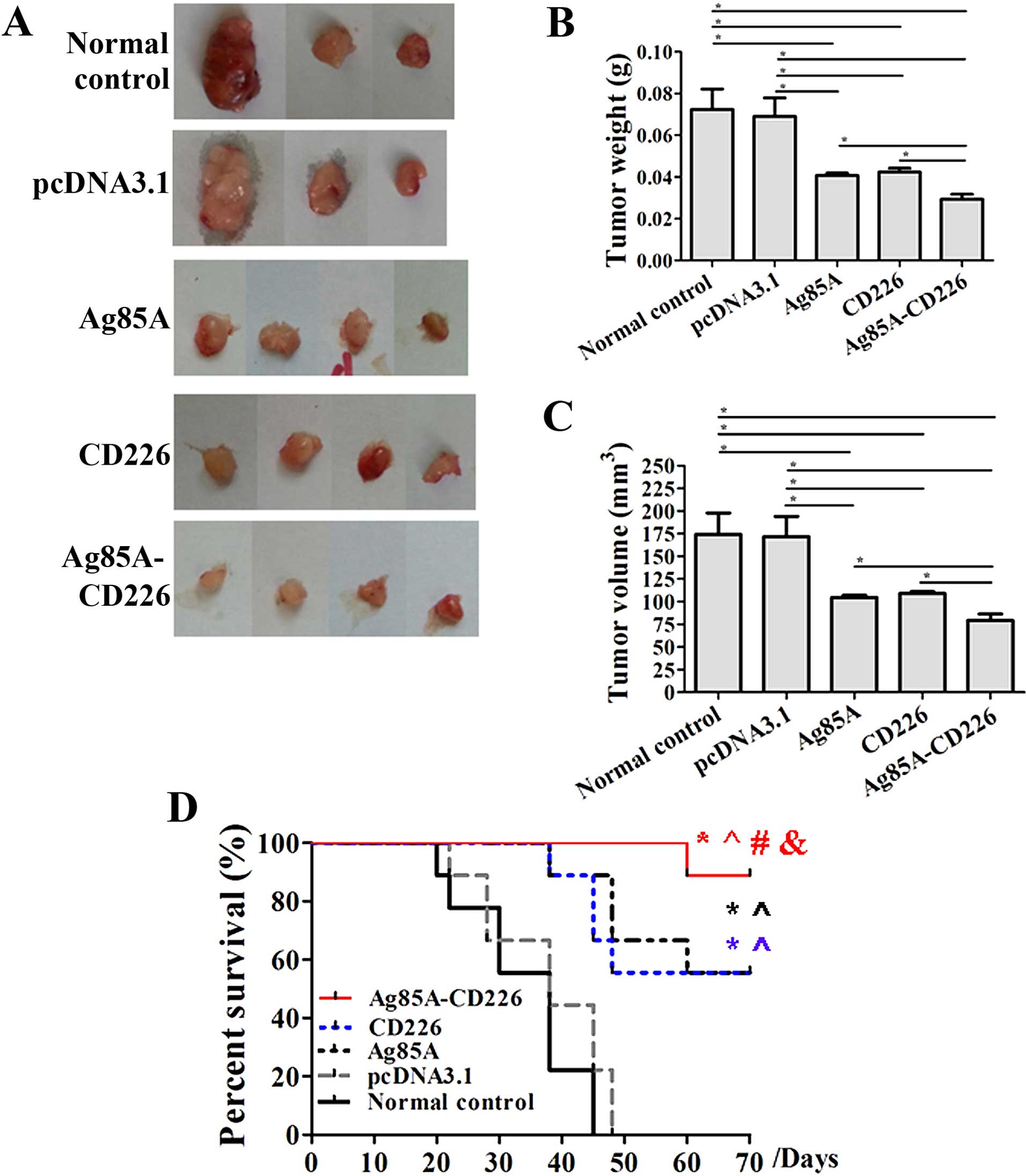
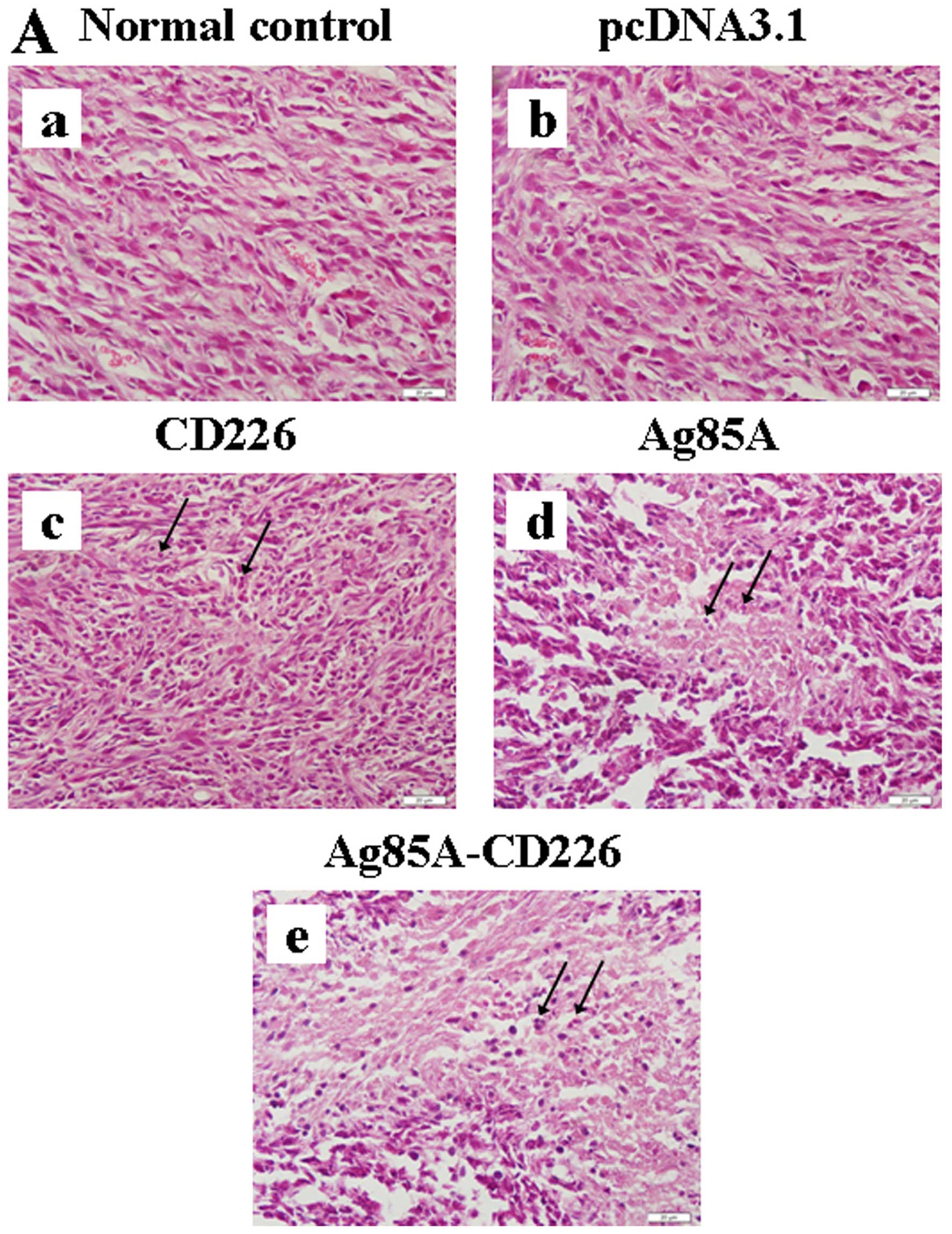
