Introduction
The Japan Clinical Oncology Group (JCOG) 9907 study
demonstrated the effectiveness of resection after neoadjuvant
chemotherapy (NAC) using a combination of 5-fluorouracil (5-FU) and
cisplatin (CDDP) for patients with cStage II or III advanced
esophageal cancer (1). On the basis
of these findings, NAC followed by surgery is now a standard
treatment strategy for patients with advanced esophageal squamous
cell carcinoma (ESCC) in Japan's Esophagus Cancer Treatment
Guidelines (2).
Although cancer stem cells (CSCs) represent only a
small fraction of the cancer cluster, they have self-renewal and
pluripotency capabilities similar to normal stem cells and
contribute to the differentiation and proliferation of cancer cells
(3). In addition, CSCs are known to
be resistant to anticancer drugs and radiation. The treatment
resistance and heterogeneity of CSCs are thought to play a
substantial role in the prognosis of patients with ESCC.
CD44 is one of the most frequently used markers to
identify a subpopulation of cells with CSC properties in various
solid tumors and is broadly accepted as a marker of poor prognosis
in various cancers (4–9). CD44 plays a central role in the
remodeling and degradation of hyaluronan that leads to cell
migration, cancer invasion, and metastasis. CD133 is another cell
surface marker of CSCs and a predictor of prognosis in ESCC
patients (10–12). Correlative studies of tumor
specimens prior to chemotherapy can provide information on
biomarkers that may predict response or resistance to chemotherapy.
However, there have been few studies examining the expression
pattern of both CD44 and CD133 in ESCC tissue. Therefore, we
conducted this study to investigate the influence of a combination
of the CSC markers CD44 and CD133 on the therapeutic response and
prognosis of ESCC patients who underwent NAC followed by radical
esophagectomy.
Materials and methods
Human tissue samples
The present study included endoscopic biopsy
specimens taken before treatment and surgically resected specimens
from 47 patients with ESCC who underwent video-assisted
thoracoscopic esophagectomy (VATS-E) or blunt esophagectomy after
preoperative chemotherapy between 2008 and 2012 at Kanazawa
University Hospital. Cancer tissue specimens were collected from
the patients after informed consent was obtained, in accordance
with the institutional guidelines of our hospital. All patients
were staged to cStage I, II, III and IV according to the 7th
edition of the Tumor-Node-Metastasis (TNM) Staging system (13). The histological types of tumors were
classified according to the WHO Classification of Tumors of the
Digestive System, 4th edition (14). All resected primary tumors and lymph
nodes were subjected to standard hematoxylin and eosin staining and
classified according to the TNM classification system (13). Histologically, all of the tumors
were squamous cell carcinomas (SCCs) (7 well differentiated, 18
moderately differentiated, and 22 poorly differentiated). Positive
lymph node metastasis was suspected in 36 cases (76.6%) prior to
treatment and 30 cases (63.8%) were proved to have pathological
lymph node metastases. The median follow-up period was 42 months
(range, 6–82 months). During this period, 20 patients (42.6%)
experienced a recurrence.
Assessment of clinical and
pathological response to NAC
The clinical response of the primary tumor was
evaluated by endoscopic examination according to the Response
Evaluation Criteria in Solid Tumors (15). The pathological response was
histopathologically diagnosed according to the evaluation criteria
of the Japan Esophageal Society (16) using a 5-grade scale (grades 0, 1a,
1b, 2 and 3) as follows: grade 0, no response or almost no change
in cancer cells after treatment; grade 1, slight response; grade
1a, mild response, mild change in cancer cells regardless of the
area, or marked changes in cancer cells in less than one-third of
total cancer cells; grade 1b, moderate response, marked changes in
one-third or more but less than two-thirds of tumor cells; grade 2,
marked response or marked changes in two-thirds or more of tumor
cells; and grade 3, no residual tumor cells, necrosis or
disappearance of all tumor cells, or replacement of all cancer
cells by granuloma-like and/or fibrous tissue. Patients were
classified into two groups according to histopathological effect as
follows: a poor response group of grades 0, 1a and 1b, and a good
response group of grades 2 and 3.
Immunohistochemistry
For immunohistochemical examination, 20%
formalin-fixed and paraffin-embedded specimens were retrieved from
the surgical pathology files of the Pathology Section of Kanazawa
University Hospital, Kanazawa, Japan. The expression of CD44 and
CD133 in endoscopic biopsy specimens and surgically resected ESCC
specimens was examined immunohistochemically using a horseradish
peroxidase (HRP)-based method. To identify the antigen in tissues,
deparaffinized sections were pretreated by autoclaving in 10%
citric acid buffer (pH 8.0) at 120°C for 15 min. After pretreatment
with protein block serum (Dako Cytomation, Kyoto, Japan) for 10 min
and 2% skim milk for 20 min to block nonspecific reactions, the
sections were incubated with mouse anti-CD44 monoclonal antibody
[diluted 1:100 in phosphate-buffered saline (PBS); R&D Systems,
Inc., Minneapolis, MN, USA] or rabbit anti-CD133 (PROM-1)
polyclonal antibody (diluted 1:250 in PBS; Abnova Corporation,
Taipei, Taiwan) at 4°C overnight. After incubation, the Envision+
polymer solution (HRP-conjugated secondary antibody; Dako
Cytomation) was applied for 1 h. The reaction products were
developed in 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB)
solution containing 0.1% H2O2. The sections
were then lightly counterstained with hematoxylin and the slides
were examined under a microscope (Olympus, Tokyo, Japan).
Evaluation of immunohistochemical
variables
Immunohistochemical staining of CD44 and CD133 in
tumor tissues was evaluated microscopically. Specimens were defined
as positive for CD44 and CD133 expression when positive staining
was noted in the cytoplasm and/or cell membrane of the tumor cells
regardless of the strength of staining and ratio of positive cells.
The interpretation of immunoreactivity was performed in a
semi-quantitative manner by analyzing both the percentage of
positive cells and the intensity of staining. The percentage of
positive staining cells in the specimens was divided into two
groups as follows: low-rate group, less than 80% of cancer cells
were positive; high-rate group, more than 80% of the cancer cells
were positive. The intensity of staining was divided into two
groups as weak or strong. We examined the correlation between CD44
and CD133 expression status and clinicopathological factors of the
tumor prior to treatment, clinicopathological therapeutic effects
after NAC, and the prognosis of ESCC patients after treatment. We
also evaluated changes in the proportion of CD44 and
CD133-expressing tumor cells in the tissue before vs. after
chemotherapy.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Immunohistochemical results of CD44 and CD133 expression
status before and after NAC and the correlation between the
histopathological effects of chemotherapy with the clinical
variables were analyzed using paired Student's t-test or
Mann-Whitney U test and assessed by the chi-square test or Fisher's
exact test as appropriate. The Kaplan-Meier method was used to
analyze survival, and the log-rank test was used to estimate
differences in survival. Prognostic factors were examined using
univariate analysis, multivariate analysis, and the Cox
proportional hazards regression model. Statistical significance was
assumed for P<0.05. All analyses were performed with SPSS IBM
Statistics ver. 22; IBM Corp., Armonk, NY, USA).
