Introduction
Gastric cancer (GC) is one of the most common types
of cancer worldwide. More than 70% of new cases and deaths occur in
developing countries, particularly in Eastern Asia and Europe, and
South America (1). In China, GC has
the second highest incidence among common types of cancers
(2). Although gastric carcinoma of
certain patients can be resected, these patients still have a poor
prognosis; their 5-year overall survival is only approximately
20–30% (3). Many patients with GC
have no chance for surgery since they were already in the advanced
or metastatic stage when their GC diagnosis was confirmed (4); the median survival time of these
patients is often no more than one year (5). Recurrence and metastasis are the main
reasons for the high mortality rate in patients with GC (6–9).
Although several anticancer drugs and different surgical modalities
have been presented, GC still cannot be cured, particularly in its
late stages. Thus, understanding the molecular mechanisms
underlying the pathogenesis of GC is important.
Ral-interacting protein of 76 kDa, also known as
RalBP1 (RLIP76) is a multifunctional protein. It is a member of the
Ras family. Ras is the downstream protein of many receptor
proteins, including vascular endothelial growth factor (VEGF)
(10) and the activation of Ras is
an important part in the pathogenesis of human malignant
carcinomas. Akt pathway plays an important role in signal
transduction from Ras. RLIP76 has a major role in endocytosis
(11,12), mitochondrial fission (13) and cell proliferation,
differentiation, apoptosis and migration (14) in normal and cancer cells. It is also
an important factor in the mechanisms of drug resistance since it
is capable of exporting the GSH-conjugates of alkylating
chemotherapy agents, such as melphalan, as well as those of
non-alkylating drugs, such as doxorubicin and vinorelbine. It
participates in the formation of multi-functional protein
complexes, including the mitotic spindle and the receptor signaling
complexes of EGF, TGF-β, insulin and clathrin-dependent endocytosis
(11,15,16)
and determines the rate of receptor-ligand signaling.
Many human tissues express RLIP76, including liver,
heart, ovary, lungs, muscles and kidneys. However, in multiple
cancers, such as melanomas and lung and ovarian carcinomas, RLIP76
is overexpressed (17–19). In mammary tumors, overexpression of
RLIP76 is positively related with advanced tumor grade and
negatively related with survival (20,21).
Blocking RLIP76 with targeting antibodies or antisense RNA is
related to increasing sensitivity to chemotherapy and radiation,
and causes pronounced tumor regression in non-small cell lung and
colon carcinomas (21), prostate
cancer (20) and B16 melanomas
(22) in mice, and leads to
apoptosis in wide varieties of histologic types of cancers in cell
culture including melanoma, prostate cancer, non-small-cell lung
cancer, small-cell lung, ovarian and colon cancer, lymphoma and
myeloid leukemia (22–29). These data suggest that therapeutic
strategies that target RLIP76 may provide a broad-spectrum
anti-neoplastic approach. However, functions of RLIP76 in GC remain
unknown.
In the present study, we detected RLIP76 expression
in GC tissues and reduced RLIP76 expression in SGC-7901 and MGC-803
cells to explore the role of RLIP76 on cell apoptosis, angiogenesis
and growth in vitro. Results of the present studies indicate
that the suppression of the RLIP76 gene by shRNA can inhibit
proliferation, induce apoptosis, reduce angiogenesis, and suppress
the invasiveness of GC cells. It suggests that RLIP76 may be a
potential candidate for the improvement of the therapeutic outcomes
of patients with GC.
Materials and methods
Cell culture
The GC cell lines SGC-7901 and MGC-803 used in the
present study were obtained from the Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), penicillin G (100
U/ml) and streptomycin (100 µg/ml), and were maintained in
monolayer culture at 37°C in humidified air with 5%
CO2.
Detection of cell viability by CCK-8
assay
The transfected GC cells were seeded into 96-well
plates (Corning Inc., Corning, New York, NY, USA) at a density of
3,000 and 5,000 cells/well for SGC-7901 and MGC-803 cells,
respectively, each well contained medium supplemented with 10% FBS.
The cultures were stained using a Cell Counting Kit-8 (CCK-8;
Beyotime Institute of Biotechnology, Haimen, Jiangsu, China) at
various time points. Briefly, 20 µl of CCK-8 solution was
added to each well, and then the solution was incubated for 4 h at
37°C. Each solution was then measured by spectrophotometry at 450
nm in a Multiskan Ascent microplate reader (Thermo Fisher
Scientific Oy, Fl-01620 Vantaa, Finland).
Colony formation assay
Cells (n=500) were seeded into 60-mm plates and were
allowed to attach overnight. The cells were incubated for 10 days
in complete medium for colony formation. The colonies formed were
washed with phosphate-buffered saline (PBS), fixed with 4%
hematoxylin and stained with crystal violet. The colonies were
counted and compared with the controls.
Hoechst 33342 staining analysis
The transfected GC cells were seeded into 6-well
plates and were incubated at 37°C. After 24 h, the cells were
stained with 0.1 µg/ml Hoechst 33342 (Sigma) for 5 min and
were then observed by fluorescence microscopy using the appropriate
filter for blue fluorescence.
Transwell assay
Cell migration and invasion were detected by
Transwell methods. In the migration assay, cells were seeded in the
upper chambers in serum-free media without a Matrigel membrane. In
contrast, the lower chambers were loaded with RPMI-1640 medium
supplemented with 10% FBS. After 24 or 36 h respectively, the
SGC-7901 or MGC-803 cells in the upper chambers that had not
migrated were removed by a cotton swab. In the invasion assay, GC
cells (2×105) were seeded in the upper chambers in
serum-free media with the Matrigel (BD) membrane, whereas the lower
chambers were loaded with RPMI-1640 medium supplemented with 10%
FBS. After 36 or 48 h respectively, the cells in the upper chambers
that had not migrated were removed by a cotton swab. Cells were
fixed with 4% polyoxymethylene. The total number of cancer cells
was counted after they were fixed and stained with 4%
hematoxylin.
Western blotting
Cells were washed with balanced salt solution (138
mmol/l NaCl, 5 mmol/l KCl, 0.3 mmol/l KH2PO4,
0.3 mmol/l Na2HPO4, 4 mmol/l
NaHCO3 and 5.6 mmol/l glucose, pH 7.4). The washed cells
were lysed by buffer containing 50 mM Tris-HCl, pH 7.6, 150 mM
NaCl, 0.1% sodium dodecylsulfate, 1% Nonidet P-40 and 0.5%
sodium-deoxycholate, 0.1 mmol/l phenylmethylsulfonyl fluoride
(PMSF) and protease inhibitor. The lysates were cooled with ice for
30 min, and then centrifuged at 4°C at 12,000 × g for 30 min.
