Introduction
Glioblastoma (GBM) is the most common primary brain
tumor characterized with high malignancy (1). Despite of the multiple therapies
developed during the recent decades, including surgery,
chemotherapy and radiation, the median survival of GBM is less than
16 months (2,3). One of the reasons for such poor
prognosis is the tendency of GBM to invade into surrounding brain
parenchyma even at early phase of tumorigenesis (4). Infiltration of cancer cells into
normal brain tissue makes it impossible for complete surgical
removal, and increases the risk of resistance to conventional
chemotherapy and radiation, finally leads to recurrence (5). Therefore, how to decrease the invasion
activity of cancer cells became an important issue in the
development of innovative strategies for GBM treatment.
Malignant cells are under the surveillance of immune
cells to resist invasion, which is an important aspect of
anticancer immunity. Although normal brain tissue is usually
acknowledged as immunologically privileged, the impaired barrier of
tumor vasculature of GBM facilitates the infiltration and
accumulation of immune cells in the tumor microenvironment
(6). Macrophages are the most
abundant immune cells in GBM tissue, up to 30% of total tumor mass
(7). GBM-infiltrated macrophages
are composed by two different subgroups with distinct origin but
similar phenotypes, including microglia arising from resident CNS
macrophages, and tumor-associated macrophages arising form
circulating monocytes (8,9). These two subgroups are shown to
promote GBM progression from multiple aspects, including
vasculogenesis, immune suppression and GBM cell invasion (10,11).
Co-cultivation with primary microglia cells enhanced the expression
and activation of matrix metalloproteinases (MMPs) of glioma cells,
and also promoted their invasion into collagen matrix (12–14).
However, the above studies only showed the invasion-promoting
effects of microglia cells, and there is little evidence concerning
the role of another major macrophage subgroup, the tumor-associated
macrophages, in the modulation of GBM invasion process. Moreover,
how macrophages adapted such phenotype and function in GBM
microenvironment still needs further investigation.
Hypoxia is one of the well-acknowledged
characteristics of GBM microenvironment, due to rapid cell
proliferation and inadequate vascularization (15). Hypoxic GBM cells showed increased
invasive capacity, resistance to therapies and poor prognosis of
patients (16,17). Besides tumor cells, hypoxia
microenvironment also modulated the functions and biological
behavior of immune cells infiltrated in the tumor, including
macrophages. Previous studies have found that hypoxia-modulated
macrophages promoted the invasive and metastatic behavior of
various types of cancer cells, such as gastric and colon cancer
(18–20). However, the effects of hypoxic
tumor-associated macrophages on GBM invasion are largely unknown.
Based on the above observations, we speculate that the hypoxia
microenvironment in GBM may modulate the functions of
tumor-associated macrophages, including their effects on GBM cell
invasion. In the present study, we verified the above speculation
by showing that hypoxia enhanced CCL4 expression in THP-1-derived
macrophages and CCR5 expression in GBM cell line U87, respectively.
The enhancement of CCL4-CCR5 axis led to increased MMP-9 expression
in U87 cells, and promoted their invasive capacity. Our results
suggested that hypoxia plays an important role in the development
of a prone-invasion microenvironment in GBM tissue by not only
directly affecting the biological behavior of tumor cells, but also
modulating the functions of tumor-associated macrophages. Hypoxia
in tumor tissue would be a potential target for the treatment of
GBM.
Materials and methods
Reagents and antibodies
Phorbol-12-myristate-13-acetate (PMA) was obtained
from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal anti-human CCL4
neutralizing antibody (NAb) were obtained from R&D Systems
(Minneapolis, MN, USA). Fluorescein isothiocyanate (FITC) or
phycoerythrin (PE)-labeled monoclonal antibodies for CCR2, CCR3,
CCR4, CCR5 and CCR7 were obtained from R&D Systems.
Cell culture and generation of
THP-1-derived macrophages
Human GBM cell line U87 and acute monocytic leukemia
cell line THP-1 was obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). The cells were cultured in
RPMI-1640 medium supplemented with heat-incubated 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C
in normoxic (21% O2, 5% CO2, 74%
N2) or hypoxic (1% O2, 5% CO2, 95%
N2) incubators. In some cases, CCR5 siRNA or scramble
control siRNA were transfected into U87 cells before the
cultivation in normoxic or hypoxic conditions, and the details are
described in the following section.
To generate THP-1-derived macrophages,
2×105 THP-1 cells were incubated in normoxic or hypoxic
incubators and treated with 100 ng/ml PMA for 48 h, as previously
described (21). Then, the medium
was washed and replaced by fresh RPMI-1640 medium with 10% FBS for
another 24 h under normoxic or hypoxic conditions to obtain
macrophage supernatant.
