Introduction
Thyroid cancer is one of the most common types of
endocrine malignancy, with a gradual increase in the incidence
rate; and it is the fifth leading cause of cancer globally,
accounting for 2.1% of all the newly detected cancers (1,2). Most
of the cases of thyroid cancers show biologically indolent
phenotype and have an excellent prognosis with survival rates of
more than 95% at 20 years although the recurrence or persistent
rate is still high (2).
As classified by World Health Organization (WHO),
most primary thyroid cancers are epithelial tumors that originate
from thyroid follicular cells, comprising three main pathological
types: papillary thyroid carcinoma (PTC), follicular thyroid
carcinoma (FTC) and anaplastic thyroid carcinoma (ATC). While
another kind of epithelial tumor, medullary thyroid carcinoma (MTC)
arises from thyroid parafollicular cells. PTC and FTC are
categorized as differentiated thyroid cancer (DTC), they are
well-differentiated and have indolent tumor growth (3). They account for >90% of thyroid
cancers, while MTC only accounts for <5% (4).
Radioiodine is a safe and effective modality used
for more than 60 years in treating thyroid cancers and has
potential uses in breast, prostate and other cancers.
131I is generally used in radiotherapy and for in
vivo experiments because its half-life of merely 8 days
(5). Ablative radioiodine therapy
exploits the ability of the thyroid to take up iodide, a process
mediated by the sodium iodide symporter (NIS), which is an integral
membrane glycoprotein located in the basolateral plasma membrane of
thyroid follicular epithelial cells (6,7).
However, thyroid tumors such as FTC have a chance of developing
aggressive thyroid carcinomas with metastatic propensity and
resistance to radioiodine (5,8–11). As
it is more likely to produce distant metastases, FTC may have a
poorer prognosis compared with PTC (10–12).
It is necessary to identify the underlying
mechanisms leading to decreased efficiency of radioiodine therapy
in those radioiodine-refractory thyroid tumors. As mentioned above,
for active iodide uptake to occur, adequate amount of NIS must be
located in the cytomembrane of thyroid follicular epithelial cells
(13). Numerous researchers have
focused on finding ways to increase NIS expression in thyroid
cancers, with expectation of improving the iodide transport ability
in tumor cells and elevating the effectiveness of radioiodine
therapy (14,15). However, according to some other
studies, NIS is overexpressed in some cases of thyroid cancers
(detected by immunohistochemistry), wherein the impaired function
of NIS may be attributed to its abnormal internalization (7,13–20).
Thus, it may be the post-translational regulation rather than the
decreased expression of NIS that robustly contribute to a reduction
in iodide uptake in some thyroid cancers (21,22).
Therefore, it is of great importance to explore the factors taking
part in regulating the NIS subcellular localization, especially in
the cases of aggressive thyroid carcinomas resistance to
radioiodine therapy.
Catenin beta-1 (β-catenin) is a dual function
protein regulating cell-cell adhesion as well as transcription of
number of genes, the latter of which is mainly controlled by Wnt
signaling molecules (canonical Wnt pathway) (23). In 2014, Sastre-Perona and
Santisteban (24) revealed the
Wnt-independent role of β-catenin in regulating the proliferation
and differentiation of rat thyroid follicular cells. They found
that direct interaction of β-catenin with Pax8 resulted in
increased activity of NIS. According to their conclusion, β-catenin
is possibly a positive regulator of NIS and iodide uptake activity
in physiological conditions. On the other hand, studies have shown
that β-catenin was overexpressed in aggressive thyroid cancers
(25–28). Whether β-catenin plays a critical
role in affecting the expression of NIS within thyroid tumor cells,
as well as regulating the abnormal subcellular localization, is
worthy of investigation.
Previous studies have discovered that β-catenin can
enhance HIF-1 mediated transcription events, thus, promoting cancer
cell survival and endowing them with better adaptability to hypoxia
(23,25,29,30).
HIF are associated with poor prognosis in many tumor types
(31,32). Like β-catenin, the overexpression of
HIF-1α was also observed in thyroid cancers, and the potential
significance of the HIF signaling pathway in progression and
aggressiveness of thyroid carcinoma has been demonstrated (33). Compared with that in normal thyroid
tissues, HIF-1α expression was significantly greater in various
types of primary thyroid tumors and ATC and FTC showed the highest
levels of expression (34).
Moreover, HIF-1α may reduce the effectiveness of cancer treatment
as it has been shown that overexpression of HIF-1α in cancer cells
plays an important role in the development of resistance to
radiotherapy and some forms of chemotherapy (35).
We presume that HIF-1α may exert its power on
desensitisation to radiotherapy in aggressive thyroid carcinomas in
β-catenin-dependent manner. This signal pathway may act through
changing membrane localization other than transcriptional level of
NIS. Therefore, in the present study, we examined the subcellular
localization of NIS in relation to β-catenin nuclear translocation,
subsequent to the activation of HIF signaling pathway in FTC cells.
