Introduction
Lung cancer is clinically classified into two major
histological types: non-small cell lung cancer (NSCLC), which
accounts for more than 80% of all lung cancer cases; and small cell
lung cancer (SCLC), which accounts for 13–15% (1). Although SCLC incidence is relatively
lower, its poor prognosis is a cause for concern. The standard
treatment for SCLC has not dramatically changed during the past 30
years, and platinum-based chemotherapy (2,3),
hyper-fractionated thoracic radiation (4), and prophylactic cranial irradiation
(5,6) are still used therapeutically. However,
multidrug resistance (MDR) following chemotherapy increases the
relapse ratio and mortality. The median survival time is only 6–8
months and the 5-year survival rate is <15% (7). In the absence of any meaningful
advances in chemotherapy or programs to improve the survival rates,
MDR has become a major clinical obstacle in the treatment of SCLC
(8), warranting investigation into
the mechanisms underlying MDR.
Previous studies suggest complex MDR mechanisms
involving multiple genes and a variety of mechanisms at the tissue,
cell and molecular levels (8–10). As
transcription regulating factors, microRNAs (miRNAs) have become
the 'hot topic' in gene regulation (11,12).
miRNAs, evolved from an RNA polymerase 2 transcript (pri-miRNA)
with a strong secondary structure (stem-loop), are classified into
diverse small RNA families. The miRNAs are 21–24 nucleotides (nt)
in length, single-stranded and non-coding (13). The miRNAs may theoretically target
any mRNA, and therefore, participate in a very broad functional
spectrum including the cell cycle, cell growth, apoptosis, cell
differentiation and stress response (14). It is no surprise that miRNAs are
involved in human cancer development and tumor metastasis. However,
only a few studies are available discussing the role of miRNAs in
the multidrug resistance of SCLC.
The aim of the present study was to elucidate the
role of miRNAs in SCLC. Using the next generation high throughput
Solexa-Illumina sequencing technology, we determined the varied
expression of known miRNAs as well as discovered novel miRNAs.
Furthermore, the possible biological significance of putative
target genes of novel miRNAs in H446 and H446/CDDP cells was
determined using Gene Ontology (GO) analysis and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway analysis. The findings should
help understand the role of miRNAs in MDR of SCLC and provide ideas
for future research.
Materials and methods
Cell lines and culture
The SCLC cell line H446 and its multidrug-resistant
cell line H446/CDDP were kindly gifted by Dr Guisheng Qian
(Institute of Human Respiratory Disease, Xinqiao Hospital, The
Third Military Medical University, China). More importantly, the
H446 cell line was purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA), and the H446/CDDP cell line
was derived from H446 cells. These cells were maintained in
RPMI-1640 medium with 10% fetal calf serum (Gibco-BRL, Grand
Island, NY, USA) in a humidified atmosphere containing 5%
CO2 at 37°C. In order to maintain the MDR phenotype,
cisplatin (at a final concentration of 0.5 µg/ml) was added
to the culture medium for the H446/CDDP cells.
Validation of drug resistance
Sensitivity of the H446 and H446/CDDP cells to
cisplatin was evaluated by a microculture tetrazolium (MTT) assay.
Briefly, the cells were seeded in 96-well plates at a density of
1×104 cell/well. The cells were treated with 5
µg/ml cisplatin for 24, 48 or 72 h, respectively. The
supernatant was discarded, and 20 µl MTT (5 mg/ml, dissolved
in PBS and filtered through a 0.22-mm membrane) was added into each
well, followed by incubation for 4 h at 37°C. Finally, absorbance
values were determined at 492 nm on an automated Bio-Rad 550
microtiter plate reader.
Total RNA extraction
Total RNA from H446 and H446/CDDP cell lines was
extracted using TRIzol reagent (Invitrogen, USA) according to the
manufacturer's protocol. Total RNA samples were subjected to an RNA
quality control process for Solexa sequencing.
Data cleaning and length
distribution
Different small RNAs vary in length. For example,
miRNAs are normally 21 or 22 nt, siRNAs are 24 nt, and piRNAs are
30 nt. Therefore, length distribution analysis was helpful to
determine the composition of small RNA samples. In the present
study, clean reads were obtained from raw reads after eliminating
contaminant reads, including low quality reads, reads with 5′
primer contaminants, reads without 3′ primer, reads without the
insert tag, reads with poly A and reads shorter than 18 nt. The
length distribution of these clean reads was summarized using
software developed by BGI (formerly known as Beijing Genomics
Institute).
Small RNA annotation
Small RNA tags from the two libraries were aligned
to associated repeat RNAs and annotated with rRNA, scRNA, snoRNA,
snRNA, tRNA exon and intron alignment in order to eliminate matched
tags. Furthermore, the small RNA tags were also annotated with
sequences from Rfam and matched tags were also discarded. Above
all, in order to map each unique sRNA to only one annotation, the
following priority rule was strictly followed: rRNA (in which
Genbank > Rfam) > known miRNA > repeat > exon >
intron. In addition, the total rRNA represented the quality of
samples, usually <40% in animal samples of high quality.
Expression analysis of known miRNAs
The two small RNA libraries were aligned to the
miRNAs in miRBase 21 in order to obtain the miRNA data in H446 and
H446/CDDP cell lines. The expression of miRNAs in the two libraries
was normalized to obtain the expression of transcript per million
(TPM), using the normalization formula: Normalized expression =
Actual miRNA count/total count of clean reads × 1,000,000.
Subsequently, fold-change and p-value of the normalized expression
were calculated. Finally, the scatter plot and fold-change table
were generated to reveal the differential expression of known
miRNAs in the H446 and H446/CDDP cells.
Validation of known miRNA expression in
the H446 and H446/CDDP cell lines
The differential expression of miRNAs in the H446
and H446/CDDP cells was validated by qPCR. Briefly, total RNA was
extracted from the H446 and H446/CDDP cells in the logarithmic
phase with TRIzol reagent (Invitrogen) according to the
manufacturer's instructions. RNA quality was monitored using
agarose electrophoresis and miRNA levels were expressed as the
ratio to U6 level in the same RNA sample. PCR primers are listed in
Table I. Amplification and
detection of chosen miRNAs were carried out using iQ™ SYBR Green
Supermix reagent and iQ™ Single-Color Real-Time PCR detection
system (both from Bio-Rad, USA).
 | Table IPrimer sequences of the genes used in
this study. |
Table I
Primer sequences of the genes used in
this study.
| miRNA name | miRNA sequence |
|---|
|
hsa-miR-323a-3p |
5′-cacauuacacggucgaccucu-3′ |
| hsa-miR-411-5p |
5′-uaguagaccguauagcguacg-3′ |
| hsa-miR-382-5p |
5′-gaaguuguucgugguggauucg-3′ |
| hsa-miR-486-5p |
5′-uccuguacugagcugccccgag-3′ |
|
hsa-miR-323b-3p |
5′-cccaauacacggucgaccucuu-3′ |
| hsa-miR-485-3p |
5′-gucauacacggcucuccucucu-3′ |
| hsa-miR-876-5p |
5′-uggauuucuuugugaaucacca-3′ |
| hsa-miR-379-5p |
5′-ugguagacuauggaacguagg-3′ |
| hsa-miR-486-3p |
5′-cggggcagcucaguacaggau-3′ |
| hsa-miR-487b |
5′-aaucguacagggucauccacuu-3′ |
|
hsa-miR-2277-5p |
5′-agcgcgggcugagcgcugccaguc-3′ |
| hsa-miR-137 |
5′-uuauugcuuaagaauacgcguag-3′ |
| hsa-miR-369-3p |
5′-aauaauacaugguugaucuuu-3′ |
| hsa-miR-3911 |
5′-uguguggauccuggaggaggca-3′ |
| hsa-miR-204-3p |
5′-gcugggaaggcaaagggacgu-3′ |
| hsa-miR-432-5p |
5′-ucuuggaguaggucauugggugg-3′ |
| hsa-miR-204-5p |
5′-uucccuuugucauccuaugccu-3′ |
| hsa-miR-485-5p |
5′-agaggcuggccgugaugaauuc-3′ |
| hsa-miR-127-3p |
5′-ucggauccgucugagcuuggcu-3′ |
| hsa-miR-494 |
5′-ugaaacauacacgggaaaccuc-3′ |
| hsa-miR-382-3p |
5′-aaucauucacggacaacacuu-3′ |
| hsa-miR-409-3p |
5′-gaauguugcucggugaaccccu-3′ |
| hsa-miR-584-5p |
5′-uuaugguuugccugggacugag-3′ |
Novel miRNA prediction
The characteristic hairpin structure of the miRNA
precursor was used in the present study to predict novel miRNAs
with the help of software, Mireap (http://sourceforge.net/projects/mireap/), developed by
BGI. Novel miRNAs were predicted by exploring the secondary
structure, the Dicer cleavage site and the minimum free energy of
the non-annotated small RNA tags.