Cultivation of U87 cells with
macrophage supernatant
For the treatment of macrophage supernatant to GBM
cells, 2×105 U87 cells in 2 ml RPMI-1640 medium with 10%
FBS were plated into 6-well plate and incubated for 24 h under
normoxic or hypoxic conditions. Then, the medium were replaced by
50% normoxic or hypoxic macrophage supernatant, respectively, and
cultured for another 24 h under normoxic or hypoxic conditions.
After the cultivation procedure, U87 cells were collected for
further invasion assay. In some cases, CCL4 (NAb, 50 µg/ml) was
added along with macrophage supernatant.
Cell invasion assay
The invasion assay of U87 cells was performed as
previously described (22) with
minor modifications. Briefly, 50 µl of diluted 1:4 Matrigel in
serum-free RPMI-1640 medium was added to the upper chamber of
24-well Transwell inserts (8-µm pores; both from BD Biosciences,
Franklin Lakes, NJ, USA) and incubated at 37°C overnight for
gelling. U87 cells (5×104), previously treated
with/without macrophage supernatant for 24 h, were resuspended in
100 µl serum-free medium and were plated into the upper chamber of
Transwell inserts coated with Matrigel. RPMI-1640 medium (600 µl)
containing 10% FBS were added to the lower chamber. After
incubating under normoxic or hypoxic conditions for 48 h, the
noninvaded cells on top of the Transwell were removed with a cotton
swab. The invaded cells were fixed by 10% formalin and stained with
eosin. Three fields of each well were photographed, and cell
numbers were determined.
Real-time quantitative RT-PCR
Total RNA was extracted by TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized through
the reverse transcription. Total RNA (1.0 µg) was transcribed into
cDNA with oligo(dT)16 primers and Moloney murine leukemia virus
reverse transcriptase according to the manufacturer's instructions
(Invitrogen). Quantitative RT-PCR was performed on the LightCycler
2.0 Instrument (Roche Diagnostic, Mannheim, Germany). GAPDH was
used as an internal control. Primers for MMP-2, MMP-9, CCL3, CCL4,
CCL5, interferon regulatory factor-8 (IRF-8) and GAPDH are listed
in Table I. Five microliters of 2X
SYBR®-Green qPCR Master mix (Thermo Fisher Scientific,
Waltham, MA, USA), 1 µl of forward primer, 1 µl of reverse primer,
1 µl of cDNA and 2 µl of ddH2O was added into the
reaction mixture and incubated at 94°C for 30 sec, 58°C for 30 sec
and 72°C for 45 sec for 40 cycles. The mRNA level of each sample
was measured by the 2−∆∆Ct method (23).
 | Table I.Primer sequences for RT-PCR. |
Table I.
Primer sequences for RT-PCR.
| Gene name |
| Primer
sequences |
|---|
| MMP-2 | F | 5′-CAA GTT TCC ATT
CCG CTT C-3′ |
|
| R | 5′-GTT CCC ACC AAC
AGT GGA CA-3′ |
| MMP-9 | F | 5′-TTG ACA GCG ACA
AGA AGT GGG-3′ |
|
| R | 5′-GCC ATT CAC GTC
GTC CTT AT-3′ |
| CCL3 | F | 5′-TGG CTC TCT GCA
ACC AGT TCT-3′ |
|
| R | 5′-GTA GCT GAA GCA
GCA GGC G-3′ |
| CCL4 | F | 5′-CCG TGT TAT TGT
ATT AGG TG-3′ |
|
| R | 5′-GAA TCA AAT GTG
TTA TCC ATG T-3′ |
| CCL5 | F | 5′-GGC AGC CCT CGC
TGT CAT CCT CA-3′ |
|
| R | 5′-CTT GAT GTG GGC
ACG GGG CAG TG-3′ |
| IRF-8 | F | 5′-AGT AGC ATG TAT
CCA GGA CTG AT-3′ |
|
| R | 5′-CAC AGC GTA ACC
TCG TCT TC-3′ |
| GAPDH | F | 5′-GGT GGT CTC CTC
TGA CTT CAA CAG-3′ |
|
| R | 5′-GTT GCT GTA GCC
AAA TTC GTT GT-3′ |
Flow cytometry
To analyze the expression of chemokine receptors
CCR2, CCR3, CCR4, CCR5 and CCR7, normoxic or hypoxic U87 cells were
stained by monoclonal antibodies labeled with FITC or PE. Isotype
controls were run in parallel. The analysis was performed by a
FACSCalibur flow cyto-meter, and the mean fluorescence intensity
was determined by CellQuest version 3.3 software (BD
Biosciences).