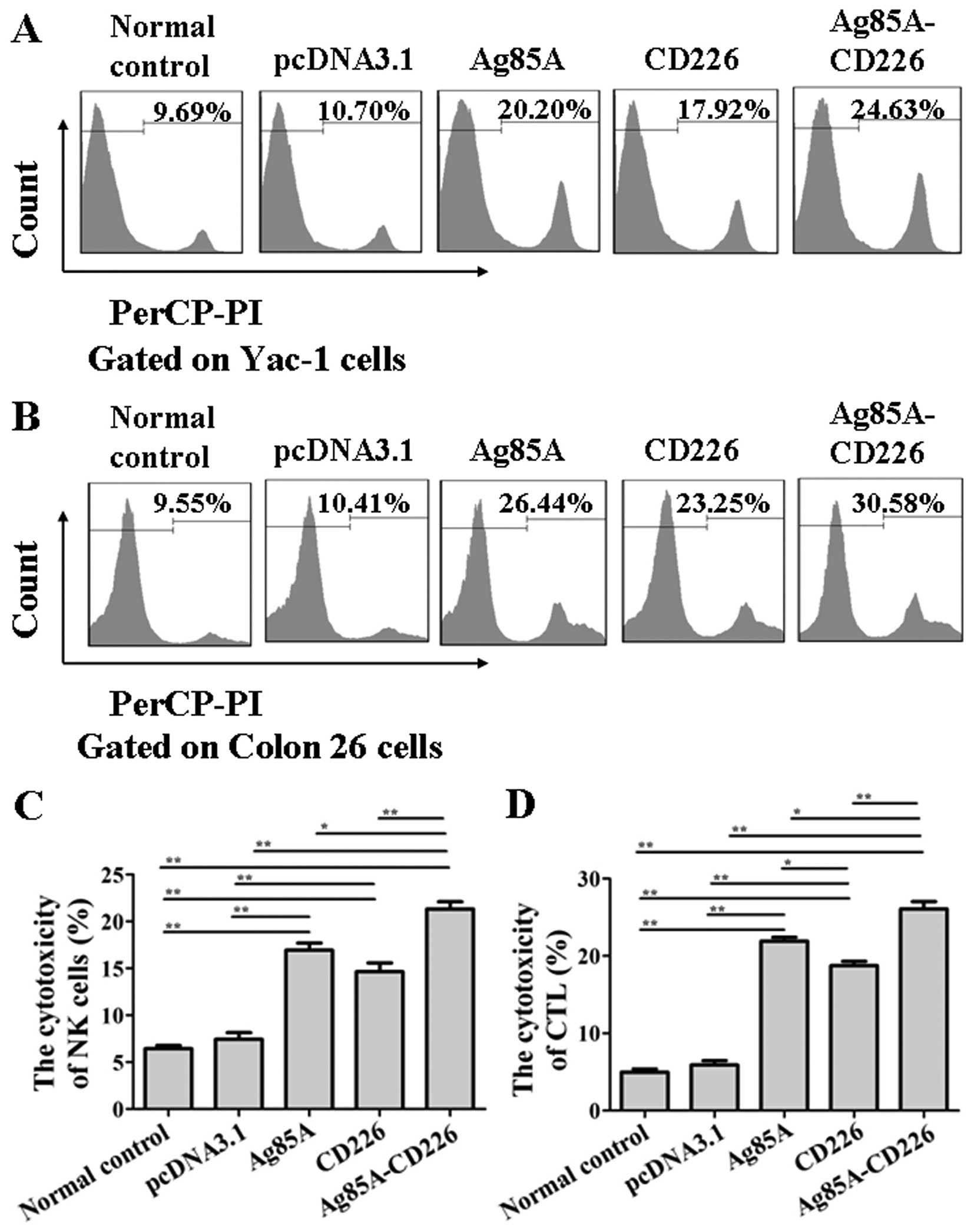
|
1
|
Esteban-Jurado C, Garre P, Vila M, Lozano
JJ, Pristoupilova A, Beltrán S, Abulí A, Muñoz J, Balaguer F, Ocaña
T, et al: New genes emerging for colorectal cancer predisposition.
