Introduction
Cancer is the cause of 25% of mortalities in
developed countries (1). The
prognosis of patients with advanced cancer is associated with the
degree of aggressive metastasis (2,3). It is
imperative to understand the mechanisms involved in the metastasis
of cancer since there are limited effective therapies available
once the cancer has spread (3–5). The
process of cancer metastasis appears to be regulated by a variety
of gene products, however, the precise mechanisms of tumor cell
dissemination are poorly understood (6,7). The
epithelial-mesenchymal transition (EMT), which has been recognized
for several decades as a fundamental process of embryogenesis, may
be involved in many pathological processes, particularly cancer
progression. During the EMT of cancer cells in situ,
epithelial cell layers lose their polarity and their cell-cell
contacts, and then undergo a marked remodeling of the cytoskeleton.
The expression of proteins that promote cell-cell contact, such as
E-cadherin and β-catenin, may be lost, and the cells may acquire
mesenchymal markers, such as vimentin, fibronectin and N-cadherin,
enhancing their ability for cell migration and invasion, which are
pivotal events in the initial step of metastasis. Therefore,
investigators have focused on inhibiting the EMT of cancer cells as
a new therapeutic strategy to prevent cancer metastasis.
Cancer chemoprevention using natural compounds,
known as 'dietary chemoprevention', prevents or suppresses the
development of cancer, and is attracting interest as a cancer
therapy. Understanding the molecular mechanisms of dietary
chemoprevention is important for the safe application of these
compounds in populations of patients at a high risk of cancer, as
well as the development of novel treatment regimens for cancer
patients.
Psolarea corylifolia L. (PC), commonly known
as 'Boh-Gol-Zhee' in Korea, is used in traditional Ayurvedic and
Chinese medicine. Six compounds (bakuchiol, psoralen, isopsoralen,
corylifolin, corylin and psoralidin) are the major components of PC
extract, and they possess significant antibacterial, antifungal,
antioxidant, antitumor, estrogenic and immunomodulatory properties
(8). It has been reported that
whole extracts of PC, in organic solvents such as methanol, ethanol
and chloroform, inhibit the proliferation of cancer cells, although
the mechanism of action remains to be elucidated (9–11).
In the present study, we hypothesized that an
aqueous extract of Psoralea corylifolia L. (PCAE), a typical
medicinal decoction, is an effective inhibitor of EMT during cancer
progression, and may therefore be used as a dietary chemopreventive
agent for malignant tumors. We reported that PCAE significantly
inhibited the invasion and migration of various cancer cells during
the LPS-induced EMT by downregulating the NF-κB-SNAIL signaling
pathway. We suggested that PCAE is an excellent candidate dietary
chemoprevention agent for use against malignant tumors since it
inhibits metastasis.
Materials and methods
Materials and reagents
The PC used in the present study was kindly supplied
by Professor Jung-Hye Choi (Kyung Hee University). Aqueous
extraction procedures were performed by boiling 100 g PC in 500 ml
distilled water for 30 min and then filtering using Whatman filter
paper no. 2 (Advantec, Tokyo, Japan). Subsequently, the filtrates
were combined and evaporated under a vacuum and lyophilized with a
freeze dryer (Ilshine Laboratory, Suwon, Korea). The dry residue
was stored at −20°C. MDA-MB231 and SKOV-3 cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin
antibiotics. The antibodies NF-κB p65 subunit, β-actin and PCNA
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Snail was purchased from Cell Signalling Technology (Beverly,
MA, USA), vimentin and β-catenin were purchased from Abcam
(Cambridge, MA, USA) and N-cadherin was purchased from BD
Biosciences (San Jose, CA, USA).
Proliferation assay
Proliferation assays were based on the
3-[4,5-dimethythiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
method. Cells were seeded in a 96-well plate, at a density of
1×104 cells/well. After overnight culture, PCAE was
added to the cells and cultured for 24 h. The medium was removed
and DMSO was added at MTT solubilization solution. Absorbance was
measured at 550 nm.
