Introduction
Lung cancer is presently the leading cause of global
cancer-related death, with an increasing prevalence and mortality.
Smoking is the predominant risk factor for lung cancer. While, in
East Asia, ~30% of patients suffering from lung cancer were never
smokers (1,2), and non-smoking-related lung cancer can
also occur in current and former smokers (3). Unfortunately, lung cancer has not been
solved in regards to prevention or diagnosis or treatment.
In addition to small cell lung cancer (SCLC)
accounting for 10–15% of lung cancer cases, non-small cell lung
cancer (NSCLC) represents ~85–90% of overall lung cancer cases
(4). NSCLC is also subdivided into
three histologic types, including adenocarcinoma, squamous cell
carcinoma and large cell undifferentiated carcinoma. Lung
adenocarcinoma accounts for almost 40% of NSCLC. Due to the
relatively high incidence of lung adenocarcinoma, much research has
been conducted to elucidate its nature and mechanisms.
Previous studies have found various genes related to
lung adenocarcinoma. Su et al (5) found that a higher level of
cyclo-oxygenase-2 decreased the survival rate of patients through
many mechanisms, such as a corresponding higher level of vascular
endothelial growth factor that stimulated the growth and migration
of cancer cells (6), a higher
lymphatic vessel density that reduced the restriction of cancer
cell invasion (7), and enhanced
lymph node metastasis that accelerated the metastasis of cancer
cells (8). Mutations of oncogene
K-ras and tumor-suppressor gene TP53 have a strong
link with lung adenocarcinoma (9).
Other fusion genes have been further studied concerning the
correlation with lung adenocarcinoma. Fusion of the kinesin family
member 5B and RET proto-oncogene was found to occur in a
subset of NSCLC (10). Fusion genes
of echinoderm microtubule associated protein like 4 - anaplastic
lymphoma receptor tyrosine kinase and kinesin light chain 1 -
anaplastic lymphoma receptor tyrosine kinase were also found in
lung adenocarcinoma (11).
To date, the pathogenesis of NSCLC and lung
adenocar-cinoma is difficult to determine. To reduce the enormous
morbidity and mortality of lung adenocarcinoma, it is critical to
identify lung adenocarcinoma-associated genes and mechanisms.
Integrated analysis of full DEGs and the expression of regulatory
factors such as methylation, mRNA splicing, transcription factors
(TFs) and microRNAs (miRNAs) is an effective method for disease
pathogenesis study. In the present study, DEGs, exons and isoforms,
as well as DEG-related methylation, TFs and miRNAs were integrated
and analyzed.
Materials and methods
Datasets
The raw experimental data under accession no. GSE
37764 (12) used in the present
study are publically available in the Gene Expression Omnibus (GEO)
database (http://www.ncbi.nlm.nih.gov/geo). These data, which
include expression profiling, methylation profiling and non-coding
RNA profiling of 6 never-smoker Korean female patients, were
produced by high throughput sequencing. The histologic origins were
cancer tissues and adjacent normal tissues of non-small cell lung
adenocarcinoma. In the present study, using normal tissues as
control, the molecular variations in tumor tissues were identified.
The platform of this data is GPL10999 (Illumina Genome Analyzer
IIx, Homo sapiens).
Methylation profiling and differentially
methylated gene screening
Trimmomatic (13)
software package, a flexible, pair-aware and efficient
preprocessing tool for Illumina sequence data, is often used to
remove low quality reads and trim adaptor sequences. In the present
study, the methylated DNA immunoprecipitation-sequencing
(MeDIP-seq) was preprocessed with the Trimmomatic (13). During the preprocessing of the
Illumina reads, a minimum quality cutoff on the first and last
bases using LEADING: 3 (trim the leading nucleotides until quality
>3) and TRAILING: 3 (trim the trailing nucleotides until quality
>3) was imposed, and a minimum sliding window quality using
SLIDINGWINDOW: 4:15 (trim the window of size four for reads with
local quality below a score of 15) was subjected. In addition, the
resulting reads shorter than 25 bases were discarded. Then the
Bowtie (14) alignment algorithm
(with default parameters) was used to align the Illumina reads to
the human reference genome (hg19), and SAM tools (15) was applied to remove PCR duplicates.
The differentially methylated regions (DMRs) were identified by
MEDIPS (16) in R. with false
discovery rate (FDR) <0.1. Each DMR contains multiple methylated
loci, and the determination of overlaps between methylation loci
and the adjacent genes were computed using the BED Tools (17) software. Briefly, differentially
methylated loci between −2,000 and +1,000 bp around transcription
start site (TSS) were selected, and the adjacent genes were defined
as differentially methylated genes (DMGs).
Gene expression profile analysis
RNA-seq reads were cleaned to remove low quality
regions and sequencing adaptors utilizing Trimmomatic (13) software package (LEADING: 3,
TRAILING: 3, SLIDINGWINDOW: 4:15, MINLEN: 36). These massively
parallel short reads were subsequently mapped to a reference genome
with TopHat (18) (no >5 bases
mismatch). Since multiexon genes can encode different transcripts
and multiple transcript variants encode different isoforms,
differentially expressed exons were analyzed by DEXSeq (19) in R. and differential expression
analysis of genes and transcript isoforms were performed with
Cufflinks (20) algorithm. The
parameters of DEXSeq and Cufflinks were default values. The
thresholds were q-value <0.05 and fold-change (FC) >2.
Comparing the cancer tissues and control, genes and exons with
average expression levels >10 FPKM (fragments/kilobase of
transcript/million mapped reads) were defined as differentially
expressed.
Screening of differentially expressed
miRNAs
High-throughput sequencing data for miRNA expression
were cleaned to remove low quality regions and sequencing adaptors
utilizing Trimmomatic [2] software package (LEADING: 3, TRAILING:
3, SLIDINGWINDOW: 4:15, MINLEN: 25). Reads of millions of miRNA
sequences were aligned to the genome and the expression value of
each miRNA was measured using miRExpress (21). Subsequently, the screening of
differentially expressed miRNAs was performed with DESeq2 (22) in R/Bioconductor FDR <0.01; FC
>2; base mean >10). Conserved miRNA targets were retrieved
from public websites (TargetScan, http://targetscan.org) (23).
Functional analysis of a variety of
differential genes
Gene Ontology (GO) functional enrichment and
annotation of differential genes, including differentially
methylated genes and DEGs, were computed using the database for
annotation, visualization and integration discovery (DAvID)
(24). The annotation of
miRNA-target DEGs was performed with TarBase 6.0 database
(capturing the exponential growth of miRNA targets with
experimental support) (25). Then
the DIANA miRPath v.2.0 (26) was
used to determine molecular pathways potentially altered by
differentially expressed miRNAs based on the Kyoto Encyclopedia of
Genes and Genomes (KEGG) database. The ChIP-X Enrichment Analysis
(ChEA) and the ENCODE ChIP-seq (28) were utilized to search for enriched
TFs located upstream of the DEGs. TF-target genes were predicted
and combined with differentially expressed miRNAs and DEGs using
mirConnX (29) with Pearson’s
correlation coefficient >0.96 and then, the integrated network
of TFs, miRNAs and TF-target DEGs were constructed and
analyzed.
