounced in the mice subjected to
CD62Lhigh T-cell transfer than in those administered
CD62Llow T cells. The CD4/CD8 ratio was lower in mice
treated with α-CTLA-4 or subjected to adoptive T-cell transfer,
either alone or in combination, than this ratio in the control
mice. The lowest CD4/CD8 ratio was observed in the mice subjected
to CD62Lhigh T-cell transfer and α-CTLA-4 combination
therapy. The frequency of Tregs increased in all mice treated with
α-CTLA-4 or subjected to adoptive T-cell transfer. The frequency of
IFN-γ-producing cells among CD4-positive lymphocytes was not
affected by the treatments. Mice injected with α-CTLA-4 or T cells,
either alone or in combination, exhibited a tendency toward a
higher frequency of IFN-γ-producing cells among CD8+
lymphocytes than control mice, yet the effect did not reach
statistical significance.
CTLA-4 inactivation promotes T-cell
migration and lowers the frequency of Foxp3-positive cells within
the tumor
We assessed the number of infiltrating T cells
within the tumor by immunohistochemistry. Quantitative analysis
represents the mean counts from three high-power fields. The number
of intratumoral CD3+ cells in mice subjected to α-CTLA-4
monotherapy, or to adoptive CD62Llow or
CD62Lhigh T-cell transfer, either alone or in
combination, was higher than in untreated controls (Fig. 4). α-CTLA-4 and CD62Llow
and CD62Lhigh adoptive transfer promoted the migration
of CD3 positive cells better than adoptive T-cell transfer combined
with administration of control IgG. The number of CD3+
cells migrating within the tumor was the highest in mice subjected
to α-CTLA-4 and CD62Lhigh adoptive transfer combination
therapy. The number of Foxp3+ cells within the tumor was
higher in mice administered either CD62Llow or
CD62Lhigh T cells than this number in the untreated
mice. Mice administered α-CTLA-4 presented a tendency toward an
increased number of intratumoral Foxp3+ cells, although
the effect did not reach statistical significance. Administration
of α-CTLA-4 reduced the number of Foxp3-positive cells in the mice
administered CD62Lhigh but not CD62Llow T
cells.
CTLA-4 inhibition alters the expression
of T-bet, GATA-3 and Foxp3 in tumors of mice subjected to adoptive
T-cell transfer
Western blot analysis was performed to investigate
helper T (Th) differentiation in the tumors. The differentiation of
Th1 lymphocytes is associated with a specific transcription factor,
T-bet, which is a key regulator of cytokine expression by Th1. The
expression of T-bet was increased under all experimental conditions
in comparison to basal conditions (Fig.
5). CTLA-4 inhibition and adoptive CD62Llow and
CD62Lhigh T-cell transfer combination therapy enhanced
the T-bet expression levels. T-bet expression was the highest in
the mice treated with α-CTLA-4 in combination with
CD62Lhigh cell transfer. Blocking CTLA-4 attenuated the
expression of the Th2 lineage transcription factor GATA3, whereas
adoptive T-cell transfer had the opposite effect. CTLA-4 inhibition
and CD62Llow or CD62Lhigh adoptive transfer
combination therapy attenuated GATA3 expression. Foxp3 expression
was higher in the mice subjected to adoptive T-cell transfer than
that in the controls. Although CTLA-4 inactivation alone did not
affect Foxp3 expression, α-CTLA-4 and adoptive T-cell transfer
combination therapy attenuated Foxp3 expression in comparison to
mice administered CD62Llow and CD62Lhigh
cells combined with control IgG.
Discussion
The present study provides evidence that blocking
CTLA-4 enhances the antitumor efficacy of adoptive T-cell transfer
therapy, particularly when CD62Lhigh T cells,
characterized by a high frequency of naïve T cells, were
administered. Our results also indicate that α-CTLA-4 and adoptive
T-cell transfer combination therapy increases the number of
CD3+ cells within the tumor, and that CTLA-4 inhibition
leads to polarization of tumor-infiltrating T cells toward the Th1
lineage. Furthermore, α-CTLA-4 combined with CD62Lhigh
yet not CD62Llow T cells decreased the frequency of
Tregs within the tumor. Although CTLA-4 suppression combined with
cancer vaccines (15–18) and therapeutic antibodies (20,21) is
effective against tumors in preclinical models, there is limited
evidence of a synergistic antitumor effect of CTLA-4 suppression
and adoptive T-cell therapy (24,25).