Results
Expression of CD44 and CD133 in
endoscopic biopsy specimens and resected esophageal cancer
specimens
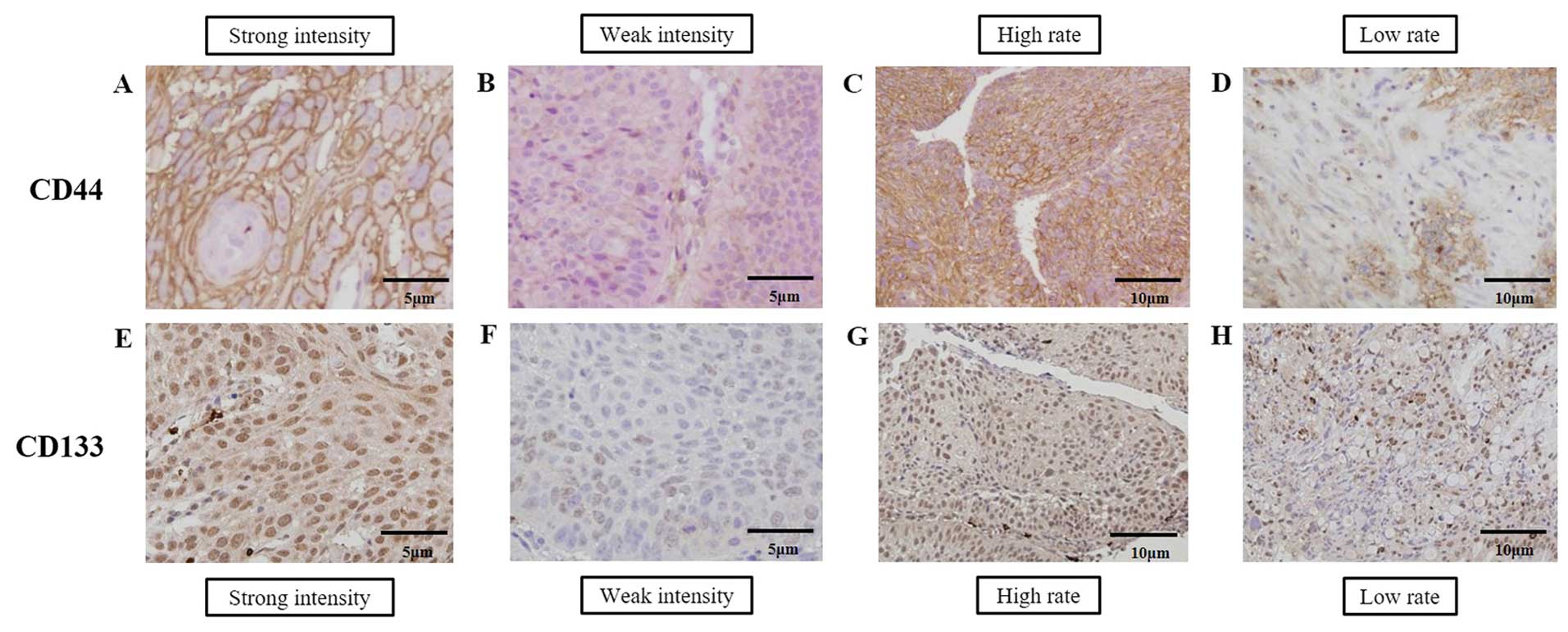
Representative expression patterns of CD44 and CD133
in ESCC biopsy specimens are shown in Fig. 1. CD44 immunoreactivity was diffusely
evident in membranous and granular cytoplasmic patterns (Fig. 1A and D) whereas CD133
immunoreactivity was diffusely evident in granular cytoplasmic and
intranuclear patterns (Fig. 1E).
Demographics of the 47 ESCC patients categorized according to CD44
and CD133 expression status in biopsied specimens before
chemotherapy are shown in Table I.
Strong or weak positive CD44 staining was observed in all cases and
only one case showed negative staining of CD133. A single case with
negative expression of CD133 was included in the weak intensity
group. The level of CD44 and CD133 expression before NAC was not
associated with preoperative assessment of tumor depth, lymph node
metastasis, degree of tumor differentiation, and cStage (Table I).
 | Table I.Demographics of the 47 ESCC patients
according to CD44 and CD133 expression status in biopsied specimens
before chemotherapy. |
Table I.
Demographics of the 47 ESCC patients
according to CD44 and CD133 expression status in biopsied specimens
before chemotherapy.