Results
Differentially methylated regions and
genes
After comparison of the MeDIP-Seq data between
cancer tissues and para-carcinoma tissues of 6 non-small cell lung
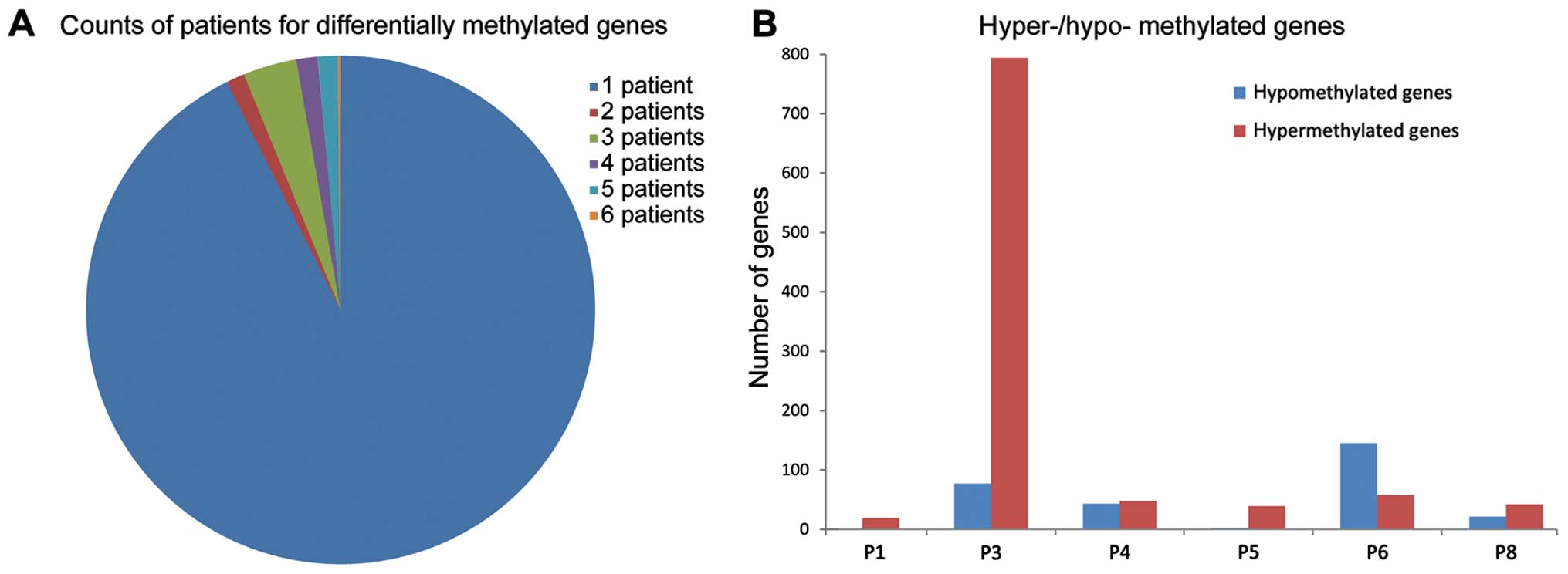
carcinoma patients, DMRs and DMGs were obtained. Most of the DMGs
(>90%) were detected in one patient only (Fig. 1A). Only 82 genes were found in 2 or
more patients and ~1/3 of the DMGs (34) were located in the mitochondrial
genome. The numbers of DMGs in 5 patients were similar except in 1
patient (P3) (Fig. 1B). In patient
P3, the hypermethylated genes were significantly more than in the
others.
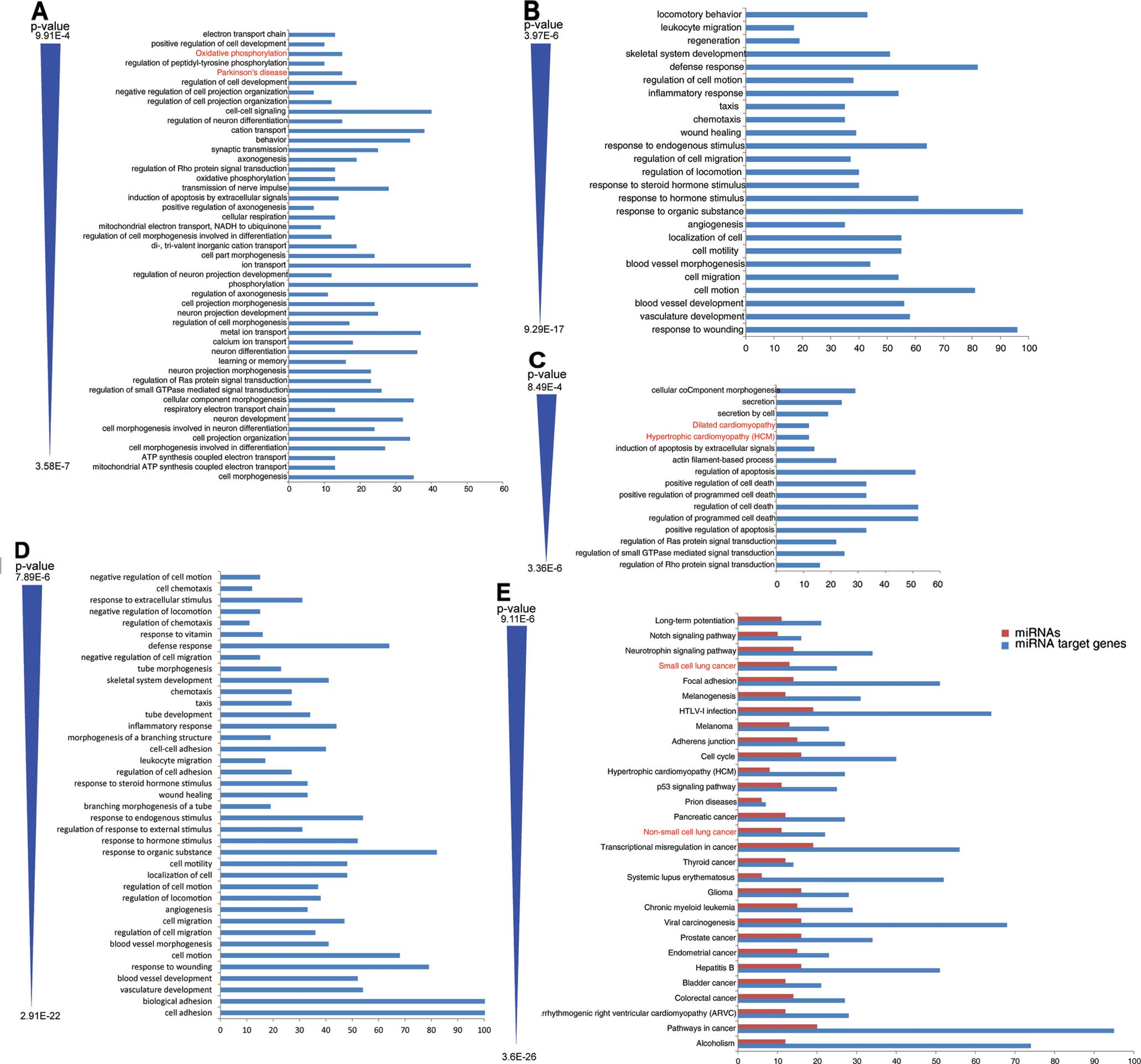
Functional GO analysis showed that the DMGs were
mostly associated with metabolic pathways (Fig. 2A). The most commonly enriched GO
terms were cell morphogenesis, mitochondrial ATP synthesis coupled
electron transport and ATP synthesis coupled electron
transport.
KEGG pathway analysis identified two enriched
pathways, respectively: Parkinson’s disease (hsa: 05012) and
oxidative phosphorylation (hsa: 00190) (Fig. 2A).
Gene expression profile analysis
DEG screening analysis found that a total of 1,498
genes were differentially expressed between the cancer tissues and
adjacent normal tissues, and 1207 isoforms of 1,103 genes were
differentially expressed. Additionally, 1,286 exons of 916 genes
were also differentially expressed.