World J Gastroenterol. 20:1961–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li HZ, Mao WM, Wang XH, Yu CD and Du LB:
Incidence and mortality of cancer in Zhejiang province in 2009.
Zhonghua Yu Fang Yi Xue Za Zhi. 47:592–596. 2013.In Chinese.
PubMed/NCBI
|
|
3
|
Yi N, Xiao MB, Ni WK, Jiang F, Lu CH and
Ni RZ: High expression of peroxiredoxin 4 affects the survival time
of colorectal cancer patients, but is not an independent
unfavorable prognostic factor. Mol Clin Oncol. 2:767–772.
2014.PubMed/NCBI
|
|
4
|
Yang Z, Bai Y, Huo L, Chen H, Huang J, Li
J, Fan X, Yang Z, Wang L and Wang J: Expression of A disintegrin
and metalloprotease 8 is associated with cell growth and poor
survival in colorectal cancer. BMC Cancer. 14:5682014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim J, Huynh R, Abraham I, Kim E and Kumar
RR: Number of lymph nodes examined and its impact on colorectal
cancer staging. Am Surg. 72:902–905. 2006.PubMed/NCBI
|
|
6
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Belisle JT, Vissa VD, Sievert T, Takayama
K, Brennan PJ and Besra GS: Role of the major antigen of
Mycobacterium tuberculosis in cell wall biogenesis. Science.
276:1420–1422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huygen K, Content J, Denis O, Montgomery
DL, Yawman AM, Deck RR, DeWitt CM, Orme IM, Baldwin S, D'Souza C,
et al: Immunogenicity and protective efficacy of a tuberculosis DNA
vaccine. Nat Med. 2:893–898. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borremans M, de Wit L, Volckaert G, Ooms
J, de Bruyn J, Huygen K, van Vooren JP, Stelandre M, Verhofstadt R
and Content J: Cloning, sequence determination, and expression of a
32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect
Immun. 57:3123–3130. 1989.PubMed/NCBI
|
|
10
|
Montgomery DL, Huygen K, Yawman AM, Deck
RR, Dewitt CM, Content J, Liu MA and Ulmer JB: Induction of humoral
and cellular immune responses by vaccination with M. tuberculosis
antigen 85 DNA. Cell Mol Biol (Noisy-le-grand). 43:285–292.
1997.
|
|
11
|
Dou J, Tang Q, Yu F, Yang H, Zhao F, Xu W,
Wang J, Hu W, Hu K and Liou C: Investigation of immunogenic effect
of the BCG priming and Ag85A-GM-CSF boosting in Balb/c mice model.
Immunobiology. 215:133–142. 2010. View Article : Google Scholar
|
|
12
|
Dou J, Wang Y, Yu F, Yang H, Wang J, He X,
Xu W, Chen J and Hu K: Protection against Mycobacterium
tuberculosis challenge in mice by DNA vaccine Ag85A-ESAT-6-IL-21
priming and BCG boosting. Int J Immunogenet. 39:183–190. 2012.
View Article : Google Scholar
|
|
13
|
Wang D, Xu J, Feng Y, Liu Y, Mchenga SS,
Shan F, Sasaki J and Lu C: Liposomal oral DNA vaccine
(mycobacterium DNA) elicits immune response. Vaccine. 28:3134–3142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CS, Wu Y, Dou J, Wen P, Zhao FS, Tang
Q, Li JL and Wang YQ: Study of anti-melanoma effect in mice
injected with melanoma cells transfected with the recombinant
expressing Ag85A and GM-CSF. J Southeast Univ. 29:57–61. 2010.
