Introduction
Leukemia is characterized by uncontrolled cell
proliferation and blockage in the differentiation of hematopoietic
cells (1,2). However, clinical trials concerning
treatment strategies for leukemia have not achieved satisfactory
outcomes, and new targets for treating leukemia are necessary. One
of the best strategies for new anti-leukemia agents are carried out
via induction of cell differentiation or apoptotic death in
leukemia cells (3–5). Regulation and/or management of cell
cycle progression and apoptosis are prominent approaches to
anti-leukemia therapy (2,6). Cyclin-dependent kinase (CDK) complexes
can modulate cell cycle progression, especially cyclin-dependent
kinase 1 (CDK1) and cyclin B are pivotal molecules in the
regulation of the cell cycle in the G2/M phase (7,8). The
cell division cycle 25C (cdc25C) phosphatase controls CDK1 activity
and accelerates mitosis entry by dephosphorylation of CDK1 on Thr14
and Tyr15 sites (9,10). Additionally, the activity of
CDK1/cyclin B complex is blocked by p21waf/cip1
signaling which serves as a CDK inhibitor (11,12).
Several agents have been shown to interfere with the activity of
CDK1 and cause subsequent cell cycle arrest, and these agents have
been developed into significant clinical anticancer drugs through
induction of cancer cell apoptosis (13,14).
When tumor cells undergo apoptosis, nuclear condensation, DNA
fragmentation and apoptotic bodies are manifested (15,16).
During the apoptotic process, caspase proteins undergo proteolytic
processing and trigger a cascade of caspase activation (17,18).
Therefore, these key factors can regulate the apoptotic process and
play a vital role in the treatment of leukemia.
Benzyloxybenzaldehyde derivatives have been reported
to have multiple biological functions, including binding to
estrogen receptors (ERα and β), arresting cell cycle progression
and inducing apoptotic cell death (19–21). A
class of 2-benzyloxybenzaldehyde derivatives was designed and
synthesized in our laboratory (20). 2-Benzyloxybenzaldehyde analog
2-[(3-methoxybenzyl)oxy]benzaldehyde (CCY-1a-E2) has been found to
be a potent compound against leukemia cells. CCY-1a-E2 exhibited an
anti-leukemic effect on a leukemia BALB/c mouse model (19). High dosage treatment (100 mg/kg) of
CCY-1a-E2 was found to have no adverse effects on renal, hepatic
and hematological parameters upon safety evaluation analysis
(19). However, the molecular
mechanism underlying the anti-leukemia effects of CCY-1a-E2 has not
been completely clarified. Here, the anti-proliferative activity of
CCY-1a-E2 was evaluated in human leukemia HL-60 cells. We found
that CCY-1a-E2 led to cell cycle arrest at the G2/M phase and
caused apoptosis in HL-60 cells through mitochondria-dependent
caspase cascade signaling.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS), L-glutamine,
penicillin-streptomycin, RPMI-1640 medium, and trypsin-EDTA were
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
The primary antibodies used in this study and their corresponding
IgG antibodies conjugated to horseradish peroxidase (HRP) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Z-IETD-FMK (a specific caspase-8 inhibitor), Z-LEHD-FMK (a
specific caspase-9 inhibitor) and Z-DEVD-FMK (a specific caspase-3
inhibitor) were obtained from R&D Systems, Inc. (Minneapolis,
MN, USA). All chemicals and reagents were of analytical grade and
purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA) unless
otherwise stated.
Cell culture
Human promyelocytic leukemia cell line HL-60 and
human acute myelogenous leukemia cell lines KG-1 and KG-1a were
purchased from the Bioresource Collection and Research Center
(BCRC) (Hsinchu, Taiwan). K562 erythroleukemia cell line was
purchased from the American Type Culture Collection (ATCC)
(Manassas, VA, USA). Peripheral blood mononuclear cells (PBMCs)
were collected from whole blood samples with the BD Vacutainer
Mononuclear Cell Preparation Tube (CPT) with sodium heparin
(Becton, Dickinson and Co., Franklin Lakes, NJ, USA) and were
isolated using Ficoll-Paque™ Plus (GE Healthcare UK, Ltd., Little
Chalfont, Buckinghamshire, UK). Cells were placed into
75-cm2 culture flasks and were grown in RPMI-1640 medium
supplemented with 10% FBS, 100 U/ml penicillin, and 100
µg/ml streptomycin at 37°C under a humidified atmosphere of
5% CO2 and 95% air.
Detection of cell number and
viability
HL-60, K562, KG-1 and KG-1a cells (1×104
cells/well) in 96-well plates were incubated with 0, 1, 2.5, 5 and
10 µM of CCY-1a-E2 for 24 and 48 h. The trypan blue dye
exclusion assay was applied to determine the number of viable cells
by using a Countess Automated Cell Counter (Thermo Fisher
Scientific, Inc.) as previously reported (22). HL-60 cells were exposed to 0, 1,
2.5, 5 and 10 µM of CCY-1a-E2 after pre-incubation with or
without 10, 25 and 50 µM of Z-IETD-FMK, Z-LEHD-FMK and
Z-DEVD-FMK for 2 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed for the quantitative analysis of cell viability
as described elsewhere (23,24).