Functional GO analysis showed that most of these
differential genes were related to cell migration and apoptosis
(Fig. 2B-D). The most commonly
enriched terms of genes were response to wounding, vasculature
development and blood vessel development. The most commonly
enriched terms of the exons were regulation of Rho protein signal
transduction, regulation of small GTPasse mediated signal
transduction and regulation of Ras protein signal transduction. The
most commonly enriched terms of transcripts were cell adhesion,
biological adhesion and vasculature development.
KEGG pathway analysis of the differentially
expressed exons found two enriched pathways named hypertrophic
cardiomyopathy (HCM) (hsa: 05410) and dilated cardiomyopathy (hsa:
05414) (Fig. 2C).
A total of 541 genes among these differential genes
possessed only 1 differentially expressed exon. As shown in
Figs. 3 and 4, the expression levels of different exons
corresponding to the same one gene had dozens of time variations.
Several exons with a lower expression level appeared almost
unanimously between the cases and control, while others displayed a
significant difference between these two groups.
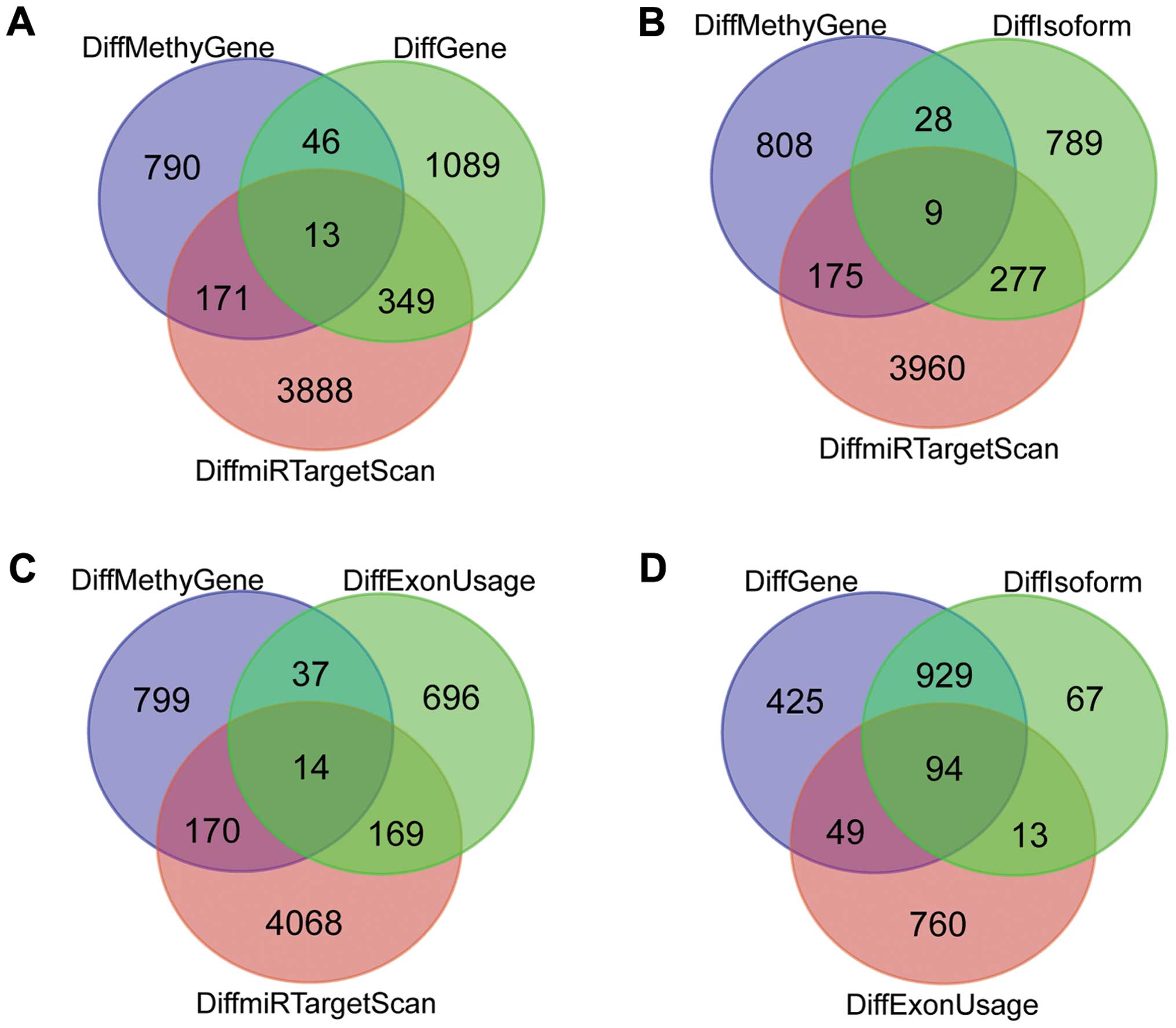
There were 94 common genes differentially expressed
at the levels of genes, isoforms and exons (Fig. 3D).
Differential expression of miRNAs
Within 50 precursors, 44 miRNAs were detected as
differentially expressed miRNAs. The most overexpressed miRNAs were
miR-6510, miR-301b and miR-183. miR-144, miR-486 and miR-451a were
the most commonly downregulated miRNAs.
GO and pathway analysis shown that the
differentially expressed miRNAs were correlated with many
disease-related pathways (Fig. 2E).
The top three enriched pathways, respectively, were alcoholism
(hsa: 05034), pathways in cancer (hsa: 05200) and arrhymogenic
right ventricular cardimyopathy (ARvC) (hsa: 05412). NSCLC (hsa:
05223) and small cell lung cancer (hsa: 05222) were also enriched
pathways.
Nine differentially expressed miRNA-target genes
with methylation were differentially expressed at levels of
isoforms (Fig. 3B) and 14 at the
level of exons (Fig. 3C).