|
| CD44 | CD133 |
|---|
|
|
|
|
|---|
| Variables | Strong intensity
group (n=24) | Weak intensity
group (n=23) | P-value | High positive rate
group (n=16)a | Low positive rate
group (n=31)a | P-value | Strong intensity
group (n=23) | Weak intensity
group (n=24) | P-value | High positive rate
group (n=15)a | Low positive rate
group (n=32)a | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 20 | 20 | 1.000 | 15 | 25 | 0.396 | 18 | 22 | 0.245 | 12 | 28 | 0.664 |
|
Female | 4 | 3 |
| 1 | 6 |
| 5 | 2 |
| 3 | 4 |
|
| Age (years) | 64.2±7.0 | 63.6±7.0 | 0.755 | 64.4±7.8 | 63.6±6.6 | 0.704 | 63.8±7.4 | 64.0±6.6 | 0.949 | 63.4±7.8 | 64.1±6.6 | 0.743 |
| Main location of
tumor |
| Upper
esophagus | 5 | 1 | 0.227 | 2 | 4 | 0.861 | 2 | 4 | 0.081 | 3 | 3 | 0.199 |
| Middle
esophagus | 13 | 14 |
| 10 | 17 |
| 17 | 10 |
| 10 | 17 |
|
| Lower
esophagus | 6 | 8 |
| 4 | 10 |
| 4 | 10 |
| 2 | 12 |
|
| Tumor
differentation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Well/moderate | 11 | 14 | 0.302 | 9 | 16 | 0.763 | 14 | 11 | 0.302 | 10 | 15 | 0.205 |
|
Poor | 13 | 9 |
| 7 | 15 |
| 9 | 13 |
| 5 | 17 |
|
| Tumor depth |
|
|
|
|
|
|
|
|
|
|
|
|
| cT1,
2 | 7 | 5 | 0.559 | 3 | 9 | 0.505 | 8 | 4 | 0.193 | 3 | 9 | 0.725 |
| cT3,
4 | 17 | 18 |
| 13 | 22 |
| 15 | 20 |
| 12 | 23 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
cN0 | 6 | 5 | 0.792 | 3 | 8 | 0.725 | 3 | 8 | 0.168 | 2 | 9 | 0.461 |
|
cN1-3 | 18 | 18 |
| 13 | 23 |
| 20 | 16 |
| 13 | 23 |
|
| Distant
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
cM0 | 23 | 23 | 1.000 | 16 | 30 | 1.000 | 23 | 23 | 1.000 | 15 | 31 | 1.000 |
|
cM1 | 1 | 0 |
| 0 | 1 |
| 0 | 1 |
| 0 | 1 |
|
| cStage, TNM
7th |
|
|
|
|
|
|
|
|
|
|
|
|
| cStage
I, II | 6 | 6 | 0.932 | 3 | 9 | 0.505 | 7 | 5 | 0.450 | 2 | 10 | 0.288 |
| cStage
III, IV | 18 | 17 |
| 13 | 22 |
| 16 | 19 |
| 13 | 22 |
|
| NAC regimen |
|
|
|
|
|
|
|
|
|
|
|
|
| FP | 23 | 21 | 0.586 | 16 | 28 | 0.272 | 23 | 21 | 0.215 | 14 | 30 | 0.680 |
|
DCF | 1 | 1 |
| 0 | 2 |
| 0 | 2 |
| 1 | 1 |
|
|
DCS | 0 | 1 |
| 0 | 1 |
| 0 | 1 |
| 0 | 1 |
|
| Surgical
procedure |
|
|
|
|
|
|
|
|
|
|
|
|
|
VATS-E | 23 | 23 | 1.000 | 16 | 30 | 1.000 | 22 | 24 | 0.489 | 14 | 32 | 0.319 |
| Blunt
esophagectomy | 1 | 0 |
| 0 | 1 |
| 1 | 0 |
| 1 | 0 |
|
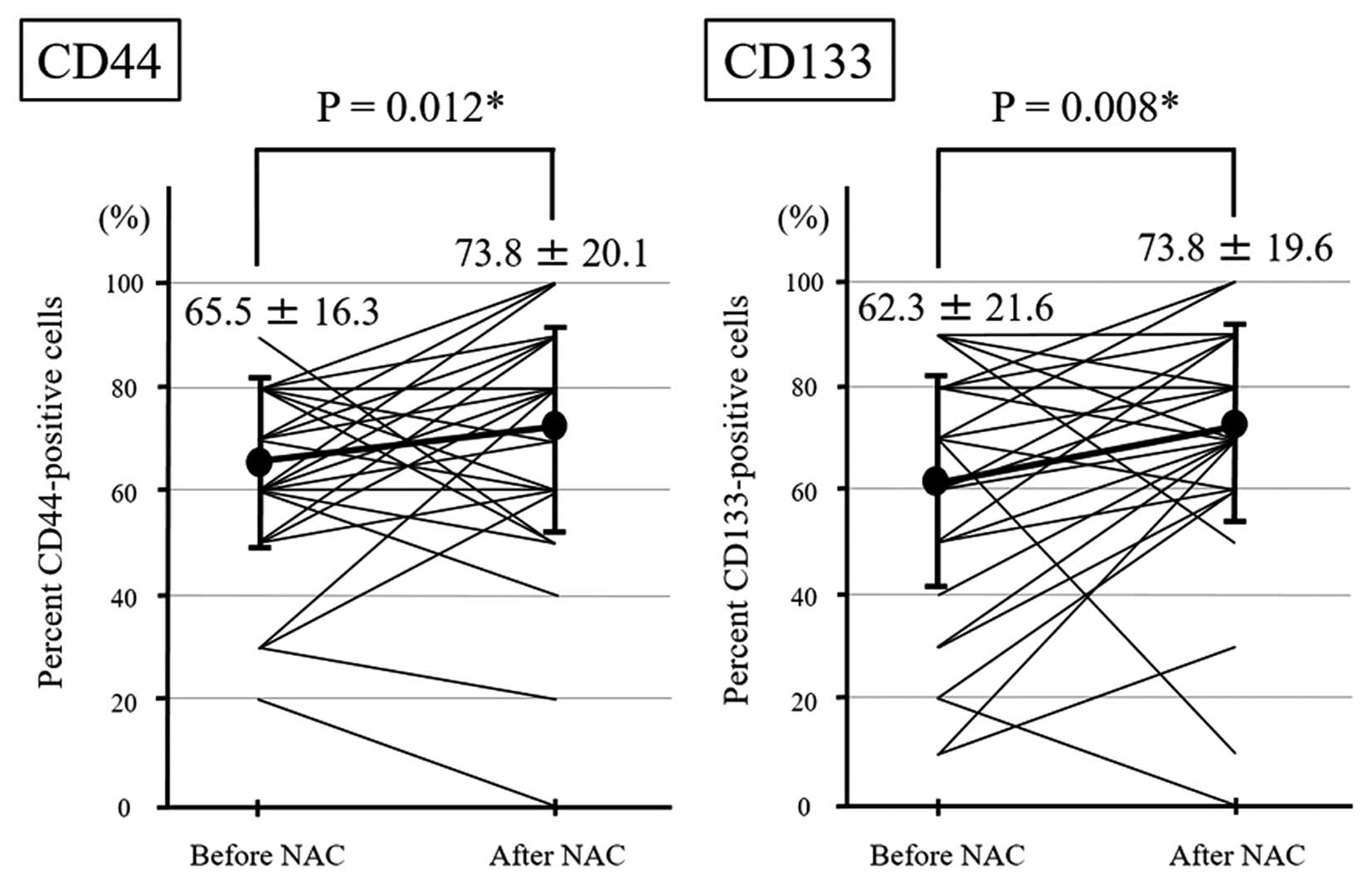
The expression status of CD44 and CD133 in tumor
cells in the biopsied specimens before NAC and the resected tissue
after NAC in same patients was analyzed (Fig. 2). CD44- or CD133-positive cell were
diffusely observed in the biopsied specimens before chemotherapy
(Fig. 2A and C, and E and G). In
some cases with high chemotherapeutic effects, CD44- or
CD133-positive cell clusters were observed in the necrotic and
fibrotic areas of resected tissues of ESCC after NAC (Fig. 2B and F). However in the cases with
poor chemotherapeutic effects, most CD44- or CD133-positive cells
distributed similarly to the pre-treatment state (Fig. 2D and H). There were almost no
obvious changes in the intensity before and after chemotherapy. The
median rate of CD44-positive and CD133-positive cells in
endoscopically biopsied specimens before chemotherapy was 65.5±16.3
and 62.3±21.6%, respectively. In contrast, the median rate of
CD44-positive and CD133-positive cells in resected specimens after
NAC was 73.8±20.1 and 73.8±19.6%, respectively. The rates of both
CD44-positive and CD133-positive cells in the tissue were
significantly increased after chemotherapy (CD44, P=0.012; CD133,
P=0.008) (Fig. 3).