Assessment of cell cycle distribution by
flow cytometric analysis
HL-60 cells (2×105 cells/well) were
seeded into 12-well plates and then treated with 5 µM
CCY-1a-E2 for 0, 3, 6, 12 and 24 h. The cells were fixed and
stained with propidium iodide (PI) solution following a previously
reported method (6). The sub-G1
peak (apoptotic population) and cell cycle profiling were
determined using a BD FACSCalibur flow cytometer (BD Biosciences,
San Jose, CA, USA), and the data were analyzed by utilizing BD
CellQuest software.
Immunoblotting analysis
HL-60 cells (1×107 cells) were placed in
T75 flasks and treated with 5 µM CCY-1a-E2 for 0, 1, 2, 4,
6, 8, 10, 12 and 16 h. After treatments, the cells were lysed with
lysis buffer, and each sample was electrophoresed as previously
detailed (15,22,25),
and the membrane was probed with an appropriate secondary antibody
for enhanced chemiluminescence (Immobilon Western HRP Substrate;
Merck Millipore, Bedford, MA, USA).
4′,6-Diamidino-2-phenylindole (DAPI)
staining and DNA fragmentation assay
HL-60 cells (2×105 cells/ml) were treated
with 5 µM CCY-1a-E2 for 24 h and thereafter stained with 1
µg/ml DAPI as previously described (16,25).
After a 48-h exposure, DNA was extracted from each sample and
electrophoresis was run according to a previous method (26).
Analyses of caspase-3, -8 and -9
activities
HL-60 cells at a density of 1×107
cells/flask were incubated with 5 µM CCY-1a-E2 for 0, 2, 4,
8 and 12 h. At the end of the incubation, the cells were lysed and
assessed according to the manufacturer's instructions provided in
the Caspase-3, -8 and -9 Colorimetric Assay kits (R&D Systems,
Inc.).
Determination of mitochondrial membrane
potential (ΔΨm)
HL-60 cells at a density of 2×105
cells/well in 12-well plates were treated with 5 µM
CCY-1a-E2 for 0, 6, 12, 18 and 24 h. Cells from each treatment were
harvested and re-suspended in 500 µl of DiOC6
(3) (Thermo Fisher Scientific,
Inc.) at 50 nM for ΔΨm. After incubation at 37°C for 30 min, the
cells were analyzed by flow cytometry as described by Lee et
al (27).
Statistical analysis
The statistical significance of the difference was
defined (p<0.05) and carried out utilizing Student's t-test, and
the data are expressed as the mean ± standard deviation (SD) from
three independent experiments.
Results
CCY-1a-E2 reduces cell viability in
leukemia cells
Four cell lines (HL-60, K562, KG-1 and KG-1a) were
used to assess the cytotoxicity of CCY-1a-E2. The cells were
treated with 0, 1, 2.5, 5 and 10 µM of CCY-1a-E2 for 24 h,
and the viable cell number was measured by trypan blue dye
exclusion assay. CCY-1a-E2 dose-dependently decreased the viability
of the HL-60, K562, KG-1 and KG-1a cells. Notaby, HL-60 cells were
more sensitive to CCY-1a-E2 than the three other cell lines
(Fig. 1A). In addition, the
inhibitory effect of CCY-1a-E2 on the proliferation of HL-60 cells
was time-dependent (Fig. 1B), and
the half maximal inhibitory concentration (IC50) value
for the 48-h treatment of CCY-1a-E2 in the HL-60 cell line was
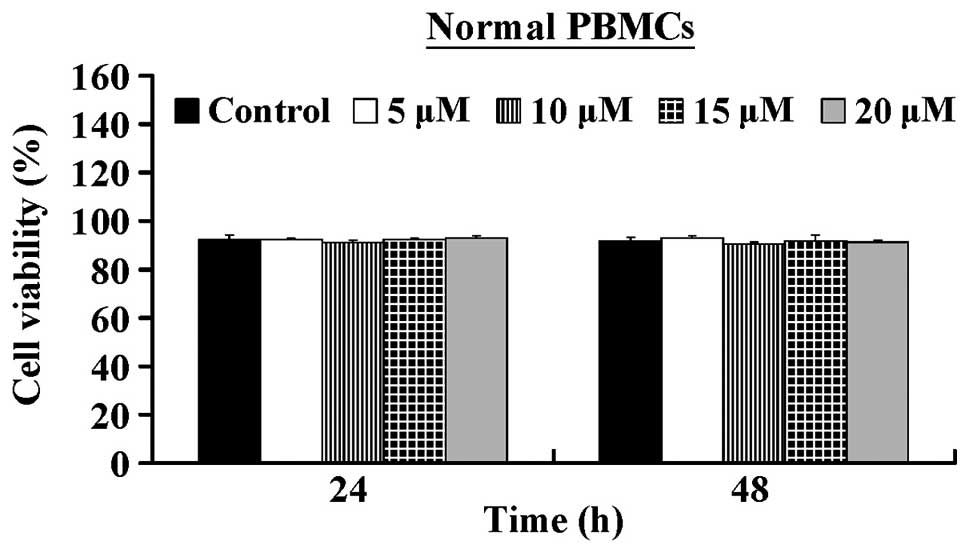
5.32±0.25 µM. In contrast, CCY-1a-E2 exerted low
cytotoxicity against normal human PBMCs (Fig. 2). Our results suggest that CCY-1a-E2
exhibits anti-leukemia action against HL-60 cells in
vitro.