Target gene prediction of the novel
miRNAs
Target genes of novel miRNAs released by Mireap were
also predicted according to previous suggestions (15,16):
i) no more than 4 mismatches between sRNA and target (G-U bases
count as 0.5 mismatches); ii) no more than 2 adjacent mismatches in
the miRNA/target duplex; iii) no adjacent mismatches in positions
2–12 of the miRNA/target duplex (5′ of miRNA); iv) no mismatches in
positions 10–11 of miRNA/target duplex; v) no more than 2.5
mismatches in positions 1–12 of the miRNA/target duplex (5′ of
miRNA); and vi) minimum free energy (MFE) of the miRNA/target
duplex at least 75% of the MFE as the miRNA bound to its
complement. The prediction process was carried out using software
developed by BGI.
Functional analysis of predicted target
genes
GO analysis is a popular standardized classification
system for gene function, which provides a set of controlled
vocabulary to comprehensively describe genes and gene products. The
GO results revealed molecular function, cellular components and
biological processes of novel miRNAs by mapping target genes to GO
terms in the database (http://www.geneontology.org/), calculating gene
numbers for each term, and determining significantly enriched GO
terms in target gene candidates.
Pathway analysis facilitates the understanding of
biological functions of genes. KEGG (http://www.genome.ad.jp/kegg/pathway.html) is a major
public pathway-related database (17,18).
KEGG pathway analysis identifies significantly enriched metabolic
pathways or signal transduction pathways in target gene candidates
compared with the whole reference gene background. Therefore, in
the present study, KEGG pathway analysis was adopted to analyze the
function of putative target genes.
Statistical analysis
Sensitivity of H446 and H446/CDDP cells to cisplatin
was determined by MTT test and the differences among groups were
compared by one-way classification ANOVA; the differential
expression of known miRNAs in H446 and H446/CDDP cells was compared
by t-test. Fold-change and p-value of normalized known miRNA
expression were determined using software developed by BGI. GO
analysis was accomplished with a hyper-geometric test to unravel
significantly enriched GO terms in target gene candidates, and the
formula is not shown in the manuscript.
Results
Evaluation of cisplatin sensitivity
The sensitivity of H446 and H446/CDDP cells to
cisplatin was evaluated to develop the basis for Solexa-Illumina
sequencing. The MTT test results (Fig.
1) revealed a differential survival rate in the H446 and
H446/CDDP cells challenged by cisplatin at each time-point
(P<0.01). Furthermore, the survival rate in both the H446 and
H446/CDDP cells decreased with prolonged stimulation, and the
difference in survival rate was statistically significant.
Length distribution of small RNAs in the
SCLC cell lines
Differential sensitivity to cisplatin indicated that
H446/CDDP cells were tolerant to cisplatin. We, therefore, explored
whether the drug resistance was related to miRNA expression. In
order to identify miRNAs in the H446 and H446/CDDP cell lines, two
small RNA libraries from the two cell lines were constructed and
sequenced independently. The sequencing results (Table II) exhibited 7,703,264 and
9,151,947 raw reads from the H446 and H446/CDDP cells,
respectively. The raw reads were filtered according to several
criteria. After removing low quality reads, 3′ adaptor sequence, 5′
adaptor contaminants and reads smaller than 18 nt, 7,346,780 and
8,618,043 clean reads for the H446 and H446/CDDP cells were
obtained, and the length distribution was analyzed to characterize
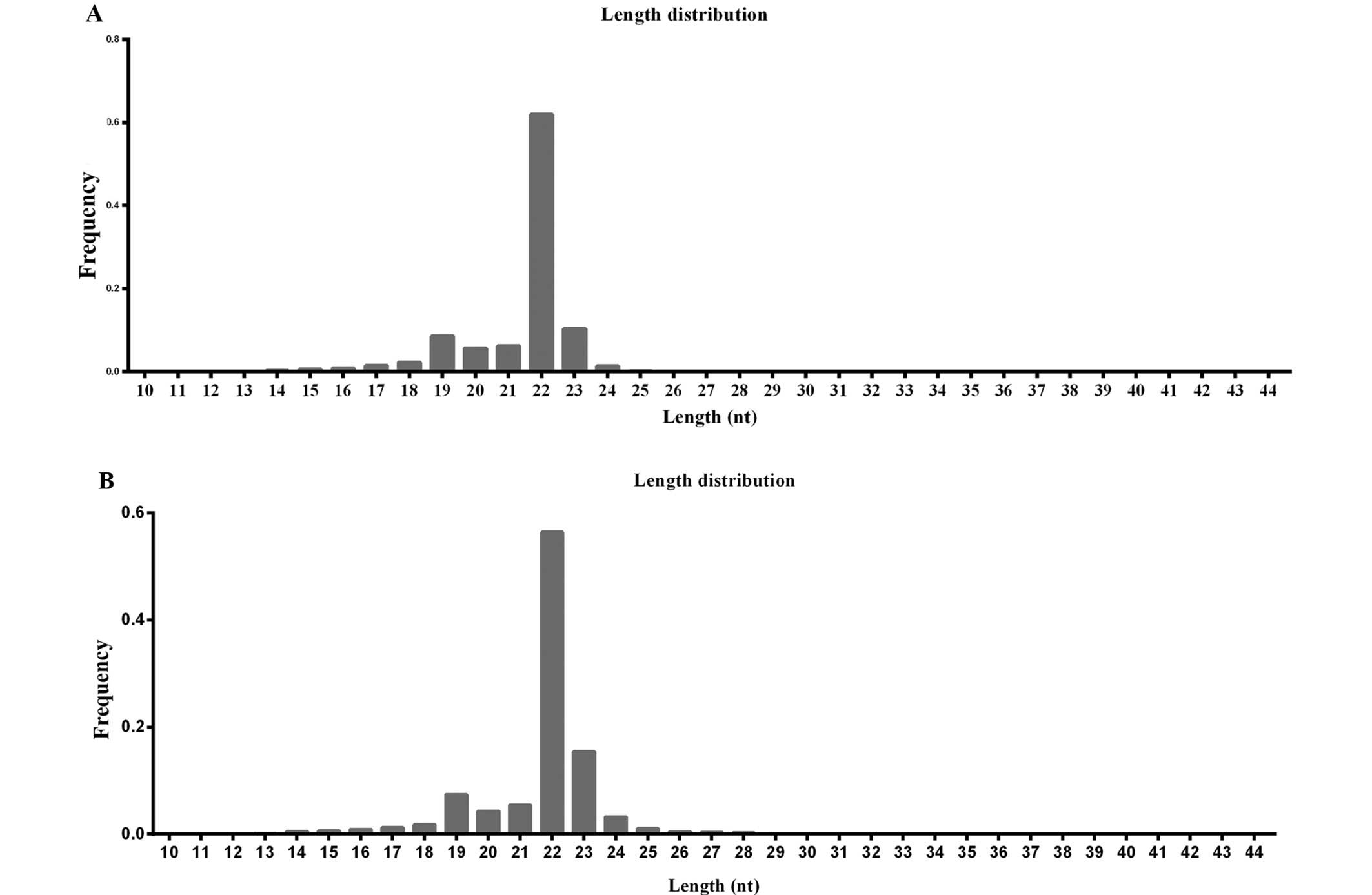
the small RNAs in SCLC. As shown in Fig. 2, the majority of reads, 79.86% for
H446 (Fig. 2A) and 80.58% for
H446/CDDP (Fig. 2B), were in the
range of 21 to 24 nt in length, with 22 nt being the most abundant
group of small RNAs. Read number and unique read number were the
highest in this range, which indicated the abundance of these
regulatory RNAs in SCLC. The 22 nt length was predominant,
consistent with previous reports involving different species
(19). We also found the second
highest diversity of 23-nt small RNAs compared with the total read
number in the H446/CDDP cells (Fig.