Colony-forming assay
Single-cell suspensions of 5×103 cells
were seeded in a 6-well plate and allowed to attach for 24 h at
37°C in culture medium. The cells were then treated with 100 or
1,000 µM PCAE. After 10 days, the colonies were fixed with
100% methanol for 10 min at room temperature and stained with 0.1%
crystal violet. Plates were washed with phosphate-buffered saline
(PBS) and images were captured.
Immunofluorescence staining
MDA-MB231 cells were grown in 4-chamber slides in
serum-free medium, and were treated with LPS (5 µg/ml) or
co-treated with LPS (5 µg/ml) and PVAE (100 µM).
After 24-h incubation, the cells were fixed with 4%
paraformaldehyde at 4°C. The cells were washed with PBS containing
0.1% BSA and incubated with anti-N-cadherin antibody for 1 h
followed by 1 h incubation with fluorescence-tagged secondary
antibody, then counter-stained with 4′,6-diamidino-2-phenylindole
(DAPI) for 5 min. Cell images were captured at a magnification of
×400 on a Leica fluorescence microscope.
Cell migration assay
Migration was assessed by a wound-healing assay. The
cells were seeded at 2×104 MDA-MB231 and SKOV-3
cells/well and were cultured for 24 h. After scraping the cell
monolayer with a sterile micropipette tip, the wells were washed
with PBS, and treated with LPS (5 µg/ml) or co-treated with
LPS (5 µg/ml) and PCAE (100 µM). The first image of
each scratch was obtained at time zero. At 24 h, each scratch was
examined and captured at the same location and the healed area was
measured.
Transwell invasion assay
The invasion of tumor cells was assessed in
Transwell chambers equipped with 8-µm pore size, 6.5 mm
diameter polyvinylpyrrolidone-free polycarbonated membranes that
were coated with 1 mg/ml fibronectin. The cells were seeded onto
the upper wells at a concentration of 1×105 and
MDA-MB231 and SKOV-3 cells/well were cultured for 24 h following
treatment with LPS (5 µg/ml) or co-treatment with LPS (5
µg/ml) and PCAE (100 µM). The bottom chambers of the
Transwell were filled with conditioned medium. After incubation for
24 h, the cells were fixed with 100% methanol for 10 min at room
temperature, stained with 0.1% crystal violet and counted under a
light microscope.
Western blotting
The MDA-MB231 and SKOV-3 cells were treated with LPS
(5 µg/ml) or co-treated with LPS (5 µg/ml) and PVAE
(100 µM) for 24 h. After lysing the cells with RIPA buffer,
the proteins were resolved by SDS-PAGE and immuno-blotted using
primary antibodies such as anti-N-cadherin, anti-β-catenin,
anti-vimentin, anti-NF-κB p65 subunit, anti-Snail and anti-β-actin
antibody. Following treatment with appropriate secondary
antibodies, the immunoreactive bands were visualized using a
standard ECL method.
Statistical analysis
The results are presented as mean ± SE. Statistical
comparisons between groups were carried out using one-way ANOVA
followed by the Student's t-test.
Results
PCAE inhibits the growth of human cancer
cell in vitro
We initially examined the effects of PCAE on the
proliferation of two human metastatic cancer cell lines, MDA-MB231
(breast cancer cells) and SKOV-3 (ovarian cancer cells). The cancer
cell lines were treated for 24 h with various concentrations of
PCAE and cell viability was measured with a
3-(4,5-dimeth-ylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. As shown in Fig. 1A, cell
proliferation was inhibited by PCAE in a dose-dependent manner,
although a different IC50 (the drug concentration that
causes 50% growth inhibition) was observed for each cell line
(417±7 µg/ml for MDA-MB231 and 690±4 µg/ml for
SKOV-3). The long-term effects of PCAE were determined with a
colony-forming assay. MDA-MB231 and SKOV-3 cells were cultured with
or without PCAE for 10-15 days. A PCAE concentration of 100
µg/ml had no significant effect on the cells, whereas 1,000
µg/ml PCAE almost completely inhibited colony formation
(Fig. 1B and C). Therefore, we
considered 100 µg/ml PCAE a suitable dose for subsequent
experiments.