CCY-1a-E2 causes G2/M phase arrest in
HL-60 cells
To explore the effect of CCY-1a-E2 on cell cycle
distribution, the cells were treated for various time periods with
CCY-1a-E2. The percentage of treated cells in phase G1, S and G2/M
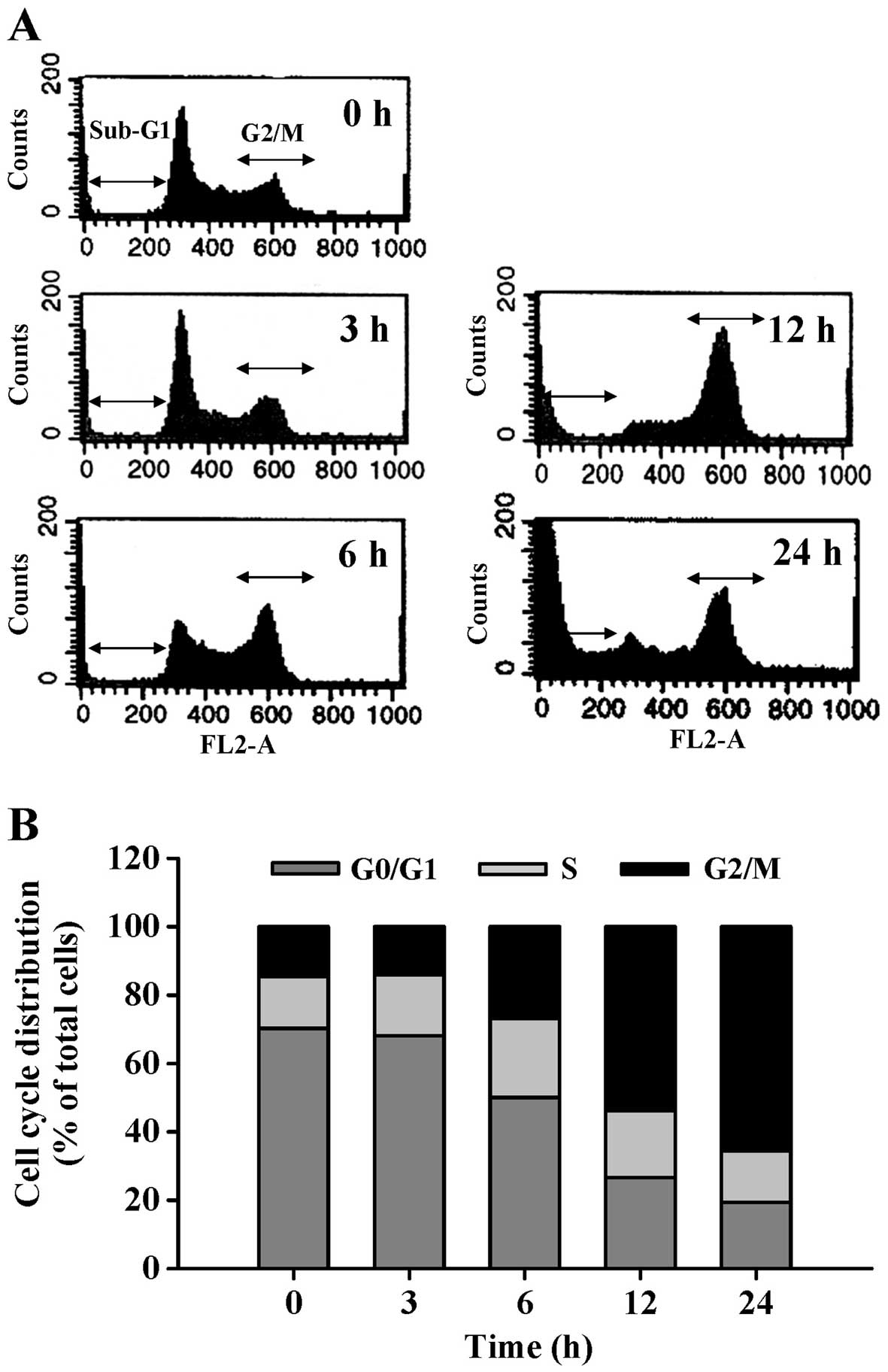
was detected by DNA content stained with PI. CCY-1a-E2 induced G2/M
phase arrest and increased the sub-G1 population (apoptotic cells)
(Fig. 3A). Exposure of HL-60 cells
to 5 µM CCY-1a-E2 for 6, 12 and 24 h resulted in a
significant increase in the percentage of cells in the G2/M phase,
while a marked decrease in the percentage of cells in the G0/G1
phase was observed (Fig. 3B). To
examine the protein levels associated with the G2/M phase,
CCY-1a-E2-treated cells were analyzed by immunoblotting. The
protein expression levels of cyclin B, CDK1, cdc25C were increased.
However, after 6 hours of treatment, cdc25C protein level gradually
decreased, but p21 protein level significantly increased (Fig. 4). These findings suggest that
CCY-1a-E2 regulated CDK1 activation and resulted in G2/M phase
arrest in the HL-60 cells.