2B). Similar small RNA length distribution was discovered in
the H446 cells with a limited significance for 23-nt small RNAs
(Fig. 2A).
 | Table IISummary of reads and unique sequences
in small RNA libraries from the H446 and H446/CDDP cells. |
Table II
Summary of reads and unique sequences
in small RNA libraries from the H446 and H446/CDDP cells.
| Types of reads | H446 | H446-CDDP |
|---|
| Raw reads | 7,703,264 | 9,151,947 |
| Adaptor
removed | 36,549 | 44,975 |
|
Exon-/intron-antisense removed | 5,405 | 12,530 |
| Exon-/intron-sense
removed | 28,987 | 69,948 |
|
r-/sc-/sn-/sno-/srp-/t-RNA removed | 216,256 | 377,967 |
| Putative small RNA
population | 7,096,132 | 8,157,598 |
Annotation of small RNAs
In order to explore the components of the two
libraries, small RNAs from the H446 and H446/CDDP cells were
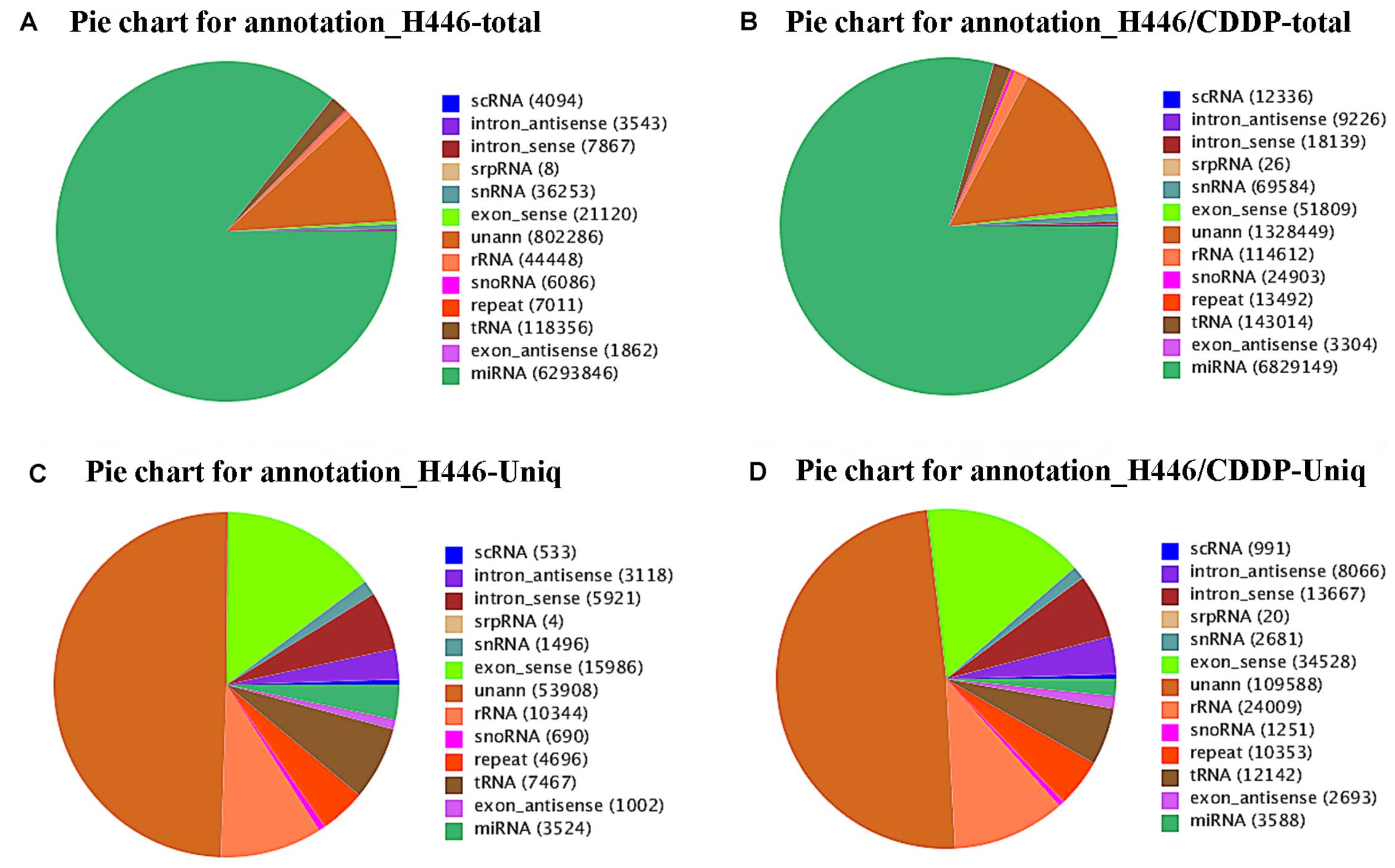
annotated. Pie charts (Fig. 3)
revealed the percentage of each type of small RNA, and the numbers
in the figure labels in parentheses indicate the different types of
small RNAs expressed. As shown in Fig.
3A and B, most small RNAs from both H446 and H446/CDDP cells
were characterized as miRNAs. The rRNA proportion in the two
libraries was <40%, which indicated that the quality of our
samples was adequate for Solexa-Illumina sequencing. Furthermore,
we also observed a relatively smaller percentage of miRNAs
expressed in the H446 and H446/CDDP cells, respectively (Fig. 3C and D).
Therefore, small RNA annotation together with length
distribution, exhibited the complexity and diversity of the small
RNAs in SCLC, and provide clues for further analysis of miRNAs in
the two cell lines. Several types of small RNAs that could be
annotated were discarded, such as rRNAS, tRNAs, snRNAs, snoRNAs,
known miRNAs, exon intron and non-annotated sRNAs. Finally putative
small RNA populations including 7,096,132 H446 cells and 8,157,598
H446/CDDP cells were obtained (Table
II).