Integrated analysis of the differential
genes
Thirteen differentially expressed miRNA-target genes
included differentially methylated genes, and also differentially
expressed exon-related genes. Furthermore, they were regulated by
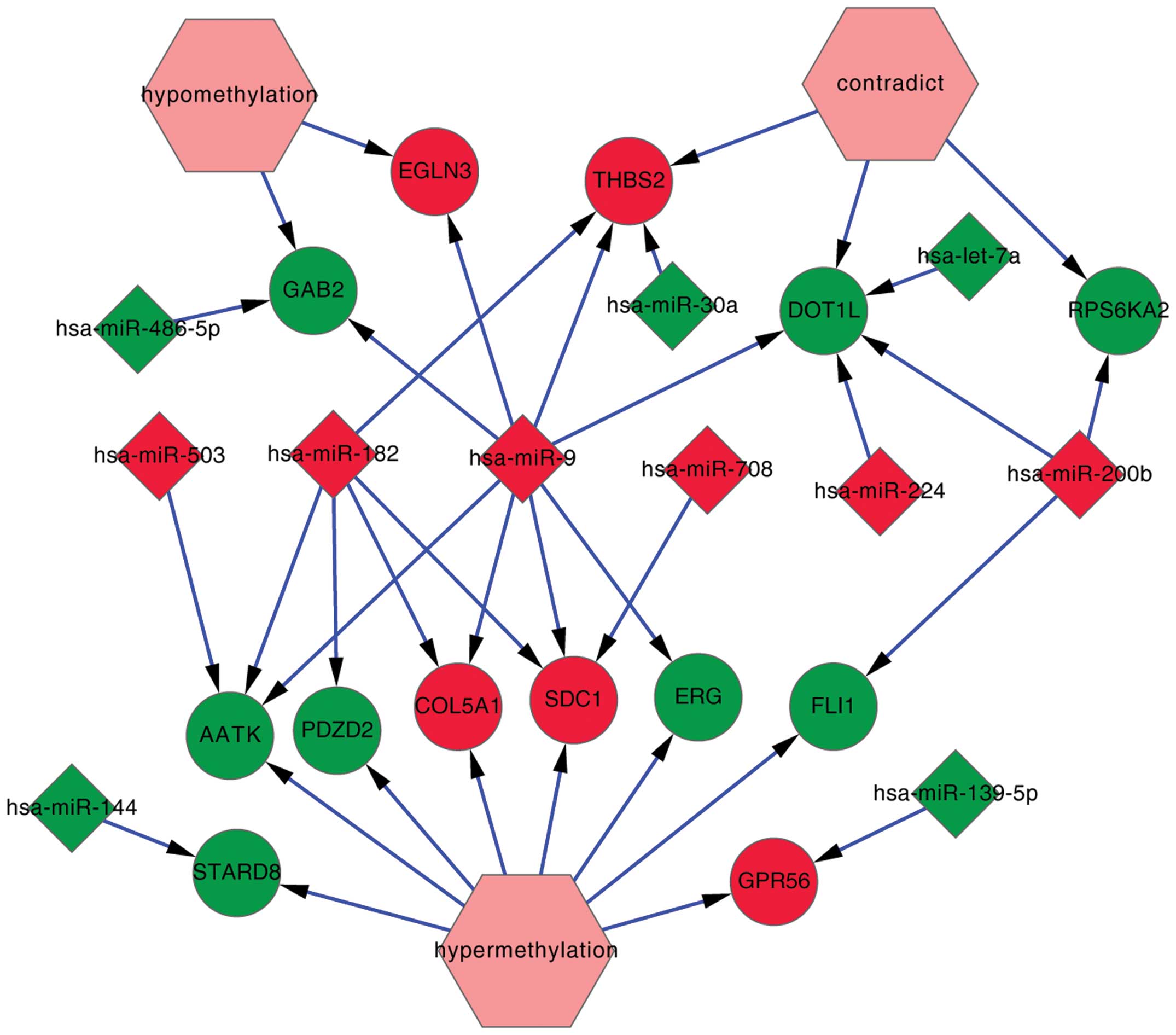
differentially expressed miRNAs (Table
I, Fig. 3A). Yet, differential
methylation of these 13 genes was observed only in one patient. In
addition, ribosomal protein S kinase, 90 kDa, polypeptide 2
(RPS6KA2), DOT1-like histone H3K79 methyltransferase
(DOT1L) and thrombospondin 2 (THBS2), included in
these 13 genes, were detected with both hypomethylation and
hypermethylation.
 | Table IThirteen differentially expressed
genes under the regulation of differentially expressed miRNAs and
methylation. |
Table I
Thirteen differentially expressed
genes under the regulation of differentially expressed miRNAs and
methylation.
| Name | miRNA | Expression | N | T | log2FC (T/N) | Hyper-methylated
sample |
|---|
| ERG | hsa-miR-9 | 1 | 30.2622 | 7.6856 | −1.97729 | 1T |
| FLI1 | hsa-miR-200b | 1 | 35.8052 | 11.0298 | −1.69876 | 1T |
| RPS6KA2 | hsa-miR-200b | 1 | 39.4554 | 19.498 | −1.01689 | 1N1T |
| STARD8 | hsa-miR-144 | −1 | 15.475 | 4.3143 | −1.84274 | 1T |
| PDZD2 | hsa-miR-182 | 1 | 17.9635 | 5.28059 | −1.76629 | 1T |
| GPR56 | hsa-miR-139-5p | −1 | 32.3344 | 65.4073 | 1.01638 | 1T |
| SDC1 | hsa-miR-182 | 1 | 67.1969 | 206.511 | 1.61975 | 1T |
| SDC1 | hsa-miR-708 | 1 | 67.1969 | 206.511 | 1.61975 | 1T |
| SDC1 | hsa-miR-9 | 1 | 67.1969 | 206.511 | 1.61975 | 1T |
| EGLN3 | hsa-miR-9 | 1 | 5.38757 | 30.8381 | 2.51701 | 1N |
| COL5A1 | hsa-miR-182 | 1 | 25.2564 | 57.9578 | 1.19835 | 1T |
| COL5A1 | hsa-miR-9 | 1 | 25.2564 | 57.9578 | 1.19835 | 1T |
| AATK | hsa-miR-182 | 1 | 15.6319 | 2.48414 | −2.65367 | 1T |
| AATK | hsa-miR-503 | 1 | 15.6319 | 2.48414 | −2.65367 | 1T |
| AATK | hsa-miR-9 | 1 | 15.6319 | 2.48414 | −2.65367 | 1T |
| GAB2 | hsa-miR-486-5p | −1 | 17.3317 | 8.056 | −1.10527 | 1N |
| GAB2 | hsa-miR-9 | 1 | 17.3317 | 8.056 | −1.10527 | 1N |
| DOT1L | hsa-let-7a | −1 | 14.0112 | 6.96755 | −1.00786 | 1N1T |