CCY-1a-E2 triggers apoptotic death in
HL-60 cells
In Fig. 3, the
percentage of cells in the sub-G1 phase (apoptotic cells) was
increased after CCY-1a-E2 exposure. To evaluate whether CCY-1a-E2
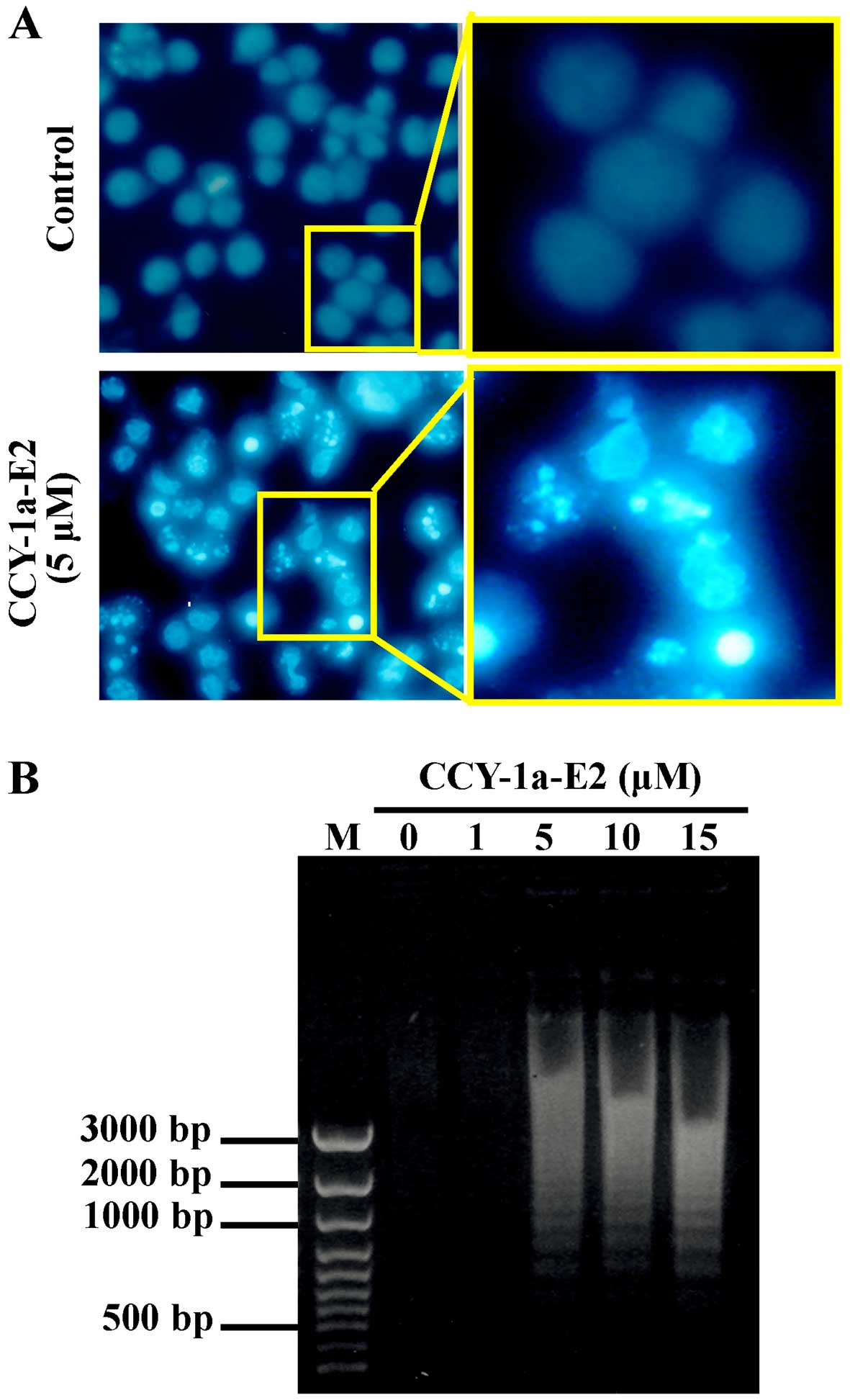
induces apoptosis, we performed DAPI staining for chromatin
condensation and a DNA fragmentation assay. The number of cells
with brighter cell nuclei were elevated in the CCY-1a-E2-treated
cells (Fig. 5A). In addition, DNA
was extracted from the cells following treatment with various
concentrations of CCY-1a-E2 for 24 h, and subjected to agarose gel
electrophoresis. DNA ladder was observed in samples treated with 5,
10 and 15 µM CCY-1a-E2 (Fig.
5B). Our results imply that CCY-1a-E2 treatment induced
apoptosis in the HL-60 cells.