RNA interference
Small-interference RNA (siRNA) targeting CCR5 and
scramble control siRNA were designed by GenePharma Co., Ltd
(Shanghai, China). For transient silencing, 5×104/ml U87
cells were seeded onto 24-well plates and transfected with siRNA
(all 80 nmol/l) using Lipofectamine RNAiMAX reagent (Invitrogen)
following the manufacturer's protocol.
Enzyme-linked immunosorbent assay
(ELISA)
The supernatant of normoxic or hypoxic THP-1-derived
macrophages was collected. The concentrations of CCL3, CCL4 and
CCL5 in supernatant were determined by ELISA kits (R&D Systems)
according to the manufacturer's instructions.
Statistical analysis
Data are primarily presented as the mean ± SD. The
SPSS software package (version 13.0; SPSS, Inc., Chicago, IL, USA)
was used for all statistical analysis. The distribution of the
samples was determined via Kolmogorov-Smirnov test. The results of
experiments were analyzed by t-test or one-way ANOVA wherever
appropriate. Tukey post hoc comparison was performed when
statistical significance (p<0.05) was found between
observations.
Results
Hypoxia and macrophage supernatant
promote invasion of U87 cells by upregulating MMP-9 expression
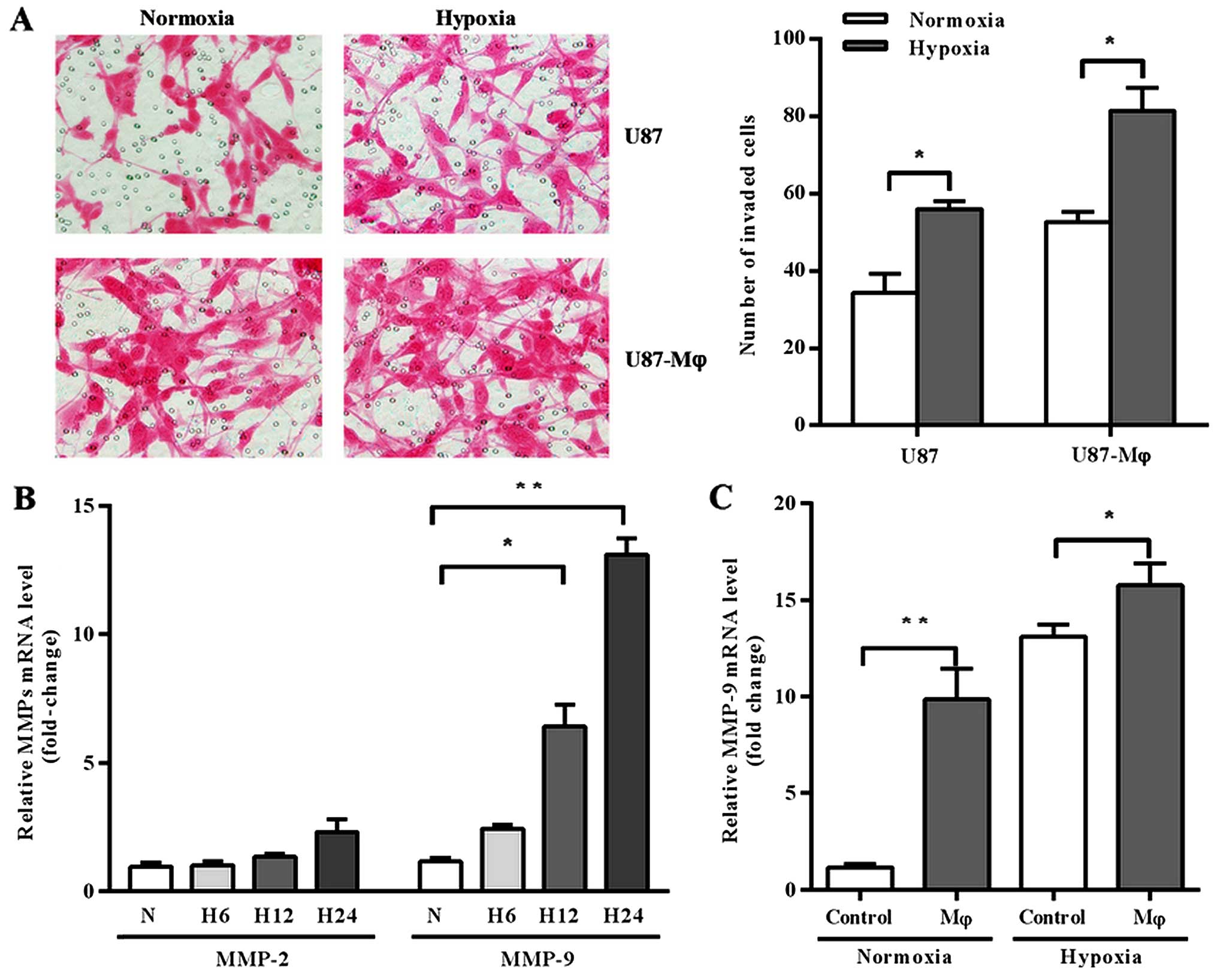
Firstly, we analyzed the effects of hypoxia and
macrophage supernatant on invasion of GBM cell line U87 by
Matrigel-coated Transwell assay. The results showed that both
hypoxia and macrophage supernatant promoted U87 invasion. Moreover,
U87 cells gained more invasive capacity when they were pre-treated
by hypoxic macrophage supernatant before cultured under hypoxia
conditions (Fig. 1A).
