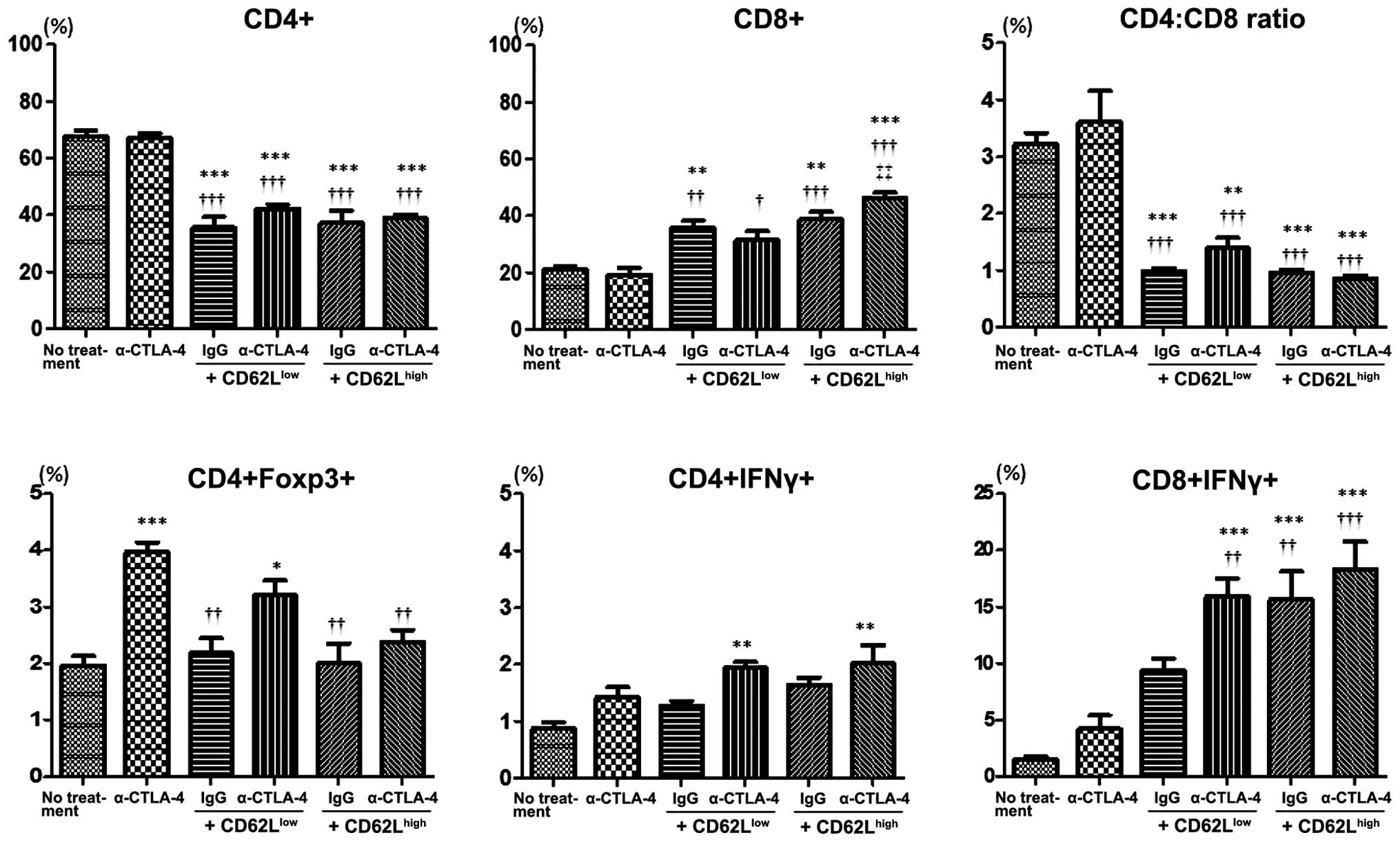
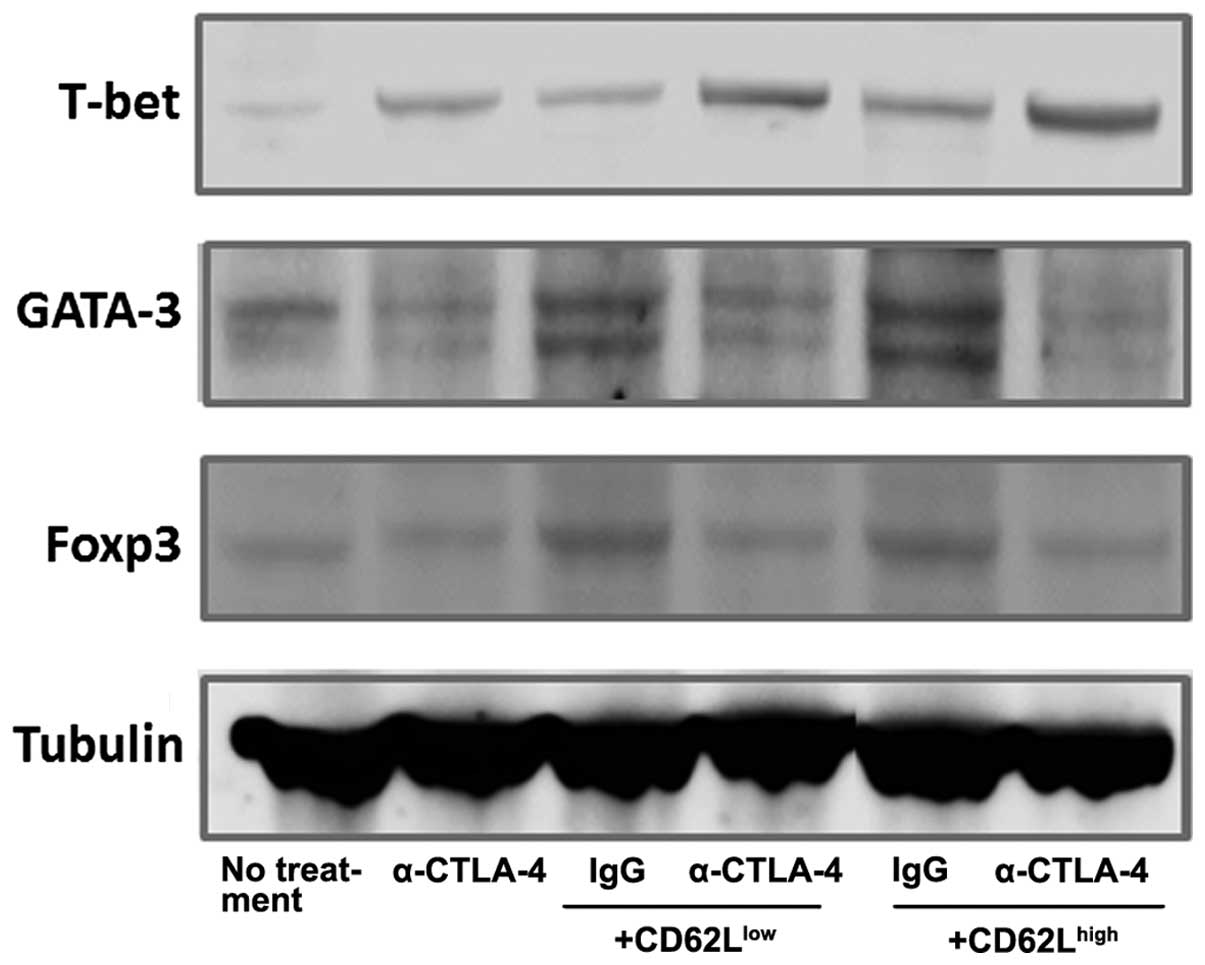
|
1
|
Krummel MF and Allison JP: CTLA-4
engagement inhibits IL-2 accumulation and cell cycle progression
upon activation of resting T cells. J Exp Med. 183:2533–2540. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alegre ML, Frauwirth KA and Thompson CB:
T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 1:220–228.