Proteins in the collected supernatant were separated by SDS-PAGE on
10 or 8% gels, and then transferred to polyvinylidene fluoride
(PVDF) membranes. The membrane, after a block with 10% skim milk
was incubated with antibodies to RLIP76. The detection of β-actin
(1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) on the
same membrane was used as the loading control. Specific antibodies
for RLIP76 (ab133549, monoclonal, 1/10,000-1/50,000; Abcam,
Cambridge, UK), caspase-3 (#9662, 1:1,000, polyclonal), Akt (#4691,
1:1,000, monoclonal), phosphorylated Akt (p-Akt) (#4060, 1:2,000,
monoclonal), mTOR (#2983, 1:1,000, monoclonal), phosphorylated mTOR
(p-mTOR) (#5536, 1:1,000, monoclonal), caspase-8 (#9746, 1:1,000,
monoclonal), caspase-9 (#9508, 1:1,000, monoclonal), PARP (#9542,
1:1,000, monoclonal) (all from Cell Signaling Technology, Bedford,
MA, USA) were used for the immunodetection of the corresponding
proteins. HRP-conjugated secondary antibodies (1:10,000; Zhongshan
Golden Bridge Biotechnology, Beijing, China), followed by enhanced
chemiluminescence (Millipore Corp., Billerica, MA, USA), were
used.
Patients and specimen selection
Paraffin-embedded pathological specimens were
obtained from the archives of the Department of Pathology of
Shandong Province Hospital Affiliated to Shandong University (P.R.
China) between January 2013 and January 2014. In all, 76 samples of
GC were obtained. In addition, 40 samples of normal gastric
epithelial tissues that were obtained from patients who underwent
surgery for gastric polyps were included in the present study. The
patients were aged 28–70 years (median, 46 years). The consent
procedure and study protocol were approved by the Medical Ethics
Committee, Shandong Provincial Hospital Affiliated to Shandong
University.
Lentiviral transfection and stable cell
line selection
Lentivirus that encoded RLIP76-specific shRNA and
the scrambled shRNA lentivirus were supplied by GenomeDitech Co.
(Shanghai, China). GC cells (SGC-7901 and MGC-803) were infected
with recombinant shRNA that was specific for RLIP76 lentiviral
stocks or scrambled shRNA lentiviral stocks, and stable cell clones
were selected after puromycin selection. Western blotting and
qRT-PCR were used to select RLIP76 knockdown cell lines (KD) and
control cell lines (NC). The lentiviral vector green fluorescent
protein (GFP) expressed in lentiviral vectors allowed for the
assessment of the infection efficiency.
Quantitative RT-PCR analysis
Total cellular RNA was isolated with TRIzol reagent
and was reverse transcribed to cDNA by M-MLV reverse transcriptase
(both from Takara, Otsu, Japan) according to the manufacturer's
protocol. qPCR products were detected with SYBR-Green in a
LightCycler® 480 Real-Time PCR system (Roche
Diagnostics, Indianapolis, IN, USA). The β-actin gene was amplified
as an internal normalization. The following primers for RLIP76 were
used: 5′-ggCATgAAgTgTgAAggCATCTAC-3′ and
5′-CTCgCAAATACTgCTTCAgCAAAC-3′.
Immunohistochemistry
RLIP76 protein expression was evaluated by the
streptavidin-peroxidase immunohistochemical method.
Paraffin-embedded surgical specimens were sequentially cut into
4-µm thick sections. Then, the sections were deparaffinized
and antigen retrieval was performed. Next, the sections were
incubated with hydrogen peroxide. Mouse monoclonal antibodies to
RLIP76 (ab56815, monoclonal; Abcam) were used at a dilution of
1:250 and incubated at 4°C overnight. PBS was used instead of the
primary antibody, which served as a negative control. Further
experimental steps were performed according to the instructions of
the secondary biotinylated antibody kit purchased from ZSGB Biotech
(Beijing, China).
Enzyme-linked immunosorbent assay
The transfected GC cells were seeded into 12-well
plates (2×105) and incubated at 37°C for 24 h. Then, the
culture medium was collected from the NC and KD cells. The medium
was then tested by ELISA to measure the level of VEGF protein,
which was performed according to the manufacturer's protocol.
Tube formation assay
Matrigel was thawed at 4°C overnight before the
experiment. A 96-well plate was coated with cold Matrigel and
incubated for 1 h at 37°C. Cell cultures were collected from GC
cells, and mixed with HUVEC, respectively. The mixture was added to
the wells and was incubated at 37°C with 5% CO2. The
cells were monitored every 2 h under a microscope for 6–12 h, and
tube formation was imaged at 8 h.
Statistical analysis
All data were evaluated with a two-tailed unpaired
Student's t-test or with a two-tailed paired Student's t-test. A
p-value <0.05 was considered to indicate a statistically
significant result.
Results
RLIP76 is overexpressed in GC
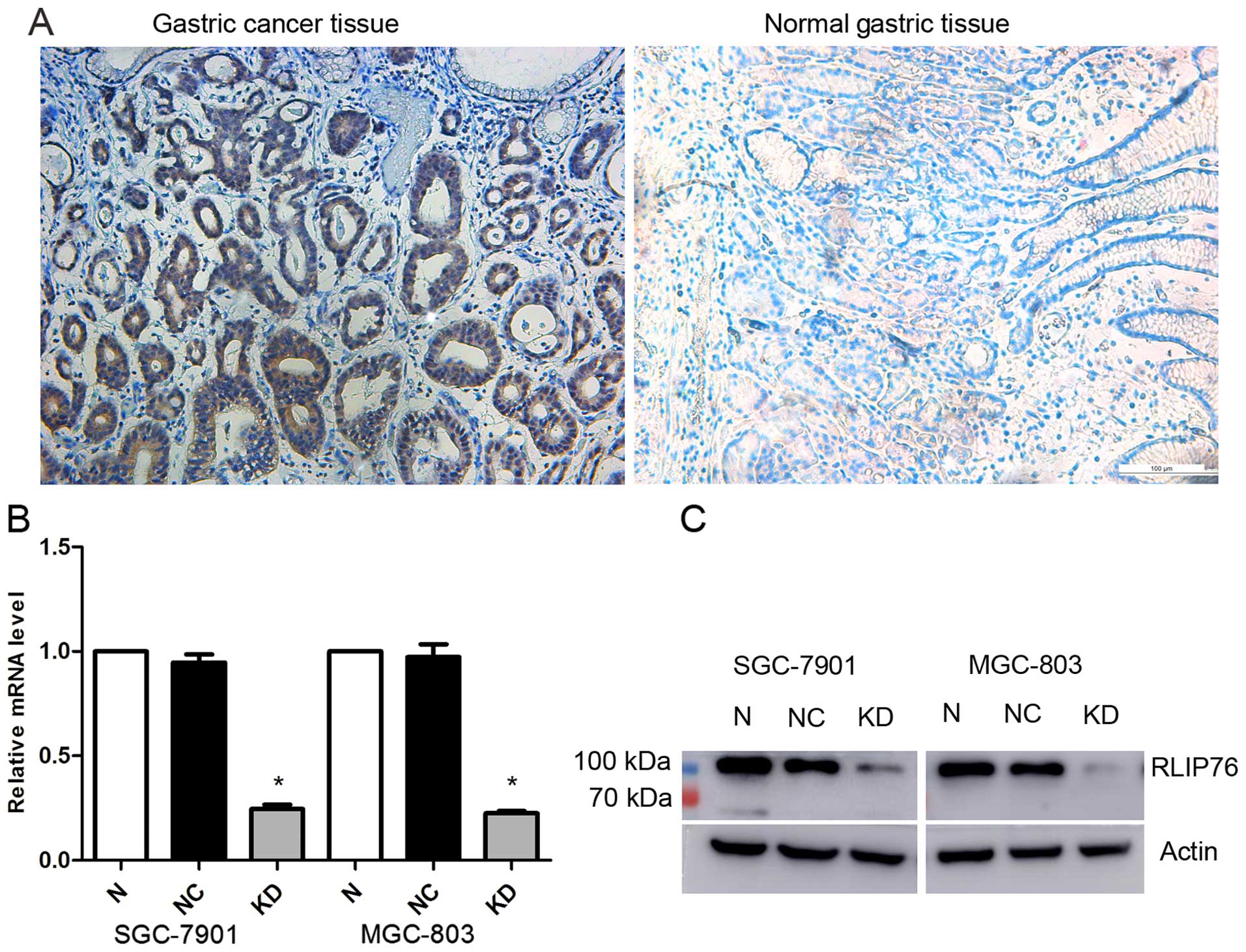
To examine RLIP76 expression in the GC tissues
included in the present study, we performed an immunohistochemical
analysis. In total, 76 samples were collected and incubated with
anti-RLIP76 IgG antibody. The results indicate relatively higher
expression of RLIP76 in tumor tissues than in normal tissues
(Fig. 1A).