CCY-1a-E2 enhances caspase-9, -8 and -3
activities in HL-60 cells
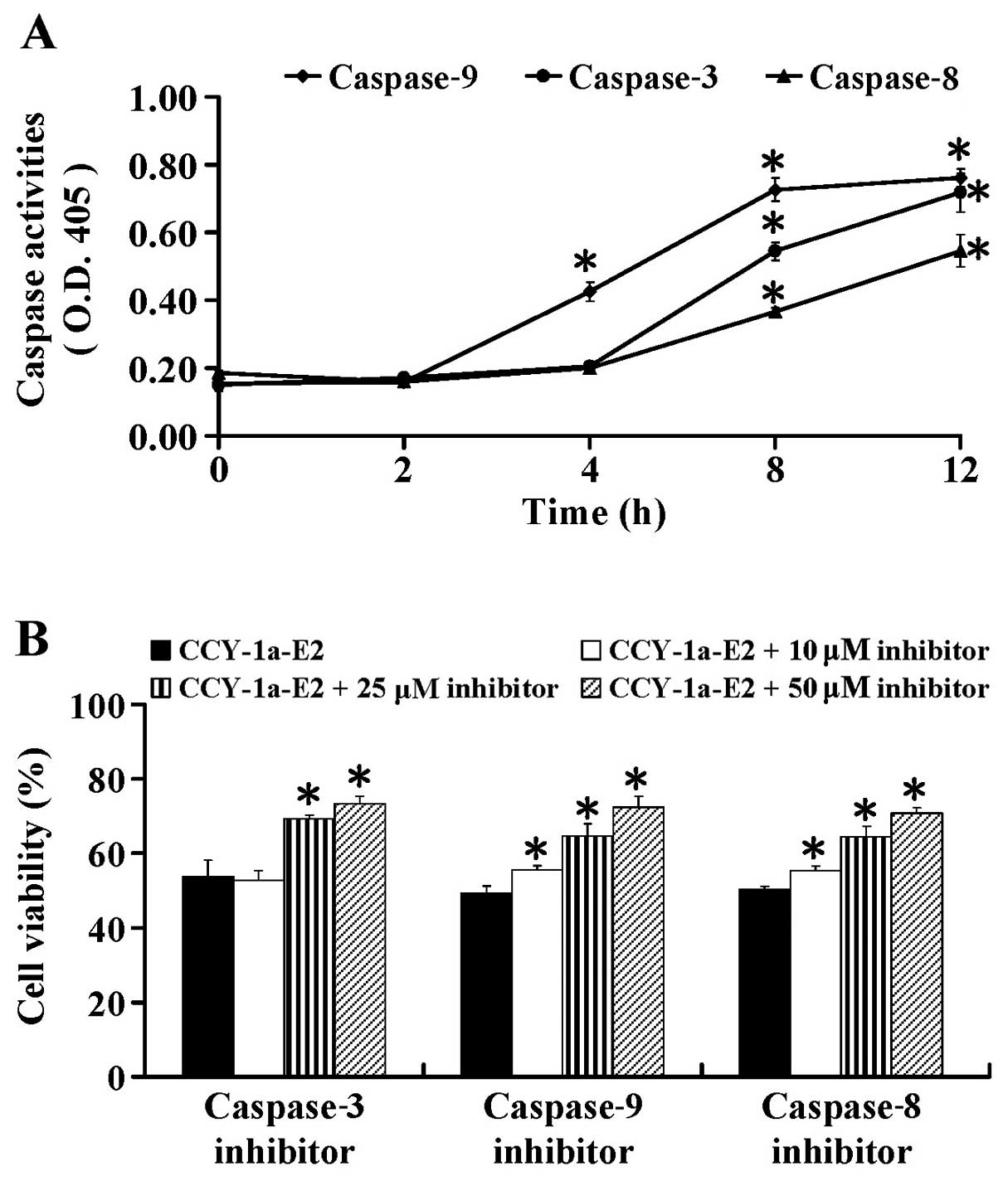
To further ascertain whether CCY-1a-E2-induced
apoptosis is caspase-dependent, we performed specific caspase
activity assays. HL-60 cells were exposed to 5 µM CCY-1a-E2
for different time periods. We found that CCY-1a-E2 enhanced the
activities of caspase-9, -3 and -8 (Fig. 6A) after 2, 4, 8 and 12 h of
exposure. To confirm the role of caspase-mediated apoptosis by
CCY-1a-E2, the cells were pre-treated with specific caspase
inhibitors. Our data showed that Z-IETD-FMK, Z-LEHD-FMK and
Z-DEVE-FMK at 10, 25 and 50 µM concentration-dependently
suppressed CCY-1a-E2-reduced cell viability (Fig. 6B). These data indicated that
CCY-1a-E2-induced apoptosis was mediated via both extrinsic and
intrinsic signaling in the HL-60 cells.
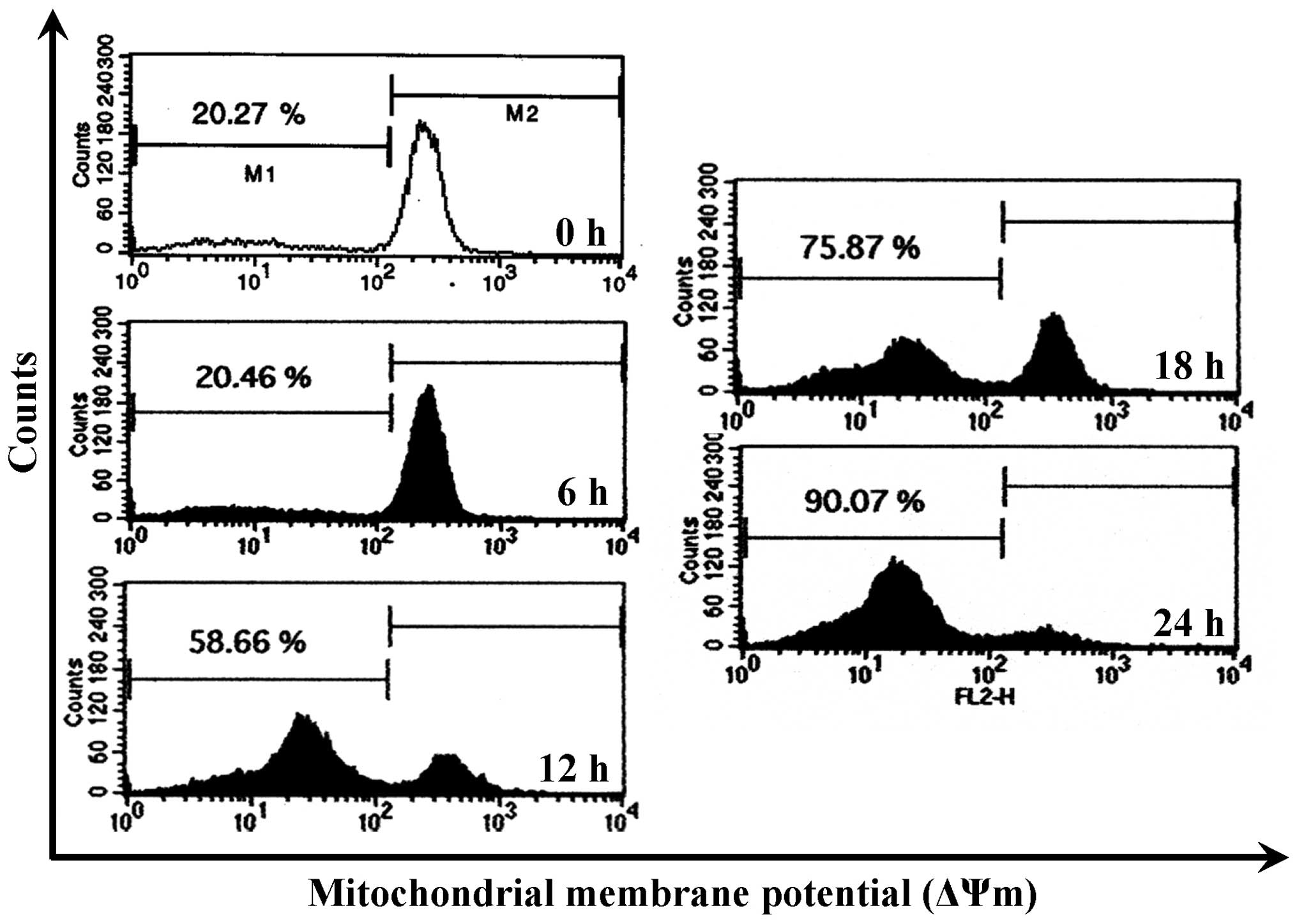
CCY-1a-E2 collapses ΔΨm and alters
mitochondria-mediated apoptosis signaling in HL-60 cells
To confirm whether the CCY-1a-E2-provoked apoptosis
was mediated via the mitochondrial pathway, the level of ΔΨm was
measured and immunoblotting was carried out. CCY-1a-E2 treatment
led to a decrease in ΔΨm in a time-course pattern (Fig. 7). CCY-1a-E2 also promoted the
protein expression of cytochrome c, Bax and PARP, while it
suppressed the level of Bcl-2 (Fig.
8). Additionally, activation of Fas/CD95 protein occurred in
the treated cells (Fig. 8).
Therefore, these results conclude that CCY-1a-E2-mediated apoptosis
of HL-60 cells was carried out through the mitochondria- and death
receptor-dependent pathway.
Discussion
2-Benzyloxybenzaldehyde (CCY-1a) has been shown to
inhibit superoxide anion generation via Akt inactivation and
phospholipase D activation in rat neutrophils (21). In addition, 2-benzyloxybenzaldehyde
can block Ca2+ entry and suppress formyl
peptide-stimulated increase in intracellular Ca2+ in
neutrophils (28). Pan et al
(29) demonstrated that
2-benzy-loxybenzaldehyde modulates vascular smooth muscle cell
proliferation by blocking the Ras/p42/44 MAPK pathway and
inhibiting NF-κB and AP-1 DNA binding activities. Our previous