This is the first report on the effects of α-CTLA-4 on Th
polarization of tumor-infiltrating T cells following adoptive
T-cell transfer, and suggests that the effects of combination
therapy depend on the state of T-cell differentiation. These data
may have important implications in the clinical application of
α-CTLA-4 combined with adoptive T-cell therapy.
The exact mechanism mediating the antitumor effects
of CTLA-4 inhibition remains undefined. Although antitumor activity
of CTLA-4 suppression may be mediated by interference with the
negative regulation of effector T-cell (Teff) function, recent
reports suggest a secondary mechanism, wherein CTLA-4 inhibition
affects Teff suppressive activity or mediates Treg depletion
(25–27). In agreement with our results
pertaining to the expression of CD3 and Foxp3, prior reports have
demonstrated that CTLA-4 suppression decreases the number of Tregs
within tumors, yet not those occurring in the draining lymph node
(25,26), and increases the Teff/Treg ratio,
which suggests an imbalanced proliferation of Tregs over Teffs
within the tumor microenvironment (28–30).
Recently, Simpson et al demonstrated in a mouse model that
α-CTLA-4 depletes tumor-infiltrating Tregs and that this effect is
dependent on the presence of Fcγ receptor-expressing macrophages
(25). These findings indicate that
antibody-dependent cellular cytotoxicity (ADCC) is likely to be
involved in Treg depletion in response to α-CTLA-4. However, a
hamster α-CTLA-4 was used in the present study, so that under these
experimental conditions, α-CTLA-4 may decrease the number of
Foxp3-positive cells by ADCC-independent mechanisms. Previous
studies have demonstrated that induced Tregs, a subset of Tregs,
develop as a consequence of activation of mature T cells under
specific conditions in the tumor periphery, at local tumor sites,
or in lymphoid organs (31,32). Furthermore,
Foxp3+CD25+CD4+ Tregs can be
present in a tumor as a result of conversion from the
CD25−CD4+ population in the adoptive transfer
system (33,34). Therefore, α-CTLA-4 may have blocked
this conversion such that the number of Foxp3-positive cells within
the tumor was decreased in the mice subjected to adoptive transfer
with CD62Lhigh T cells, represented mostly by naïve T
cells.
In agreement with our results, CTLA-4 inhibition has
been found to enhance the Th1 response (35–37).
van Elsas et al reported that T cells from mice treated with
α-CTLA-4 in combination with a GM-CSF-producing tumor cell vaccine
exhibited enhanced IFN-γ secretion in vivo. In addition, the
severity of experimental allergic encephalomyelitis, a classical
Th1-mediated autoimmune disease model, is exacerbated by CTLA-4
suppression (36,37). Contrary to these findings, it was
demonstrated that engagement of CTLA-4 with B7 led to polarization
of naïve CD4+ cells toward the Th1 subset and that the
Th1 polarization was inhibited by CTLA-4 suppression in
vitro (38). However, our
findings support the notion that blocking CTLA-4 caused
polarization of transferred naïve CD4+ T cells toward
the Th1 subset. Differences in these studies may be explained by
the use of different experimental models and by the complexity of
the events that regulate Th cell subset polarization and
interactions of the immune system with tumors.
It is not clear whether the effects of blocking
CTLA-4 on Th-cell subset polarization are mediated by an effect on
the transferred T cells or on endogenous T cells, as these cells
cannot be distinguished within the tumor site. Although analysis of
the behavior of transferred cells is important, this is a
significant challenge, since in light of our findings, the efficacy
of antitumor therapy and the effects on Th-cell subset polarization
by α-CTLA-4 are determined by the state of T-cell differentiation.
Recently, we reported that expansion of T cells in the presence of
fibronectin CH296 (FN-CH296) leads to higher yields of naïve T
cells, and that FN-CH296-stimulated T-cell adoptive transfer
therapy was very well tolerated with a level of efficacy in a phase
1 clinical trial (39). Based on
these results, we intend to conduct a clinical trial to clarify the
efficacy of α-CTLA-4 and adoptive transfer with FN-CH296-stimulated
T-cell combination therapy.
In conclusion, α-CTLA-4 enhances the antitumor
activity of adoptive T-cell transfer therapy, and the effects are
more pronounced in the context of naïve T-cell administration.
CTLA-4 suppression may enhance Th1 polarization and attenuate Treg
differentiation of T cells infiltrating the tumor. These findings
suggest that α-CTLA-4 and FN-CH296-stimulated T-cell adoptive
transfer combination therapy holds potential as an effective
antitumor clinical intervention.