shRNA decreases the RLIP76 expression in
human GC SGC-7901 and MGC-803 cell lines
To evaluate the effect of the knockdown of RLIP76 on
the biological behavior of GC cell lines, RNA interference vectors:
lentivirus encoding shRNA specific for RLIP76 (KD) and the control
(NC), were designed for specific interference of the endogenous
RLIP76 gene. RLIP76 expression was detected by qRT-PCR and western
blot analysis. As shown in Fig. 1B,
no significant difference of RLIP76 mRNA level was found between
the normal and the NC cells, but a significantly lower level of
RLIP76 mRNA expression in KD cells (p<0.05). The relative RLIP76
mRNA level reduced to 0.245722±0.021077 in KD SGC-7901 and
0.225389±0.00974 in KD MGC-803, respectively. Western blot analysis
also displayed a significant reduction in RLIP76 expression in both
transfected cell lines (Fig.
1B).
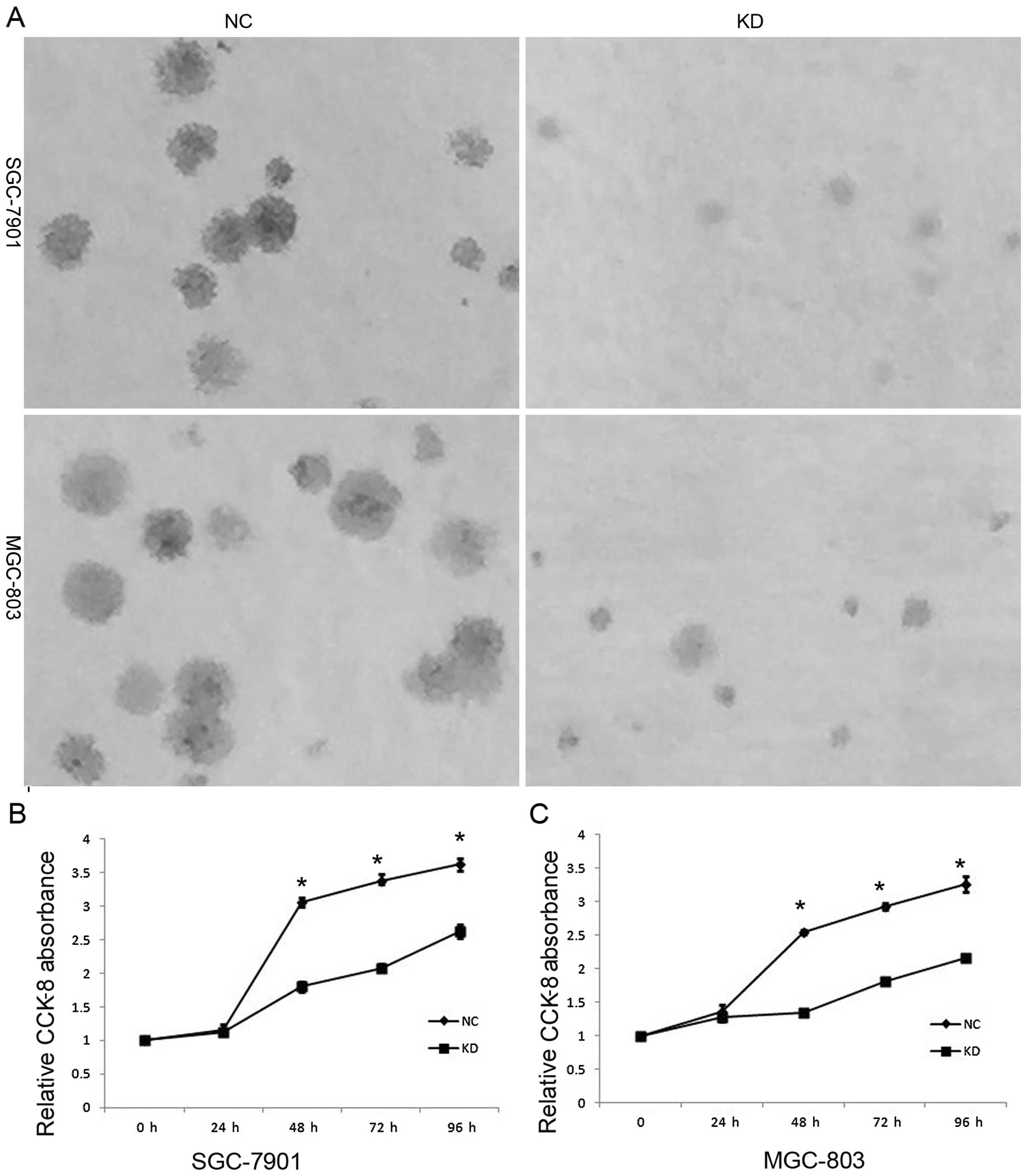
Knockdown of RLIP76 decreases cell
proliferation and increases apoptosis of GC cells through Akt/mTOR
signaling pathway
To investigate the biological function of RLIP76 in
the development and progression of GC, we performed a colony
formation and a CCK-8 assay after transfection of GC cells. The
SGC-7901 and MGC-803 cells that were transfected with RLIP76 shRNA
formed fewer and smaller colonies than the NC cells (Fig. 2A). As shown in Fig. 2B and C, the KD GC cells displayed
significant growth decrease compared with the NC cells after 24 h,
including 48, 72 and 96 h (p<0.05).