study showed that CCY-1a-E2 reduced the percentage of viable murine
leukemia WEHI-3 cells, and the IC50 value of CCY-1a-E2
was 5 µM for a 24-h treatment (19). To evaluate the anticancer effect of
CCY-1a-E2 on other leukemia cell lines, we treated HL-60, K562,
KG-1 and KG-1a cells with various concentrations of CCY-1a-E2. A
significant concentration-dependent decrease of cell viability was
observed in all cell lines after 24 h exposure to 2.5-10 µM
of CCY-1a-E2. CCY-1a-E2 not only decreased the cell viability in
HL-60 cells, but also exerted low cytotoxicity in PBMCs (Figs. 1 and 2). The clinical applicability of an
anti-leukemia drug depends on its being distinct in both its
potency and therapeutic index between leukemia and normal blood
cells (6). Our result was in
agreement with the previous study of CCY-1a-E2 on reducing
viability of WEHI-3 cells (19).
Overall, CCY-1a-E2 represents a promising candidate as an
anticancer agent for leukemia due to its low toxicity to normal
cells.
CCY-1a-E2 significantly inhibited cell viability and
induced cell apoptosis in the HL-60 cells (Figs. 1 and 3). However, the molecular mechanisms of
its anti-leukemia activity remain unknown. Our data demonstrated
that CCY-1a-E2 triggered cell cycle arrest at the G2/M phase after
a 6-h exposure, while its effect on the sub-G1 cell population
appeared following a 12-h treatment (Fig. 3A). This result indicated that
CCY-1a-E2-induced G2/M phase arrest occurred before the onset of
apoptosis. The CDK1/cyclin B complex is one of the main regulators,
resulting in G2/M progression and apoptosis. G2/M checkpoints can
be exerted by inactivating cdc25C and activating CDK inhibitor
(p21waf/cip), which subsequently inactivates CDK1, and prevents
cells from entering mitosis (9,12). In
the present study, we investigated the expression levels of G2/M
phase-related proteins. Our results demonstrated that CCY-1a-E2
induced the expression of cyclin B, CDK1, cdc25C and p21 in a
time-dependent manner (Fig. 4). Our
finding implicates a novel role for the 2-benzyloxybenzaldehyde
derivative.