We also disrupted the β-catenin signal in the regulatory mechanism
to see if the efficacy of radioiodine treatment in HIF-1α
overexpressing aggressive thyroid cancers is promoted. Our data
suggest an entirely novel mechanism that alters NIS subcellular
localization, thereby regulating cell ability to aggressively
uptake iodide in cancer cells.
Materials and methods
Cell culture
Human thyroid carcinoma cell line FTC133, which was
derived from the primary tumor of a 42-year-old male patient with
metastatic FTC (36) was kindly
provided by Professor Karl-M. Derwahl (Humboldt University, Berlin,
Germany). The FTC133 cells were cultured in Dulbeccos modified
Eagles medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO,
USA) and penicillin/streptomycin in a humidified incubator (Thermo
Scientific Forma 370; Thermo Fisher Scientific, Shanghai, China) at
37°C and 5% CO2.
Plasmids transfection
Recombinant plasmid pcDNA3.1(−)/HIF-1α was kindly
provided by Professor Leland W.K. Chung from the Winship Cancer
Center at Emory University (Atlanta, GA, USA). Recombinant plasmids
pcDNA3.1(−)/β-catenin and pSUPER/β-catenin were constructed by Dr
Yong Luo in our laboratory, the latter of which was used for shRNA
mediated knockdown of β-catenin as previously described (37).
FTC133 cells were transfected with pcDNA3.1
(−)/HIF-1α, pcDNA3.1 (−)/β-catenin by means of Lipofectamine 2000
system (Invitrogen, Carlsbad, CA, USA). After a 6 h co-incubation,
the cells were then cultured for 2 days with DMEM (containing 10%
FBS); after that, the cells were passaged (1:3) and then screened
with the selective medium (containing 400 µg/ml G418); selective
medium was replaced every 5 days. Four weeks later, all the
untransfected cells died by apoptosis and transfected cells formed
into monoclonal cell clusters; the cells were maintained in the
medium containing 200 µg/ml G418. The acquired stably transfected
lines were named as FTC133-HIF-1α and FTC133-β-catenin,
respectively.
For shRNA-mediated knockdown, FTC133-HIF-1α and
FTC133-β-catenin cells were transfected with pSUPER/β-catenin by
means of Lipofectamine 2000 (Invitrogen). After a 6 h
co-incubation, the cells were then cultured for 3 days with DMEM
(containing 10% FBS); then the cells were passaged (1:3) and
screened with the selective medium (containing 400 µg/ml puromycin)
for 4 weeks. All the untransfected cells died by apoptosis and
transfected cells formed into monoclonal cell clusters; the silent
cells were maintained in the medium containing 200 µg/ml
puromycin.
Western blotting
Cell lysate preparation and blotting conditions have
been previously described (4).
Antibodies (Gibco, Waltham, MA, USA) against HIF-1α, β-catenin, NIS
and GAPDH (as an internal control) were used for western blot
analyses as per instructions of the manufacturers.
Immunofluorescent staining
Cells were fixed in 10% paraformaldehyde for 30 min
and blocked with goat serum (Gibco) for 30 min, respectively. Cells
were then incubated at 37°C for 1 h with the rabbit anti-human
β-catenin primary antibody or the rabbit anti-human NIS antibody
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), at a dilution of
1:100 in 0.5% BSA in phosphate-buffered saline (PBS). After washing
3 times with PBS, cells were co-incubated with the appropriate
rhodamine or fluorescein-tagged goat anti-rabbit antibody
(Sigma-Aldrich), diluted at 1:50 in 0.5% BSA in PBS at 37°C for 1
h. After final washes with PBS, the coverslips were mounted on a
microscope slide. The fluorescence staining intensity and
intracellular localization were then examined by Olympus
immunofluorescence microscope (BX43).
Iodide uptake assay
125I is generally applied during iodide
uptake assay since its half-life is as long as 60 days (5,10).
Cells were grown as monolayer on 6-well plates at a
concentration of 5×104 cells/well. To measure the iodide
uptake activity, after 3–4 days of culture, cells were washed with
HBSS and incubated in the reaction buffer containing
125I (3.7 KBq) (Yuanzi, China) for 20 min at 37°C, and
cells incubated with the solvent (water-free alcohol) alone was
used as control. After incubation, cells were washed with ice-cold
HBSS 3 times, and then incubated with 1 ml ice-cold water-free
alcohol for 20 min. The radioactivity was measured for the
incubation water-free alcohol in a γ-counter (Wizard 2480;
Perkin-Elmer, Waltham, MA, USA), as previously described (38). 125I-uptake was expressed
as counts per minute.