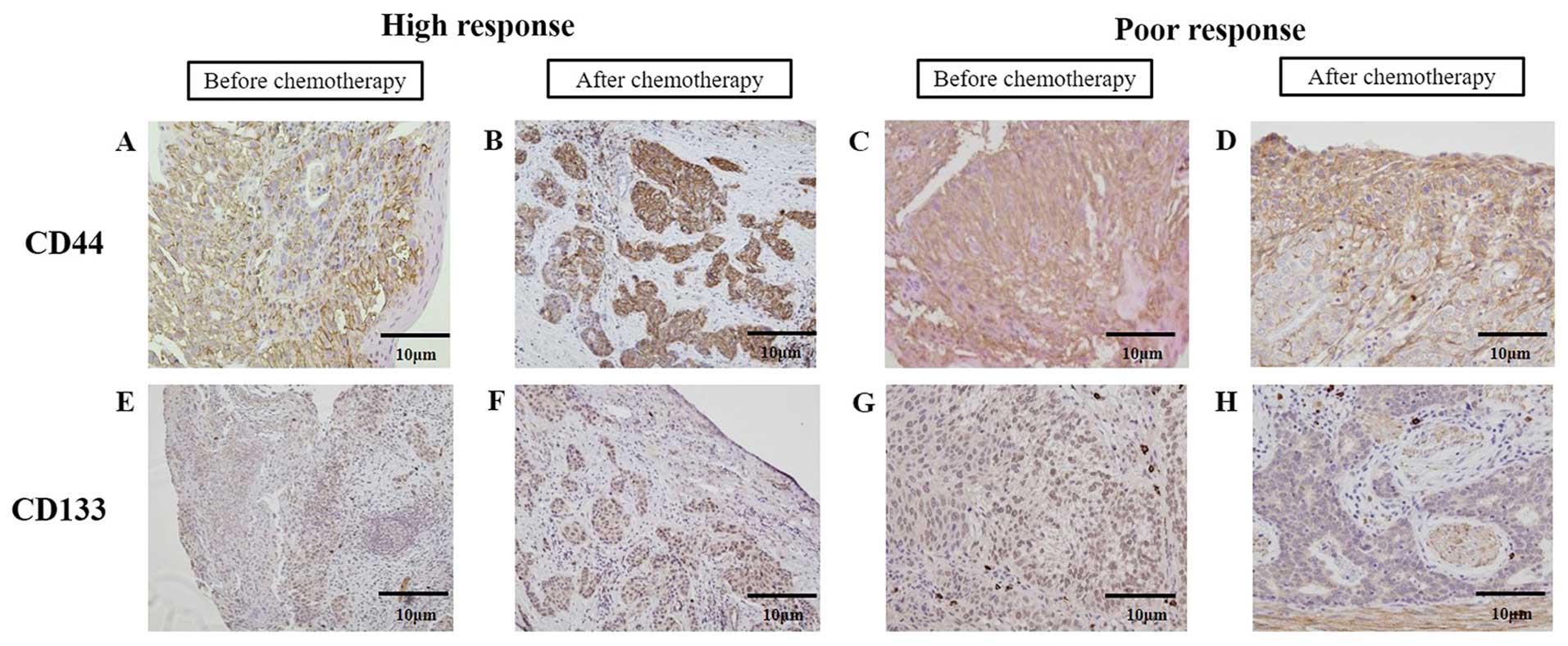 | Figure 2.Changes in CD44 and CD133 expression
in esophageal cancer tissues before and after chemotherapy. (A-D)
Representative photomicrographs of CD44 and (E-H) CD133
immunohistochemistry (A, C, E and G) before and (B, D, F and H)
after chemotherapy in the cases with high or poor therapeutic
response. Immunohistochemical staining before and after
chemotherapy was performed in the same patient (B, D, F and H
correspond to A, C, E and G, respectively). (B and F) In the cases
with high therapeutic response to chemotherapy, relatively strong
intensity and high positive rate for CD44 or CD133-expressing tumor
cells were observed before chemotherapy and some clusters of
residual cancer cells with strong CD44 or CD133 expression were
observed in tumor tissue after chemotherapy. (D and H) In the cases
with poor therapeutic response to chemotherapy, strong intensity
and a high positive rate for CD44 or CD133 expression were observed
before chemotherapy and many CD44- or CD133-positive cells
remained. |
Correlations between CSC marker
expression before chemotherapy and clinicopathological effects of
chemotherapy
There was no significant correlation between the
status of each CSC marker before NAC and the clinicopathological
effects of NAC (Table II).
However, the histopathological effect of NAC in resected specimens
was significantly correlated with clinical response after NAC,
tumor depth, and cStage (Table
III).
 | Table II.Correlation between CSC marker
expression before chemotherapy and clinicopathological variables
after chemotherapy. |
Table II.
Correlation between CSC marker
expression before chemotherapy and clinicopathological variables
after chemotherapy.
|
| CD44 | CD133 |
|---|
|
|
|
|
|---|
| Variables | Strong intensity
group (n=24) | Weak intensity
group (n=23) | P-value | High positive rate
group(n=16) | Low positive rate
group(n=31) | P-value | Strong intensity
group (n=23) | Weak intensity
group (n=24) | P-value | High positive rate
group(n=15) | Low positive rate
group(n=32) | P-value |
|---|
| Reduction ratio in
tumor major axis |
|
|
|
|
|
|
|
|
|
|
|
|
|
≥100% | 9 | 9 | 0.908 | 8 | 10 | 0.236 | 11 | 7 | 0.188 | 8 | 10 | 0.147 |
|
≤99% | 15 | 14 |
| 8 | 21 |
| 12 | 17 |
| 7 | 22 |
|
| Reductive response
in the primary tumora |
|
|
|
|
|
|
|
|
|
|
|
|
| PD,
SD | 13 | 8 | 0.181 | 8 | 13 | 0.598 | 9 | 12 | 0.454 | 7 | 14 | 0.851 |
| PR,
CR | 11 | 15 |
| 8 | 18 |
| 14 | 12 |
| 8 | 18 |
|
| Histopathological
effects |
|
|
|
|
|
|
|
|
|
|
|
|
| Grade
0, 1a, 1b | 20 | 18 | 0.724 | 12 | 26 | 0.466 | 20 | 18 | 0.461 | 13 | 25 | 0.697 |
| Grade
2, 3 | 4 | 5 |
| 4 | 5 |
| 3 | 6 |
| 2 | 7 |
|
 | Table III.Correlation between the
histopathological effects of chemotherapy and the clinical
variables. |
Table III.