Differentially expressed known miRNAs in
H446 and H446/CDDP cells
Putative small RNA populations from the two cell
lines were aligned to the precursor/mature miRNAs in miRBase 21 and
known miRNA data in H446 and H446/CDDP cells were collected. The
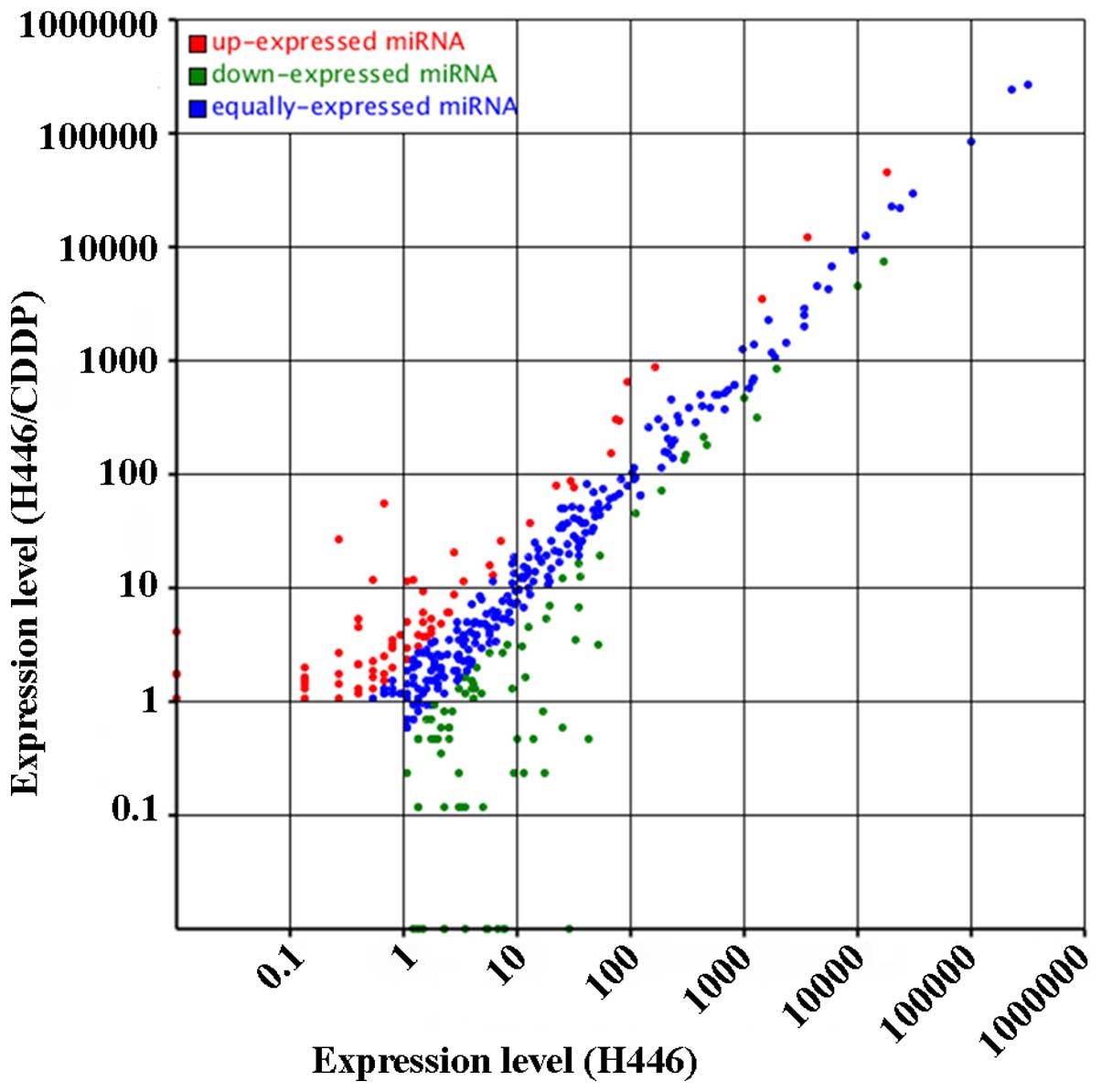
analysis revealed more than 400 miRNAs, which were differentially
expressed in the H446 and H446-CDDP cells (Fig. 4). More importantly, there were 72
de-regulated and 55 upregulated miRNAs in the H446/CDDP cells; the
difference was statistically significant (P<0.01) compared with
that of the H446 cells. We also identified the most markedly
changed 10 miRNAs from the H446 and H446/CDDP cells (Tables III and IV). The counts of different miRNAs in the
H446 cells changed from 1 (hsa-miR-3131, hsa-miR-3124-5p,
hsa-miR-4647, hsa-miR-216b, hsa-miR-769-3p and hsa-miR-4750) to
2,368,107 (hsa-let-7a-5p). The expression changes of known miRNAs
in the H446/CDDP cells ranged from 1 (hsa-miR-3148, hsa-miR-671-3p,
hsa-miR-495, hsa-miR-2682-5p, hsa-miR-433, hsa-miR-493-5p,
hsa-miR-584-5p and hsa-miR-494) to 2,278,377 (hsa-let-7a-5p)
suggesting drastic variation in the expression of miRNAs in
SCLC.
 | Table IIITop 10 most abundant miRNAs in the
H446 cells. |
Table III
Top 10 most abundant miRNAs in the
H446 cells.
| miR name | H446 expressed | H446/CDDP
expressed | H446-std | H446/CDDP-std | Fold-change (log2
H446-CDDP/H446) | P-value |
|---|
| hsa-miR-452-5p | 9,604 | 2,667 | 1,307.2394 | 309.467 | −2.07866589 | 0 |
| hsa-miR-21-5p | 127,238 | 63,455 | 17,318.8798 | 7,363.0405 | −1.23397208 | 0 |
| hsa-miR-29a-3p | 14,327 | 7,210 | 1,950.106 | 836.6169 | −1.2209135 | 0 |
| hsa-miR-222-3p | 74,587 | 38,395 | 10,152.3388 | 4,455.1878 | −1.18825397 | 0 |
| hsa-miR-23a-3p | 7,412 | 3,941 | 1,008.8774 | 457.2964 | −1.1415494 | 0 |
| hsa-miR-224-5p | 3,457 | 1,543 | 470.5463 | 179.043 | −1.39403057 | 8.89E-239 |
| hsa-miR-100-5p | 3,275 | 1,788 | 445.7735 | 207.4717 | −1.10339629 | 5.24E-157 |
|
hsa-miR-181a-2-3p | 2,205 | 1,131 | 300.1315 | 131.2363 | −1.19342792 | 1.96E-120 |
|
hsa-miR-125b-5p | 2,301 | 1,273 | 313.1984 | 147.7133 | −1.08427711 | 7.75E-108 |
| hsa-miR-27b-5p | 1,403 | 608 | 190.968 | 70.5497 | −1.43661906 | 1.36E-102 |
 | Table IVTop 10 most abundant miRNAs in the
H446/CDDP cells. |
Table IV
Top 10 most abundant miRNAs in the
H446/CDDP cells.
| miR name | H446 expressed | H446/CDDP
expressed | H446-std | H446/CDDP-std | Fold-change (log2
H446-CDDP/H446) | P-value |
|---|
|
hsa-miR-1307-3p | 696 | 5,475 | 94.7354 | 635.295 | 2.74545114 | 0 |
| hsa-miR-10a-5p | 1,243 | 7,504 | 169.1898 | 870.7313 | 2.36358499 | 0 |
| hsa-miR-423-5p | 27,054 | 104,330 | 3,682.4296 | 12,105.9967 | 1.71699201 | 0 |
| hsa-miR-320a | 136,101 | 385,405 | 18,525.259 | 44,720.7098 | 1.2714494 | 0 |
| hsa-miR-320b | 10,816 | 29,499 | 1,472.2096 | 3,422.9349 | 1.21725077 | 0 |
| hsa-miR-92b-5p | 547 | 2,557 | 74.4544 | 296.7031 | 1.99459099 | 1.47E-243 |
|
hsa-miR-92a-1-5p | 592 | 2,508 | 80.5795 | 291.0173 | 1.85262016 | 8.82E-217 |
| hsa-miR-204-5p | 5 | 474 | 0.6806 | 55.0009 | 6.33650426 | 5.69E-118 |
| hsa-miR-7-5p | 166 | 682 | 22.5949 | 79.1363 | 1.80834244 | 2.23E-58 |
| hsa-miR-204-3p | 2 | 225 | 0.2722 | 26.108 | 6.58368107 | 3.40E-57 |
Validation of miRNA expression in the
H446 and H446/CDDP cells
Among the differentially expressed miRNAs, 23 miRNAs
were randomly selected and validated by qPCR. As shown in Fig. 5, expression of 15 miRNAs was
significantly different including five miRNAs (miR-485-3p,
miR-2277-5p, miR-3911, miR-204-3p and miR-204-5p) elevated
>5-fold in the H446/CDDP cells. Markedly, the miR-204-3p
expression was elevated 16 times and miR-204-5p was increased
>300-fold. By contrast, the expression of 6 miRNAs (miR-127-3p,
miR-137, miR-382-5p, miR-3911, miR-409-3p, miR-432-5p, miR-485-3p,
miR-486-5p and miR-494) in the H446/CDDP cells was decreased
>5-fold. In addition, the miR-127-3p expression was decreased
~50 times. Above all, the expression trends of the selected miRNAs
in the H446 and H446/CDDP were similar to the Solexa sequencing
results.
Prediction of novel miRNAs in the H446
and H446/CDDP cells
The stem-loop hairpin secondary structure is an
important feature, which can be utilized to distinguish miRNAs from
each other. Furthermore, Solexa sequencing enables identification
of novel transcripts. Therefore, our next objective was to predict
novel miRNAs in the H446 and H446/CDDP cell lines by investigating
the stem-loop hairpin structure using the Solexa sequencing method.