MTT assay
Cells (1×104) were seeded into each well
of 96-well tissue culture plates. At various time-points (24, 48
and 72 h) of cell culture in DMEM, 50 µl MTT solution (2.5 mg/ml)
was added into the plate and incubated with cells for an additional
4 h. The media were collected separately from each chamber, and
cell-associated MTT crystals were dissolved separately in dimethyl
sulfuroxide (DMSO; 150 µl/well) on a shaker at room temperature.
The color intensity was measured at 570 nm against the appropriate
blank controls (0.1% BSA/RPMI-1640 medium with MTT solution and 150
µl DMSO) using a Bio-Rad Technologies microplate reader.
Invasion assay
Cells were cultured in 6-well plates to 90%
confluency and then collected after trypsinization. An analysis
assessing the invasion of cells was performed using 6-well
Transwell inserts with 6.5-mm diameters and 8-µM pores (Corning,
Corning, NY, USA). In brief, the filters were precoated for 30 min
at 37°C with 25 µl extracellular matrix (Sigma-Aldrich) gel mixed
with dimethyl sulfoxide (1:1). The trypsinised cells
(7×104) were washed with PBS, resuspended in the
serum-free medium, and placed in the upper chamber, and a medium
containing 10% FBS was used as a chemoattractant in the lower
chamber. Cells were incubated at 37°C in 5% CO2 for 24,
48 and 72 h, respectively, and the number of cells that invaded
across the membranes were fixed and stained with Giemsa. The
non-migratory cells on the upper chamber were removed with cotton
swabs, and the migratory cells present on the lower surface were
counted in 10 random fields and photographed at ×100 magnification
under an inverted microscope (Olympus).
Xenograft experiments
This part of the study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals in the Weatherall Report. In addition,
the protocol was approved by the Ethics Committee for Animal
Experiments of the Beijing University. Surgery was performed under
sodium pentobarbital (Merk, Darmstadt, Germany) anesthesia, and all
efforts were made to ameliorate suffering.
Six-week-old male SCID mice (Charles River
Laboratories, Wilmington, MA, USA) were injected subcutaneously
with 2×106 cells in the left forelimb. Animals (8–10 per
group) were monitored daily, and tumor volumes were measured by
vernier caliper with the formula: V = (LW2)/2, where L
is the length and W is the width. When xenograft tumor grew to the
volume of 50 mm3, mice of the experiment groups (see the
corresponding figure legends for the specific group settings) were
treated with peritoneal injection of 37MBq 131I, whereas
those of control groups were injected with 0.1 ml physical saline.
Thirty-five days after the radioiodine treatment, tumors were
weighed and fixed overnight at 4°C in 10% paraformaldehyde and
embedded in paraffin for histological analysis.
Immunohistochemical staining
Paraffin-embedded sections of xenograft cancer
tissues were used for immunohistochemical staining, using a
commercially available kit (Boshide, Wuhan, China). Primary
antibodies (Sigma-Aldrich) against β-catenin, NIS, cell cycle
protein (Ki-67), anti-apoptosis protein (survivin) and apoptosis
protein (caspase-3) were used. The samples were then counter
stained with hematoxylin to indicate the nucleus.
The results were observed by three physicians, who
were blinded to the grouping. For every index, 3 pictures of cancer
tissues from each animal (n=10, per group) were selected for
quantification. Five high power fields were selected from each
picture to assess the percentage of positively staining cells in
all fields of observation.
Statistical analysis
Statistical analyses were performed using the SPSS
statistical software 13.0. The data are represented as mean ±
standard derivation (SD). Statistical significance was evaluated by
one-way analysis of variance (ANOVA) with the Student-Newman-Keuls
(SNK) test for post hoc test or Student's t-test. P<0.05 was
considered statistically significant.
Results
HIF-1α overexpression endowed FTC
cells with aggressiveness in a β-catenin-dependent manner
As shown clearly by western blotting (Fig. 1A), HIF-1α overexpression upregulated
β-catenin in FTC cells, but not vice versa. Application of
β-catenin shRNA can successfully reduce the protein amount of
β-catenin.
FTC cells overexpressing either HIF-1α or β-catenin
were compared for changes in the content of several markers
concerned with epithelial-mesenchymal transitions (EMT) (Fig. 2A). There is a decrease in the levels
of epithelial markers E-cadherin and CK18 but an increase in the
levels of vimentin, VEGF, fibronectin and MMP-2 in the FTC cells
overexpressing either HIF-1α or β-catenin. However, these EMT
phenotypes were all restored after knockdown of β-catenin.
We further found that HIF-1α/β-catenin
overexpression will favor FTC cells with stronger growth ability as
well as metastatic propensity (Fig. 2B
and C). Whereas, once the β-catenin expression was reduced by
shRNA knockdown, the changes in the aggressiveness of the thyroid
cancer cells were reversed.