2001. View
Article : Google Scholar
|
|
3
|
Chambers CA, Sullivan TJ and Allison JP:
Lymphoproliferation in CTLA-4-deficient mice is mediated by
costimulation-dependent activation of CD4+ T cells.
Immunity. 7:885–895. 1997. View Article : Google Scholar
|
|
4
|
Greenwald RJ, Oosterwegel MA, van der
Woude D, Kubal A, Mandelbrot DA, Boussiotis VA and Sharpe AH:
CTLA-4 regulates cell cycle progression during a primary immune
response. Eur J Immunol. 32:366–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waterhouse P, Penninger JM, Timms E,
Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H and Mak TW:
Lymphoproliferative disorders with early lethality in mice
deficient in Ctla-4. Science. 270:985–988. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribas A, Kefford R, Marshall MA, et al:
Phase III randomized clinical trial comparing tremelimumab with
standard-of-care chemotherapy in patients with advanced melanoma. J
Clin Oncol. 31:616–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grosso JF and Jure-Kunkel MN: CTLA-4
blockade in tumor models: An overview of preclinical and
translational research. Cancer Immun. 13:52013.PubMed/NCBI
|
|
10
|
Mokyr MB, Kalinichenko T, Gorelik L and
Bluestone JA: Realization of the therapeutic potential of CTLA-4
blockade in low-dose chemotherapy-treated tumor-bearing mice.
Cancer Res. 58:5301–5304. 1998.PubMed/NCBI
|
|
11
|
Pilones KA, Kawashima N, Yang AM, Babb JS,
Formenti SC and Demaria S: Invariant natural killer T cells
regulate breast cancer response to radiation and CTLA-4 blockade.
Clin Cancer Res. 15:597–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005.PubMed/NCBI
|
|
13
|
Waitz R, Solomon SB, Petre EN, Trumble AE,
Fassò M, Norton L and Allison JP: Potent induction of tumor
immunity by combining tumor cryoablation with anti-CTLA-4 therapy.
Cancer Res. 72:430–439. 2012. View Article : Google Scholar
|
|
14
|
Kwon ED, Foster BA, Hurwitz AA, Madias C,
Allison JP, Greenberg NM and Burg MB: Elimination of residual
metastatic prostate cancer after surgery and adjunctive cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy.
Proc Natl Acad Sci USA. 96:15074–15079. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurwitz AA, Yu TF, Leach DR and Allison
JP: CTLA-4 blockade synergizes with tumor-derived
granulocyte-macrophage colony-stimulating factor for treatment of
an experimental mammary carcinoma. Proc Natl Acad Sci USA.
95:10067–10071. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pedersen AE, Buus S and Claesson MH:
Treatment of transplanted CT26 tumour with dendritic cell vaccine
in combination with blockade of vascular endothelial growth factor
receptor 2 and CTLA-4. Cancer Lett. 235:229–238. 2006. View Article : Google Scholar
|
|
17
|
Met O, Wang M, Pedersen AE, Nissen MH,
Buus S and Claesson MH: The effect of a therapeutic dendritic
cell-based cancer vaccination depends on the blockage of CTLA-4
signaling. Cancer Lett. 231:247–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davila E, Kennedy R and Celis E:
Generation of antitumor immunity by cytotoxic T lymphocyte epitope
peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4
blockade. Cancer Res. 63:3281–3288. 2003.PubMed/NCBI
|
|
19
|
Daftarian P, Song GY, Ali S, Faynsod M,
Longmate J, Diamond DJ and Ellenhorn JD: Two distinct pathways of
immuno-modulation improve potency of p53 immunization in rejecting
established tumors. Cancer Res. 64:5407–5414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kocak E, Lute K, Chang X, et al:
Combination therapy with anti-CTL antigen-4 and anti-4-1BB
antibodies enhances cancer immunity and reduces autoimmunity.
Cancer Res. 66:7276–7284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gattinoni L, Klebanoff CA, Palmer DC,
Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR,
Rosenberg SA and Restifo NP: Acquisition of full effector function
in vitro paradoxically impairs the in vivo antitumor efficacy of
adoptively transferred CD8+ T cells. J Clin Invest.