We introduced 21 to 25 novel miRNAs into the H446 and H446/CDDP
cells (Tables V and VI), and found the read number of the
novel miRNAs was much lower than that of the known miRNAs,
indicating that novel miRNAs were usually expressed at lower
levels.
 | Table VNovel miRNAs predicted in the H446
cells. |
Table V
Novel miRNAs predicted in the H446
cells.
| Name | Sequence
(5′-3′) | Length (nt) | Arm (5′/3′) | Chromosome
location | Precursor length
(nt) | MEF (kcal/mol) | Count |
|---|
| H446-m0001 |
AGGGAGGGGTGGCAGGGATT | 20 | 5′ |
chr11:66638660:66638740:− | 81 | −30.7 | 6 |
| H446-m0002 |
TACTTACCTGTCCCCTACCCCAC | 23 | 3′ |
chr12:53292676:53292766:− | 91 | −34.1 | 5 |
| H446-m0003 |
GCTGGGGATGGAAGCTGAAGCC | 22 | 3′ |
chr13:111102941:111103018:+ | 78 | −25.7 | 23 |
| H446-m0004 |
TTGGCCTGTAGCCCGGTCCGGT | 22 | 3′ |
chr17:61850673:61850755:− | 83 | −43.1 | 5 |
| H446-m0005 |
CAAAATGATGAGGTACCTGATA | 22 | 5′ |
chr20:3194751:3194835:+ | 85 | −20.4 | 33 |
| H446-m0006 |
CTGGGAGTGGAGGGGAGGGTA | 21 | 3′ |
chr20:60944769:60944849:+ | 81 | −32.9 | 8 |
| H446-m0007 |
GGAGGAACCTTGGAGCTTCGGCA | 23 | 3′ |
chr22:31556037:31556127:− | 91 | −45.3 | 45 |
| H446-m0008 |
TGGGGAGGTGTGGAGTCAGCAT | 22 | 5′ |
chr3:127294107:127294179:− | 73 | −46.3 | 14 |
| H446-m0009 |
TCTGTTTGTCGTAGGCAGATGG | 22 | 3′ |
chr5:99385174:99385256:+ | 83 | −18 | 9 |
| H446-m0010 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6:28918820:28918902:+ | 83 | −21.5 | 3,647 |
| H446-m0011 |
GCAAATGATGTGAGAGATTC | 20 | 3′ |
chr6:168343859:168343952:+ | 94 | −20.2 | 9 |
| H446-m0012 |
CGCGCCTGCAGGAACTGGTAGA | 22 | 3′ |
chr6:1390549:1390646:− | 98 | −39.62 | 14 |
| H446-m0013 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_cox_hap2:437556:437638:+ | 83 | −21.5 | 3,647 |
| H446-m0014 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_mann_hap4:222225:222307:+ | 83 | −21.5 | 3,647 |
| H446-m0015 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_mcf_hap5:222373:222455:+ | 83 | −21.5 | 3,647 |
| H446-m0016 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_qbl_hap6:222220:222302:+ | 83 | −21.5 | 3,647 |
| H446-m0017 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_ssto_hap7:259727:259809:+ | 83 | −21.5 | 3,647 |
| H446-m0018 |
CCGGGCGGGCGAGGAGCGGG | 20 | 5′ |
chr7:1200062:1200125:+ | 64 | −31.2 | 6 |
| H446-m0019 |
TGGGCGGCCACTTGACATCCTC | 22 | 5′ |
chr7:128587281:128587362:− | 82 | −34.8 | 6 |
| H446-m0020 |
AACTGGGCATAGCTGTACTTTT | 22 | 3′ |
chr8:141538823:141538913:− | 91 | −19.1 | 9 |
| H446-m0021 |
AGGGCCGAAGGGTGGAAGCT | 20 | 5′ |
chr9:135927378:135927447:+ | 70 | −35 | 13 |
 | Table VINovel miRNAs predicted in the
H446/CDDP cells. |
Table VI
Novel miRNAs predicted in the
H446/CDDP cells.
| Name | Sequence
(5′-3′) | Length (nt) | Arm (5′/3′) | Chromosome
location | Precursor length
(nt) | MEF (kcal/mol) | Count |
|---|
|
H446-CDDP-m0001 |
AGCTGGGGATGGAAGCTGAAGCC | 23 | 3′ |
chr13:111102941:111103018:+ | 78 | −25.7 | 9 |
|
H446-CDDP-m0002 |
AGCCGCCTCCACCAAGCCTGGA | 22 | 3′ |
chr14:70449623:70449708:+ | 86 | −35.1 | 6 |
|
H446-CDDP-m0003 |
GGAGGGAGGGGGACGAGCGCGCG | 23 | 5′ |
chr14:32545963:32546039:− | 77 | −45.2 | 7 |
|
H446-CDDP-m0004 |
TGAGTGCGGGCTGGGCACAAGT | 22 | 5′ |
chr17:6347800:6347869:+ | 70 | −31.7 | 15 |
|
H446-CDDP-m0005 |
CAGGGCTGGGAGGGAGTGGGA | 21 | 3′ |
chr1:153585537:153585627:− | 91 | −34.1 | 8 |
|
H446-CDDP-m0006 |
CGGCGGCTCCCCGCTCCCCGGA | 22 | 3′ |
chr1:214454448:214454532:− | 85 | −55.1 | 6 |
|
H446-CDDP-m0007 |
GCAAAATGATGAGGTACCTGATA | 23 | 5′ |
chr20:3194750:3194836:+ | 87 | −20.4 | 23 |
|
H446-CDDP-m0008 |
TGCGGGTGAGGATGAGGGTGC | 21 | 3′ |
chr20:61276739:61276835:+ | 97 | −43.5 | 15 |
|
H446-CDDP-m0009 |
GGAGGAACCTTGGAGCTTCGGCA | 23 | 3′ |
chr22:31556037:31556127:− | 91 | −45.3 | 56 |
|
H446-CDDP-m0010 |
TGGGGAGGTGTGGAGTCAGCAT | 22 | 5′ |
chr3:127294107:127294179:− | 73 | −46.3 | 48 |
|
H446-CDDP-m0011 |
TGGGGGACGGTGGGCACCGAG | 21 | 5′ |
chr4:184329416:184329499:− | 84 | −49.6 | 15 |
|
H446-CDDP-m0012 |
TCAGACACAGGTATGGCTGGCT | 22 | 5′ |
chr5:171831829:171831908:− | 80 | −30.6 | 14 |
|
H446-CDDP-m0013 |
TAGCTCTGATGATGGTGGTTTCT | 23 | 3′ |
chr5:176878879:176878970:− | 92 | −23.2 | 7 |
|
H446-CDDP-m0014 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6:28918820:28918902:+ | 83 | −21.5 | 7,600 |
|
H446-CDDP-m0015 |
AGCAAATGATGTGAGAGATTC | 21 | 3′ |
chr6:168343859:168343952:+ | 94 | −20.2 | 17 |
|
H446-CDDP-m0016 |
CGCGCCTGCAGGAACTGGTAGA | 22 | 3′ |
chr6:1390549:1390646:− | 98 | −39.62 | 22 |
|
H446-CDDP-m0017 |
TGAGATCAAGACCAGGGAAAAT | 22 | 3′ |
chr6:134609915:134609990:− | 76 | −30.3 | 5 |
|
H446-CDDP-m0018 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_cox_hap2:437556:437638:+ | 83 | −21.5 | 7,600 |
|
H446-CDDP-m0019 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_mann_hap4:222225:222307:+ | 83 | −21.5 | 7,600 |
|
H446-CDDP-m0020 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_mcf_hap5:222373:222455:+ | 83 | −21.5 | 7,600 |
|
H446-CDDP-m0021 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_qbl_hap6:222220:222302:+ | 83 | −21.5 | 7,600 |
|
H446-CDDP-m0022 |
TCGGGCGGGAGTGGTGGCTTT | 21 | 3′ |
chr6_ssto_hap7:259727:259809:+ | 83 | −21.5 | 7,600 |
|
H446-CDDP-m0023 |
TGAGTGTGTGTGTGTGAGTGTGA | 23 | 3′ |
chr8:79679467:79679541:+ | 75 | −20.3 | 21 |
|
H446-CDDP-m0024 |
AGGGCCGAAGGGTGGAAGCT | 20 | 5′ |
chr9:135927378:135927447:+ | 70 | −35 | 19 |
|
H446-CDDP-m0025 |
CCCTGGGGTTCTGAGGACATG | 21 | 5′ |
chr9:35710651:35710743:− | 93 | −39 | 7 |
Target prediction of novel miRNAs
Target genes were predicted according to the
aforementioned rules and principles discussed above. The results
indicated the presence of 130,566 candidate target genes for 21
novel miRNAs in the H446 cell line and 153,092 putative target
genes for the 25 novel miRNAs from the H446/CDDP cell line. We also
annotated the biological processes, molecular functions and
cellular components of the target genes using GO analysis (20,21).