We further investigated whether the modification of
U87 cell invasive activity was dependent on MMPs expression, since
they are acknowledged for the involvement in cell invasion process.
We analyzed the expression of MMP-2 and MMP-9 in U87 cells, which
are two of the most studied MMPs and closely associated with cell
invasive capacity. Firstly, we explored the effect of hypoxia on
MMP-2 and MMP-9 expression at different time point. As shown in
Fig. 1B, the expression of MMP-2 in
U87 cells remained relatively constant in hypoxic condition, but
MMP-9 was significantly upregulated under hypoxia stimulation in a
time-related fashion (p=0.0108 at 12 h, p=0.0006 at 24 h).
Secondly, we analyzed the expression of MMP-9 under the treatment
of macrophage supernatant and found that macrophage supernatant
upregulated MMP-9 expression either in normoxic or hypoxic
conditions (Fig. 1C; p=0.0114 and
0.0118, respectively).
Hypoxia increases CCR5 expression
regulated MMP-9 expression and invasion of U87 cells
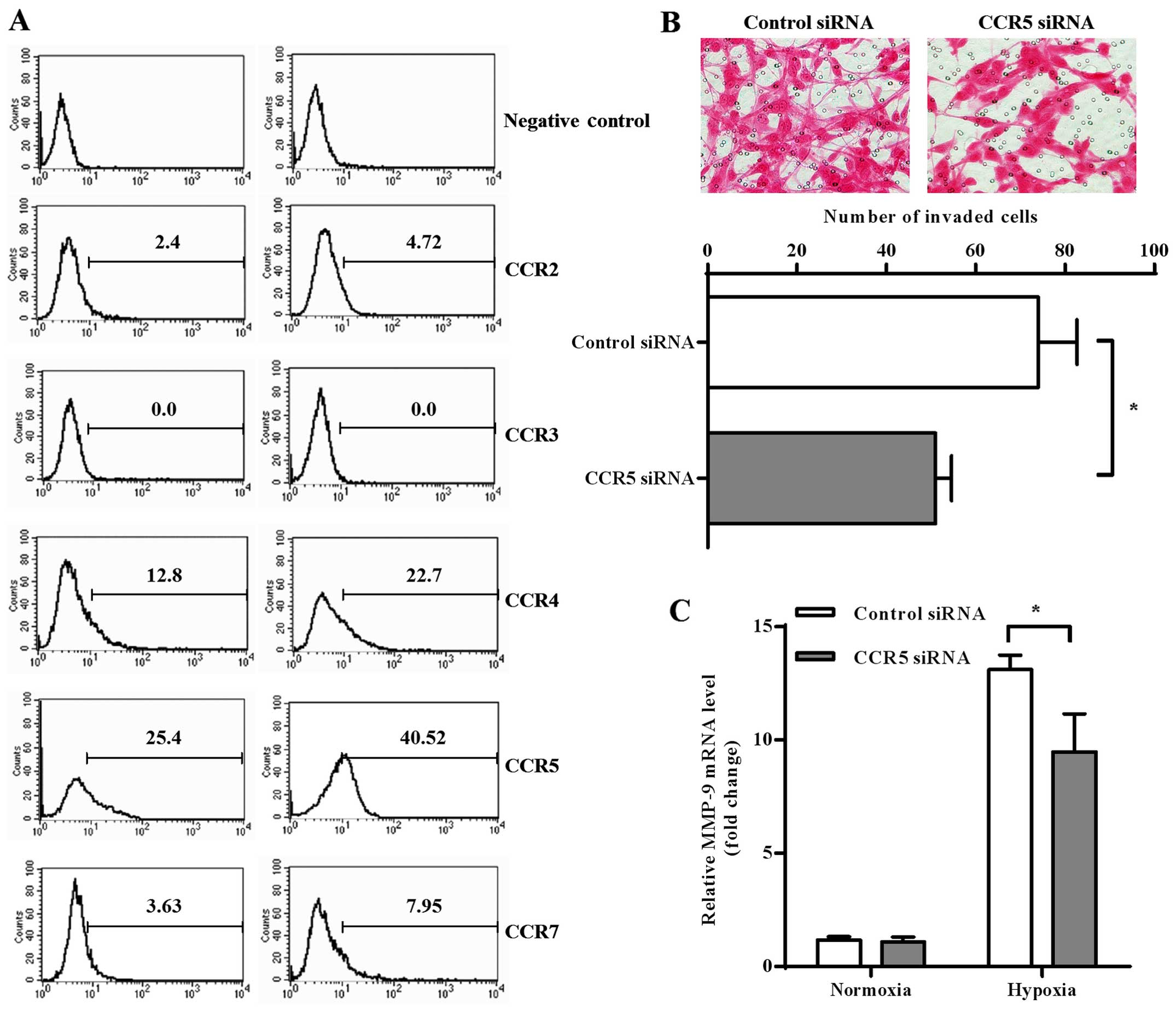
Chemokine receptors have been shown as important
regulators for invasion process of cancer cells (21). In order to investigate whether the
axis of chemokine-chemokine receptors was involved in
hypoxia-modulated invasion of GBM cells, we measured expression of
several CC chemokine receptors (CCRs) on normoxic and hypoxic U87
cells. The results of flow cytometry found relatively high
expression of CCR4 and CCR5 on U87 cells, while low expression of
CCR2 and CCR7 and no CCR3 was found. Both CCR4 and CCR5 expression
was significantly increased after hypoxia treatment, but CCR5
showed higher expression than CCR4 in both normoxic and hypoxic U87
cells (Fig. 2A).
Based on the above observations, we chose CCR5 for
further investigation into its possible role in GBM invasion. We
knocked down CCR5 expression on U87 cells by siRNA transfection and
found that CCR5 downregulation significantly decreased invasion
activity of hypoxic U87 cells (Fig.
2B; p=0.0237). We also found that CCR5 knockdown decreased
MMP-9 transcription in hypoxic U87 cells (p=0.0100), while no
significant downregulation was observed under normoxia condition
(Fig. 2C).
Hypoxia increases CCL4 secretion in