Acknowledgments
The present study was partially supported by
Grant-in-Aid for Scientific Research (no. 23590891 and 26460914)
from the Japanese Ministry of Education, Culture, Sports, Science
and Technology.
Abbreviations:
|
CTLA
|
cytotoxic T lymphocyte-associated
antigen 4
|
|
Treg
|
regulatory T cell
|
|
Th
|
helper T
|
|
Teff
|
effector T cell
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
References
|
1
|
Krummel MF and Allison JP: CTLA-4
engagement inhibits IL-2 accumulation and cell cycle progression
upon activation of resting T cells. J Exp Med. 183:2533–2540. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alegre ML, Frauwirth KA and Thompson CB:
T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 1:220–228.
2001. View
Article : Google Scholar
|
|
3
|
Chambers CA, Sullivan TJ and Allison JP:
Lymphoproliferation in CTLA-4-deficient mice is mediated by
costimulation-dependent activation of CD4+ T cells.
Immunity. 7:885–895. 1997. View Article : Google Scholar
|
|
4
|
Greenwald RJ, Oosterwegel MA, van der
Woude D, Kubal A, Mandelbrot DA, Boussiotis VA and Sharpe AH:
CTLA-4 regulates cell cycle progression during a primary immune
response. Eur J Immunol. 32:366–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waterhouse P, Penninger JM, Timms E,
Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H and Mak TW:
Lymphoproliferative disorders with early lethality in mice
deficient in Ctla-4. Science. 270:985–988. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribas A, Kefford R, Marshall MA, et al:
Phase III randomized clinical trial comparing tremelimumab with
standard-of-care chemotherapy in patients with advanced melanoma. J
Clin Oncol. 31:616–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grosso JF and Jure-Kunkel MN: CTLA-4
blockade in tumor models: An overview of preclinical and
translational research. Cancer Immun. 13:52013.PubMed/NCBI
|
|
10
|
Mokyr MB, Kalinichenko T, Gorelik L and
Bluestone JA: Realization of the therapeutic potential of CTLA-4
blockade in low-dose chemotherapy-treated tumor-bearing mice.
Cancer Res. 58:5301–5304. 1998.PubMed/NCBI
|
|
11
|
Pilones KA, Kawashima N, Yang AM, Babb JS,
Formenti SC and Demaria S: Invariant natural killer T cells
regulate breast cancer response to radiation and CTLA-4 blockade.
Clin Cancer Res. 15:597–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005.PubMed/NCBI
|
|
13
|
Waitz R, Solomon SB, Petre EN, Trumble AE,
Fassò M, Norton L and Allison JP: Potent induction of tumor
immunity by combining tumor cryoablation with anti-CTLA-4 therapy.
Cancer Res. 72:430–439. 2012. View Article : Google Scholar
|
|
14
|
Kwon ED, Foster BA, Hurwitz AA, Madias C,
Allison JP, Greenberg NM and Burg MB: Elimination of residual
metastatic prostate cancer after surgery and adjunctive cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy.
Proc Natl Acad Sci USA. 96:15074–15079. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurwitz AA, Yu TF, Leach DR and Allison
JP: CTLA-4 blockade synergizes with tumor-derived
granulocyte-macrophage colony-stimulating factor for treatment of
an experimental mammary carcinoma. Proc Natl Acad Sci USA.
95:10067–10071. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pedersen AE, Buus S and Claesson MH:
Treatment of transplanted CT26 tumour with dendritic cell vaccine
in combination with blockade of vascular endothelial growth factor
receptor 2 and CTLA-4. Cancer Lett. 235:229–238. 2006. View Article : Google Scholar
|
|
17
|
Met O, Wang M, Pedersen AE, Nissen MH,
Buus S and Claesson MH: The effect of a therapeutic dendritic
cell-based cancer vaccination depends on the blockage of CTLA-4
signaling. Cancer Lett. 231:247–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davila E, Kennedy R and Celis E:
Generation of antitumor immunity by cytotoxic T lymphocyte epitope
peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4
blockade. Cancer Res. 63:3281–3288. 2003.PubMed/NCBI
|
|
19
|
Daftarian P, Song GY, Ali S, Faynsod M,
Longmate J, Diamond DJ and Ellenhorn JD: Two distinct pathways of
immuno-modulation improve potency of p53 immunization in rejecting
established tumors. Cancer Res. 64:5407–5414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kocak E, Lute K, Chang X, et al:
Combination therapy with anti-CTL antigen-4 and anti-4-1BB
antibodies enhances cancer immunity and reduces autoimmunity.