Correlation between the
histopathological effects of chemotherapy and the clinical
variables.
|
| Histopathological
effects |
|---|
|
|
|
|---|
| Variables | Grades 0, 1a and 1b
(n=38) | Grades 2 and 3
(n=9) | P-value |
|---|
| Response to
NAC |
|
|
|
|
Reduction ratio in tumor major
axis |
|
|
|
|
≤99% | 21 | 8 | 0.124 |
|
≥100% | 17 | 1 |
|
|
Reductive response in the
primary tumorb |
|
|
|
|
PR, CR | 18 | 8 | 0.030a |
|
PD, SD | 20 | 1 |
|
| Clinical
parameters |
|
|
|
| cT |
|
|
|
|
cT1, 2 | 7 | 5 | 0.035a |
|
cT3, 4 | 31 | 4 |
|
| cN |
|
|
|
|
cN0 | 8 | 3 | 0.419 |
|
cN1-3 | 30 | 6 |
|
|
cStage |
|
|
|
|
cStage I, II | 6 | 6 | 0.005a |
|
cStage III,
IV | 32 | 3 |
|
| Tumor
differentiation |
|
|
|
|
Well/moderately | 21 | 4 | 0.715 |
|
Poor | 17 | 5 |
|
Prognostic significance of CD44 and
CD133 expression
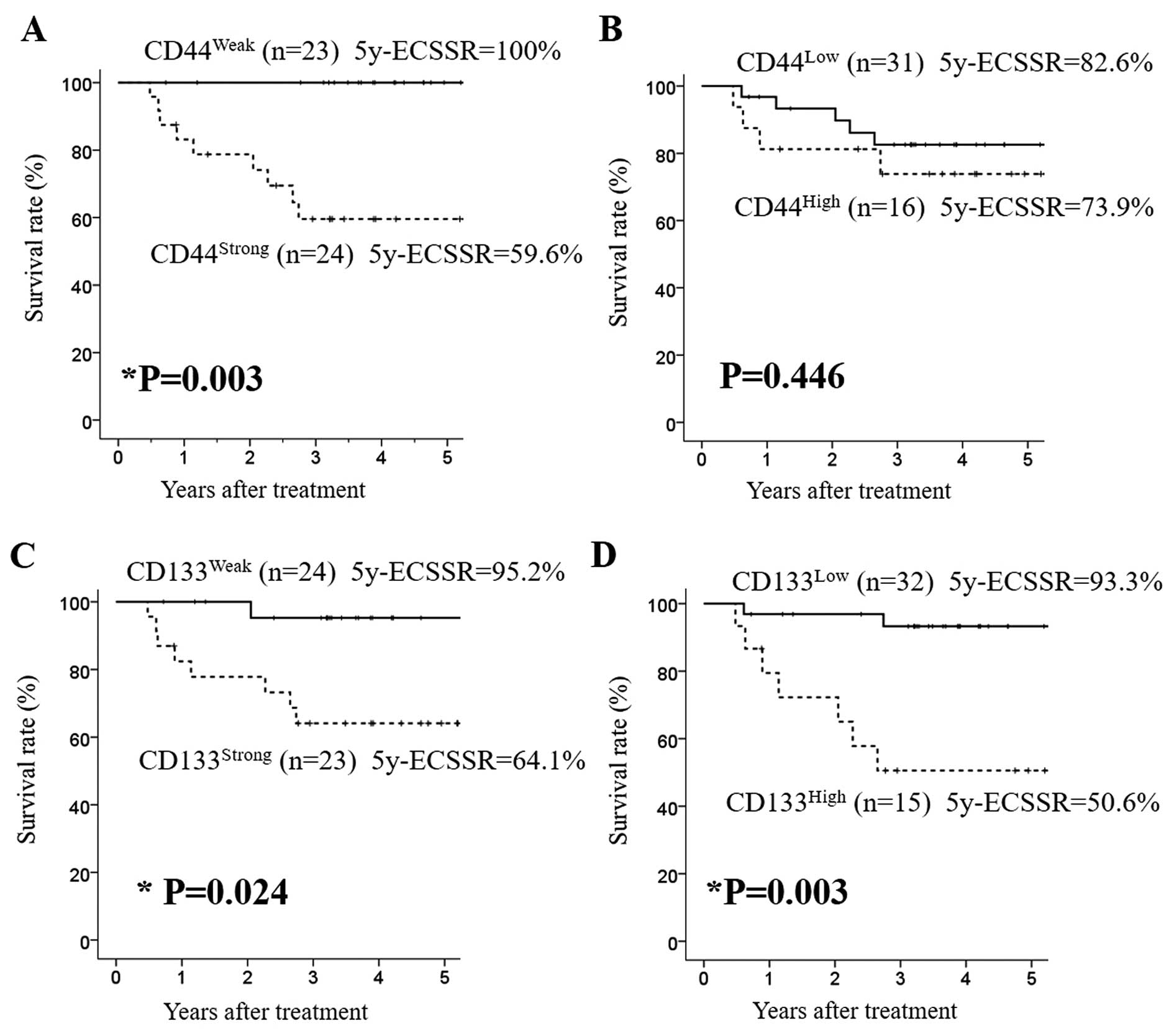
Survival analyses showed that strongly positive
expression of CD44 or CD133 before NAC was significantly correlated
with poor esophageal cancer-specific survival (ECSS) (P=0.003 and
P=0.024, respectively) (Fig. 4A and
C). Fifteen ESCC patients with a high positive rate of CD133
expression before NAC showed significantly poorer ECSS than
patients with a low positive rate of CD133 expression (P=0.003)
(Fig. 4D). However, the positivity
rate of CD44 expression before NAC had no significant prognostic
impact on ECSS (P=0.446) (Fig. 4B).