|
|
15
|
Zhang P, Wang J, Wang D, Wang H, Shan F,
Chen L, Hou Y, Wang E and Lu CL: Dendritic cell vaccine modified by
Ag85A gene enhances anti-tumor immunity against bladder cancer. Int
Immunopharmacol. 14:252–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibuya A, Campbell D, Hannum C, Yssel H,
Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR,
Lanier LL, et al: DNAM-1, a novel adhesion molecule involved in the
cytolytic function of T lymphocytes. Immunity. 4:573–581. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirakawa J, Shibuya K and Shibuya A:
Requirement of the serine at residue 329 for lipid raft recruitment
of DNAM-1 (CD226). Int Immunol. 17:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seth S, Georgoudaki AM, Chambers BJ, Qiu
Q, Kremmer E, Maier MK, Czeloth N, Ravens I, Foerster R and
Bernhardt G: Heterogeneous expression of the adhesion receptor
CD226 on murine NK and T cells and its function in NK-mediated
killing of immature dendritic cells. J Leukoc Biol. 86:91–101.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Yang F, Zhu J, Sang L, Han X, Wang
D, Shan F, Li S, Sun X and Lu C: CD226 as a genetic adjuvant to
enhance immune efficacy induced by Ag85A DNA vaccination. Int
Immunopharmacol. 25:10–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuominen VJ, Ruotoistenmäki S, Viitanen A,
Jumppanen M and Isola J: ImmunoRatio: A publicly available web
application for quantitative image analysis of estrogen receptor
(ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res.
12:R562010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marcusson-Ståhl M and Cederbrant K: A
flow-cytometric NK-cytotoxicity assay adapted for use in rat
repeated dose toxicity studies. Toxicology. 193:269–279. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao F, Dou J, Wang J, Chu L, Tang Q, Wang
Y, Li Y, Cao M, Hu W and Hu K: Investigation on the anti-tumor
efficacy by expression of GPI-anchored mIL-21 on the surface of
B16F10 cells in C57BL/6 mice. Immunobiology. 215:89–100. 2010.
View Article : Google Scholar
|
|
23
|
Clive KS, Tyler JA, Clifton GT, Holmes JP,
Mittendorf EA, Ponniah S and Peoples GE: Use of GM-CSF as an
adjuvant with cancer vaccines: Beneficial or detrimental? Expert
Rev Vaccines. 9:519–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miguel A, Herrero MJ, Sendra L, Botella R,
Algás R, Sánchez M and Aliño SF: Comparative antitumor effect among
GM-CSF, IL-12 and GM-CSF+IL-12 genetically modified tumor cell
vaccines. Cancer Gene Ther. 20:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olivares J, Kumar P, Yu Y, Maples PB,
Senzer N, Bedell C, Barve M, Tong A, Pappen BO, Kuhn J, et al:
Phase I trial of TGF-beta 2 antisense GM-CSF gene-modified
autologous tumor cell (TAG) vaccine. Clin Cancer Res. 17:183–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agarwalla P, Barnard Z, Fecci P, Dranoff G
and Curry WT Jr: Sequential immunotherapy by vaccination with
GM-CSF-expressing glioma cells and CTLA-4 blockade effectively
treats established murine intracranial tumors. J Immunother.
35:385–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seth S, Qiu Q, Danisch S, Maier MK, Braun
A, Ravens I, Czeloth N, Hyde R, Dittrich-Breiholz O, Förster R, et
al: Intranodal interaction with dendritic cells dynamically
regulates surface expression of the co-stimulatory receptor CD226
protein on murine T cells. J Biol Chem. 286:39153–39163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burgdorf SK, Claesson MH, Nielsen HJ and
Rosenberg J: Changes in cytokine and biomarker blood levels in
patients with colorectal cancer during dendritic cell-based
vaccination. Acta Oncol. 48:1157–1164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishimura T, Nakui M, Sato M, Iwakabe K,
Kitamura H, Sekimoto M, Ohta A, Koda T and Nishimura S: The
critical role of Th1-dominant immunity in tumor immunology. Cancer
Chemother Pharmacol. 46(Suppl): S52–S61. 2000. View Article : Google Scholar : PubMed/NCBI
|