β-catenin activation subsequent to
HIF-1α overexpression changes NIS subcellular localization and
impair cell radioiodine uptake
As evident in western blotting (Fig. 1A), HIF-1α or β-catenin
overexpression results in a mild decrease in the content of NIS.
From immunofluorescent staining (Fig.
1B), we found that additional to the protein amount, HIF-1α
overexpression also promoted the nuclear translocation of
β-catenin, and relocated NIS from the originally diffuse
distribution or location near the cytomembrane into intracellular
localization near the nucleus. These changes of protein
localization also appear when β-catenin was directly overexpressed,
and were reversed by β-catenin knockdown.
Additionally, cell radioiodine uptake assay
(Fig. 1C) showed that
overexpression of either HIF-1α or β-catenin decreased radioiodine
uptake ability of FTC cells ~50 and 37.5%, respectively (P<0.05
or P<0.01, respectively). While after β-catenin knockdown, the
impaired iodide uptake capacity was restored (all P<0.05).
β-catenin knockdown can improve the
growth inhibitory effect of radioiodine treatment in HIF-1α
overexpressing FTC cells
In the xenograft tumor model, we found that
overexpression of HIF-1α and β-catenin could both promote the
growth of FTC cells (Fig. 3A),
while 131I treatment inhibited such tumor growth.
Quantification of tumor mass at 35 days after radiotherapy (right
in Fig. 3A) also showed that
131I treatment could markedly kill the HIF-1α
overexpressing cancer cells or inhibit their growth, but further
combined with β-catenin knockdown significantly promoted the
therapeutic efficacy (P<0.05).
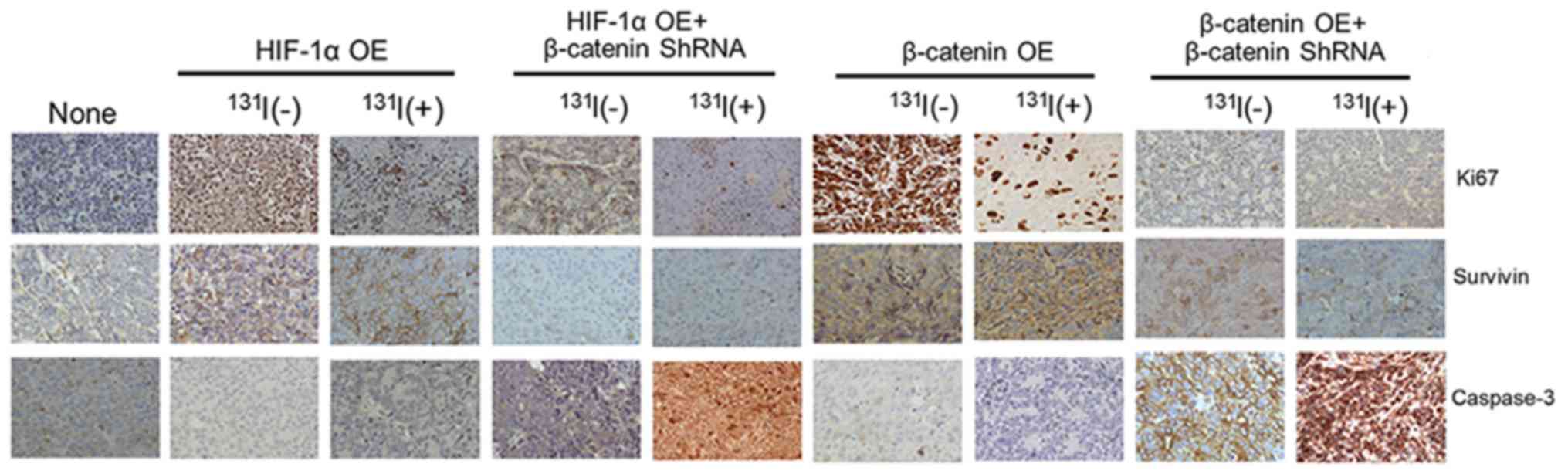
 | Figure 3.β-catenin knockdown can improve the
growth inhibitory effect of radioiodine treatment in HIF-1α
overexpressing FTC cells. FTC133 cells with different treatments
(grouping as Fig. 1) were injected
into male SCID mice to measure the tumor growth. When xenograft
tumor grew to the volume of 50 mm3, peritoneal injection
of 37MBq 131I was introduced, while 0.1 ml physical
saline was used as control in all the groups that did not receive
radioiodine treatment. There were altogether 9 groups as shown in
the horizontal axis in right panel of (A). (A) From left to right
are the representative images of tumors, the tumor growth curves,
and the tumor mass 35 days after the 131I treatment (or
saline injection) in each group. The images were taken on the same
day as tumor mass measurement. (B) Quantification (based on
immunohistochemical staining, with representative images shown in
Fig. 4) and statistics of the rate
of Ki-67, survivin or caspase-3 expression in tumor tissues from
different groups. For every index, 3 images of cancer tissues from
each animal were selected for quantification. In addition, 5 high
power fields were selected from each image for the analysis. y-axis
indicates the average percentage of the positively stained cells
from all fields of observation. For each assay, n=10 (number of
animals) in each group. *P<0.05 and **P<0.01, vs. the None
group. #P<0.05, ##P<0.01 and
###P<0.001, vs. the same OE groups without β-catenin
shRNA knockdown nor 131I treatment.