| DOT1L | hsa-miR-200b | 1 | 14.0112 | 6.96755 | −1.00786 | 1N1T |
| DOT1L | hsa-miR-224 | 1 | 14.0112 | 6.96755 | −1.00786 | 1N1T |
| DOT1L | hsa-miR-9 | 1 | 14.0112 | 6.96755 | −1.00786 | 1N1T |
| THBS2 | hsa-miR-182 | 1 | 10.5524 | 64.5186 | 2.61214 | 1N1T |
| THBS2 | hsa-miR-30a | −1 | 10.5524 | 64.5186 | 2.61214 | 1N1T |
| THBS2 | hsa-miR-9 | 1 | 10.5524 | 64.5186 | 2.61214 | 1N1T |
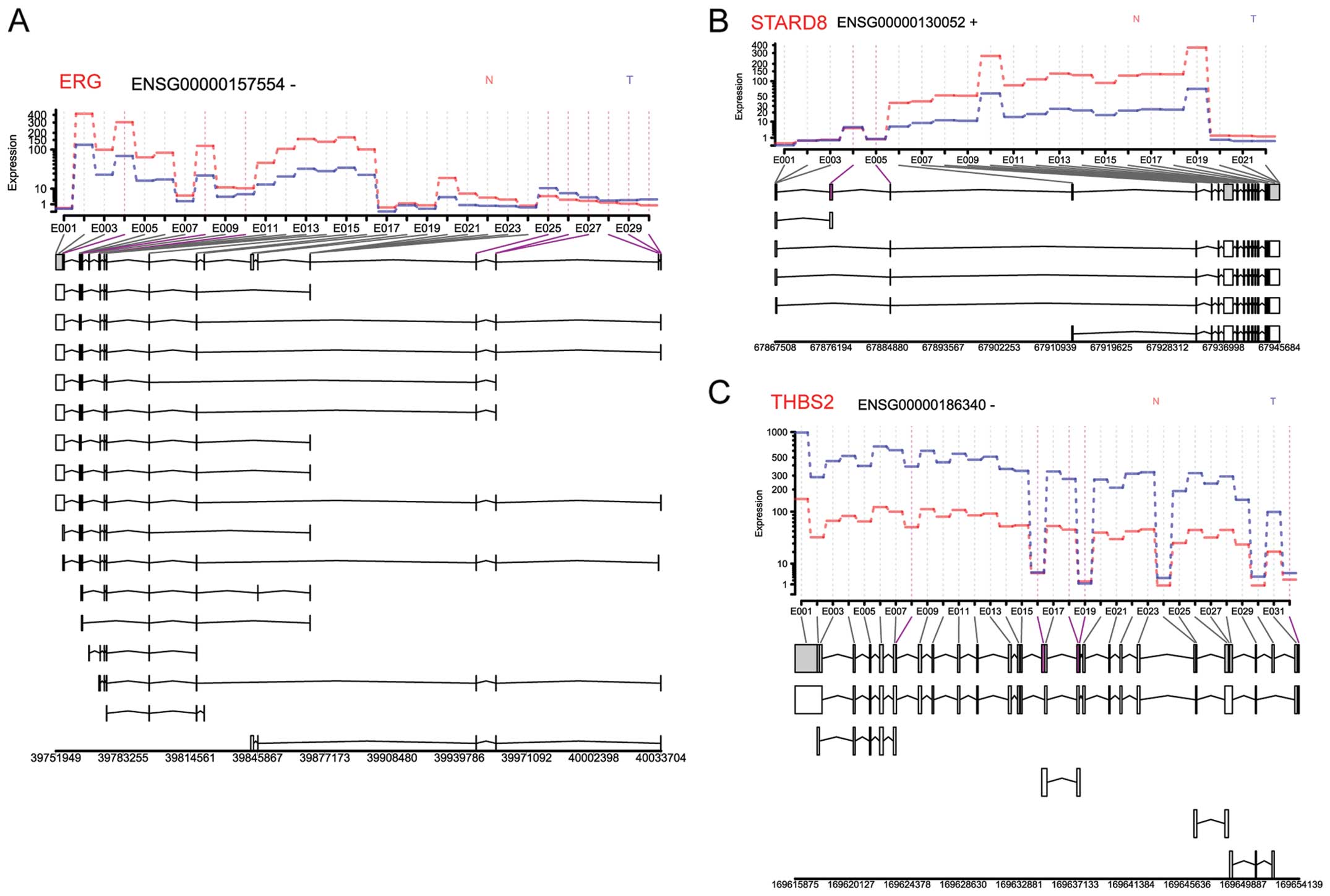
The overlapping genes among the differentially
methylated genes, DEGs and isoforms, differentially expressed
exon-related genes, differentially expressed miRNA-targeted genes
were v-ets avian erythroblastosis virus E26 Oncogene Homolog
(ERG), StAR-related lipid transfer domain containing 8
(STARD8) and THBS2. Isoforms and expression levels of
these 3 genes are shown in Fig.
4
The possible regulatory networks of methylation,
miRNA expression and gene expression are shown in Fig. 5. There were 6 overexpressed and 5
downregulated miRNAs, 5 upregulated and 8 downregulated genes, 2
genes with hypomethylation, 8 genes with hypermethylation and 3
genes with contradiction. In the 11 miRNAs, miR-9 (degree= 8) and
miR-182 (degree=5) had more degrees than the others. Genes
including DOT1L, apoptosis-associated tyrosine kinase
(AATK), syndecan 1 (SDC1) and THBS2 had more
degrees.
Transcription analysis of DEGs
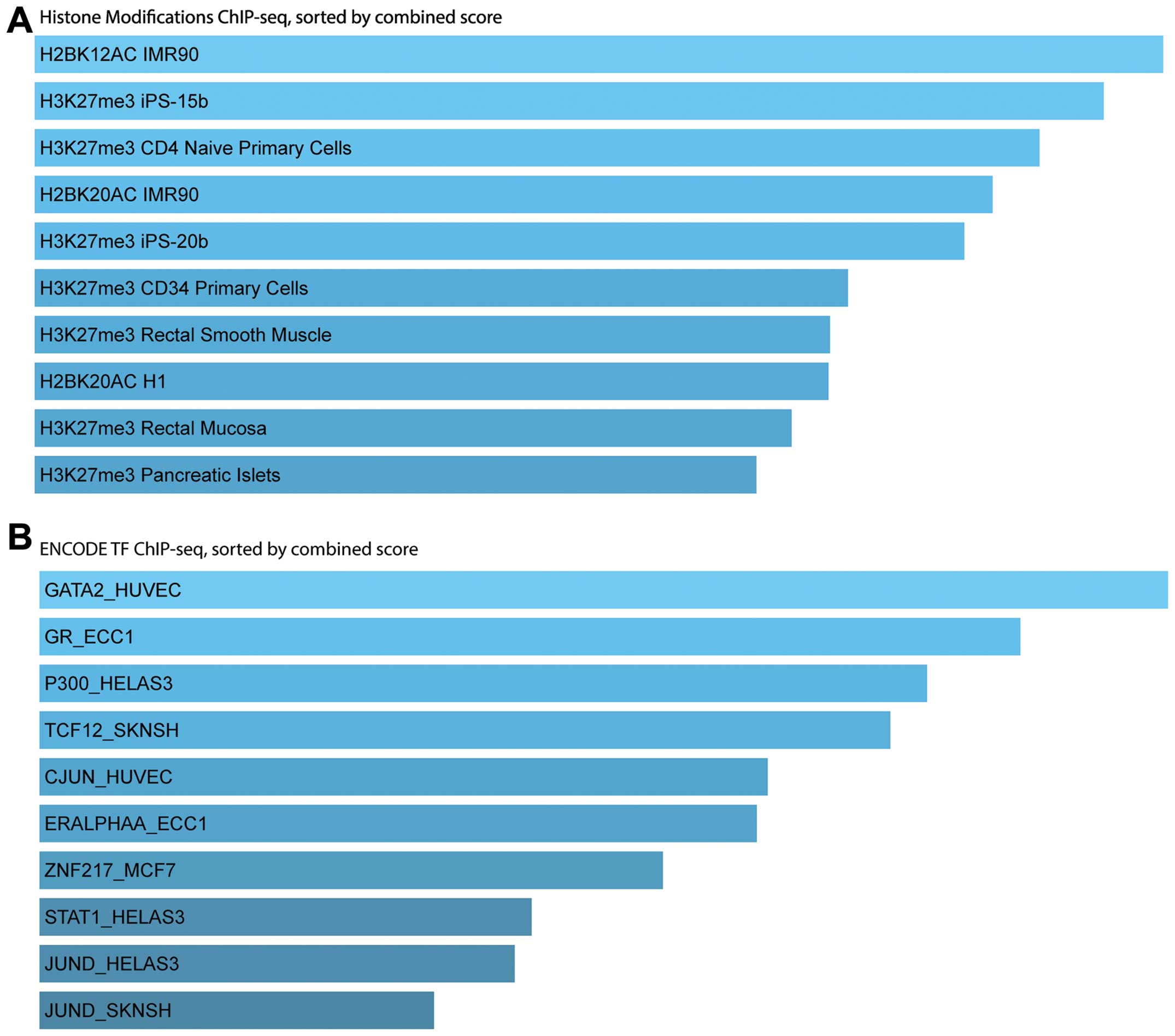
ChEA2 analysis results indicated that the screened
DEGs were modified and regulated by multi-cancer cell line histones
including tri-methylation of lysine 27 on histone H3 (H3K27me3) and
di-acetylation of lysine 12 or 20 on histone H2 (H2BK12/20AC),
which pertained to the ENCODE database (Fig. 6A). The upstream TF binding patterns
were not as clustered as that of the histone modification; they
were enriched in different ChIP-seq clusters of TFs in different
cell lines (Fig. 6B), such as GATA2
and CJUN in human umbilical vein endothelial cells (HUvECs),
glucocorticoid receptors (GRs) and estrogen receptor (ER)α in
endometrial cells (ECC1), while, P300, signal transducer and
activation of transcription (STAT1) and JUND in HeLaS3 cells.