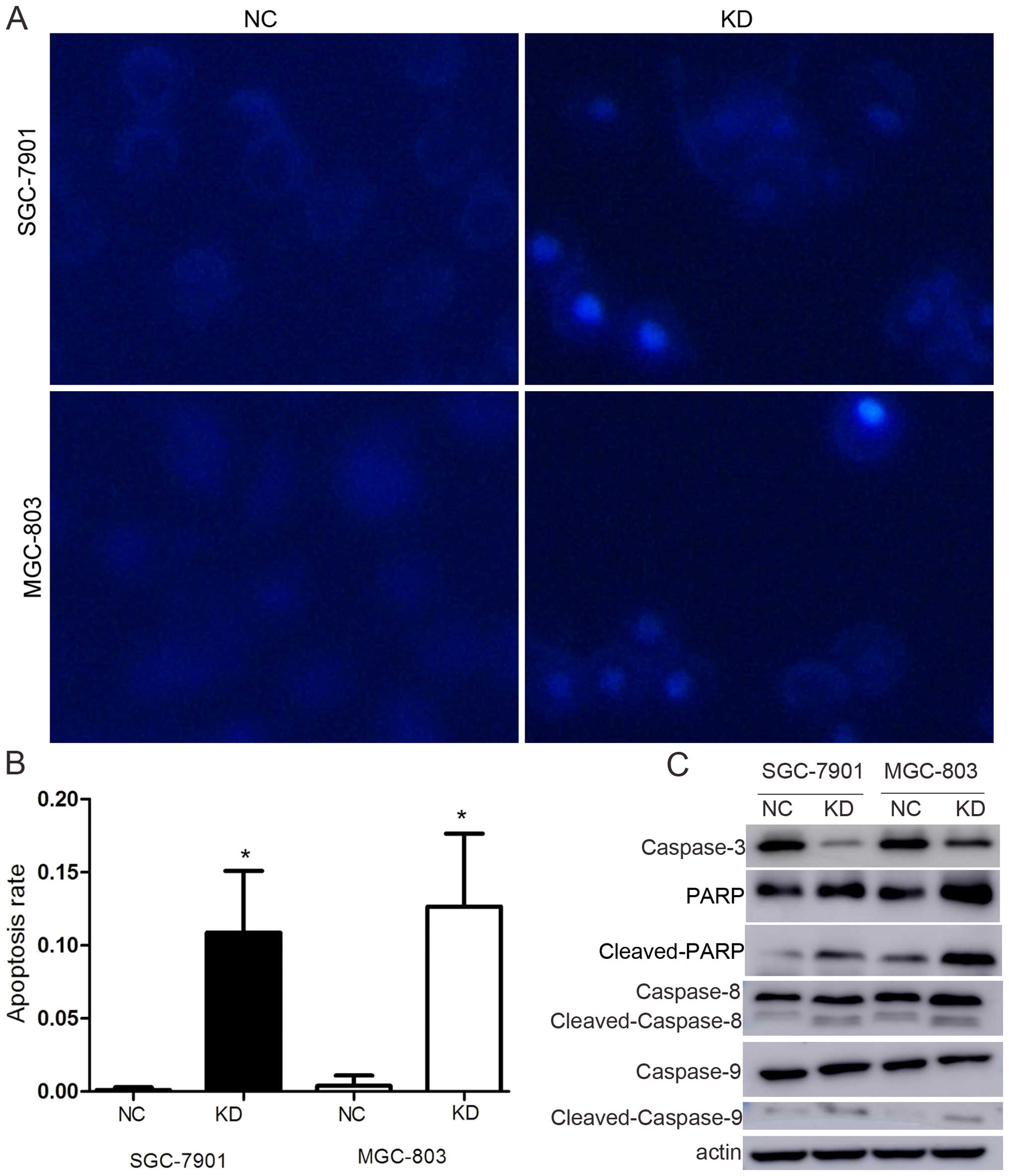
Apoptosis is one of the predominant types of
programmed cell death that involves a series of biochemical events
that lead to specific cell morphological changes, including cell
shrinkage, nuclear fragmentation, chromatin condensation and
chromosomal DNA fragmentation. As shown in Fig. 3A and B, the degrees of nuclear
fragmentation and chromatin condensation were greater in the KD
cells than in the NC cells in both cell lines. The apoptosis rate
increased from 0.11±0.18 to 10.87±4.2% in SGC-7901 and from
0.40±0.69 to 12.67±4.98% in MGC-803 (p<0.05).
To confirm the influence of RLIP76 knockdown
relative to apoptosis in GC cells, the expression of
apoptosis-associated proteins, such as caspase-3, PARP, caspase-8
and -9, were detected by western blotting. The results showed that
caspase-3 expression decreased, whereas cleaved PARP,
cleaved-caspase-8 and cleaved-caspase-9 were significantly
increased (Fig. 3C).
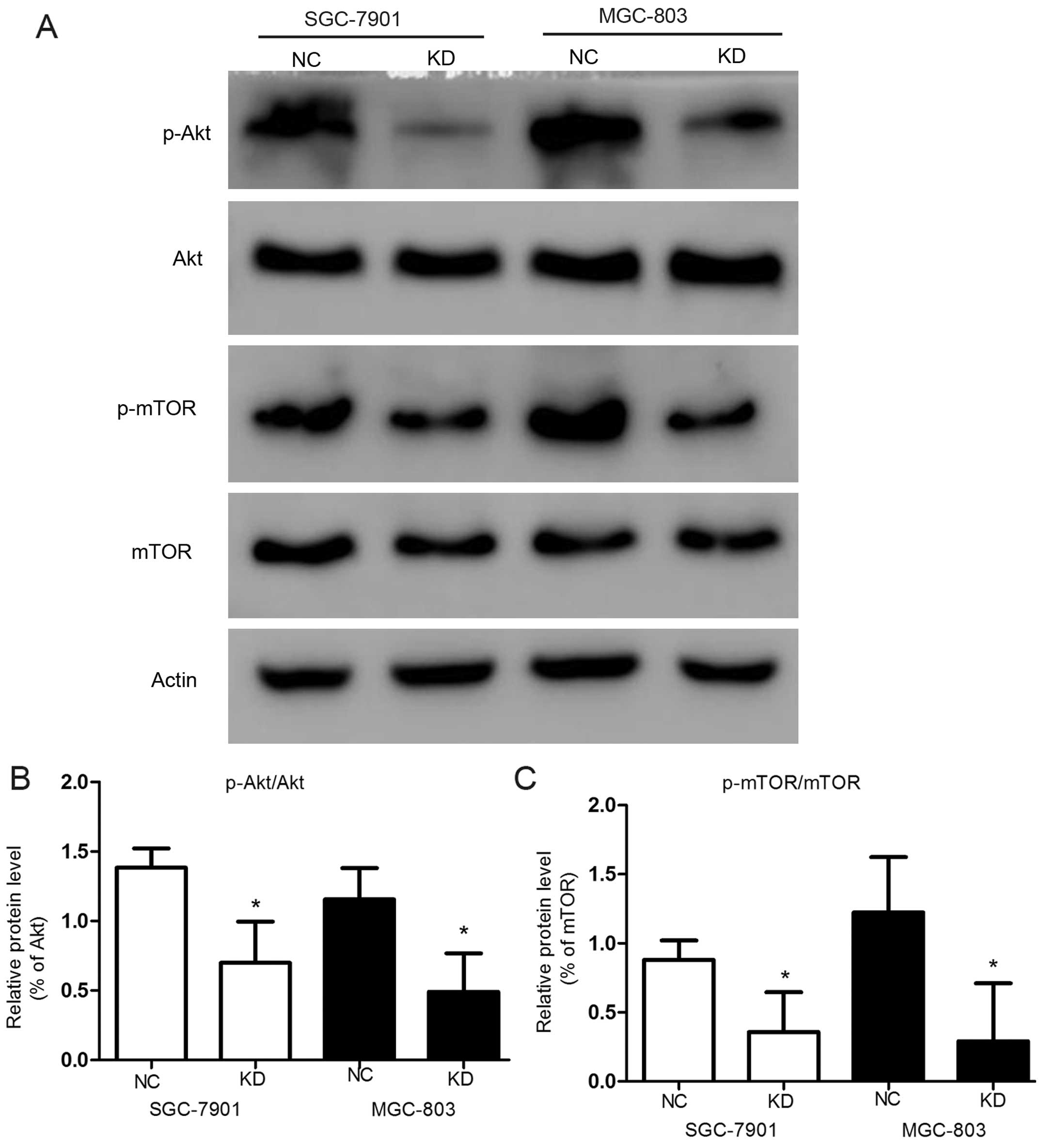
In contrast, in order to identify which signaling
pathway was associated with apoptosis, we analyzed the levels of
Akt and mTOR and their phosphorylation levels in the
transfected GC cells by western blot analysis (Fig. 4A). The knockdown of RLIP76
significantly reduced phosphorylation of Akt [from
138.45±13.8 to 69.9±29.7% in SGC-7901 (p<0.05), and from
115.5±26.6 to 49.07±27% in MGC-803 (p<0.05)] (Fig. 4B) and mTOR in both GC cell
lines significantly (Fig. 4C)
(p<0.05). The activation suppression of p-Akt leads to
further suppression of mTOR, which suppresses the expression
of pro-apoptotic Bim and favors the survival and proliferation of
cancer cells (30).