PCAE inhibits LPS-induced cell migration
and invasion during EMT
Chen et al have reported that, LPS (5
µg/ml) independently triggers the EMT process (12). We investigated the effects of PCAE
on cell migration and invasion to verify that PCAE inhibits
LPS-induced EMT since the EMT is associated with enhanced tumor
progression. Two cancer cell lines were treated with LPS-alone, a
combination of LPS plus PCAE or PCAE-alone, and were monitored with
a wound-healing assay and a fibronectin-based Transwell invasion
assay. The LPS-treated cancer cells exhibited a ≥1.2-fold increase
in migration, whereas treatment with 100 µg/ml PCAE
inhibited this LPS-induced migration by 60% (Fig. 2A and B). We also examined whether
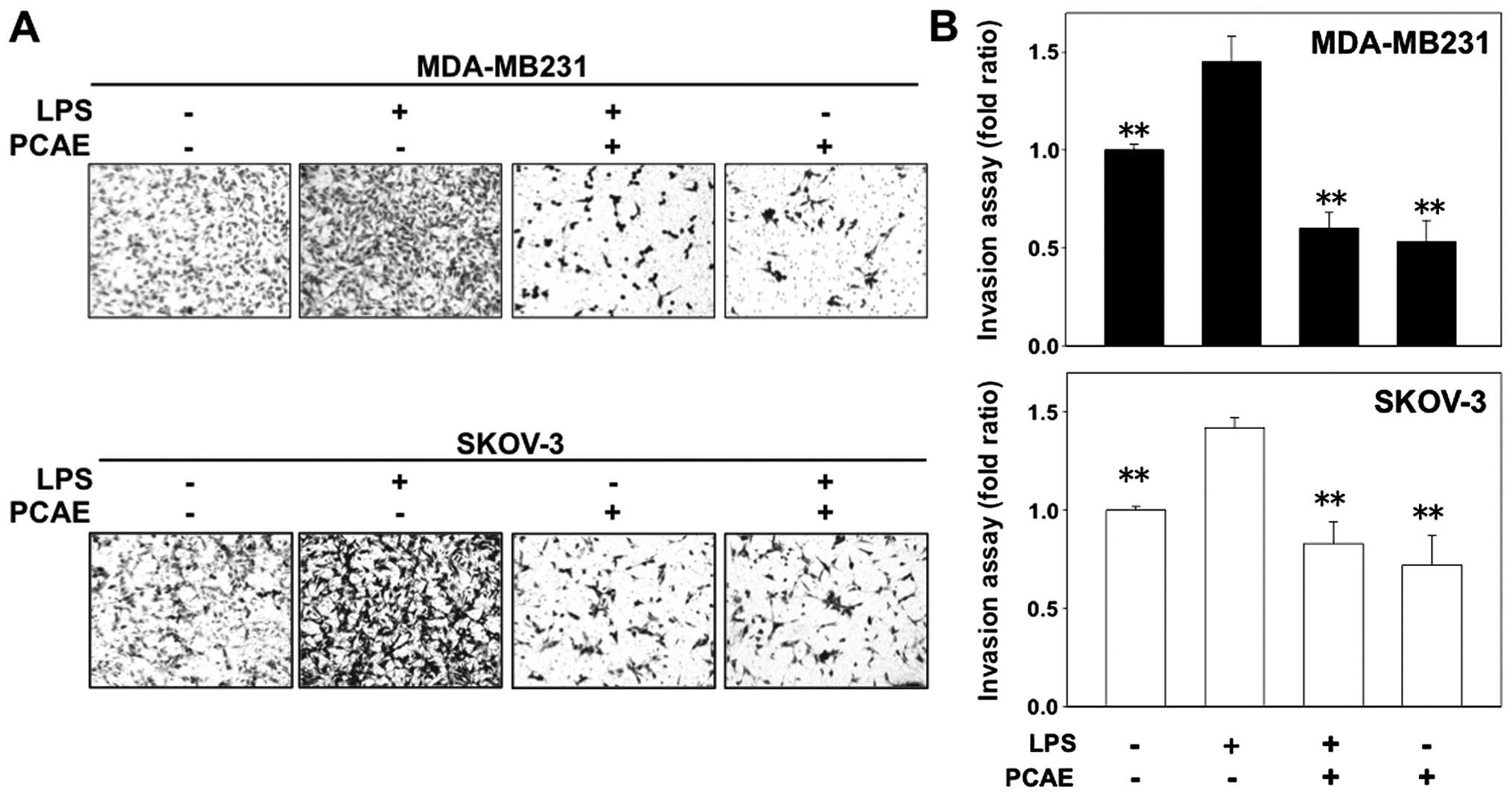
PCAE inhibits the LPS-induced invasiveness of cancer cells.