115:1616–1626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang J, Khong HT, Dudley ME, El-Gamil M,
Li YF, Rosenberg SA and Robbins PF: Survival, persistence, and
progressive differentiation of adoptively transferred
tumor-reactive T cells associated with tumor regression. J
Immunother. 28:258–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe A, Hara M, Chosa E, Nakamura K,
Sekiya R, Shimizu T and Onitsuka T: Combination of adoptive cell
transfer and antibody injection can eradicate established tumors in
mice - an in vivo study using anti-OX40mAb, anti-CD25mAb and
anti-CTLA4mAb-. Immunopharmacol Immunotoxicol. 32:238–245. 2010.
View Article : Google Scholar
|
|
25
|
Simpson TR, Li F, Montalvo-Ortiz W, et al:
Fc-dependent depletion of tumor-infiltrating regulatory T cells
co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J
Exp Med. 210:1695–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selby MJ, Engelhardt JJ, Quigley M,
Henning KA, Chen T, Srinivasan M and Korman AJ: Anti-CTLA-4
antibodies of IgG2a isotype enhance antitumor activity through
reduction of intratumoral regulatory T cells. Cancer Immunol Res.
1:32–42. 2013. View Article : Google Scholar
|
|
27
|
Takahashi T, Tagami T, Yamazaki S, Uede T,
Shimizu J, Sakaguchi N, Mak TW and Sakaguchi S: Immunologic
self-tolerance maintained by CD25+CD4+
regulatory T cells constitutively expressing cytotoxic T
lymphocyte-associated antigen 4. J Exp Med. 192:303–310. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Curran MA and Allison JP: Tumor vaccines
expressing flt3 ligand synergize with ctla-4 blockade to reject
preimplanted tumors. Cancer Res. 69:7747–7755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liakou CI, Kamat A, Tang DN, Chen H, Sun
J, Troncoso P, Logothetis C and Sharma P: CTLA-4 blockade increases
IFNγ-producing CD4+ICOShi cells to shift the
ratio of effector to regulatory T cells in cancer patients. Proc
Natl Acad Sci USA. 105:14987–14992. 2008. View Article : Google Scholar
|
|
30
|
Hodi FS, Butler M, Oble DA, et al:
Immunologic and clinical effects of antibody blockade of cytotoxic
T lymphocyte-associated antigen 4 in previously vaccinated cancer
patients. Proc Natl Acad Sci USA. 105:3005–3010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bluestone JA and Abbas AK: Natural versus
adaptive regulatory T cells. Nat Rev Immunol. 3:253–257. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akbar AN, Taams LS, Salmon M and
Vukmanovic-Stejic M: The peripheral generation of CD4+
CD25+ regulatory T cells. Immunology. 109:319–325. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25− naive T cells to
CD4+CD25+ regulatory T cells by TGF-β
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikemoto T, Yamaguchi T, Morine Y, Imura S,
Soejima Y, Fujii M, Maekawa Y, Yasutomo K and Shimada M: Clinical
roles of increased populations of Foxp3+CD4+
T cells in peripheral blood from advanced pancreatic cancer
patients. Pancreas. 33:386–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentation. J Exp Med. 190:355–366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perrin PJ, Maldonado JH, Davis TA, June CH
and Racke MK: CTLA-4 blockade enhances clinical disease and
cytokine production during experimental allergic encephalomyelitis.
J Immunol. 157:1333–1336. 1996.PubMed/NCBI
|
|
37
|
Karandikar NJ, Vanderlugt CL, Walunas TL,
Miller SD and Bluestone JA: CTLA-4: A negative regulator of
autoimmune disease. J Exp Med. 184:783–788. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouchi N, Kihara S, Arita Y, et al: Novel
modulator for endothelial adhesion molecules: Adipocyte-derived
plasma protein adiponectin. Circulation. 100:2473–2476. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishikawa T, Kokura S, Enoki T, et al:
Phase I clinical trial of fibronectin CH296-stimulated T cell
therapy in patients with advanced cancer. PLoS One. 9:e837862014.
View Article : Google Scholar : PubMed/NCBI
|