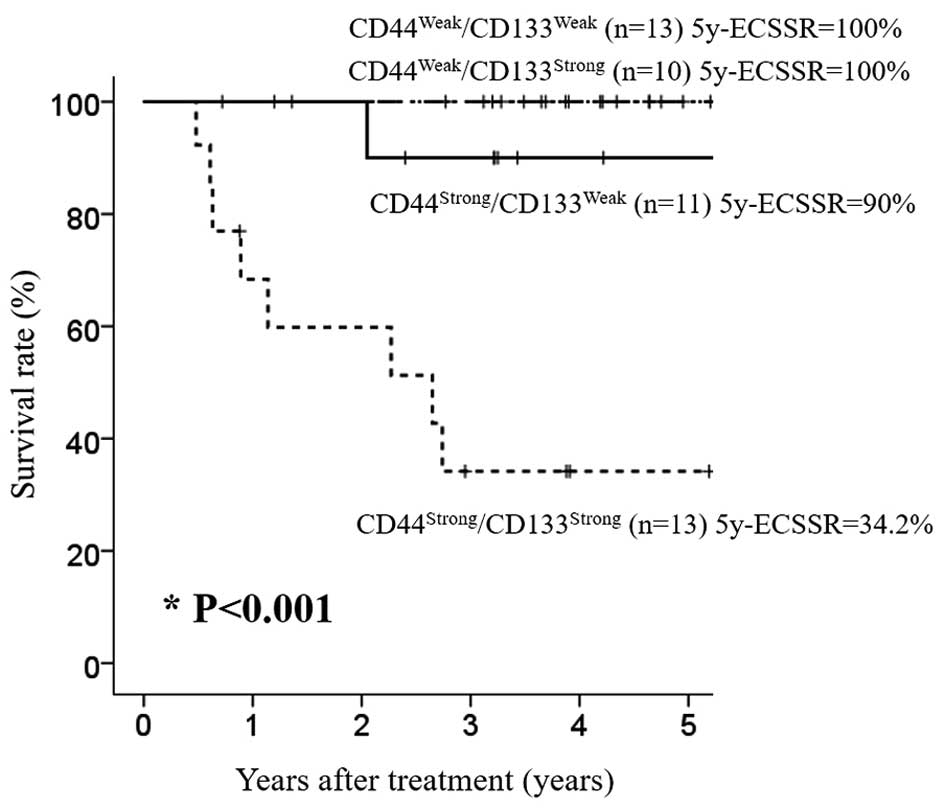
We also analyzed the impact of combined expression of CD44 and
CD133 on the outcome of ESCC and found that ESCC patients with
CD44strong/CD133strong expression showed
significantly poorer ECSS than those with CD44weak or
CD44strong/CD133weak expression (P<0.001)
(Fig. 5).
The results of univariate and multivariate survival
analysis for CSC markers and each clinicopathological factor are
shown in Table IV. We found that
CD44strong/CD133strong expression, higher
positive rate of CD133 expression before NAC, and progressive
response in the primary tumor evaluated by endoscopy were
significant unfavorable prognosticators for ECSS (Table IV).
 | Table IV.Univariate and multivariate analyses
of clinicopathological variables associated with esophageal
cancer-specific survival. |
Table IV.
Univariate and multivariate analyses
of clinicopathological variables associated with esophageal
cancer-specific survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| CSC markers |
|
|
|
|
|
|
|
Intensity of CD expression
before NAC (CD44strong/CD133strong vs.
other) | 5.618 | 1.980–15.87 | 0.001a | 25.641 | 3.021–200 | 0.003a |
|
Positive rate of CD expression
before NAC (CD133high vs. CD133low) | 5.015 | 1.060–23.716 | 0.042a | 6.778 | 1.327–34.624 | 0.021a |
| Response to
NAC |
|
|
|
|
|
|
|
Reduction ratio in major axis
of the tumor (≥100 vs. ≤99%) | 4.202 | 1.067–16.393 | 0.040a |
|
|
|
|
Reductive response in the
primary tumor (PD, SD vs. PR, CR)b | 6.329 | 1.337–29.412 | 0.020a | 10.101 | 1.799–55.556 | 0.009a |
| Clinicopathological
parameters |
|
|
|
|
|
|
| cT (3,
4 vs. 1, 2) | 1.181 | 0.591–2.363 | 0.638 |
|
|
|
| cN (≥1
vs. 0) | 5.618 | 0.226–142.86 | 0.292 |
|
|
|
| Degree of
differentiation | 1.200 | 0.644–2.237 | 0.566 |
|
|
|
| Poor
vs. well/moderate |
|
|
|
|
|
|
| Histopathological
effects | 1.101 | 0.502–2.421 | 0.809 |
|
|
|
| Grade
(0, 1a, 1b vs. 2, 3) |
|
|
|
|
|
|
| cStage
(III, IV vs. I, II) | 1.058 | 0.482–2.326 | 0.888 |
|
|
|
Discussion
In the present study, we found that the combination
of CD44 and CD133 expression status before NAC was a novel
predictor of the prognosis of ESCC patients who underwent surgical
resection after chemotherapy.
Despite the development of treatment strategies the
prognosis of ESCC remains poor because of its high rate of
recurrence and metastasis. The prognosis of ESCC patients cannot be
accurately estimated based only on clinicopathological factors such
as pretreatment TNM stage or the degree of tumor differentiation;
it is also critical to classify the effectiveness of chemotherapy
and the probability of recurrence or metastasis in pretreatment
ESCC patients.
It has been reported that NAC has numerous
advantages in various solid tumors and pathological response data
for NAC are thought to be very beneficial as surrogate markers for
long-term clinical outcome (17,18).
To estimate the therapeutic response and prognosis after therapy,
we investigated the CSC profile in primary ESCC tissue because
CSC-like cells are known to be resistant to chemotherapy or
radiation and are often characterized by elevated expression of the
stem cell surface markers CD44 and CD133 (18–21).
Stem cells are characterized by the properties of self-renewal and
pluripotency. CSC-like cells are thought to be tumor-initiating
cells (TICs) and exhibit characteristics of low rates of
proliferation, a high capacity for self-renewal, and a propensity
to differentiate into actively proliferating cancer cells (3,22). To
date, several markers have been used to identify CSCs, including
CD44, CD133, aldehyde dehydrogenase 1 (ALDH1), adenosine
triphosphate-binding cassette superfamily G member 2 (ABCG2), and
Bmi-1 (4,11,23–27).
Among these, CD44 and CD133 are known to be representative markers
of CSCs. In the present study, we performed immunohistochemical
analyses for the detection and the assessment of CSC-like cells by
examining CD44 and CD133 expression in ESCC tissues before and
after chemotherapy.
CD44 is the transmembrane adhesion receptor for
hyaluronan and plays a central role in the remodeling and
degradation processes of hyaluronan that lead to cell migration, as
well as to cancer invasion and metastasis through cell-cell and
cell-extracellular matrix adhesion. Many researchers have
demonstrated that CSCs express CD44-related surface markers. CD44
is one of the most frequently used markers to identify a
subpopulation of cells with CSC properties in solid tumors,
including colon cancer, head and neck SCC, lung cancer, gastric
cancer, pancreatic cancer, and ESCC, and is broadly accepted as a
marker of poor prognosis in various cancers (4–9).
Biddle et al (28) reported
that CD44 and epithelial cell adhesion molecule (EpCAM) expression
in CSCs correlated with the switch between mesenchymal phenotype
and epithelial phenotype in association with the
epithelial-to-mesenchymal transition (EMT) in head and neck SCC. It
has also been reported that CD44-positive CSCs exhibit molecular
characteristics associated with EMT in SCC (29–31).