Following treatment with LPS-alone, the number of invasive cells
significantly increased compared with the untreated cells
(≥1.6-fold increase). However, the number of invasive cells was
significantly reduced in the cells treated with the combination of
LPS plus PCAE (Fig. 3A and B).
These data suggested that PCAE inhibited the effect of LPS, thus
increasing the invasiveness of human cancer cells, as occurs for
the EMT.
PCAE regulates the expression of
EMT-related proteins in human cancer cells
We investigated the expression of EMT-related
proteins, such as N-cadherin, β-catenin and vimentin, to clarify
the effects of PCAE on the LPS-induced EMT. The protein expression
was measured using western blotting and quantitatively analyzed
(Fig. 4A and B). The expression of
β-catenin was significantly downregulated, whereas that of
N-cadherin and vimentin was upregulated in the LPS-treated cancer
cells compared with their expression in the controls. However, PCAE
reversed the LPS-induced EMT by reinducing the expression of
β-catenin, and inhibiting N-cadherin and vimentin expression. We
also examined the expression of N-cadherin in cancer cells with
fluorescent imaging (Fig. 4C).
Consistent with the western blotting results, N-cadherin was highly
expressed after LPS treatment, although its expression decreased
significantly after PCAE treatment. The western blotting and
fluorescent imaging results suggested that PCAE inhibits the
cellular EMT.
NF-κB-SNAIL signaling is required to
alter EMT marker expression
Previous findings showed numerous drugs inhibit the
invasion and migration of cancer cells by suppressing NF-κB
expression (13,14). Therefore, the NF-κB signaling
pathway is critically involved in the acquisition of the EMT,
mediated by SNAIL, a transcription factor directly downstream from
NF-κB. To determine whether the effect of PCAE described above is
associated with the inhibition of the NF-κB-SNAIL pathway, we
investigated the expression of the NF-κB p65 subunit and SNAIL
protein using western blotting and quantitative analysis (Fig. 5A and B). As shown in Fig. 5, LPS significantly upregulated the
expression of NF-κB p65 and SNAIL protein, with the exception of
NF-κB p65 in MDA-MB231, which reduced the expression of E-cadherin
and β-catenin, and increased the expression of N-cadherin and
vimentin as identified in previous western blotting results.
However, PCAE inhibited this effect, counteracting the effect of
LPS on NF-κB p65 and SNAIL expression. PCAE even decreased NF-κB
p65 and SNAIL expression compared to the control group. These
results indicated that PCAE inhibits the activation of NF-κB-SNAIL,
which plays a critical role in the EMT.
Discussion
The EMT is the best-known example of the changes
that occur in the patterns and functions of cancer cells (15). Epithelial cells acquire mesenchymal
fibroblast-like phenotypes, with reduced cell-cell adhesion, loss
of polarity and increased migration and invasiveness (16). As a prerequisite for EMT, cells lose
their cell-cell junctions via different mechanisms, including
loss-of-function mutations in the cadherins or catenins, or the
upregulation of proteases that cleave the cadherins (17). The EMT is considered to be a
significant step in the invasive cascade, facilitating the
migration of tumor cells from their site of origin and their
dissemination to distant tissues (18). When the tumor reaches the
dedifferentiated stage, at which single cells are disseminated,
metastatic spread increases, resulting in a poor prognosis.
Therefore, blocking or reversing this process with exogenous agents
is a promising therapeutic strategy to limit cancer spread.
In a previous study, LPS induced the EMT of cancer
cells, increasing their invasiveness and migration, which enhanced
the metastasis of breast cancer cells to the lung (19). In the present study, we have shown
that various cancer cells can be induced by LPS to undergo a
stimulated EMT, reducing β-catenin expression, and increasing
N-cadherin and vimentin expression. PCAE inhibits the action of LPS
in inducing EMT, reversing the altered expression of proteins
associated with cell invasion and migration. We also found that
NF-κB-SNAIL signaling is required for the LPS-induced EMT in
various cancer cells, which clarifies the mechanism by which PCAE
inhibits cancer cell metastasis.