GO analysis revealed that in the cellular component category
(Table VII), most target genes in
the H446 cell line were localized in insoluble cell fraction,
intracellular areas and dendrites (P<0.05), while target genes
in the H446/CDDP cells were only restricted to the membrane
(P<0.05). In the category of molecular function, the majority of
targets in the H446 cells were significantly involved (P<0.05)
(Table VIII) in transcriptional
regulation, transferase and binding activities involving
nucleosides and nucleotides (purine), adenyl nucleotides and
ribonucleotides, cations, metal ions, sequence-specific DNA binding
and enzymes. The target gene functions in the H446/CDDP cells were
limited to transition metal ion binding and DNA binding (P<0.05)
(Table VIII). The target genes
in the H446 cell line participated in more biological processes
(statistically significant processes are listed in Table IX) than those of putative target
genes in the H446/CDDP cells (all listed in Table IX).
 | Table VIIDistribution of putative target genes
in cell component: Gene Ontology - cellular component term. |
Table VII
Distribution of putative target genes
in cell component: Gene Ontology - cellular component term.
| Gene Ontology term
component | Cluster frequency
(%) | Corrected
p-value |
|---|
| H446 cells | | |
| Cell | 96.90 | 0.00158 |
| Cell part | 96.90 | 0.00158 |
| Intracellular | 74.00 | 0.01422 |
| Intracellular
part | 73.60 | 0.02138 |
| Cell fraction | 10.40 | 0.00015 |
| Insoluble
fraction | 7.60 | 0.00047 |
| Neuron
projection | 3.60 | 0.01808 |
| H446/CDDP
cells | | |
| Cell | 96.90 | 5.89E-05 |
| Cell part | 96.90 | 5.89E-05 |
| Integral to
membrane | 9.90 | 0.00278 |
 | Table VIIITop 10 most frequently enriched
molecular functions by putative target genes of novel miRNAs in the
H446 and H446/CDDP cells: Gene ontology - molecular function
term. |
Table VIII
Top 10 most frequently enriched
molecular functions by putative target genes of novel miRNAs in the
H446 and H446/CDDP cells: Gene ontology - molecular function
term.
| Gene Ontology term
function | Cluster frequency
(%) | Corrected
p-value |
|---|
| H446 cells | | |
| Binding | 84.90 | 2.37E-05 |
| Ion binding | 26.00 | 0.00803 |
| Cation
binding | 25.70 | 0.00676 |
| Metal ion
binding | 20.40 | 0.01386 |
| Purine nucleotide
binding | 13.50 | 0.02562 |
| Transferase
activity | 12.70 | 0.04526 |
| Nucleoside
binding | 11.50 | 0.0012 |
| Purine nucleoside
binding | 11.50 | 0.00144 |
| Adenyl nucleotide
binding | 11.20 | 0.00296 |
| Adenyl
ribonucleotide binding | 10.60 | 0.01851 |
| Transcription
regulator activity | 7.70 | 0.00271 |
| Enzyme
binding | 5.00 | 0.03061 |
| Sequence-specific
DNA binding | 1.80 | 0.02915 |
| H446/CDDP
cells | | |
| Binding | 84.90 | 3.53E-05 |
| Transition metal
ion binding | 14.10 | 0.00056 |
| DNA binding | 5.80 | 0.00135 |
 | Table IXDistribution of putative target genes
in biological processes: Gene ontology - biological process
term. |
Table IX
Distribution of putative target genes
in biological processes: Gene ontology - biological process
term.
| Gene Ontology
term-process | Cluster frequency
(%) | Corrected
p-value |
|---|
| H446 cells | | |
| Regulation of
metabolic process | 22.50 | 5.27E-08 |
| Regulation of
macromolecule metabolic process | 18.10 | 2.55E-07 |
| Regulation of
macromolecule metabolic process | 17.90 | 5.30E-07 |
| Biopolymer
modification | 14.60 | 8.55E-07 |
| Developmental
process | 30.80 | 1.38E-06 |
| Intracellular
signaling cascade | 10.20 | 2.06E-06 |
| Anatomical
structure development | 23.30 | 2.78E-06 |
| Protein
modification process | 13.70 | 3.35E-06 |
| Multicellular
organismal development | 22.80 | 5.67E-06 |
| Regulation of gene
expression | 16.30 | 3.30E-05 |
| H446/CDDP
cells | | |
| Anatomical
structure development | 23.20 | 2.80E-06 |
| System
development | 20.80 | 1.39E-05 |
| Multicellular
organismal development | 22.70 | 5.19E-05 |
| RNA metabolic
process | 18.20 | 6.46E-05 |
| Cellular
developmental process | 15.60 | 8.70E-05 |
| Organ
development | 14.80 | 9.03E-05 |
| Transcription | 15.80 | 9.66E-05 |
| Nitrogen compound
metabolic process | 29.70 | 0.00014 |
| Nucleobase,
nucleoside, nucleotide and nucleic acid metabolic process | 27.20 | 0.00019 |
| Cellular
process | 82.90 | 0.00096 |
Pathway annotation of target genes
We were also interested in the signaling pathways of
the target genes. KEGG analysis was adopted to determine the
biological pathway of the novel miRNAs from the H446 and H446/CDDP
cell lines. Results revealed that target genes of miRNAs from the
H446 cell line participated in greater number of biological
pathways than those of miRNAs from the H446/CDDP cells. A few
pathways were shared by target genes of novel miRNAs from both the
cell lines (Table X). The target
genes of novel miRNAs from the H446 cells mediate a few pathways
closely related to cancer, such as the ErbB signaling pathway, mTOR
signaling pathway and Notch signaling pathway in chronic myeloid
leukemia colorectal cancer, melanoma, pancreatic cancer, prostate
cancer and thyroid cancer as well as non-small cell lung cancer.
However, only the Wnt signaling pathway was closely related to
cancer in the pathway list of target genes mediated by miRNAs from
the H446/CDDP cells (Table X).
 | Table XPathways mediated by putative target
genes of novel miRNAs from the H446 and H446/CDDP cell lines. |
Table X
Pathways mediated by putative target
genes of novel miRNAs from the H446 and H446/CDDP cell lines.
| Pathways mediated
by target genes |
|---|
| H446 cells
only |
| Acute myeloid
leukemia |
| Amoebiasis |
| Axon guidance |
| β-alanine
metabolism |
| Chagas disease
(American trypanosomiasis) |
| Chronic myeloid
leukemia |
| Colorectal
cancer |
| ECM-receptor
interaction |
| Epithelial cell
signaling in Helicobacter pylori infection |
| ErbB signaling
pathway |
| Fc γ R-mediated
phagocytosis |
| Glycerolipid
metabolism |
| Inositol phosphate
metabolism |
| MAPK signaling
pathway – yeast |
| Measles |
| Melanoma |
| mTOR signaling
pathway |
| Non-small cell
lung cancer |
| Notch signaling
pathway |
| Pancreatic
cancer |
|
Phosphatidylinositol signaling
system |
| Prostate
cancer |
| Thyroid
cancer |
| Tryptophan
metabolism |
| Ubiquinone and
other terpenoid-quinone biosynthesis |
| H446/CDDP cells
only |
| Cardiac muscle
contraction |
| Hematopoietic cell
lineage |
| Natural killer
cell mediated cytotoxicity |
| Neurotrophin
signaling pathway |
| NOD-like receptor
signaling pathway |
| Osteoclast
differentiation |
| Type II diabetes
mellitus |
| Wnt signaling
pathway |
| Common
pathways |
|
Aldosterone-regulated sodium
reabsorption |
| African
trypanosomiasis |
| Bile
secretion |
| Biosynthesis of
secondary metabolites |
| Carbohydrate
digestion and absorption |
| Cysteine and
methionine metabolism |
| Endocytosis |
| Gastric acid
secretion |
| MAPK signaling
pathway |
| Metabolic
pathways |
| Pancreatic
secretion |
| Pathways in
cancer |
| Protein digestion
and absorption |
| Proximal tubule
bicarbonate reclamation |
| Purine
metabolism |
Discussion
Small RNAs (sRNAs) from the next generation high
throughput Solexa sequencing cover almost every type of sRNAs,
including miRNAs, siRNAs, piRNAs, rRNAs, tRNAs, snRNAs, snoRNAs,
repeat associated sRNAs and degraded tags of exons or introns. The
sRNAs were annotated into different categories by comparing the
collected tags with those in databases. In the present study,
digitalized sRNAs were obtained and initially subjected to data
cleaning including elimination of low-quality tags and other
contaminants. The length distribution was summarized to explore the
possible composition of sRNA libraries from the H446 and H446/CDDP
cells. Subsequently, standard bioinformatic analysis was carried
out to annotate the clean tags into different categories in order
to identify known miRNAs and predict novel miRNAs expressed in the
H446 and H446/CDDP cell lines. On the one hand, the expression
level of known miRNAs in the two cell lines was summarized and
compared to identify the varied expression of the miRNAs. On the
other hand, biological information of novel miRNAs from the H446
and H446/CDDP cells was analyzed by GO and KEGG analysis. The
results revealed differences in target gene expression, functions,
processes and pathways, which may be linked to MDR of H446/CDDP
cells.