CD44 downregulates E-cadherin expression, upregulates vimentin and
matrix metalloproteinase (MMP), and inhibits the formation of
membrane-associated E-cadherin-β-catenin complex, resulting in cell
invasion and migration (30). It
has also been reported that CD44 is a novel surface marker of TICs,
a subpopulation of cells with the ability to self-renew as well as
drive the initiation and progression of cancer (9,22).
These findings suggested that CD44 expression may correlate with
cancer cell viability, postoperative recurrence, and metastasis of
solid tumors through the process of EMT and resistance to
chemotherapy and radiation.
CD133 protein encoded by the PROM1 gene is
known as a cell surface marker of stem cells, including embryonic
stem cells and progenitor cells (32,33).
It has been reported that CD133 is also expressed on CSCs, such as
leukemic stem cells and liver CSCs (10,11).
It has also been reported that CD133 immunoreactivity is a good
predictor of prognosis in ESCC patients and that CD133 may play a
role in the regulation of the tumor cell cycle through p27 and p16
in ESCC (12). In the present study
we observed strongly positive expression of CD44 and CD133 in 51.1
and 48.9%, respectively, of the biopsied specimens before NAC.
Little change in the intensity of CD44 and CD133 expression was
observed in resected specimens after NAC, but enrichment for CD44-
or CD133-positive cells was observed in post-NAC tumor specimens
compared with pre-NAC specimens. The results of survival analyses
showed that strongly positive expression of CD44 or CD133 and
higher positive rate of CD133 before NAC were significantly
correlated with a poorer prognosis. In addition, combination
analysis of CD44 and CD133 was useful for predicting relapse-free
survival. Multivariate analysis showed that simultaneous strong
expression of CD44 and CD133, a high positive rate of
CD133-expressing tumor cells, and a progressive response in the
primary tumor were independent prognosticators for ECSS.
In the present study, CD44 and CD133 positive ratio
before chemotherapy was 65.5 and 62.3%, respectively. We tried to
examine the proportion and distribution of CSCs in tumor tissue by
CD44 and CD133 immunoreactivity. It is considered that CD44- and/or
CD133-positive cells does not represent actual CSCs, because the
ratio of CD44- and/or CD133-positive cells is much higher than the
actual proportion of exact CSCs in tumor tissue. However, it is
considered that CD44 and/or CD133-expressing tumor cells have the
characteristics of cancer stem-like cell properties. Histological
chemo-resistance and prognostic significance of these markers
before treatment may support this hypothesis. Generally, previous
studies have reported that the histopathological response reflects
the prognosis of solid cancers and that CSC marker expression was
correlated with treatment resistance (17). Aomatsu et al (18) reported that the cCR rate of
CD133-positive tumors was significantly lower than that of
CD133-negative tumors and that CD133 expression before NAC was an
independent predictive factor for pCR in breast cancer. Other
studies also showed that higher expression of CD133 was a poor
prognostic factor and was correlated with resistance to
chemotherapy and radiation in colorectal cancer (23,34).
Sahlberg et al (21)
demonstrated that colon cancer cells with high CD133 and CD44
expression were associated with AKT expression and increased
radiation resistance, and that the various AKT isoforms had
different effects on the expression of CSC markers. In the present
study, we found that ESCC patients with strong expression of CSC
markers in pre-NAC specimens had a poor prognosis regardless of the
effect of chemotherapy. From these results, it was inferred that
non-CSC like cells were consigned to cell death after chemotherapy
whereas highly chemoresistant CSC marker-positive cells survived.
Our finding that patients with CD44strong or
CD133strong expression had poorer prognosis (Fig. 4) was consistent with the results of
previous studies (18,23,34).
In addition, our present study also revealed the value of the
combined CD44 and CD133 expression status as a more sensitive
prognostic predictor in ESCC patients than CD44 or CD133 expression
status alone.
Assessment of the chemoresistance of each tumor is
very important for the selection of effective treatment methods,
but no definitive biomarkers have been shown to predict the
therapeutic effects. Correlative studies of tumor specimens before
and after NAC may provide further information on markers that
predict the response or resistance to chemotherapy. However, only a
few studies have previously examined the histopathological
responses in tumors before and after chemotherapy (35). The present study investigating the
expression status of CSCs markers before and after chemotherapy
provides the prognostic significance of CSC marker expression
status before chemotherapy, indicating that the high population of
CSC-like tumor cells shows chemoresistance.
Our study revealed that CSCs that were originally
present in tumor tissues reflect the high risk of metastasis and
recurrence and the poor prognosis of ESCC patients because of the
refractoriness of these cells to chemotherapy. Deregulation of the
refractoriness of CSCs to anticancer drugs may have an immense
clinical benefit on the prognosis of patients with ESCC.
In the future, it will be possible to predict the
optimal combination of anticancer drugs using the expression of a
combination of key proteins in tumor tissues as a criterion to
decide treatment selection and predict adverse effects. ALDH1 has
been indicated as a possible candidate for a CSC marker (23). The expression of ALDH1 was
correlated with clinicopathological factors and clinical outcomes
of patients with ESCC (24). ALDH1
was also found to be a more significantly predictive marker than
CD44+/CD24− for the identification of breast
CSCs in terms of resistance to chemotherapy (35). In a study of the prognostic value of
ABCG2 and CD133 expression in ESCC patients, ABCG2 expression was
found to be significantly correlated with survival whereas CD133
expression was not (26). If it is
possible to use combinations of various biomarkers for more
effective prediction of therapeutic effects, we may expect the
development of tailor-made treatments and an improvement in
prognosis in not only patients with ESCC, but also in those with
various other tumors. Understanding the molecular biology of
CSC-associated molecules is now essential for the development of
more effective cancer treatments. Moreover, an understanding of CSC
characteristics associated with the development of cancer and
carcinogenesis may have clinical applications for the prediction of
refractoriness to radiation or chemotherapy. Finally, although CSCs
are considered to play a pivotal role in tumor progression and the
metastatic process, other mechanisms that impair the malignant
potential and chemoresistance should also be investigated.
In conclusion, combination analysis of CD44 and
CD133 expression can be useful to predict prognosis of ESCC
patients. Immunohistochemical assessment of CSC markers in
pretreatment ESCC specimens may help physicians determine the
malignant potential of an individual patient's tumor and their
likely prognosis.
References
|
1
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuwano H, Nishimura Y, Oyama T, Kato H,
Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al:
Guidelines for Diagnosis and Treatment of Carcinoma of the
Esophagus April 2012 edited by the Japan Esophageal Society.