Cells undergo apoptosis in response to cell
death-inducing signals from death receptors on the cell surface
[such as Fas, death receptor 4 (DR4) or DR5], mitochondria, or
endoplasmic reticulum (ER) stress (30–32).
During apoptosis, caspases are activated and arranged in a
proteolytic cascade to convey the apoptotic signal (17,18).
Apoptotic evidence of chromatin condensation and DNA fragmentation
was observed in the CCY-1a-E2-treated HL-60 cells, indicating that
cells underwent apoptosis in response to CCY-1a-E2 treatment
(Fig. 5). Moreover, induction of
caspase-9, -8 and -3 activities (Fig.
6) as well as the specific cleavage of PARP (Fig. 8) were detected in the
CCY-1a-E2-treated cells. Application of the intrinsic and extrinsic
caspase specific inhibitors blocked the CCY-1a-E2-reduced cell
viability (Fig. 6). This result
further confirmed the effect of CCY-1a-E2 on apoptotic pathways.
The caspase-independent factors such as apoptosis-inducing factor
(AIF) and endonuclease G (Endo G) can be released from mitochondria
into the cytosol (33,34). Our results fail to exclude the
possibility of a caspase-independent pathway involved in the
CCY-1a-E2-induced apoptosis.
Loss of ΔΨm is one of the features of apoptosis
(35,36). Indeed, we detected a significant
loss of ΔΨm in the CCY-1a-E2-treated HL-60 cells (Fig. 7). Moreover, cytochrome c
release is related to the change in Bcl-2 family proteins during
cell apoptosis. Bcl-2 and Bax are located in the mitochondrial
outer membrane, and their ratio (Bcl-2/Bax) regulates the release
of apoptotic elements (cytochrome c, pro-caspase-9, AIF and
Endo G) to the cytosol (18,31,35).
Our data indicated that the protein expression levels of cytochrome
c, Bax and PARP (p85) were increased, whereas the protein
expression level of Bcl-2 was decreased in the CCY-1a-E2-treated
HL-60 cells (Fig. 8). Our results
are consistent with these available observations as evidenced by
the upregulation of Bax protein and the downregulation of Bcl-2
protein in the CCY-1a-E2-treated cells. These results suggest that
the activation of the caspase cascade contributed to
CCY-1a-E2-induced apoptosis of HL-60 cells.
In conclusion, we demonstrated that CCY-1a-E2 exerts
cytotoxic activity against leukemia cells, while it is less toxic
to normal PBMCs. CCY-1a-E2 induced G2/M phase arrest followed by
caspase-mediated apoptosis in the human leukemia HL-60 cells. Taken
together, these findings provide important new insights into the
possible molecular mechanisms of the anti-leukemia activity of
CCY-1a-E2.
References
|
1
|
Riether C, Schürch CM and Ochsenbein AF:
Regulation of hematopoietic and leukemic stem cells by the immune
system. Cell Death Differ. 22:187–198. 2015. View Article : Google Scholar :
|
|
2
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Lv M, Zhao X and Zhang J:
Developing a novel indolocarbazole as histone deacetylases
inhibitor against leukemia cell lines. J Anal Methods Chem.
2015:6750532015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maioral MF, Gaspar PC, Rosa Souza GR,
Mascarello A, Chiaradia LD, Licínio MA, Moraes AC, Yunes RA, Nunes
RJ and Santos-Silva MC: Apoptotic events induced by synthetic
naphthylchalcones in human acute leukemia cell lines. Biochimie.
95:866–874. 2013. View Article : Google Scholar
|
|
5
|
Padma VV: An overview of targeted cancer
therapy. Biomedicine (Taipei). 5:192015. View Article : Google Scholar
|
|
6
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Timofeev O, Cizmecioglu O, Settele F,
Kempf T and Hoffmann I: Cdc25 phosphatases are required for timely
assembly of CDK1-cyclin B at the G2/M transition. J Biol Chem.
285:16978–16990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castedo M, Perfettini JL, Roumier T and
Kroemer G: Cyclin-dependent kinase-1: Linking apoptosis to cell
cycle and mitotic catastrophe. Cell Death Differ. 9:1287–1293.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang JS, Hour MJ, Kuo SC, Huang LJ and Lee
MR: Selective induction of G2/M arrest and apoptosis in HL-60 by a
potent anticancer agent, HMJ-38. Anticancer Res. 24:1769–1778.
2004.PubMed/NCBI
|
|
12
|
Elsayed YA and Sausville EA: Selected
novel anticancer treatments targeting cell signaling proteins.
Oncologist. 6:517–537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: An emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
14
|
Chan KS, Koh CG and Li HY:
Mitosis-targeted anti-cancer therapies: Where they stand. Cell
Death Dis. 3:e4112012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung JG, Yang JS, Huang LJ, Lee FY, Teng
CM, Tsai SC, Lin KL, Wang SF and Kuo SC: Proteomic approach to
studying the cytotoxicity of YC-1 on U937 leukemia cells and
antileukemia activity in orthotopic model of leukemia mice.