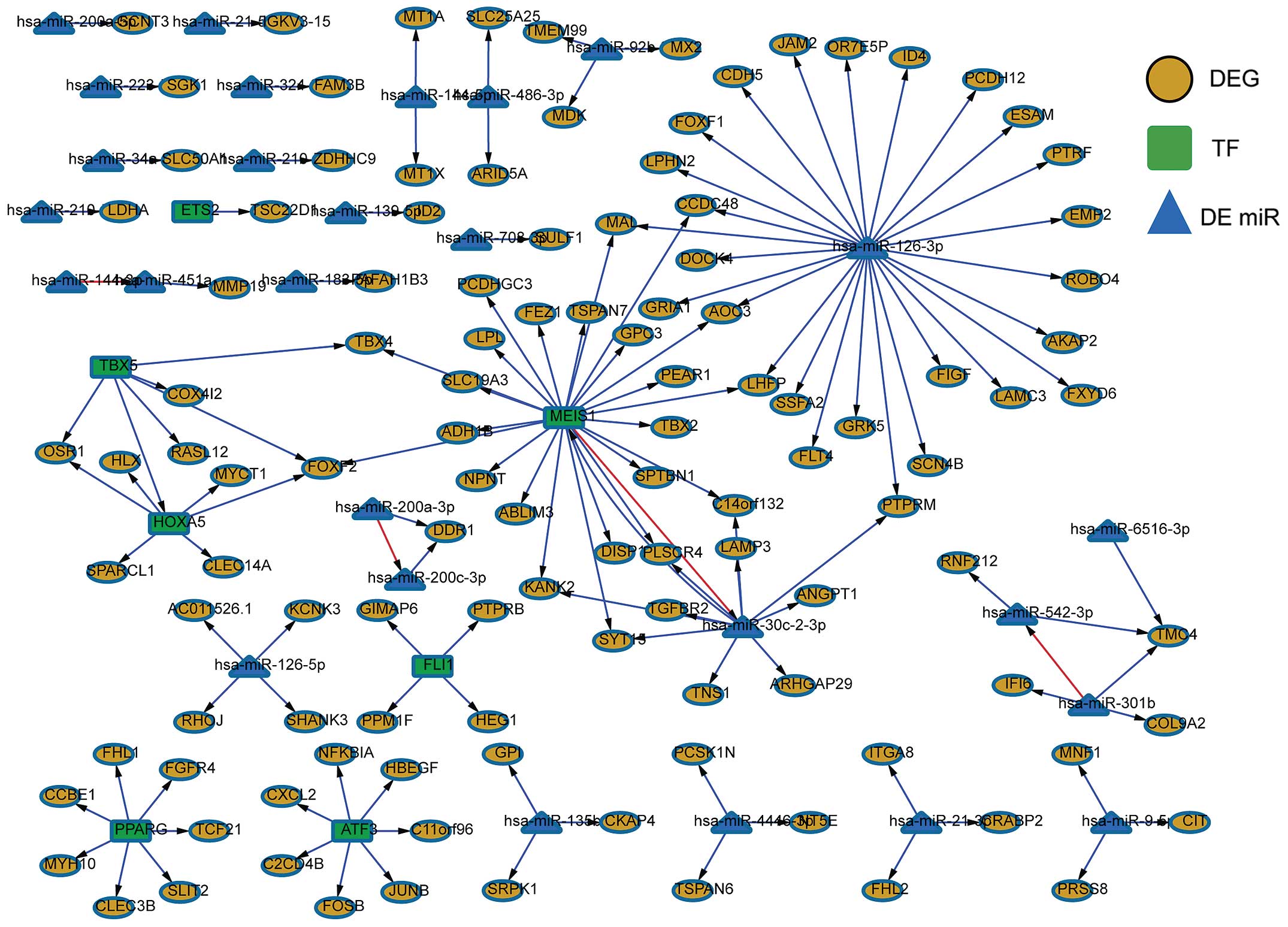
DEGs were regulated by TFs and miRNAs, and the
regulatory network was constructed as shown in Fig. 7. There were 116 DEGs, 72 TFs and 7
differentially expressed miRNAs. miR-126-3p served as a ‘hub’ in
the gene regulatory network which regulated 26 DEGs. The TF MEIS1
was another ‘hub’, which also regulated 22 DEGs and miR-30c-2-3p.
Several sub-networks with homeobox A5 (HOXA5), Meis homeobox 1
(MEIS1), T-box 5 (TBX5), miR-126-3p and miR-30c-2-3p as centers
shared several nodes and then formed another greater regulatory
network. The remaining sub-networks were detached from each
other.
Discussion
NSCLC accounts for ~85% of all lung cancer cases
(30) and remains the leading cause
of cancer-related death worldwide (31). Lung adenocarcinoma, the major
subtype of NSCLC responsible for more than 500,000 mortalities/year
worldwide (32), is associated with
a poor prognosis. In the present study, differentially methylated
regions, differentially expressed miRNAs and transcriptomics
between different tissues from 6 non-small cell lung adenocarcinoma
patients were analyzed. Several DEGs, miRNAs and TFs were screened,
which were expected to be associated with metabolism, cell
apoptosis or various diseases; thus, they may be important in the
progression of lung adenocarcinoma. Kim et al (12) identified various novel genetic
aberrations, gene network modules and miRNA-target interactions
within the same dataset, yet, it is distinct from ours. In
addition, the pathogenesis of lung adenocarcinoma is far from
clear. With the different bioinformatics tools, the results of the
same analysis were slightly different from those of Kim et
al (12). The new information
obtained from the present study may help to illuminate the
molecular mechanisms of this disease.
The methylation analysis results of the 6 patients
were relatively diverse. Since a relatively large number of
methylation sites are concentrated in the mitochondrial genome, it
is diffi-cult to research the connections between methylation and
gene expression. Additionally, RPS6KA2, DOT1L and
THBS2 were detected with both hypomethylation and
hypermethylation. This may be relatively related to the great
individual differences of the methylated regions in the patients.
However, previous research has confirmed that DNA methylation is
critical in lung cancer (33).
Combined with the subsequent analysis of differential expression of
genes, isoforms and exons, screening of miRNA-related genes, three
overlapping genes were obtained. ERG, a member of the ETS
oncogene family (34), is
intimately involved in the development of multiple cancers
including prostate cancer (35).
The TMPRSS2-ERG gene fusion is now a specific biomarker of
prostate cancer (36). There is
little research on ERG and lung adenocarcinoma. As cancer develops
from glands which are the same as prostate cancer, ERG may also be
related with lung adenocarcinoma. STARD8 was found
downregulated and highly methylated in the present study. Durkin
et al (37) suggested that
STARD8 is a tumor-suppressor gene encoding DLC-3 to suppress
tumor cell growth. It has a higher level of methylation in
colorectal cancer than other types of cancer (38). THBS2, an upregulated gene,
encodes a protein belonging to the thrombospondin family. This
protein has been shown to function as a potent inhibitor of tumor
growth and angiogenesis, and it may be involved in cell adhesion
and migration (39). THBS2
also has CpG island methylation in malignant ovarian tumors
(40). Therefore, STARD8 and THBS2
may also be involved in lung adenocarcinoma.