THP-1-derived macrophage-regulated MMP-9 expression and U87
invasion
Macrophages are important targets and sources of
chemokines. Since the above results found that hypoxic macrophage
supernatant promoted U87 cells invasion, and hypoxia upregulated
CCR5 expression affected the invasion process, we further
investigated whether CCR5-related chemokines, including CCL3, CCL4
and CCL5 in hypoxic macrophage supernatant were involved in this
modulation. As showed by our results, the concentrations of
CCR5-related chemokines secreted by THP-1-derived macrophages were
higher than those secreted by U87 cells (Fig. 3A), and they exhibited different
expression profile under hypoxia stimulation. Only the expression
of CCL4 was significantly upregulated (p=0.0149) in hypoxic
THP-1-derived macrophages, while CCL5 was decreased and CCL3
remained relatively unchanged (Fig.
3B). The results of ELISA were consistent with RT-PCR results
(Fig. 3C; p=0.0385).
In order to verify whether upregulated CCL4 secreted
by hypoxic macrophages promoted U87 invasion process, CCL4 NAb (50
µg/ml) was added into hypoxic macrophage supernatant before its
stimulation toward U87 cells. We found that CCL4 NAb in hypoxic
macrophage supernatant significantly inhibited both MMP-9
expression and invasion capacity of U87 cells (Fig. 3D and E; p=0.0086 and 0.0032,
respectively).
Hypoxia promotes IRF8 involved in CCL4
expression in THP-1-derived macrophages
We went on to explore the mechanism by which hypoxia
regulated CCL4 expression in THP-1-derived macrophages. Interferon
regulatory factors (IRFs) are important transcription factors in
the maturation and differentiation of myeloid cells, and are
closely associated with the function of macrophages. We first
investigated whether hypoxia may regulate expression of IRFs in
THP-1-derived macrophages, and surprisingly found that hypoxia
greatly increased IRF-8 transcription in macrophages by ~300 times
(Fig. 4A). To verify whether
increased IRF-8 was involved in CCL4 expression of THP-1-derived
macrophages, we knocked down its transcription by siRNA
transfection. As Fig. 4B shows
IRF-8 downregulation significantly decreased CCL4 secretion of
hypoxic THP-1-derived macrophages (p=0.0053), while had little
effects on normoxic CCL4 secretion (p=0.2396).