Proteomics. 7:3305–3317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanjiv K, Su TL, Suman S, Kakadiya R, Lai
TC, Wang HY, Hsiao M and Lee TC: The novel DNA alkylating agent
BO-1090 suppresses the growth of human oral cavity cancer in
xenografted and orthotopic mouse models. Int J Cancer.
130:1440–1450. 2012. View Article : Google Scholar
|
|
19
|
Lin C, Yang JS, Tsai SC, Lin CF and Lee
MR: In vivo evaluation of the synthesized novel
2-benzyloxybenzaldehyde analog CCY-1a-E2 for the treatment of
leukemia in the BALB/c mouse WEHI-3 allograft model. Oncol Lett.
5:777–782. 2013.PubMed/NCBI
|
|
20
|
Chang C, Kuo S, Lin Y, Wang J and Huang L:
Benzyloxybenzaldehyde analogues as novel adenylyl cyclase
activators. Bioorg Med Chem Lett. 11:1971–1974. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JP, Chang LC, Hsu MF, Huang LJ and
Kuo SC: 2-Benzyloxy-benzaldehyde inhibits
formyl-methionyl-leucyl-phenylalanine stimulation of phospholipase
D activation in rat neutrophils. Biochim Biophys Acta. 1573:26–32.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Gibson Wood W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kao TC, Shyu MH and Yen GC:
Neuroprotective effects of glycyrrhizic acid and
18beta-glycyrrhetinic acid in PC12 cells via modulation of the
PI3K/Akt pathway. J Agric Food Chem. 57:754–761. 2009. View Article : Google Scholar
|
|
24
|
Lu CC, Yang SH, Hsia SM, Wu CH and Yen GC:
Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis
and liver fibrosis in vitro. J Funct Foods. 20:20–30. 2016.
View Article : Google Scholar
|
|
25
|
Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC,
Shieh TM, Wu TS, Tu MG, Chen MY and Yang JS: Curcumin-loaded
nanoparticles induce apoptotic cell death through regulation of the
function of MDR1 and reactive oxygen species in cisplatin-resistant
CAR human oral cancer cells. Int J Oncol. 43:1141–1150.
2013.PubMed/NCBI
|
|
26
|
Lin CC, Chuang YJ, Yu CC, Yang JS, Lu CC,
Chiang JH, Lin JP, Tang NY, Huang AC and Chung JG: Apigenin induces
apoptosis through mitochondrial dysfunction in U-2 OS human
osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth
in vivo. J Agric Food Chem. 60:11395–11402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CY, Chien YS, Chiu TH, Huang WW, Lu
CC, Chiang JH and Yang JS: Apoptosis triggered by vitexin in U937
human leukemia cells via a mitochondrial signaling pathway. Oncol
Rep. 28:1883–1888. 2012.PubMed/NCBI
|
|
28
|
Wang JP, Chang LC, Kuan YH, Tsao LT, Huang
LJ and Kuo SC: 2-Benzyloxybenzaldehyde inhibits formyl
peptide-stimulated increase in intracellular Ca2+ in
neutrophils mainly by blocking Ca2+ entry. Naunyn
Schmiedebergs Arch Pharmacol. 370:353–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan SL, Guh JH, Huang YW, Chang YL, Chang
CY, Huang LJ, Kuo SC and Teng CM: Inhibition of Ras-mediated cell
proliferation by benzyloxybenzaldehyde. J Biomed Sci. 9:622–630.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lavrik IN, Golks A and Krammer PH:
Caspases: Pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orrenius S: Reactive oxygen species in
mitochondria-mediated cell death. Drug Metab Rev. 39:443–455. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ho TF and Chang CC: A promising 'TRAIL' of
tanshinones for cancer therapy. Biomedicine (Taipei). 5:232015.
View Article : Google Scholar
|
|
33
|
Kuo HM, Tsai HC, Lin YL, Yang JS, Huang
AC, Yang MD, Hsu SC, Chung MC, Gibson Wood W and Chung JG:
Mitochondrial-dependent caspase activation pathway is involved in
baicalein-induced apoptosis in human hepatoma J5 cells. Int J
Oncol. 35:717–724. 2009.PubMed/NCBI
|
|
34
|
Strauss G, Westhoff MA, Fischer-Posovszky
P, Fulda S, Schanbacher M, Eckhoff SM, Stahnke K, Vahsen N, Kroemer
G and Debatin KM: 4-Hydroperoxy-cyclophosphamide mediates
caspase-independent T-cell apoptosis involving oxidative
stress-induced nuclear relocation of mitochondrial apoptogenic
factors AIF and EndoG. Cell Death Differ. 15:332–343. 2008.
View Article : Google Scholar
|
|
35
|
Tsai SC, Huang WW, Huang WC, Lu CC, Chiang
JH, Peng SF, Chung JG, Lin YH, Hsu YM, Amagaya S, et al:
ERK-modulated intrinsic signaling and G(2)/M phase arrest
contribute to the induction of apoptotic death by allyl
isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int
J Oncol. 41:2065–2072. 2012.PubMed/NCBI
|
|
36
|
Huang TT, Lin HC, Chen CC, Lu CC, Wei CF,
Wu TS, Liu FG and Lai HC: Resveratrol induces apoptosis of human
nasopharyngeal carcinoma cells via activation of multiple apoptotic
pathways. J Cell Physiol. 226:720–728. 2011. View Article : Google Scholar
|