Knockdown of RLIP76 decreases migration
and invasion of GC cells
To assess the effect of RLIP76 knockdown on SGC-7901
and MGC-803 cell lines with respect to migration and invasion, we
performed an in vitro migration and invasion assay using NC
and KD GC cells. A decrease in cell migration and invasion was
observed in KD cells (Fig. 5),
which suggests that RLIP76 knockdown significantly suppressed
invasion and migration of GC cells in vitro (in migration
assay, SGC-7901, NC 486.7±128.8, KD 219.7±43.6; MGC-803, NC 630±95,
KD 333.7±46.5; in invasion assay, SGC-7901, NC 306±33.5, KD
97.7±24.3; MGC-803, NC 350±50.9, KD 163.3±87.5) (p<0.05).
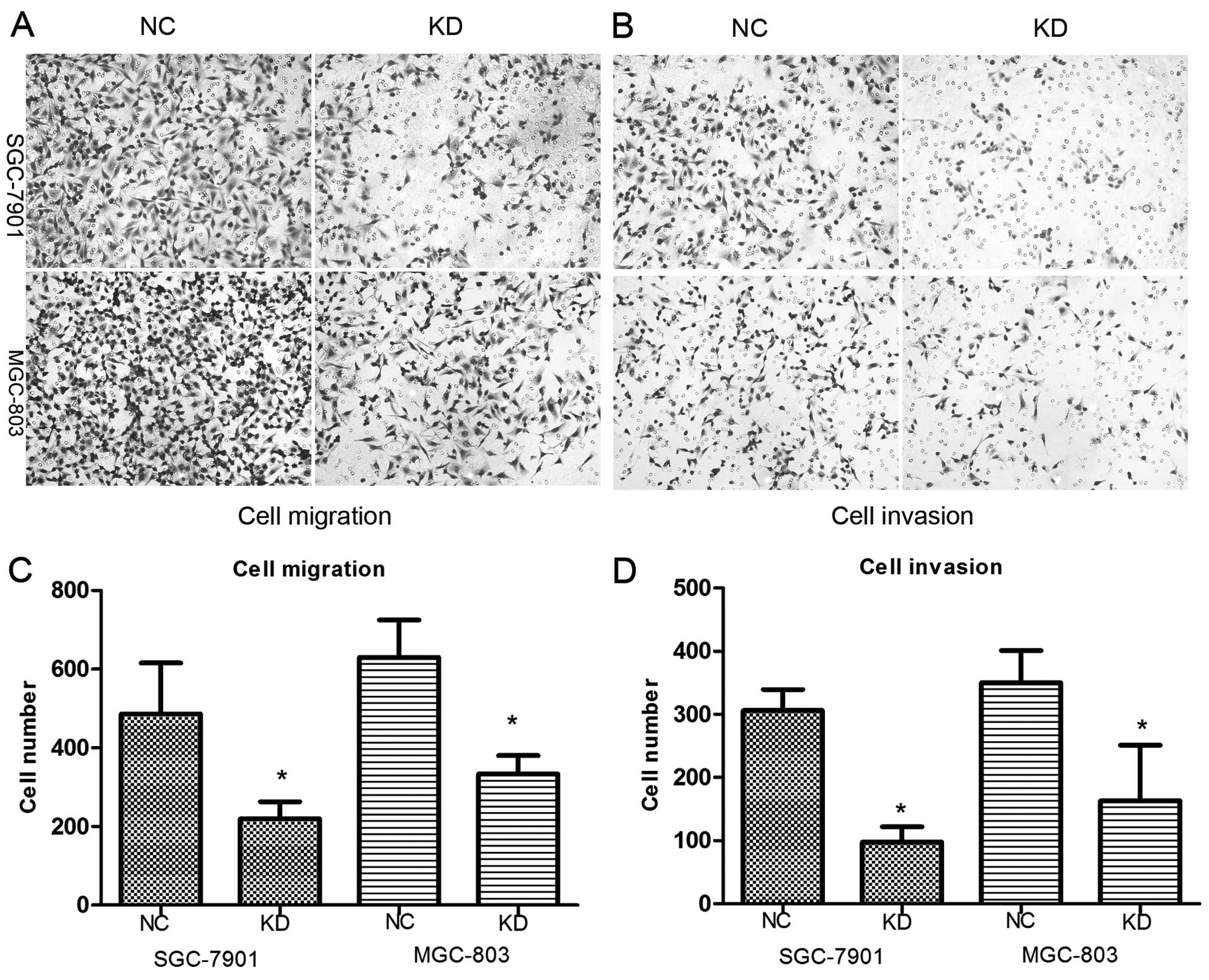 | Figure 5(A–D) Migration and invasion were
decreased after shRNA transfection. The number of cells that
migrated across the membrane with or without cold Matrigel
demonstrated that fewer KD cells can migrate compared with control
cells. In migration assay, SGC-7901, NC 486.7±128.8, KD 219.7±43.6;
MGC-803, NC 630±95, KD 333.7±46.5 (*p<0.05). In
invasion assay, SGC-7901, NC 306±33.5, KD 97.7±24.3; MGC-803, NC
350±50.9, KD 163.3±87.5) (*p<0.05). |
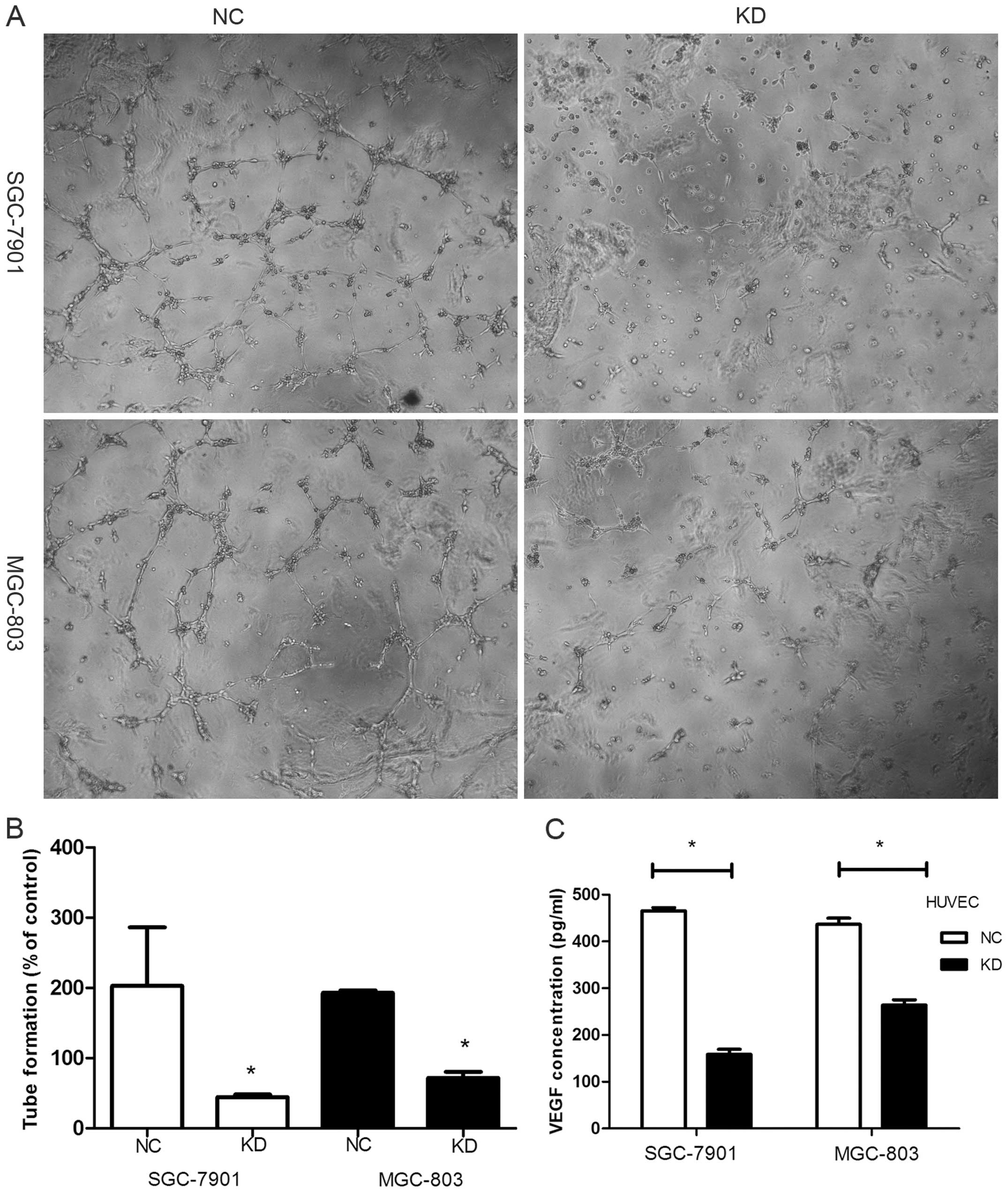
Knockdown of RLIP76 downregulates VEGF
secretion in GC cells
We observed that knockdown of RLIP76 reduced VEGF
section from 465±2.12 to 158.6 ±6.93 in SGC-7901 and from 463.5
±13.6 to 264 ±11.2 in MGC-803 (pg/ml) (Fig. 6C) (p<0.05). Tube formation by
endothelial cells, which serves as an in vitro measure of
angiogenesis, was assessed after HUVEC cells were incubated with
supernatant of GC cells for 8 h. Photomicrographs of cells showed
that tube formation was inhibited more strongly in the KD cell
lines (Fig. 6A). The average tube
length in supernatant of the KD cells was markedly shorter than
that in the NC cells (% of control) (Fig. 6B) [SGC-7901 NC, 202.8±83.3; KD,
44.5±3.69 (p<0.05); MGC-803 NC, 193±3.5; KD, 71.8±8.83
(p<0.05)].
Discussion
RLIP76 is overexpressed in a variety of solid
tumors, such as kidney and prostate cancer, among others. The
proposed mechanism of action for RLIP76-targeted therapy is
completely different from most current approaches, which mainly
concentrate on chemicals that modify kinases or phosphatases
(31). In the present study, we
determined that the expression and specific activity of the RLIP76
protein are relatively higher in gastric cancer (GC) tissues than
in normal tissues. The present study was designed to elucidate the
functional role and regulation of critical pro-survival signaling
pathways of RLIP76 in the biological activities of GC cells. shRNA
was used to suppress the RLIP76 expression and the connections