Discussion
In the present study, we analyzed the effects of
macrophage supernatant on invasive capacity of glioblastoma (GBM)
cells, and the modulation of hypoxia in this process. We found that
THP-1-derived macrophage-secreted CCL4 promoted MMP-9 expression on
U87 cells by interacting with CCR5. Hypoxia enhanced CCL4 secretion
in THP-1-derived macrophages by upregulating the transcriptional
factor IRF8, and also promoted CCR5 expression on GBM U87 cells,
therefore promoting the interaction between these two cell lines.
The enhanced CCL4-CCR5 axis further increased the invasive
activities and MMP-9 transcription of U87 cells. The results of the
present study revealed the effects of hypoxia on the interactions
and possible mechanisms that occurred between macrophages and GBM
cells, and the role of this interaction on GBM invasion. The
present study also suggested that hypoxia microenvironment may be a
potential target for resisting the invasion in the treatment of
GBM.
The axis of chemokines and chemokine receptors has
been demonstrated as important regulators in the invasion and
metastasis process of cancer cells. CCR5, whose ligands include
CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES), was reported to be
highly expressed in GBM tissue (24,25). A
recent study showed that the overexpression of CCR5 was associated
with poor prognosis of GBM patients, and activation of CCR5
signaling promoted the invasion of U87 and U251 cell lines in
vitro by upregulating MMP-9 expression (24). These observations were consistent
with our findings, as we found that CCR5 knockdown in U87 cells
reduced their invasive capacity as well as MMP-9 transcription.
However, until now there are few clues to explain the formation of
such CCR5 overexpressed characteristics occurring in GBM tissue. In
the present study, we occasionally found that hypoxia significantly
upregulated CCR5 expression in U87 cells. The upregulation of
hypoxia on CCR5 expression was also found in breast cancer cells by
a series of transcriptional and post-transcriptional modulations
(26). Taken together, these
results suggested that hypoxia may be a common regulator for CCR5
expression in tumor microenvironment.
Macrophages are important sources of chemokines.
According to our results, THP-1-derived macrophages expressed
relatively high concentration of all three CCR5-associated
chemokines, including CCL3, CCL4 and CCL5, whereas the expression
of these chemokines was low in U87 cells. Hence, we speculated that
macrophage-derived chemokines may at least partially explain why
macrophage supernatant could promote GBM cell invasion, and why
hypoxia supernatant had higher promoting effects. In the present
study, we found that CCL4 was the only one that was promoted by
hypoxia in all three CCR5-associated chemokines produced by
THP-1-derived macrophages, and its expression in supernatant
enhanced the invasion and MMP-9 expression of U87 cells. Several
chemokine/chemokine receptors have been shown to promote an
invasive phenotype in glioma cells. One of the most acknowledged
was the stromal cell-derived factor-1α (SDF-1α)-CXCR4 system
(27). The CXCR4 expression in
glioma cell lines was upregulated under hypoxia stimulation, and
enhanced their migrating or invasive activities in respond to
SDF-1α (28). Our studies found a
new axis, the CCL4-CCR5 system, which played key roles in hypoxic
GBM cell invasion. As macrophages were one of the major CCL4
sources in GBM tissue, this axis also emerged as important mediator
in the interactions between tumor-associated macrophages and GBM
cells, and hypoxia may enhance this interaction by promoting both
CCL4 and CCR5 expression in the two cell types, respectively.
The mechanism by which hypoxia promoted CCL4
expression in macrophages was also preliminarily explored in the
present study. Interferon regulatory factors (IRFs) are an
important family of intracellular protein that regulate maturation
and polarization of macrophages (29). Various members of this family, such
as IRF-1 and IRF-3, have been shown to induce chemokines expression
in macrophages (30–32). In the present study, we found for
the first time that hypoxia greatly enhanced the transcription of
IRF-8 in THP-1-derived macrophages, and IRF-8 was involved in CCL4
expression. Previous studies found that IRF-8 plays a dominant role
not only in the differentiation of macrophages from their immature
progenitors, but also in their phenotypes and functions. For
example, IRF-8 induces a M1-type gene profile upon TLR stimulation,
enhancing the expression of a number of pro-inflammatory cytokines,
including IFN-β, IL-12p40 and IL-12p35 (33). Our findings provided more evidence
on the modulation of IRF-8 in chemokines secretion of macrophage.