PC has been widely used in Ayurvedic and Chinese
medicine as a cardiac tonic, vasodilator and antitumor,
antibacterial, cytotoxic and antihelminthic agent (8). Extracts of PC produced with various
solvents, such as methanol, ethanol and chloroform, have
significant antitumor activity since they inhibit DNA replication,
the induction of hypoxia-inducible factor-1 activation by hypoxia,
mitochondrial complex I and proteasomal activities (11,20,21).
However, PC has not been associated with cancer metastasis via the
EMT, although its strong antitumor effects have been reported. To
the best of our knowledge, this is the first study to demonstrate
that the antimetastatic effects of PC are associated with the EMT
in cultured human cancer cells. Therefore, our results suggest a
new dietary chemopreventive role for PC in inhibiting the
progression of cancer metastasis.
In the present study, we have shown that PCAE
inhibits the LPS-induced EMT, and thus cell migration and invasion,
which result from the dysregulation of cell-cell adhesion proteins
and the expression of EMT-related proteins, such as N-cadherin.
Cadherins are transmembrane glycoproteins that mediate
Ca2+-dependent cell-cell adhesion (22). N-cadherin is associated with the
heightened invasive potential of cancer cells. N-cadherin is
typically expressed by mesenchymal cells, is overexpressed in some
cancer cells and correlates with cell invasiveness (23). Therefore, the gain of N-cadherin
expression in cancer cells has a functional significance in cancer
progression and metastasis (24).
PCAE also regulates the expression of other EMT-related proteins,
such as β-catenin and vimentin, inhibiting cell migration and
invasion. β-catenin is a transcription factor in the WNT signaling
pathway and is involved in the regulation of cell adhesion. It is
typically most abundant in epithelial-like cells (25,26).
Vimentin is an intermediate filament typically found in
non-epithelial and mesenchymal cells (27). When drugs inhibit the EMT of cancer
cells, β-catenin expression increases and vimentin expression
decreases.
Our results suggest that the mechanism of action of
Psoralea corylifolia L. involves the suppression of
NF-κB-SNAIL signaling. NF-κB is a structurally conserved family of
dimeric transcription factors that play pivotal roles in
maintaining the invasive phenotype and promoting the metastasis of
cancer cells (28–30). It is also an essential central
mediator of the EMT since the SNAIL transcription factor plays a
critical role in the EMT and its expression is directly induced by
NF-κB (31). The expression of
SNAIL mrNA at the EMT may be reversed by the inhibition of NF-κB,
which is the upstream regulator of SNAIL expression at the EMT
(32). Our results support previous
findings that NF-κB is the upstream regulator of SNAIL and
indirectly mediates the EMT, explaining the inhibition of tumor
progression by Psoralea corylifolia L. and PCAE.
In conclusion, we have shown that PCAE inhibits
tumor cell invasion and migration, which are associated with the
EMT during tumor progression, possibly by inhibiting the activation
of NF-κB-SNAIL signaling and regulating the expression of important
downstream EMT markers, such as E-cadherin, β-catenin, N-cadherin
and vimentin. Although further in vivo studies are required
to determine the potential utility of PCAE, inhibiting the
migration and invasion of tumor cells, as a cancer therapy, we
suggest that PCAE is an effective dietary chemopreventive agent for
malignant tumors because it inhibits metastasis.
Acknowledgments
The present study was supported by the Traditional
Korean Medicine R&D Program funded by the Ministry of Health
and Welfare through the Korea Health Industry Development Institute
(HI14C05830000), and by the Basic Science Research Program through
the National Research Foundation of Korea (NRF) funded by the
Ministry of Education (2014R1A1A2057861).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sleeman JP, Nazarenko I and Thiele W: Do
all roads lead to Rome? Routes to metastasis development. Int J
Cancer. 128:2511–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collado M and Serrano M: Senescence in
tumours: Evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View
Article : Google Scholar
|
|
5
|
Klein CA: Parallel progression of primary
tumours and metastases. Nat Rev Cancer. 9:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta GP, Minn AJ, Kang Y, Siegel PM,
Serganova I, Cordón-Cardo C, Olshen AB, Gerald WL and Massagué J:
Identifying site-specific metastasis genes and functions. Cold
Spring Harb Symp Quant Biol. 70:149–158. 2005. View Article : Google Scholar
|
|
7
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chopra B, Dhingra AK and Dhar KL: Psoralea
corylifolia L. (Buguchi) - folklore to modern evidence: Review.