∆P<0.05 and ∆∆∆P<0.001, vs. the same OE
groups with 131I treatment but without β-catenin shRNA
knockdown. |
Importantly, immunohistochemical analysis
demonstrated that (Figs. 3C and
4), overexpression of HIF-1α will
significantly promote the expression of Ki-67 and survivin in the
thyroid cancer cells as well as inhibit the expression of
caspase-3. After 131I treatment in the HIF-1α
overexpressing FTC cells, Ki-67 was significantly reduced, and
survivin had a moderate decline, but the level of caspase-3 was
still very low. While the HIF-1α overexpressing xenograft tumors
were knocked down of β-catenin, the caspase-3 expression showed a
remarkable increase upon 131I treatment (P<0.001).
Moreover, the content of survivin was reduced more markedly
(P<0.05) and the level of Ki-67 was even lower (P<0.05).
Also, the role of β-catenin in affecting the efficacy of
radioactive iodine treatment was confirmed by directly using
β-catenin overexpressing FTC cells instead of the HIF-1α
overexpressing ones in this immunohistochemical analysis.
Taken together, these results suggested how the
genuinely effective radioiodine treatment, which may guarantee
normal functioning of NIS in iodide uptake (Fig. 1C), would intervene in the
proliferative and apoptotic regulation of thyroid cancer cells.
Cell distribution of β-catenin and NIS
are negatively correlated in xenograft tumors generated from FTC
cells
To further verify in vivo that the impact of
HIF-1α overexpression on the efficiency of radioiodine treatment
should rely on the localization regulation of NIS in a
β-catenin-dependent manner, we analyzed the distribution of NIS or
β-catenin in xenograft tumors generated from FTC cells with
HIF-1α/β-catenin overexpression, as well as those having further
knockdown of β-catenin.
Immunohistochemistry showed that the nuclear
translocation of β-catenin was significantly enhanced in
HIF-1α/β-catenin overexpressing tumor cells (P<0.05) (Fig. 5). Moreover, cytomembrane
localization of NIS was markedly decreased (P<0.05). In
addition, all these changes in the subcellular distribution of
proteins were erased by shRNA-mediated knockdown of β-catenin.
Thus, we infer more confidently that the restored localization of
NIS, owing to reduced expression together with lessened nuclear
localization of β-catenin, was indeed essential for the superior
efficiency of 131I treatment (Fig. 3A) as a provider of sufficient
radioiodine uptake.
Discussion
In the present study, we paralleled the
interventions of HIF-1α overexpression and β-catenin overexpression
in thyroid carcinoma cell line FTC133, so as to demonstrate their
impacts on the aggressiveness of cancer cells, the radioiodine
uptake ability which is mediated by NIS, and the efficiency of
radiotherapy towards xenograft tumors. By means of β-catenin
knockdown subsequent to HIF-1α overexpression, we also confirmed
the dependent relationship of these two factors in above-mentioned
regulatory mechanisms.
In FTC133 cells which were originally derived from
metastatic FTC (36) we hardly
detected any expression of HIF-1α (Fig.
1A). Yet, previous study had revealed that HIF-1α was highly
expressed even in primary thyroid cancers including FTC (34). We inferred that culture conditions
in vitro may lead to impaired metastatic ability through
inactivation of relevant signaling pathways. Once the expression of
HIF-1α was artificially upregulated, FTC cells cultured in
vitro exhibited stronger growth ability together with more
obvious metastatic propensity (Fig.
2). We deemed that overexpression of HIF-1α may have made the
FTC133 cells better mimicking tumor cells of aggressive thyroid
carcinomas in vivo. In such FTC cells, we had observed that
HIF-1α acted upstream of β-catenin signaling (Fig. 1A), and confirmed by interference
experiments that the aggressiveness of FTC cells endowed with
HIF-1α overexpression was β-catenin-dependent (Figs. 2 and 3). Those were supplementary proofs to
previous discoveries on the relationship of the two pathways in
tumor development (23,25,29,30).