Our data showed differential expression of known
miRNAs in the H446 and H446/CDDP cells. Further sequence tag
analysis revealed that the expression of 72 miRNAs was markedly
decreased and that of 55 significantly increased in the H446/CDDP
cells compared with that of the H446 cells (Fig. 4), respectively. These datasets
showed that downregulation of miRNAs was more common than
upregulation in MDR cells of SCLC, which was consistent with
previously published miRNA profiling studies involving bladder
tumor tissues (22,23). Validation of the differentially
expressed miRNAs, in the present study by real-time PCR, yielded
results consistent with those obtained with Solexa sequencing. Our
previous studies demonstrated that miRNA-137 was downregulated in
H446/CDDP cells, which participated in the MDR of this cell line
(24). Bier et al (25) demonstrated that decreased expression
of miRNA-137 enhanced the stem-cell features of glioblastoma cells.
Furthermore, deregulated miRNA-494 was related to the oncogenesis
of pancreatic ductal adenocarcinoma and decreased miRNA-494 was
considered as a therapeutic or prognostic marker for this cancer
(26), which was also consistent
with our results. Furthermore, a few miRNAs discussed in our study
were similarly deregulated in other cancers: absence of miR-329
enhanced proliferation of cancer cells in neuroblastoma and glioma
(27,28); renal childhood neoplasm was
associated with de-regulated miR-215 (29) and its upregulation may inhibit the
growth of osteosarcoma and colon cancer cells (30). Indeed, the varied expression of
miRNAs provided reliable data to establish the role of miRNAs in
the MDR mechanisms of SCLC.
After analyzing the expression of known miRNAs and
exploring their possible relationship to MDR, we explored novel
miRNAs in the H446 and H446/CDDP cells, which may also be
associated with MDR. As described above, clean reads of digitalized
sRNAs were annotated into different categories, such as rRNAS,
tRNAs, snRNAs, snoRNAs, known miRNAs, exons/introns and unannotated
sRNAs. Novel miRNAs were predicted from tags that were not
annotated to any category. We discovered 21 and 25 novel miRNAs in
the H446 and H446/CDDP cells, respectively. Although the expression
of a majority of the novel miRNAs from both H446 and H446/CDDP cell
lines was low, target genes of the novel miRNAs exhibited
differences in distribution and pathophysiology.
Putative target genes of novel miRNAs from the H446
and H446/CDDP cells were analyzed by GO analysis (31). The results showed that a majority of
novel miRNA target genes in the H446 cells were involved in
regulation of a broad range of metabolic and physiological
processes including metabolism of macromolecules, biopolymer
modification, developmental processes, intracellular signaling
cascades, anatomical structure, protein modification, tissue
development, and regulation of gene expression. Compared with
targets of novel miRNAs in the H446/CDDP cells, novel miRNA targets
in the H446 cells participated in a greater number of processes,
most of which were related to transcription. A few novel miRNAs in
the H446 cells were predicted to be involved in programmed cell
death, cell differentiation, cell proliferation and cell adhesion.
Those transcription factors were known to be targeted by conserved
miRNAs and play a major role in various aspects of cancer cell
growth and tumor metastasis (32,33).
By contrast, target genes of novel miRNAs in the H446/CDDP cells
were not involved in these regulation processes. Thus, in addition
to the different expression levels of known miRNAs, the limited
functional role of novel miRNAs in the H446/CDDP cells also
highlights their MDR characteristics. Further studies are essential
to explain the functional significance of the novel miRNAs and
their targets in MDR of SCLC.
Based on the knowledge of putative target genes in
regards to distribution range, pathophysiologic process and
function type, we further analyzed possible pathways mediated by
the novel miRNAs. The KEGG pathway analysis was adopted to identify
significantly enriched metabolic pathways and signal transduction
pathways. The results indicated that target genes of novel miRNAs
from both the H446 and H446/CDDP cells participated in hundreds of
pathways, but most target genes were limited to significantly
enriched in metabolic pathways. Novel miRNAs from the H446/CDDP
cells concentrated in fewer pathways than that of the H446 cells,
and most of those pathways were shared with target genes of novel
miRNAs from the H446 cells. Among the shared pathways, endocytosis,
MAPK signaling pathway, metabolic pathways, pathways in cancer and
purine metabolism pathways are believed to be associated with
cancer metastasis (34,35), especially pathways in cancer.
According to a map of pathways in cancer (which was not shown) most
of the molecules were partially regulated by predicted genes, and
most of them were involved in classic signal transduction pathways
regulating the occurrence and metastasis of cancers (36–40). A
few pathways were modulated only by target genes of novel miRNAs
from H446 cells, such as the ErbB signaling pathway, mTOR signaling
pathway, Notch signaling pathway, and pathways mediating acute
myeloid leukemia, chronic myeloid leukemia, colorectal cancer,
melanoma, non-small cell lung cancer, pancreatic cancer and
prostate cancer. Interestingly, all those pathways were not
mediated by target genes of novel miRNAs from the H446/CDDP cells.
We believe that the differential regulation of the pathways may be
associated with MDR of H446/CDDP cells. On the other hand, a few
pathways were regulated only by target genes of novel miRNAs from
the H446/CDDP cells, including neurotrophin signaling pathway,
NOD-like receptor signaling pathway, Wnt signaling pathway and
natural killer cell-mediated cytotoxicity. However, only the Wnt
signaling pathway has been associated with carcinogenesis (41,42).
In brief, KEGG analysis revealed pathways mediated by target genes
of novel miRNAs from H446 and H446/CDDP cells, and the differential
regulation of cancer-related pathways may be associated with MDR
mechanisms in SCLC.
We analyzed the differential expression of known
miRNAs in H446 and H446/CDDP cells and discovered a set of miRNAs
with markedly altered expression in H446/CDDP cells. Furthermore,
we predicted novel miRNAs in the two cell lines and analyzed their
biological roles. The altered expression of known miRNAs and
different functions of novel miRNAs may be related to MDR of
SCLC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81071933).
References
|
1
|
Pillai RN and Owonikoko TK: Small cell
lung cancer: Therapies and targets. Semin Oncol. 41:133–142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rutledge MR, Waddell JA and Solimando DA
Jr: Carboplatin (renally dosed) and etoposide regimen for
small-cell lung cancer. Hosp Pharm. 48:274–279. 2013. View Article : Google Scholar
|
|
3
|
Chen YT, Feng B and Chen LB: Update of
research on drug resistance in small cell lung cancer chemotherapy.
Asian Pac J Cancer Prev. 13:3577–3581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia B, Hong LZ, Cai XW, Zhu ZF, Liu Q,
Zhao KL, Fan M, Mao JF, Yang HJ, Wu KL, et al: Phase 2 study of
accelerated hypofractionated thoracic radiation therapy and
concurrent chemotherapy in patients with limited-stage small-cell
lung cancer. Int J Radiat Oncol Biol Phys. 91:517–523. 2015.