Fitoterapia. 90:44–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Hong C, Zhou C, Xu D and Qu HB:
Screening antitumor compounds psoralen and isopsoralen from
Psoralea corylifolia L. seeds. Evid Based Complement Alternat Med.
2011:3630522011.
|
|
10
|
Latha PG, Evans DA, Panikkar KR and
Jayavardhanan KK: Immunomodulatory and antitumour properties of
Psoralea corylifolia seeds. Fitoterapia. 71:223–231. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang SY, Gruber J, Wong KP and Halliwell
B: Psoralea corylifolia L. inhibits mitochondrial complex I and
proteasome activities in SH-SY5Y cells. Ann NY Acad Sci.
1100:486–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim A, Im M and Ma JY: Anisi stellati
fructus extract attenuates the in vitro and in vivo metastatic and
angiogenic potential of malignant cancer cells by downregulating
proteolytic activity and pro-angiogenic factors. Int J oncol.
45:1937–1948. 2014.PubMed/NCBI
|
|
14
|
Liu YC, Chiang IT, Hsu FT and Hwang JJ:
Using NF-κB as a molecular target for theranostics in radiation
oncology research. Expert Rev Mol Diagn. 12:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whelan LC and Ryan MF: Ethanolic extracts
of Euphorbia and other ethnobotanical species as inhibitors of
human tumour cell growth. Phytomedicine. 10:53–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CZ, Cai XF, Dat NT, Hong SS, Han AR,
Seo EK, Hwang BY, Nan JX, Lee D and Lee JJ: Bisbakuchiols A and B,
novel dimeric meroterpenoids from Psoralea corylifolia. Tetrahedron
Lett. 48:8861–8864. 2007. View Article : Google Scholar
|
|
22
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen PT, Kudo Y, Yoshida M, Kamata N,
Ogawa I and Takata T: N-cadherin expression is involved in
malignant behavior of head and neck cancer in relation to
epithelial-mesenchymal transition. Histol Histopathol. 26:147–156.
2011.
|
|
24
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangiocarcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan
W, Hou F, Ge J, Zhong M, Tang Y, et al: EphA2 promotes
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in gastric cancer cells. Oncogene. 33:2737–2747. 2014. View Article : Google Scholar
|
|
26
|
Yan D, Avtanski D, Saxena NK and Sharma D:
Leptin-induced epithelial-mesenchymal transition in breast cancer
cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1
protein-dependent pathways. J Biol Chem. 287:8598–8612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Chen L, Zhang X, Xu X, Xing H,
Zhang Y, Li W, Yu H, Zeng J and Jia J: RUNX3 regulates vimentin
expression via miR-30a during epithelial-mesenchymal transition in
gastric cancer cells. J Cell Mol Med. 18:610–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y,
Zhu G, Yang L, Wang X and He D: Silibinin reverses
epithelial-to-mesenchymal transition in metastatic prostate cancer
cells by targeting transcription factors. Oncol Rep. 23:1545–1552.
2010.PubMed/NCBI
|
|
29
|
Xiao LJ, Chen YY, Lin P, Zou HF, Lin F,
Zhao LN, Li D, Guo L, Tang JB, Zheng XL, et al: Hypoxia increases
CX3Cr1 expression via HIF-1 and NF-κB in androgen-independent
prostate cancer cells. Int J Oncol. 41:1827–1836. 2012.PubMed/NCBI
|
|
30
|
Ennen M, Klotz R, Touche N, Pinel S,
Barbieux C, Besancenot V, Brunner E, Thiebaut D, Jung AC,
Ledrappier S, et al: DDB2: A novel regulator of NF-κB and breast
tumor invasion. Cancer Res. 73:5040–5052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar
|
|
32
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|