Previous observations pointed out that, FTC with
aggressiveness may show resistance to radiotherapy. In addition,
the fact that in MTC, inhibitors of HIF like 2ME2 is responsible
for promoting the efficacy of anticancer drugs such as
cabozantinib, had proved reversely the role of HIF-1α in
progression of tumors, increased aggressiveness and poor response
to therapy (39). In the present
study, we also found unsatisfactory efficiency of radioiodine
treatment towards xenograft tumors generated from HIF-1α/β-catenin
overexpressing FTC cells, which had exhibited metastatic propensity
in vitro. While reducing the expression of β-catenin
promoted the killing effect of 131I, reflecting the more
rapid shrinkage of tumor volume together with tumor mass (Fig. 3A). We confirmed that the progression
of xenograft tumors as a consequence of excessive proliferation and
blocked apoptosis was thoroughly reduced when radiotherapy was
introduced after β-catenin knockdown (Fig. 3B). Given that NIS subcellular
localization and radioiodine uptake ability of FTC cells was
profoundly subjected to HIF-1α overexpression in a
β-catenin-dependent manner (Fig. 1B and
C), it is a reasonable explanation that in the xenograft
thyroid tumors, the activated HIF1α signaling and the impaired
efficiency of radiotherapy was bridged by β-catenin induced NIS
translocation. We corroborated this by revealing the relevance of
β-catenin nuclear localization and NIS cytomembrane localization
only in the xenograft tumor cells (Fig.
5).
Previous studies have paid some attention to the
expression level of NIS in cancer cells, which allows therapeutic
application and prognosis estimation of radioiodine treatment, and
our results suggested the location of NIS may likewise indicate the
efficacy of radioiodine therapy. In fact, we even found that direct
activation of β-catenin, as well as secondary to strengthened
HIF-1α signaling, would reduce the protein amount of NIS to a
certain extent (Fig. 1A). While in
a recent study, Sastre-Perona and Santisteban (24) found that in normal thyroid cells TSH
and IGF-1 can facilitate β-catenin release from E-cadherin at the
adherence junctions, and β-catenin will increase expression of NIS
by elevating the transcriptional activity of Pax8. We supposed the
discrepancy between our results and theirs may emerge on account of
different downstream effectors, respectively, involved in
physiological and pathological conditions.
Additionally, part from adequate protein content,
the correct localization of NIS on thyroid cancer cells seems more
pivotal in determining the capabilities and sensitivity of
radiotherapy, for it has been reported that abundance of NIS was
observed in differentiated thyroid cancer while the majority was in
the cytoplasm with little on the cell surface membrane (40). Similar observations have also been
obtained in breast cancers (13–15,19).
Therefore, post-translational regulatory mechanisms, especially
translocation of NIS, have been proposed as an important factor
determining the functionality of NIS, and is of interest as a
target to augment iodide uptake in NIS-expressing cancer cells.
Riedel et al (41) explored
the mechanism by which TSH regulates NIS distribution and found
that NIS exhibits several consensus sites for the cAMP-dependent
protein kinase, protein kinase C and CK-2 kinases. They also
revealed that the NIS phosphorylation pattern changes play a role
in regulating its targeting from membrane to the intracellular
compartments.
Yet overall, the exact regulatory mechanism of NIS
especially on its localization is still not fully elucidated. To
date, the present study is the first to connect the regulation of
NIS both in subcellular localization and expression level, with the
activated HIF-1α signaling in tumor cells. We established a
regulatory axis wherein β-catenin increased in response to HIF-1α
activation and plays an essential role in affecting NIS
localization. Although in the context of thyroid tumors, the
detailed regulatory mechanisms of NIS, downstream of β-catenin
signaling remained unclear. We have evidence strongly suggesting
the reduced efficiency of radioiodine treatment towards HIF-1α
overexpressing thyroid tumor cells may be heavily due to abnormal
NIS localization caused by β-catenin activation. Thus, the
radioiodine resistance of aggressive thyroid carcinomas which
displayed highly activated HIF-1α/β-catenin signaling may be
explained. Nevertheless, whether the translocation of NIS occurred
parallelly or subsequently to the acquirement of aggressiveness
(i.e. achieving EMT and possessing a high metastatic capacity), and
indeed to what extent the efficiency of radioiodine therapy was
determined by the NIS subcellular localization need to be answered
in future studies.
In conclusion, this study made a preliminary
exploration to elucidate the signals taking part in the regulation
of NIS function as related to thyroid cancers. Dissection of such
signaling pathways may lead to resolution of the treatment of
radioiodine-refractory thyroid cancer. Our results will contribute
to improving the postoperative prognosis of thyroid cancers such as
FTC, and these advances might also have profound implications for
the treatment of breast cancers and other non-thyroidal cancers
currently being assessed for potential NIS-mediated radioiodine
therapy (20).