between RLIP76 and proliferation, apoptosis, cellular migration,
invasion and VEGF secretion were investigated. A significant
finding of the present study is that the knockdown of RLIP76
effectively suppresses the level of Akt phosphorylation,
decreases the expression of p-mTOR, and activates a sequence
of apoptotic signaling proteins. This may provide a collective
rationale for the effective regulation of the
Akt/mTOR pathway and the activation of apoptosis
following RLIP76 depletion.
In the present study, we found that the
downregulation of RLIP76 expression reduces the proliferation of GC
cells while increasing apoptosis, which is consistent with previous
studies. We performed colony formation assay and measured the
expression levels of caspase-3, -8 and -9 and PARP1 proteins. The
knockdown of RLIP76 decreased colony formation and increased the
expression of cleaved-caspase-3, cleaved-caspase-8,
cleaved-caspase-9 and cleaved-PARP at the protein level, which
implies a functional interaction between RLIP76 and caspase
pathways in GC. These data strongly support the conclusion that one
of the physiological functions of RLIP76 is to downregulate
apoptotic signaling. This process possibly occurs via the control
of intracellular levels of pro-apoptotic endogenous
lipid-peroxidation byproducts, followed by the catalysis of the
efflux of GS-E. 4HNE (and other alkenals) alone triggers apoptosis
(32–39), and the inhibition of RLIP76 has been
shown to increase the intracellular accumulation of 4HNE (40,41).
However, other potential RLIP76-mediated mechanisms for the
inhibition of apoptosis remain unclear. As RLIP76 is a member of
Ras family, Akt pathway may play an important role in signal
transduction. The present study showed a significant relationship
between the Akt/mTOR signaling pathway and RLIP76. The
decreased expression of p-Akt and p-mTOR after RLIP76
knockdown demonstrates that RLIP76 plays a vital role in regulating
GC cell apoptosis. RLIP76 has also been shown to exhibit GAP
activity toward cdc42. Activation of the Rho family G-protein,
cdc42, has been shown to induce apoptosis (42). It has been reported that
overexpression of POB1, which is the partner of RLIP76 triggers
apoptosis in prostate cancer cells (43). POB1 has been demonstrated to be a
specific inhibitor of the transport of DOX and GS-E by RLIP76. The
fact that the augmentation of POB1 also triggers apoptosis in the
absence of any chemotherapy drug in lung cancer cells has been
established. The inhibition of cellular RLIP76 via an increase in
POB1 also results in the increased accumulation and decreased
efflux of DOX, as well as significant sensitization to DOX
(44).