Gene expression is under the elaborate control of
interrelated factors including TFs and histone modification. In the
present study, comparative analysis of histone modifications in
tumor and normal tissues was conducted. This revealed that DEGs
were centrally regulated by H3K27me3 and H2BK12/20AC in several
cancer cell lines. H3K27me3 is regarded as related to gene
silencing (41). The H3K27me3
marker is associated with promoters of all hypermethylated genes
associated with tumor suppressors in cancer cells (42). With the prevalent regulation
executed by H3K27me3 on DEGs screened in the present study,
abnormal modification of H3K27me3 may play an important role in
lung adenocarcinoma. Studies have shown that high expression of
histone H3K27me3 is related with a good prognosis of patients with
NSCLC; namely, the higher expression of histone H3K27me3, the
better the prognosis of patients (43). Functional analysis of DEGs and
isoforms revealed that they were enriched in the process of
hormonal responses. Enrichment analysis of ChIP-seq presented that
DEGs were enriched in different ChIP-seq clusters including GR and
ERα of TFs. This indicated that regulation of ER and GR may be
associated with lung adenocarcinoma. Studies have shown that ERα
and ERβ, especially ERβ, are expressed in NSCLC to induce tumor
cell proliferation (44). It was
found that midkine plays a pivotal role in epithelial-mesenchymal
transition in lung adenocarcinoma (45). Enhanced ERβ-mediated estradiol
dysregulates midkine expression (46). In previous research, GR, a member of
the nuclear hormone receptor family, mediated cancer cell apoptosis
and thereby slowed tumor growth (47). GR is down-regulated by increased
promoter methylation, which is similar to mechanisms associated
with common tumor-suppressor genes (48).
The constructed TF and miRNA regulatory networks
showed that hub nodes including miR-126-3p, miR-30c-2-3p, HOXA5,
MEIS1 and TBX5 were markedly different in two separate TF and miRNA
enrichment analyses, and their levels were significantly decreased
in cancer tissues. As crucial upstream genes, their significant
change in the expression level may affect a plurality of downstream
target genes. Expression of most of the HOX family member are
altered in NSCLC cells significantly (49). Contradictory results were found by
Abe et al (49) who detected
the downregulation of HOXA5 in NSCLC. Whether HOXA5 regulates
various lung cancer-related genes or what changes it undergoes in
lung adenocarcinoma, remains to be elucidated. The relationships of
two other TFs including MEIS1 and TBX5 with lung cancer are
unclear. It is known that MEIS1 is one of the co-factors of the HOX
family (especially for HOXA7 and HOXA9) (50). Together, they are involved in human
leukemogenesis (51). TBX5
regulates cell proliferation during cardiogenesis (52) and it is related to cell migration as
well as cell proliferation in cancers (53). miR-126 has a clearer relationship
with NSCLC and could inhibit the proliferation and invasion of
NSCLC cells (54). Downregulation
of miR-30c promotes cell migration and invasion of NSCLC cells
(55). Despite the fact that
altered expression of these two miRNAs has long been known, the
exact regulatory mechanisms remain to be studied. The present
integrated analysis found that possible target expression levels of
these two miRNAs may also undergo significant changes. This
indicates one direction for further study.
Differentially methylated regions, differentially
expressed miRNAs and transcriptomics of normal and cancer tissues
were analyzed. Three possible lung adenocarcinoma-related DEGs
including ERG, STARD8 and THBS2 were
identified. Moreover, DEG-related histone modifications and TFs
were screened and underwent integrated analysis. Lung
adenocar-cinoma-related DEGs may be under comparable regulation of
histones. Moreover, several TFs and miRNAs may play critical roles
in the tumorigenesis of lung adenocarcinoma. These results provide
the foundation for further lung adenocarci-noma research, and these
results must be confirmed through additional experiments.
References
|
1
|
Toh CK, Gao F, Lim WT, Leong SS, Fong KW,
Yap SP, Hsu AA, Eng P, Koong HN, Thirugnanam A, et al:
Never-smokers with lung cancer: Epidemiologic evidence of a
distinct disease entity. J Clin Oncol. 24:2245–2251. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Subramanian J and Govindan R: Lung cancer
in never smokers: A review. J Clin Oncol. 25:561–570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yano T, Haro A, Shikada Y, Maruyama R and
Maehara Y: Non-small cell lung cancer in never smokers as a
representative ‘non-smoking-associated lung cancer̓: Epidemiology
and clinical features. Int J Clin Oncol. 16:287–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su JL, Shih JY, Yen ML, Jeng YM, Chang CC,
Hsieh CY, Wei LH, Yang PC and Kuo ML: Cyclooxygenase-2 induces
EP1-and HER-2/Neu-dependent vascular endothelial growth factor-C
up-regulation: A novel mechanism of lymphangiogenesis in lung
adenocarcinoma. Cancer Res. 64:554–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skobe M, Hawighorst T, Jackson DG, Prevo
R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K and Detmar
M: Induction of tumor lymphangiogenesis by vEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heaney AP, Singson R, McCabe CJ, Nelson V,
Nakashima M and Melmed S: Expression of pituitary-tumour
transforming gene in colorectal tumours. Lancet. 355:716–719. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bevilacqua G, Sobel ME, Liotta LA and
Steeg PS: Association of low nm23 RNA levels in human primary
infiltrating ductal breast carcinomas with lymph node involvement
and other histopathological indicators of high metastatic
potential. Cancer Res. 49:5185–5190. 1989.PubMed/NCBI
|
|
9
|
Billah S, Stewart J, Staerkel G, Chen S,
Gong Y and Guo M: EGFR and KRAS mutations in lung carcinoma:
Molecular testing by using cytology specimens. Cancer Cytopathol.
119:111–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ju YS, Lee WC, Shin JY, Lee S, Bleazard T,
Won JK, Kim YT, Kim JI, Kang JH and Seo JS: A transforming KIF5B
and RET gene fusion in lung adenocarcinoma revealed from
whole-genome and transcriptome sequencing. Genome Res. 22:436–445.
2012. View Article : Google Scholar :
|
|
11
|
Togashi Y, Soda M, Sakata S, Sugawara E,
Hatano S, Asaka R, Nakajima T, Mano H and Takeuchi K: KLC1-ALK: A
novel fusion in lung cancer identified using a formalin-fixed
paraffin-embedded tissue only. PLoS One. 7:e313232012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SC, Jung Y, Park J, Cho S, Seo C, Kim
J, Kim P, Park J, Seo J, Kim J, et al: A high-dimensional,
deep-sequencing study of lung adenocarcinoma in female
never-smokers. PLoS One. 8:e555962013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for Illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Langmead B and Salzberg SL: Fast
gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R: 1000 Genome
Project Data Processing Subgroup: The sequence alignment/map format
and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lienhard M, Grimm C, Morkel M, Herwig R
and Chavez L: MEDIPS: Genome-wide differential coverage analysis of
sequencing data derived from DNA enrichment experiments.
Bioinformatics. 30:284–286. 2014. View Article : Google Scholar :
|
|
17
|
Quinlan AR and Hall IM: BEDTools: A
flexible suite of utilities for comparing genomic features.
Bioinformatics. 26:841–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anders S, Reyes A and Huber W: Detecting
differential usage of exons from RNA-seq data. Genome Res.
22:2008–2017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang WC, Lin FM, Chang WC, Lin KY, Huang
HD and Lin NS: miRExpress: Analyzing high-throughput sequencing
data for profiling microRNA expression. BMC Bioinformatics.
10:3282009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anders S, McCarthy DJ, Chen Y, Okoniewski
M, Smyth GK, Huber W and Robinson MD: Count-based differential
expression analysis of RNA sequencing data using R and
Bioconductor. Nat Protoc. 8:1765–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, et al: DAvID
Bioinformatics Resources: Expanded annotation database and novel
algorithms to better extract biology from large gene lists. Nucleic
Acids Res. 35(Web Server issue): W169–W175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vergoulis T, Vlachos IS, Alexiou P,
Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N,
Dalamagas T and Hatzigeorgiou AG: TarBase 6.0: Capturing the
exponential growth of miRNA targets with experimental support.