These findings will help to refine the roles of IRF-8 in various
physiological or pathological processes, such as tumor development,
inflammation and tissue repair, since all these processes are
characterized with change of oxygen tension and infiltration of
immunocytes, particularly macrophages. However, how hypoxia
modulated IRF-8 expression in macrophages was not demonstrated in
the present study. As the interaction between IRF-8 and chromatin
is repressed by small ubiquitin-like modifiers (SUMO), and hypoxia
is an important regulator of sumoylation status in variant types of
cells (34–36), the post-transcriptional modulation
of IRF-8 could be an important mechanisms in hypoxic
macrophages.
In conclusion, the present study suggested for the
first time that macrophage-secreted CCL4 would promote the invasive
capacity of GBM cells by interacting with CCR5 receptor, and
hypoxia enhanced this interaction by upregulating both CCL4 and
CCR5 expression in these two cell types. Our findings will define
additional roles of tumor-infiltrated macrophages in GBM
development, and contributed to better understanding of how tumor
hypoxia microenvironment modifies local immune system in the
pathophysiology of GBM.
Acknowledgements
The present study was supported by the Foundation of
Shandong Provincial Science and Technology Development Plan
(2014GGH218017) and the Special Foundation for Taishan Scholars
(no. ts20110814).
Glossary
Abbreviations
Abbreviations:
|
GBM
|
glioblastoma
|
|
CNS
|
central nervous system
|
|
TAM
|
tumor associated macrophage
|
|
MMP
|
matrix metalloproteinase
|
|
PMA
|
phorbol-12-myristate-13-acetate
|
|
NAb
|
neutralizing antibody
|
|
FBS
|
fetal bovine serum
|
|
IRF
|
interferon regulatory factor
|
References
|
1
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davis FG, McCarthy BJ, Freels S, Kupelian
V and Bondy ML: The conditional probability of survival of patients
with primary malignant brain tumors: Surveillance, epidemiology,
and end results (SEER) data. Cancer. 85:485–491. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyer MA: Malignant gliomas in adults. N
Engl J Med. 359:18502008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanu OO, Mehta A, Di C, Lin N, Bortoff K,
Bigner DD, Yan H and Adamson DC: Glioblastoma multiforme: A review
of therapeutic targets. Expert Opin Ther Targets. 13:701–718. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hussain SF, Yang D, Suki D, Aldape K,
Grimm E and Heimberger AB: The role of human glioma-infiltrating
microglia/macrophages in mediating antitumor immune responses.
Neuro Oncol. 8:261–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Graeber MB, Scheithauer BW and Kreutzberg
GW: Microglia in brain tumors. Glia. 40:252–259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kushchayev SV, Kushchayeva YS, Wiener PC,
Scheck AC, Badie B and Preul MC: Monocyte-derived cells of the
brain and malignant gliomas: The double face of Janus. World
Neurosurg. 82:1171–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang I, Han SJ, Kaur G, Crane C and Parsa
AT: The role of microglia in central nervous system immunity and
glioma immunology. J Clin Neurosci. 17:6–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Charles NA, Holland EC, Gilbertson R,
Glass R and Kettenmann H: The brain tumor microenvironment. Glia.
60:502–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
da Fonseca AC and Badie B: Microglia and
macrophages in malignant gliomas: Recent discoveries and
implications for promising therapies. Clin Dev Immunol.
2013:2641242013.PubMed/NCBI
|
|
12
|
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen
L, Xiao HL, Wang B, Yi L, Wang QL, et al: Tumor-associated
microglia/macrophages enhance the invasion of glioma stem-like
cells via TGF-β1 signaling pathway. J Immunol. 189:444–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Markovic DS, Vinnakota K, Chirasani S,
Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, van
Rooijen N, et al: Gliomas induce and exploit microglial MT1-MMP
expression for tumor expansion. Proc Natl Acad Sci USA.
106:12530–12535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin HC, Song TY and Hu ML:
S-Adenosylhomocysteine promotes the invasion of C6 glioma cells via
increased secretion of matrix metalloproteinase-2 in murine
microglial BV2 cells. Toxicol Sci. 112:322–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans SM, Judy KD, Dunphy I, Jenkins WT,
Hwang WT, Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM,
et al: Hypoxia is important in the biology and aggression of human
glial brain tumors. Clin Cancer Res. 10:8177–8184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
16. Kessler J, Hahnel A, Wichmann H, Rot
S, Kappler M, Bache M and Vordermark D: HIF-1α inhibition by siRNA
or chetomin in human malignant glioma cells: Effects on hypoxic
radioresistance and monitoring via CA9 expression. BMC Cancer.