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81372858,
30700968, 30901725 and 30800416).
References
|
1
|
Giannoula E, Iakovou I and Chatzipavlidou
V: Risk factors and the progression of thyroid malignancies. Hell J
Nucl Med. 18:275–284. 2015.(In Greek). PubMed/NCBI
|
|
2
|
Mohamed AF, González JM and Fairchild A:
Patient benefit-risk tradeoffs for radioactive iodine-refractory
differentiated thyroid cancer treatments. J Thyroid Res.
2015:4382352015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrari SM, Fallahi P, Politti U,
Materazzi G, Baldini E, Ulisse S, Miccoli P and Antonelli A:
Molecular targeted therapies of aggressive thyroid cancer. Front
Endocrinol (Lausanne). 6:1762015.PubMed/NCBI
|
|
4
|
Luo Y, He DL, Ning L, Shen SL, Li L, Li X,
Zhau HE and Chung LW: Over-expression of hypoxia-inducible
factor-1alpha increases the invasive potency of LNCaP cells in
vitro. BJU Int. 98:1315–1319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinert HC and Aberle S: CME: Radioactive
iodine therapy in thyroid cancer. Praxis (Bern 1994).
104:1235–1243; quiz 1244–1235. 2015.[(In German)]. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai G, Levy O and Carrasco N: Cloning and
characterization of the thyroid iodide transporter. Nature.
379:458–460. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smanik PA, Liu Q, Furminger TL, Ryu K,
Xing S, Mazzaferri EL and Jhiang SM: Cloning of the human sodium
lodide symporter. Biochem Biophys Res Commun. 226:339–345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuno A, Murakami M, Hoya K, Yamada SM,
Miyamoto S, Yamada S, Son JH, Nishido H, Ide F, Nagashima H, et al:
Clinicopathological and molecular histochemical review of skull
base metastasis from differentiated thyroid carcinoma. Acta
Histochem Cytochem. 46:129–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauer AJ: Thyroid nodules and
differentiated thyroid cancer. Endocr Dev. 26:183–201.
2014.PubMed/NCBI
|
|
10
|
Dadu R and Cabanillas ME: Optimizing
therapy for radioactive iodine-refractory differentiated thyroid
cancer: Current state of the art and future directions. Minerva
Endocrinol. 37:335–356. 2012.PubMed/NCBI
|
|
11
|
Rahmani N, Hashemi S Abbas, Fazli M and
Raisian M: Clinical management and outcomes of papillary,
follicular and medullary thyroid cancer surgery. Med Glas (Zenica).
10:164–167. 2013.PubMed/NCBI
|
|
12
|
Phay JE and Ringel MD: Metastatic
mechanisms in follicular cell-derived thyroid cancer. Endocr Relat
Cancer. 20:R307–R319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dohán O, Baloch Z, Bánrévi Z, Livolsi V
and Carrasco N: Rapid communication: Predominant intracellular
overexpression of the Na+/I− symporter (NIS)
in a large sampling of thyroid cancer cases. J Clin Endocrinol
Metab. 86:2697–2700. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito T, Endo T, Kawaguchi A, Ikeda M,
Katoh R, Kawaoi A, Muramatsu A and Onaya T: Increased expression of
the sodium/iodide symporter in papillary thyroid carcinomas. J Clin
Invest. 101:1296–1300. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wapnir IL, van de Rijn M, Nowels K, Amenta
PS, Walton K, Montgomery K, Greco RS, Dohán O and Carrasco N:
Immunohistochemical profile of the sodium/iodide symporter in
thyroid, breast, and other carcinomas using high density tissue
microarrays and conventional sections. J Clin Endocrinol Metab.
88:1880–1888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Venkataraman GM, Yatin M, Marcinek R and
Ain KB: Restoration of iodide uptake in dedifferentiated thyroid
carcinoma: Relationship to human Na+/I-symporter gene
methylation status. J Clin Endocrinol Metab. 84:2449–2457. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arturi F, Russo D, Schlumberger M, du
Villard JA, Caillou B, Vigneri P, Wicker R, Chiefari E, Suarez HG
and Filetti S: Iodide symporter gene expression in human thyroid
tumors. J Clin Endocrinol Metab. 83:2493–2496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dohán O, De la Vieja A, Paroder V, Riedel
C, Artani M, Reed M, Ginter CS and Carrasco N: The sodium/iodide
Symporter (NIS): Characterization, regulation, and medical
significance. Endocr Rev. 24:48–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arturi F, Russo D, Giuffrida D,
Schlumberger M and Filetti S: Sodium-iodide symporter (NIS) gene
expression in lymph-node metastases of papillary thyroid
carcinomas. Eur J Endocrinol. 143:623–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boelaert K and Franklyn JA: Sodium iodide
symporter: A novel strategy to target breast, prostate, and other
cancers? Lancet. 361:796–797. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Liu D, Murugan AK, Liu Z and Xing
M: Histone deacetylation of NIS promoter underlies BRAF
V600E-promoted NIS silencing in thyroid cancer. Endocr Relat
Cancer. 21:161–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo H, Xing Y, Liu Y, Luo Y, Deng F, Yang
T, Yang K and Li Y: Wnt/β-catenin signaling pathway activates
melanocyte stem cells in vitro and in vivo. J Dermatol Sci.
83:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sastre-Perona A and Santisteban P:
Wnt-independent role of β-catenin in thyroid cell proliferation and
differentiation. Mol Endocrinol. 28:681–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan L, Deng W, Chen HL, Huo LL, Deng LL,
Zhang GY and Luo Y: All-trans retinoic acid improves iodine uptake
of thyroid cancer cells via repressing transcriptional activity of
β-catenin. Zhonghua Yi Xue Za Zhi. 96:553–558. 2016.(In Chinese).
PubMed/NCBI
|
|
26
|
Rossi ED, Straccia P, Palumbo M, Stigliano
E, Revelli L, Lombardi CP, Santeusanio G, Pontecorvi A and Fadda G:
Diagnostic and prognostic role of HBME-1, galectin-3, and β-catenin
in poorly differentiated and anaplastic thyroid carcinomas. Appl
Immunohistochem Mol Morphol. 21:237–241. 2013.PubMed/NCBI
|
|
27
|
Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo
LL, Chen HL, Zhang GY and Deng LL: Epithelial-mesenchymal
transition induces cancer stem cell generation in human thyroid
cancer cells in vitro. Zhonghua Yi Xue Za Zhi. 93:1261–1265.
2013.(In Chinese). PubMed/NCBI
|
|
28
|
Pagni F, Manzoni M, Buscone S and Leone
BE: β-catenin as a morpho-immunohistochemical marker for the
diagnosis of papillary thyroid carcinoma. Arch Pathol Lab Med.
139:572–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lan L, Deng W, Chen H, Huo L, Deng L,
Zhang G and Luo Y: Nuclear translocation of β-catenin represses
membrane localization of NIS in human thyroid cancer cells.
Zhonghua Yi Xue Za Zhi. 96:891–896. 2016.(In Chinese). PubMed/NCBI
|
|
30
|
Jin S, Borkhuu O, Bao W and Yang YT:
Signaling pathways in thyroid cancer and their therapeutic
implications. J Clin Med Res. 8:284–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang
C, Xie S, Chen C, Hu L, Xu S, et al: Wnt/β-catenin signaling
enhances hypoxia-induced epithelial-mesenchymal transition in
hepatocellular carcinoma via crosstalk with hif-1α signaling.
Carcinogenesis. 34:962–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burrows N, Babur M, Resch J, Williams KJ
and Brabant G: Hypoxia-inducible factor in thyroid carcinoma. J
Thyroid Res. 2011:7629052011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burrows N, Resch J, Cowen RL, von
Wasielewski R, Hoang-Vu C, West CM, Williams KJ and Brabant G:
Expression of hypoxia-inducible factor 1 alpha in thyroid
carcinomas. Endocr Relat Cancer. 17:61–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goretzki PE, Frilling A, Simon D and
Roeher HD: Growth regulation of normal thyroids and thyroid tumors
in man. Recent Results Cancer Res. 118:48–63. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao JH, Luo Y, Jiang YG, He DL and Wu CT:
Knockdown of β-catenin through shRNA cause a reversal of EMT and
metastatic phenotypes induced by HIF-1α. Cancer Invest. 29:377–382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kraiem Z, Sadeh O and Yosef M: Iodide
uptake and organification, tri-iodothyronine secretion, cyclic AMP
accumulation and cell proliferation in an optimized system of human
thyroid follicles cultured in collagen gel suspended in serum-free
medium. J Endocrinol. 131:499–506. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin H, Jiang X, Zhu H, Jiang W, Dong X,
Qiao H, Sun X and Jiang H: 2ME2 inhibits the activated
hypoxia-inducible pathways by cabozantinib and enhances its
efficacy against medullary thyroid carcinoma. Tumour Biol.
37:381–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang R, Wang H, Zhao J, Yao J, Shang H,
Zhu H, Liao L and Dong J: Association between sodium iodide
symporter and differentiated thyroid cancer: A meta-analysis of 9
studies. Int J Clin Exp Med. 8:17986–17994. 2015.PubMed/NCBI
|
|
41
|
Riedel C, Levy O and Carrasco N:
Post-transcriptional regulation of the sodium/iodide symporter by
thyrotropin. J Biol Chem. 276:21458–21463. 2001. View Article : Google Scholar : PubMed/NCBI
|