View Article : Google Scholar
|
|
5
|
Rule WG, Foster NR, Meyers JP, Ashman JB,
Vora SA, Kozelsky TF, Garces YI, Urbanic JJ, Salama JK and Schild
SE: Prophylactic cranial irradiation in elderly patients with small
cell lung cancer: Findings from a North Central Cancer Treatment
Group pooled analysis. J Geriatr Oncol. 6:119–126. 2015. View Article : Google Scholar
|
|
6
|
Zhu H, Guo H, Shi F, Zhu K, Luo J, Liu X,
Kong L and Yu J: Prophylactic cranial irradiation improved the
overall survival of patients with surgically resected small cell
lung cancer, but not for stage I disease. Lung Cancer. 86:334–338.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schabath MB, Nguyen A, Wilson P, Sommerer
KR, Thompson ZJ and Chiappori AA: Temporal trends from 1986 to 2008
in overall survival of small cell lung cancer patients. Lung
Cancer. 86:14–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knez L, Sodja E, Kern I, Košnik M and
Cufer T: Predictive value of multidrug resistance proteins,
topoisomerases II and ERCC1 in small cell lung cancer: A systematic
review. Lung Cancer. 72:271–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinehara Y, Minami T, Kijima T, Hoshino S,
Morimura O, Otsuka T, Hayama Y, Fukushima K, Takeuchi Y,
Higashiguchi M, et al: Favorable response to trastuzumab plus
irinotecan combination therapy in two patients with HER2-positive
relapsed small-cell lung cancer. Lung Cancer. 87:321–325. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ushijima R, Takayama K, Izumi M, Harada T,
Horiuchi Y, Uchino J, Hara N and Nakanishi Y: Immunohistochemical
expression of MRP2 and clinical resistance to platinum-based
chemotherapy in small cell lung cancer. Anticancer Res.
27:4351–4358. 2007.
|
|
11
|
Eda A, Tamura Y, Yoshida M and Hohjoh H:
Systematic gene regulation involving miRNAs during neuronal
differentiation of mouse P19 embryonic carcinoma cell. Biochem
Biophys Res Commun. 388:648–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tindall MJ and Clerk A: Modelling negative
feedback networks for activating transcription factor 3 predicts a
dominant role for miRNAs in immediate early gene regulation. PLOS
Comput Biol. 10:e10035972014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang R, Marshall D, Bryan GJ and Hornyik
C: Identification and characterization of miRNA transcriptome in
potato by high-throughput sequencing. PLoS One. 8:e572332013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zha W, Cao L, Shen Y and Huang M: Roles of
miR-144-ZFX pathway in growth regulation of non-small-cell lung
cancer. PLoS One. 8:e741752013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshikawa M: Biogenesis of trans-acting
siRNAs, endogenous secondary siRNAs in plants. Genes Genet Syst.
88:77–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwab R, Palatnik JF, Riester M, Schommer
C, Schmid M and Weigel D: Specific effects of microRNAs on the
plant transcriptome. Dev Cell. 8:517–527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altermann E and Klaenhammer TR:
PathwayVoyager: Pathway mapping using the Kyoto Encyclopedia of
Genes and Genomes (KEGG) database. BMC Genomics. 6:602005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wrzodek C, Dräger A and Zell A:
KEGGtranslator: Visualizing and converting the KEGG PATHWAY
database to various formats. Bioinformatics. 27:2314–2315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukunaga R, Han BW, Hung JH, Xu J, Weng Z
and Zamore PD: Dicer partner proteins tune the length of mature
miRNAs in flies and mammals. Cell. 151:533–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blake JA and Harris MA: The Gene Ontology
(GO) project: Structured vocabularies for molecular biology and
their application to genome and expression analysis. Curr Protoc
Bioinformatics Chapter. 7:2–7. 2008.
|
|
21
|
Smid M and Dorssers LC: GO-Mapper:
Functional analysis of gene expression data using the expression
level as a score to evaluate Gene Ontology terms. Bioinformatics.
20:2618–2625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Zhang H, He H, Tong W, Wang B,
Liao G, Chen Z and Du C: Up-regulation of microRNA in bladder tumor
tissue is not common. Int Urol Nephrol. 42:95–102. 2010. View Article : Google Scholar
|
|
23
|
Chen YH, Wang SQ, Wu XL, Shen M, Chen ZG,
Chen XG, Liu YX, Zhu XL, Guo F, Duan XZ, et al: Characterization of
microRNAs expression profiling in one group of Chinese urothelial
cell carcinoma identified by Solexa sequencing. Urol Oncol.
31:219–227. 2013. View Article : Google Scholar
|
|
24
|
Li P, Ma L, Zhang Y, Ji F and Jin F:
MicroRNA-137 down-regulates KIT and inhibits small cell lung cancer
cell proliferation. Biomed Pharmacother. 68:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bier A, Giladi N, Kronfeld N, Lee HK,
Cazacu S, Finniss S, Xiang C, Poisson L, deCarvalho AC, Slavin S,
et al: Micro-RNA-137 is downregulated in glioblastoma and inhibits
the stemness of glioma stem cells by targeting RTVP-1. Oncotarget.
4:665–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W,
Cui J, Du Y, Wei D, Huang S, et al: Down-regulation of microRNA-494
via loss of SMAD4 increases FOXM1 and β-catenin signaling in
pancreatic ductal adenocarcinoma cells. Gastroenterology.
147:485–497.e18. 2014. View Article : Google Scholar
|
|
27
|
Yang H, Li Q, Zhao W, Yuan D, Zhao H and
Zhou Y: miR-329 suppresses the growth and motility of neuroblastoma
by targeting KDM1A. FEBS Lett. 588:192–197. 2014. View Article : Google Scholar
|
|
28
|
Xiao B, Tan L, He B, Liu Z and Xu R:
MiRNA-329 targeting E2F1 inhibits cell proliferation in glioma
cells. J Transl Med. 11:1722013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Senanayake U, Das S, Vesely P, Alzoughbi
W, Fröhlich LF, Chowdhury P, Leuschner I, Hoefler G and Guertl B:
miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated
and their common target ACVR2B is strongly expressed in renal
childhood neoplasms. Carcinogenesis. 33:1014–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song B, Wang Y, Titmus MA, Botchkina G,
Formentini A, Kornmann M and Ju J: Molecular mechanism of
chemoresistance by miR-215 in osteosarcoma and colon cancer cells.
Mol Cancer. 9:962010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Götz S, García-Gómez JM, Terol J, Williams
TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J and Conesa A:
High-throughput functional annotation and data mining with the
Blast2GO suite. Nucleic Acids Res. 36:3420–3435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagaraju GP, Dontula R, El-Rayes BF and
Lakka SS: Molecular mechanisms underlying the divergent roles of
SPARC in human carcinogenesis. Carcinogenesis. 35:967–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Cremoux P and Robert J: Cell signalling
and cancer: Characterisation of therapeutic targets. Pathol Biol
(Paris). 60:217–222. 2012.In French. View Article : Google Scholar
|
|
34
|
Allard B, Turcotte M and Stagg J:
CD73-generated adenosine: Orchestrating the tumor-stroma interplay
to promote cancer growth. J Biomed Biotechnol. 2012:4851562012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dollé L, Depypere HT and Bracke ME:
Anti-invasive/anti-metastasis strategies: New roads, new tools and
new hopes. Curr Cancer Drug Targets. 6:729–751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang G, Nishimoto K, Yang Y and
Kleinerman ES: Participation of the Fas/FasL signaling pathway and
the lung microenvironment in the development of osteosarcoma lung
metastases. Adv Exp Med Biol. 804:203–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giubellino A, Burke TR Jr and Bottaro DP:
Grb2 signaling in cell motility and cancer. Expert Opin Ther
Targets. 12:1021–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miliani de Marval PL and Zhang Y: The
RP-Mdm2-p53 pathway and tumorigenesis. Oncotarget. 3:234–238. 2011.
View Article : Google Scholar
|
|
41
|
Cai Y, Cai T and Chen Y: Wnt pathway in
osteosarcoma, from oncogenic to therapeutic. J Cell Biochem.
115:625–631. 2014. View Article : Google Scholar
|
|
42
|
Li X, Jia Y, Zhang W, Zhang Y, Li B, Huang
M, Bao F, Wu J and Lou Y: The research progress about Wnt pathway
of lung cancer stem cells. Zhongguo Fei Ai Za Zhi. 14:695–698.
2011.In Chinese. PubMed/NCBI
|