10:6052010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen Z, Kauttu T, Seppänen H, Vainionpää
S, Ye Y, Wang S, Mustonen H and Puolakkainen P: Both macrophages
and hypoxia play critical role in regulating invasion of gastric
cancer in vitro. Acta Oncol. 52:852–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen Z, Seppänen H, Vainionpää S, Ye Y,
Wang S, Mustonen H and Puolakkainen P: IL10, IL11, IL18 are
differently expressed in CD14+ TAMs and play different
role in regulating the invasion of gastric cancer cells under
hypoxia. Cytokine. 59:352–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mu L, Wang J, Chen Y, Li L, Guo X, Zheng S
and Jing C: Hypoxia-inducible factor-1α and semaphorin4D genes
involved with tumor-associated macrophage-induced metastatic
behavior and clinical significance in colon cancer. Chin Med J.
127:3568–3575. 2014.PubMed/NCBI
|
|
21
|
Zhao P, Gao D, Wang Q, Song B, Shao Q, Sun
J, Ji C, Li X, Li P and Qu X: Response gene to complement 32
(RGC-32) expression on M2-polarized and tumor-associated
macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4.
Cell Mol Immunol. 12:692–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Han M, Chen W, He Y, Huang B, Zhao
P, Huang Q, Gao L, Qu X and Li X: KIF1B promotes glioma migration
and invasion via cell surface localization of MT1-MMP. Oncol Rep.
35:971–977. 2016.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Wang Y, Xue Y, Lv W, Zhang Y and
He S: Critical roles of chemokine receptor CCR5 in regulating
glioblastoma proliferation and invasion. Acta Biochim Biophys Sin.
47:890–898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pham K, Luo D, Liu C and Harrison JK:
CCL5, CCR1 and CCR5 in murine glioblastoma: Immune cell
infiltration and survival rates are not dependent on individual
expression of either CCR1 or CCR5. J Neuroimmunol. 246:10–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin S, Wan S, Sun L, Hu J, Fang D, Zhao R,
Yuan S and Zhang L: Chemokine C-C motif receptor 5 and C-C motif
ligand 5 promote cancer cell migration under hypoxia. Cancer Sci.
103:904–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Sarkar S, Cua R, Zhou Y, Hader W
and Yong VW: A dialog between glioma and microglia that promotes
tumor invasiveness through the CCL2/CCR2/interleukin-6 axis.
Carcinogenesis. 33:312–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zagzag D, Lukyanov Y, Lan L, Ali MA,
Esencay M, Mendez O, Yee H, Voura EB and Newcomb EW:
Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in
glioblastoma: Implications for angiogenesis and glioma cell
invasion. Lab Invest. 86:1221–1232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Günthner R and Anders HJ:
Interferon-regulatory factors determine macrophage phenotype
polarization. Mediators Inflamm. 2013:7310232013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Génin P, Algarté M, Roof P, Lin R and
Hiscott J: Regulation of RANTES chemokine gene expression requires
cooperativity between NF-kappa B and IFN-regulatory factor
transcription factors. J Immunol. 164:5352–5361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Guan X and Ma X: Interferon
regulatory factor 1 is an essential and direct transcriptional
activator for interferon γ-induced RANTES/CCl5 expression in
macrophages. J Biol Chem. 280:24347–24355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yarilina A, Park-Min KH, Antoniv T, Hu X
and Ivashkiv LB: TNF activates an IRF1-dependent autocrine loop
leading to sustained expression of chemokines and STAT1-dependent
type I interferon-response genes. Nat Immunol. 9:378–387. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holtschke T, Löhler J, Kanno Y, Fehr T,
Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF,
et al: Immunodeficiency and chronic myelogenous leukemia-like
syndrome in mice with a targeted mutation of the ICSBP gene. Cell.
87:307–317. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koh MY, Nguyen V, Lemos R Jr, Darnay BG,
Kiriakova G, Abdelmelek M, Ho TH, Karam J, Monzon FA, Jonasch E, et
al: Hypoxia-induced SUMOylation of E3 ligase HAF determines
specific activation of HIF2 in clear-cell renal cell carcinoma.
Cancer Res. 75:316–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun L, Li H, Chen J, Iwasaki Y, Kubota T,
Matsuoka M, Shen A, Chen Q and Xu Y: PIASy mediates hypoxia-induced
SIRT1 transcriptional repression and epithelial-to-mesenchymal
transition in ovarian cancer cells. J Cell Sci. 126:3939–3947.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu YC, Ling TY, Lu SH, Kuo HC, Ho HN, Yeh
SD, Shen CN and Huang YH: Chemotherapeutic sensitivity of
testicular germ cell tumors under hypoxic conditions is negatively
regulated by SENP1-controlled sumoylation of OCT4. Cancer Res.
72:4963–4973. 2012. View Article : Google Scholar : PubMed/NCBI
|