Esophagus. 12:1–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012.PubMed/NCBI
|
|
5
|
Chen J, Zhou J, Lu J, Xiong H, Shi X and
Gong L: Significance of CD44 expression in head and neck cancer: A
systemic review and meta-analysis. BMC Cancer. 14:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao S, He JL, Qiu ZX, Chen NY, Luo Z,
Chen BJ and Li WM: Prognostic value of CD44 variant exon 6
expression in non-small cell lung cancer: A meta-analysis. Asian
Pac J Cancer Prev. 15:6761–6766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nosrati A, Naghshvar F and Khanari S:
Cancer stem cell markers CD44, CD133 in primary gastric
adenocarcinoma. Int J Mol Cell Med. 3:279–286. 2014.PubMed/NCBI
|
|
8
|
Mizukami T, Kamachi H, Mitsuhashi T,
Tsuruga Y, Hatanaka Y, Kamiyama T, Matsuno Y and Taketomi A:
Immunohistochemical analysis of cancer stem cell markers in
pancreatic adenocarcinoma patients after neoadjuvant
chemoradiotherapy. BMC Cancer. 14:6872014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu
CL, Ji XD, Guan DX, Gao H, Xu LY, et al: Tumor initiating cells in
esophageal squamous cell carcinomas express high levels of CD44.
PLoS One. 6:e214192011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horn PA, Tesch H, Staib P, Kube D, Diehl V
and Voliotis D: Expression of AC133, a novel hematopoietic
precursor antigen, on acute myeloid leukemia cells. Blood.
93:1435–1437. 1999.PubMed/NCBI
|
|
11
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto H, Fujishima F, Nakamura Y,
Zuguchi M, Ozawa Y, Takahashi Y, Miyata G, Kamei T, Nakano T,
Taniyama Y, et al: Significance of CD133 expression in esophageal
squamous cell carcinoma. World J Surg Oncol. 11:512013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumors. 7th. Wiley-Blackwell;
Oxford: 2010
|
|
14
|
Fléjou JF: WHO Classification of digestive
tumors: the fourth edition. Ann Pathol. 31:(Suppl 5). S27–S31.
2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japan Esophageal Society, . Japanese
Classification of Esophageal Cancer, tenth edition: part I.
Esophagus. 6:1–25. 2009. View Article : Google Scholar
|
|
17
|
Meredith KL, Weber JM, Turaga KK, Siegel
EM, McLoughlin J, Hoffe S, Marcovalerio M, Shah N, Kelley S and
Karl R: Pathologic response after neoadjuvant therapy is the major
determinant of survival in patients with esophageal cancer. Ann
Surg Oncol. 17:1159–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aomatsu N, Yashiro M, Kashiwagi S,
Takashima T, Ishikawa T, Ohsawa M, Wakasa K and Hirakawa K: CD133
is a useful surrogate marker for predicting chemosensitivity to
neoadjuvant chemotherapy in breast cancer. PLoS One. 7:e458652012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HH, Seo KJ, An CH, Kim JS and Jeon HM:
CD133 expression is correlated with chemoresistance and early
recurrence of gastric cancer. J Surg Oncol. 106:999–1004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piao LS, Hur W, Kim TK, Hong SW, Kim SW,
Choi JE, Sung PS, Song MJ, Lee BC, Hwang D, et al:
CD133+ liver cancer stem cells modulate radioresistance
in human hepatocellular carcinoma. Cancer Lett. 315:129–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sahlberg SH, Spiegelberg D, Glimelius B,
Stenerlöw B and Nestor M: Evaluation of cancer stem cell markers
CD133, CD44, CD24: Association with AKT isoforms and radiation
resistance in colon cancer cells. PLoS One. 9:e946212014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou F, Mu YD, Liang J, Liu ZX, Chen HS
and Zhang JF: Expression and prognostic value of tumor stem cell
markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett.
7:507–512. 2014.PubMed/NCBI
|
|
24
|
Wang Y, Zhe H, Gao P, Zhang N, Li G and
Qin J: Cancer stem cell marker ALDH1 expression is associated with
lymph node metastasis and poor survival in esophageal squamous cell
carcinoma: A study from high incidence area of northern China. Dis
Esophagus. 25:560–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hang D, Dong HC, Ning T, Dong B, Hou DL
and Xu WG: Prognostic value of the stem cell markers CD133 and
ABCG2 expression in esophageal squamous cell carcinoma. Dis
Esophagus. 25:638–644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shien K, Toyooka S, Ichimura K, Soh J,
Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, et al:
Prognostic impact of cancer stem cell-related markers in non-small
cell lung cancer patients treated with induction chemoradiotherapy.
Lung Cancer. 77:162–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biddle A, Liang X, Gammon L, Fazil B,
Harper LJ, Emich H, Costea DE and Mackenzie IC: Cancer stem cells
in squamous cell carcinoma switch between two distinct phenotypes
that are preferentially migratory or proliferative. Cancer Res.
71:5317–5326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geng S, Guo Y, Wang Q, Li L and Wang J:
Cancer stem-like cells enriched with CD29 and CD44 markers exhibit
molecular characteristics with epithelial-mesenchymal transition in
squamous cell carcinoma. Arch Dermatol Res. 305:35–47. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Bras GF, Allison GL, Richards NF,
Ansari SS, Washington MK and Andl CD: CD44 upregulation in
E-cadherin-negative esophageal cancers results in cell invasion.
PLoS One. 6:e270632011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen C, Zimmermann M, Tinhofer I, Kaufmann
AM and Albers AE: Epithelial-to-mesenchymal transition and cancer
stem(−like) cells in head and neck squamous cell carcinoma. Cancer
Lett. 338:47–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin AH, Miraglia S, Zanjani ED,
Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J and Buck
DW: AC133, a novel marker for human hematopoietic stem and
progenitor cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
33
|
Katoh Y and Katoh M: Comparative genomics
on PROM1 gene encoding stem cell marker CD133. Int J Mol Med.
19:967–970. 2007.PubMed/NCBI
|
|
34
|
Jao SW, Chen SF, Lin YS, Chang YC, Lee TY,
Wu CC, Jin JS and Nieh S: Cytoplasmic CD133 expression is a
reliable prognostic indicator of tumor regression after neoadjuvant
concurrent chemoradiotherapy in patients with rectal cancer. Ann
Surg Oncol. 19:3432–3440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanei T, Morimoto K, Shimazu K, Kim SJ,
Tanji Y, Taguchi T, Tamaki Y and Noguchi S: Association of breast
cancer stem cells identified by aldehyde dehydrogenase 1 expression
with resistance to sequential Paclitaxel and epirubicin-based
chemotherapy for breast cancers. Clin Cancer Res. 15:4234–4241.
2009. View Article : Google Scholar : PubMed/NCBI
|