RLIP76 deletion in mice inhibiting angiogenesis in
xenografted tumors has been demonstrated. As Ras is the downstream
protein of VEGF, the relationship between RLIP76 and VEGF was
explored. In the present study, RLIP76 suppression decreased VEGF
section and VEGF-induced tube formation in vitro. Since VEGF
is important in tumor cells, such as regulating receptor surface
expression, it could influence the tumor cell function (45). PI3K has been shown involved
in VEGF secretion in many cells, and may be a major regulator of
VEGF secretion in angiogenic environments (46–49).
However, the mechanisms by which RLIP76 regulates VEGF secretion
remain to be explored.
In conclusion, the present study shows that RLIP76
is overexpressed in GC tissues and knockdown of RLIP76 in GC cells
significantly suppresses growth, decreases VEGF section and tube
formation, and increases apoptosis through Akt/mTOR
signaling pathway. Although further studies are needed, results of
the present study suggest that RLIP76 may be a potential therapy
target for GC treatment and may be used with conventional
therapeutics to increase treatment efficacy.
Acknowledgments
The present study was supported in part by grants
from the National Natural Science Foundation of China (81472685),
the Science and Technology Development Project of Shandong Province
(2013GSF11852), the Postdoctoral Innovation Project Special
Foundation of Shandong Province (201302031), the Major Science and
Technology Projects of Shandong Province (2015ZDXX0802A01) and the
Promotive Research Fund for Excellent Young and Middle-Aged
Scientists of Shandong Province (BS2014YY037). Thanks to Zhaoping
Li for statistical analysis.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
3
|
Quéro L, Guillerm S and Hennequin C:
Neoadjuvant or adjuvant therapy for gastric cancer. World J
Gastrointest Oncol. 7:102–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dassen AE, Lemmens VE, van de Poll-Franse
LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Vd Wurff AA, Bosscha K
and Coebergh JW: Trends in incidence, treatment and survival of
gastric adenocarcinoma between 1990 and 2007: A population-based
study in the Netherlands. Eur J Cancer. 46:1101–1110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oba K, Paoletti X, Bang YJ, Bleiberg H,
Burzykowski T, Fuse N, Michiels S, Morita S, Ohashi Y, Pignon JP,
et al GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research
International Collaboration) Group: Role of chemotherapy for
advanced/recurrent gastric cancer: An individual-patient-data
meta-analysis. Eur J Cancer. 49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buchner AM, Shahid MW, Heckman MG, Krishna
M, Ghabril M, Hasan M, Crook JE, Gomez V, Raimondo M, Woodward T,
et al: Comparison of probe-based confocal laser endomicroscopy with
virtual chromoendoscopy for classification of colon polyps.
Gastroenterology. 138:834–842. 2010. View Article : Google Scholar
|
|
7
|
Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, Yu T,
Chu CL, Zhang TG and Li YQ: Diagnostic value of confocal laser
endomicroscopy for gastric superficial cancerous lesions. Gut.
60:299–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanduleanu S, Driessen A, Gomez-Garcia E,
Hameeteman W, de Bruïne A and Masclee A: In vivo diagnosis and
classification of colorectal neoplasia by chromoendoscopy-guided
confocal laser endomicroscopy. Clin Gastroenterol Hepatol.
8:371–378. 2010. View Article : Google Scholar
|
|
9
|
Wallace M, Lauwers GY, Chen Y, Dekker E,
Fockens P, Sharma P and Meining A: Miami classification for
probe-based confocal laser endomicroscopy. Endoscopy. 43:882–891.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jullien-Flores V, Mahé Y, Mirey G,
Leprince C, Meunier-Bisceuil B, Sorkin A and Camonis JH, Gacon G
and Camonis JH: RLIP76, an effector of the GTPase Ral, interacts
with the AP2 complex: Involvement of the Ral pathway in receptor
endocytosis. J Cell Sci. 113:2837–2844. 2000.PubMed/NCBI
|
|
12
|
Nakashima S, Morinaka K, Koyama S, Ikeda
M, Kishida M, Okawa K, Iwamatsu A, Kishida S and Kikuchi A: Small G
protein Ral and its downstream molecules regulate endocytosis of
EGF and insulin receptors. EMBO J. 18:3629–3642. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kashatus DF, Lim KH, Brady DC, Pershing
NL, Cox AD and Counter CM: RALA and RALBP1 regulate mitochondrial
fission at mitosis. Nat Cell Biol. 13:1108–1115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldfinger LE, Ptak C, Jeffery ED,
Shabanowitz J, Hunt DF and Ginsberg MH: RLIP76 (RalBP1) is an R-Ras
effector that mediates adhesion-dependent Rac activation and cell
migration. J Cell Biol. 174:877–888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quaroni A and Paul EC: Cytocentrin is a
Ral-binding protein involved in the assembly and function of the
mitotic apparatus. J Cell Sci. 112:707–718. 1999.PubMed/NCBI
|
|
16
|
Awasthi S, Singhal SS, Sharma R, Zimniak P
and Awasthi YC: Transport of glutathione conjugates and
chemotherapeutic drugs by RLIP76 (RALBP1): A novel link between
G-protein and tyrosine kinase signaling and drug resistance. Int J
Cancer. 106:635–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Awasthi S, Singhal SS, Awasthi YC, Martin
B, Woo JH, Cunningham CC and Frankel AE: RLIP76 and cancer. Clin
Cancer Res. 14:4372–4377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Awasthi S, Singhal SS, Srivastava SK,
Zimniak P, Bajpai KK, Saxena M, Sharma R, Ziller SA III, Frenkel EP
and Singh SV: Adenosine triphosphate-dependent transport of
doxorubicin, daunomycin, and vinblastine in human tissues by a
mechanism distinct from the P-glycoprotein. J Clin Invest.
93:958–965. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Awasthi YC, Singhal SS, Gupta S, Ahmad H,
Zimniak P, Radominska A, Lester R and Sharma R: Purification and
characterization of an ATPase from human liver which catalyzes ATP
hydrolysis in the presence of the conjugates of bilirubin bile
acids and glutathione. Biochem Biophys Res Commun. 175:1090–1096.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singhal SS, Roth C, Leake K, Singhal J,
Yadav S and Awasthi S: Regression of prostate cancer xenografts by
RLIP76 depletion. Biochem Pharmacol. 77:1074–1083. 2009. View Article : Google Scholar :
|
|
21
|
Singhal SS, Singhal J, Yadav S, Dwivedi S,
Boor PJ, Awasthi YC and Awasthi S: Regression of lung and colon
cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding
protein 1). Cancer Res. 67:4382–4389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singhal SS, Awasthi YC and Awasthi S:
Regression of melanoma in a murine model by RLIP76 depletion.