Cancer Res. 66:7276–7284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gattinoni L, Klebanoff CA, Palmer DC,
Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR,
Rosenberg SA and Restifo NP: Acquisition of full effector function
in vitro paradoxically impairs the in vivo antitumor efficacy of
adoptively transferred CD8+ T cells. J Clin Invest.
115:1616–1626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang J, Khong HT, Dudley ME, El-Gamil M,
Li YF, Rosenberg SA and Robbins PF: Survival, persistence, and
progressive differentiation of adoptively transferred
tumor-reactive T cells associated with tumor regression. J
Immunother. 28:258–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe A, Hara M, Chosa E, Nakamura K,
Sekiya R, Shimizu T and Onitsuka T: Combination of adoptive cell
transfer and antibody injection can eradicate established tumors in
mice - an in vivo study using anti-OX40mAb, anti-CD25mAb and
anti-CTLA4mAb-. Immunopharmacol Immunotoxicol. 32:238–245. 2010.
View Article : Google Scholar
|
|
25
|
Simpson TR, Li F, Montalvo-Ortiz W, et al:
Fc-dependent depletion of tumor-infiltrating regulatory T cells
co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J
Exp Med. 210:1695–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selby MJ, Engelhardt JJ, Quigley M,
Henning KA, Chen T, Srinivasan M and Korman AJ: Anti-CTLA-4
antibodies of IgG2a isotype enhance antitumor activity through
reduction of intratumoral regulatory T cells. Cancer Immunol Res.
1:32–42. 2013. View Article : Google Scholar
|
|
27
|
Takahashi T, Tagami T, Yamazaki S, Uede T,
Shimizu J, Sakaguchi N, Mak TW and Sakaguchi S: Immunologic
self-tolerance maintained by CD25+CD4+
regulatory T cells constitutively expressing cytotoxic T
lymphocyte-associated antigen 4. J Exp Med. 192:303–310. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Curran MA and Allison JP: Tumor vaccines
expressing flt3 ligand synergize with ctla-4 blockade to reject
preimplanted tumors. Cancer Res. 69:7747–7755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liakou CI, Kamat A, Tang DN, Chen H, Sun
J, Troncoso P, Logothetis C and Sharma P: CTLA-4 blockade increases
IFNγ-producing CD4+ICOShi cells to shift the
ratio of effector to regulatory T cells in cancer patients. Proc
Natl Acad Sci USA. 105:14987–14992. 2008. View Article : Google Scholar
|
|
30
|
Hodi FS, Butler M, Oble DA, et al:
Immunologic and clinical effects of antibody blockade of cytotoxic
T lymphocyte-associated antigen 4 in previously vaccinated cancer
patients. Proc Natl Acad Sci USA. 105:3005–3010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bluestone JA and Abbas AK: Natural versus
adaptive regulatory T cells. Nat Rev Immunol. 3:253–257. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akbar AN, Taams LS, Salmon M and
Vukmanovic-Stejic M: The peripheral generation of CD4+
CD25+ regulatory T cells. Immunology. 109:319–325. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25− naive T cells to
CD4+CD25+ regulatory T cells by TGF-β
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikemoto T, Yamaguchi T, Morine Y, Imura S,
Soejima Y, Fujii M, Maekawa Y, Yasutomo K and Shimada M: Clinical
roles of increased populations of Foxp3+CD4+
T cells in peripheral blood from advanced pancreatic cancer
patients. Pancreas. 33:386–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentation. J Exp Med. 190:355–366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perrin PJ, Maldonado JH, Davis TA, June CH
and Racke MK: CTLA-4 blockade enhances clinical disease and
cytokine production during experimental allergic encephalomyelitis.
J Immunol. 157:1333–1336. 1996.PubMed/NCBI
|
|
37
|
Karandikar NJ, Vanderlugt CL, Walunas TL,
Miller SD and Bluestone JA: CTLA-4: A negative regulator of
autoimmune disease. J Exp Med. 184:783–788. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouchi N, Kihara S, Arita Y, et al: Novel
modulator for endothelial adhesion molecules: Adipocyte-derived
plasma protein adiponectin. Circulation. 100:2473–2476. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishikawa T, Kokura S, Enoki T, et al:
Phase I clinical trial of fibronectin CH296-stimulated T cell
therapy in patients with advanced cancer. PLoS One. 9:e837862014.
View Article : Google Scholar : PubMed/NCBI
|