Nucleic Acids Res. 40:D222–D229. 2012. View Article : Google Scholar :
|
|
26
|
Vlachos IS, Kostoulas N, Vergoulis T,
Georgakilas G, Reczko M, Maragkakis M, Paraskevopoulou MD,
Prionidis K, Dalamagas T and Hatzigeorgiou AG: DIANA miRPath v.2.0:
Investigating the combinatorial effect of microRNAs in pathways.
Nucleic Acids Res. 40:W498–W504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lachmann A, Xu H, Krishnan J, Berger SI,
Mazloom AR and Ma’ayan A: ChEA: Transcription factor regulation
inferred from integrating genome-wide ChIP-X experiments.
Bioinformatics. 26:2438–2444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Consortium EP: ENCODE Project Consortium:
The ENCODE (ENCyclopedia Of DNA Elements) Project. Science.
306:636–640. 2004. View Article : Google Scholar
|
|
29
|
Huang GT, Athanassiou C and Benos PV:
mirConnX: Condition-specific mRNA-microRNA network integrator.
Nucleic Acids Res. 39(suppl): W416–W423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joshi M, Liu X and Belani CP: Taxanes,
past, present, and future impact on non-small cell lung cancer.
Anticancer Drugs. 25:571–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joseph P, Bowman B, Galban S and
Rehemtulla A: A Kras driven murine model elucidates oncogenic role
of FADD in lung cancer. Department of Radiation Oncology,
University of Michigan; Ann Arbor, MI: 2013, Honor’s Bachelor’s
Thesis. http://deepblue.lib.umich.edu/bitstream/handle/2027.42/98916/parijo.pdf?sequence=1.
|
|
33
|
Rauch TA, Wang Z, Wu X, Kernstine KH,
Riggs AD and Pfeifer GP: DNA methylation biomarkers for lung
cancer. Tumour Biol. 33:287–296. 2012. View Article : Google Scholar
|
|
34
|
Reddy ES, Rao VN and Papas TS: The erg
gene: A human gene related to the ets oncogene. Proc Natl Acad Sci
USA. 84:6131–6135. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thoms JA, Birger Y, Foster S, Knezevic K,
Kirschenbaum Y, Chandrakanthan V, Jonquieres G, Spensberger D, Wong
JW, Oram SH, et al: ERG promotes T-acute lymphoblastic leukemia and
is transcriptionally regulated in leukemic cells by a stem cell
enhancer. Blood. 117:7079–7089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leyten GH, Hessels D, Jannink SA, Smit FP,
de Jong H, Cornel EB, de Reijke TM, Vergunst H, Kil P, Knipscheer
BC, et al: Prospective multicentre evaluation of PCA3 and
TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary
biomarkers for prostate cancer. Eur Urol. 65:534–542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Durkin ME, Ullmannova V, Guan M and
Popescu NC: Deleted in liver cancer 3 (DLC-3), a novel Rho
GTPase-activating protein, is downregulated in cancer and inhibits
tumor cell growth. Oncogene. 26:4580–4589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mokarram P, Kumar K, Brim H,
Naghibalhossaini F, Saberi-firoozi M, Nouraie M, Green R, Lee E,
Smoot DT and Ashktorab H: Distinct high-profile methylated genes in
colorectal cancer. PLoS One. 4:e70122009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bornstein P: Thrombospondins function as
regulators of angiogenesis. J Cell Commun Signal. 3:189–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brezillon S, Zeltz C, Schneider L, Terryn
C, Vuillermoz B, Ramont L, Perrau C, Pluot M, Diebold MD, Radwanska
A, et al: Lumican inhibits B16F1 melanoma cell lung metastasis. J
Physiol Pharmacol. 60(Suppl 4): 15–22. 2009.
|
|
41
|
Barski A, Cuddapah S, Cui K, Roh TY,
Schones DE, Wang Z, Wei G, Chepelev I and Zhao K: High-resolution
profiling of histone methylations in the human genome. Cell.
129:823–837. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hussain M, Rao M, Humphries AE, Hong JA,
Liu F, Yang M, Caragacianu D and Schrump DS: Tobacco smoke induces
polycomb-mediated repression of Dickkopf-1 in lung cancer cells.
Cancer Res. 69:3570–3578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen X, Song N, Matsumoto K, Nanashima A,
Nagayasu T, Hayashi T, Ying M, Endo D, Wu Z and Koji T: High
expression of trimethylated histone H3 at lysine 27 predicts better
prognosis in non-small cell lung cancer. Int J Oncol. 43:1467–1480.
2013.PubMed/NCBI
|
|
44
|
Bogush TA, Dudko EA, Beme AA, Bogush EA,
Kim AI, Polotsky BE, Tjuljandin SA and Davydov MI: Estrogen
receptors, antiestrogens, and non-small cell lung cancer.
Biochemistry (Mosc). 75:1421–1427. 2010. View Article : Google Scholar
|
|
45
|
Mak P, Chang C, Pursell B and Mercurio AM:
Estrogen receptor β sustains epithelial differentiation by
regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci
USA. 110:4708–4713. 2013. View Article : Google Scholar
|
|
46
|
Zhao G, Nie Y, Lv M, He L, Wang T and Hou
Y: ERβ-mediated estradiol enhances epithelial mesenchymal
transition of lung adenocarcinoma through increasing transcription
of midkine. Mol Endocrinol. 26:1304–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schlossmacher G, Stevens A and White A:
Glucocorticoid receptor-mediated apoptosis: Mechanisms of
resistance in cancer cells. J Endocrinol. 211:17–25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Koutsimpelas D, Pongsapich W, Heinrich U,
Mann S, Mann WJ and Brieger J: Promoter methylation of MGMT, MLH1
and RASSF1A tumor suppressor genes in head and neck squamous cell
carcinoma: Pharmacological genome demethylation reduces
proliferation of head and neck squamous carcinoma cells. Oncol Rep.
27:1135–1141. 2012.PubMed/NCBI
|
|
49
|
Abe M, Hamada J, Takahashi O, Takahashi Y,
Tada M, Miyamoto M, Morikawa T, Kondo S and Moriuchi T: Disordered
expression of HOX genes in human non-small cell lung cancer. Oncol
Rep. 15:797–802. 2006.PubMed/NCBI
|
|
50
|
Svingen T and Tonissen KF: Altered HOX
gene expression in human skin and breast cancer cells. Cancer Biol
Ther. 2:518–523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Argiropoulos B, Yung E and Humphries RK:
Unraveling the crucial roles of Meis1 in leukemogenesis and normal
hematopoiesis. Genes Dev. 21:2845–2849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hatcher CJ, Kim M-S, Mah CS, Goldstein MM,
Wong B, Mikawa T and Basson CT: TBX5 transcription factor regulates
cell proliferation during cardiogenesis. Dev Biol. 230:177–188.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aherne NJ, Rangaswamy G and Thirion P:
Prostate cancer in a male with Holt-Oram syndrome: First clinical
association of the TBX5 mutation. Case Rep Urol.
2013:4053432013.PubMed/NCBI
|
|
54
|
Miko E, Margitai Z, Czimmerer Z, Várkonyi
I, Dezso B, Lányi A, Bacsó Z and Scholtz B: miR-126 inhibits
proliferation of small cell lung cancer cells by targeting SLC7A5.
FEBS Lett. 585:1191–1196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou H, Xu X, Xun Q, Yu D, Ling J, Guo F,
Yan Y, Shi J and Hu Y: microRNA-30c negatively regulates
endometrial cancer cells by targeting metastasis-associated gene-1.
Oncol Rep. 27:807–812. 2012.
|