Cancer Res. 66:2354–2360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Awasthi S, Singhal SS, Singhal J, Cheng J,
Zimniak P and Awasthi YC: Role of RLIP76 in lung cancer doxorubicin
resistance: II. Doxorubicin transport in lung cancer by RLIP76. Int
J Oncol. 22:713–720. 2003.PubMed/NCBI
|
|
24
|
Awasthi S, Singhal SS, Singhal J, Yang Y,
Zimniak P and Awasthi YC: Role of RLIP76 in lung cancer doxorubicin
resistance: III. Anti-RLIP76 antibodies trigger apoptosis in lung
cancer cells and synergistically increase doxorubicin cytotoxicity.
Int J Oncol. 22:721–732. 2003.PubMed/NCBI
|
|
25
|
Yadav S, Singhal SS, Singhal J,
Wickramarachchi D, Knutson E, Albrecht TB, Awasthi YC and Awasthi
S: Identification of membrane-anchoring domains of RLIP76 using
deletion mutant analyses. Biochemistry. 43:16243–16253. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stuckler D, Singhal J, Singhal SS, Yadav
S, Awasthi YC and Awasthi S: RLIP76 transports vinorelbine and
mediates drug resistance in non-small cell lung cancer. Cancer Res.
65:991–998. 2005.PubMed/NCBI
|
|
27
|
Singhal SS, Yadav S, Singhal J, Zajac E,
Awasthi YC and Awasthi S: Depletion of RLIP76 sensitizes lung
cancer cells to doxorubicin. Biochem Pharmacol. 70:481–488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Awasthi S, Cheng J, Singhal SS, Saini MK,
Pandya U, Pikula S, Bandorowicz-Pikula J, Singh SV, Zimniak P and
Awasthi YC: Novel function of human RLIP76: ATP-dependent transport
of glutathione conjugates and doxorubicin. Biochemistry.
39:9327–9334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Awasthi S, Cheng JZ, Singhal SS, Pandya U,
Sharma R, Singh SV, Zimniak P and Awasthi YC: Functional reassembly
of ATP-dependent xenobiotic transport by the N- and C-terminal
domains of RLIP76 and identification of ATP binding sequences.
Biochemistry. 40:4159–4168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sugatani T and Hruska KA: Akt1/Akt2 and
mammalian target of rapamycin/Bim play critical roles in osteoclast
differentiation and survival, respectively, whereas Akt is
dispensable for cell survival in isolated osteoclast precursors. J
Biol Chem. 280:3583–3589. 2005. View Article : Google Scholar
|
|
31
|
Singhal SS, Singhal J, Figarola J, Horne D
and Awasthi S: RLIP76 targeted therapy for kidney cancer. Pharm
Res. 32:3123–3136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Sharma A, Sharma R, Patrick B,
Singhal SS, Zimniak P, Awasthi S and Awasthi YC: Cells
preconditioned with mild, transient UVA irradiation acquire
resistance to oxidative stress and UVA-induced apoptosis: Role of
4-hydroxynonenal in UVA-mediated signaling for apoptosis. J Biol
Chem. 278:41380–41388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, He Q, Chan LL, Zhou F, El Naghy
M, Thompson EB and Ansari NH: Involvement of caspases in
4-hydroxy-alkenal-induced apoptosis in human leukemic cells. Free
Radic Biol Med. 30:699–706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng JZ, Singhal SS, Saini M, Singhal J,
Piper JT, Van Kuijk FJ, Zimniak P, Awasthi YC and Awasthi S:
Effects of mGST A4 transfection on 4-hydroxynonenal-mediated
apoptosis and differentiation of K562 human erythroleukemia cells.
Arch Biochem Biophys. 372:29–36. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng JZ, Singhal SS, Sharma A, Saini M,
Yang Y, Awasthi S, Zimniak P and Awasthi YC: Transfection of mGSTA4
in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis
by inhibiting JNK-mediated signaling. Arch Biochem Biophys.
392:197–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma R, Brown D, Awasthi S, Yang Y,
Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, et
al: Transfection with 4-hydroxynonenal-metabolizing glutathione
S-transferase isozymes leads to phenotypic transformation and
immortalization of adherent cells. Eur J Biochem. 271:1690–1701.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Camandola S, Poli G and Mattson MP: The
lipid peroxidation product 4-hydroxy-2,3-nonenal increases
AP-1-binding activity through caspase activation in neurons. J
Neurochem. 74:159–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC
and Song BJ: Selective activation of the c-Jun N-terminal protein
kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12
cells. Mol Pharmacol. 58:535–541. 2000.PubMed/NCBI
|
|
39
|
Liu W, Kato M, Akhand AA, Hayakawa A,
Suzuki H, Miyata T, Kurokawa K, Hotta Y, Ishikawa N and Nakashima
I: 4-hydroxynonenal induces a cellular redox status-related
activation of the caspase cascade for apoptotic cell death. J Cell
Sci. 113:635–641. 2000.PubMed/NCBI
|
|
40
|
Cheng JZ, Sharma R, Yang Y, Singhal SS,
Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S and Awasthi YC:
Accelerated metabolism and exclusion of 4-hydroxynonenal through
induction of RLIP76 and hGST5.8 is an early adaptive response of
cells to heat and oxidative stress. J Biol Chem. 276:41213–41223.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Awasthi YC, Yang Y, Tiwari NK, Patrick B,
Sharma A, Li J and Awasthi S: Regulation of
4-hydroxynonenal-mediated signaling by glutathione S-transferases.
Free Radic Biol Med. 37:607–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su JL, Lin MT, Hong CC, Chang CC, Shiah
SG, Wu CW, Chen ST, Chau YP and Kuo ML: Resveratrol induces
FasL-related apoptosis through Cdc42 activation of
ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells.
Carcinogenesis. 26:1–10. 2005. View Article : Google Scholar
|
|
43
|
Oosterhoff JK, Penninkhof F, Brinkmann AO,
Anton Grootegoed J and Blok LJ: REPS2/POB1 is downregulated during
human prostate cancer progression and inhibits growth factor
signalling in prostate cancer cells. Oncogene. 22:2920–2925. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yadav S, Zajac E, Singhal SS, Singhal J,
Drake K, Awasthi YC and Awasthi S: POB1 over-expression inhibits
RLIP76-mediated transport of glutathione-conjugates, drugs and
promotes apoptosis. Biochem Biophys Res Commun. 328:1003–1009.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moriya R, Takahashi K, Kitahara A, Onuma
H, Handa K, Sumitani Y, Tanaka T, Katsuta H, Nishida S, Itagaki E,
et al: Possible involvement of PI3K-dependent pathways in the
increased VEGF120 release from osteoblastic cells preloaded with
palmitate in vitro. Biochem Biophys Res Commun. 445:275–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao W, Guo W, Zhou Q, Ma SN, Wang R, Qiu
Y, Jin M, Duan HQ and Kong D: In vitro antimetastatic effect of
phosphatidylinositol 3-kinase inhibitor ZSTK474 on prostate cancer
PC3 cells. Int J Mol Sci. 14:13577–13591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takahashi K, Miyokawa-Gorin K, Handa K,
Kitahara A, Moriya R, Onuma H, Sumitani Y, Tanaka T, Katsuta H,
Nishida S, et al: Endogenous oxidative stress, but not ER stress,
induces hypoxia-independent VEGF120 release through
PI3K-dependent pathways in 3T3-L1 adipocytes. Obesit. 21:1625–1634.
2013. View Article : Google Scholar
|