|
1
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bird A: Perceptions of epigenetics.
Nature. 447:396–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peschansky VJ and Wahlestedt C: Non-coding
RNAs as direct and indirect modulators of epigenetic regulation.
Epigenetics. 9:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goll MG and Bestor TH: Eukaryotic cytosine
methyltransferases. Annu Rev Biochem. 74:481–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weber M, Hellmann I, Stadler MB, Ramos L,
Pääbo S, Rebhan M and Schübeler D: Distribution, silencing
potential and evolutionary impact of promoter DNA methylation in
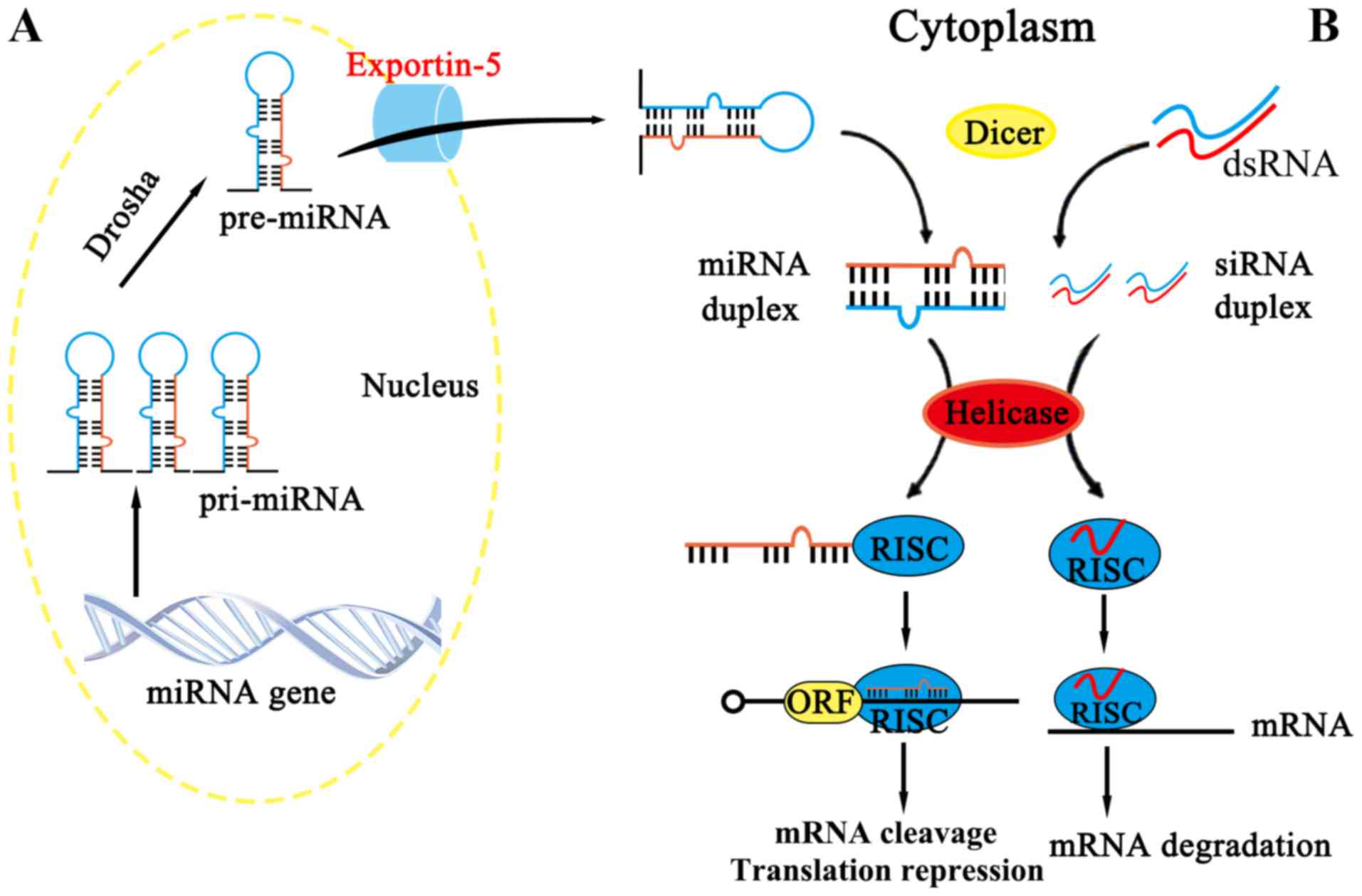
the human genome. Nat Genet. 39:457–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okano M, Bell DW, Haber DA and Li E: DNA
methyltransferases Dnmt3a and Dnmt3b are essential for de novo
methylation and mammalian development. Cell. 99:247–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berger SL: The complex language of
chromatin regulation during transcription. Nature. 447:407–412.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernstein BE, Meissner A and Lander ES:
The mammalian epigenome. Cell. 128:669–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldberg AD, Allis CD and Bernstein E:
Epigenetics: A landscape takes shape. Cell. 128:635–638. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harr JC, Gonzalez-Sandoval A and Gasser
SM: Histones and histone modifications in perinuclear chromatin
anchoring: From yeast to man. EMBO Rep. 17:139–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jenuwein T and Allis CD: Translating the
histone code. Science. 293:1074–1080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu C, Jain SU, Hoelper D, Bechet D, Molden
RC, Ran L, Murphy D, Venneti S, Hameed M, Pawel BR, et al: Histone
H3K36 mutations promote sarcomagenesis through altered histone
methylation landscape. Science. 352:844–849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mellor J, Dudek P and Clynes D: A glimpse
into the epigenetic landscape of gene regulation. Curr Opin Genet
Dev. 18:116–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grewal SI and Elgin SC: Transcription and
RNA interference in the formation of heterochromatin. Nature.
447:399–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reik W: Stability and flexibility of
epigenetic gene regulation in mammalian development. Nature.
447:425–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tchurikov NA: Molecular mechanisms of
epigenetics. Biochemistry (Mosc). 70:406–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaratiegui M, Irvine DV and Martienssen
RA: Noncoding RNAs and gene silencing. Cell. 128:763–776. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Costa FF: Non-coding RNAs, epigenetics and
complexity. Gene. 410:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amaral PP, Dinger ME, Mercer TR and
Mattick JS: The eukaryotic genome as an RNA machine. Science.
319:1787–1789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghildiyal M and Zamore PD: Small silencing
RNAs: An expanding universe. Nat Rev Genet. 10:94–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H: Epigenetics: Advances of non-coding
RNAs regulation in mammalian cells. Yi Chuan. 31:1077–1086.
2009.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moazed D: Small RNAs in transcriptional
gene silencing and genome defence. Nature. 457:413–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jinek M and Doudna JA: A three-dimensional
view of the molecular machinery of RNA interference. Nature.
457:405–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris KV, Chan SW, Jacobsen SE and Looney
DJ: Small interfering RNA-induced transcriptional gene silencing in
human cells. Science. 305:1289–1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawasaki H and Taira K: Induction of DNA
methylation and gene silencing by short interfering RNAs in human
cells. Nature. 431:211–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bayne EH and Allshire RC: RNA-directed
transcriptional gene silencing in mammals. Trends Genet.
21:370–373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou W, Wang J, Man WY, Zhang QW and Xu
WG: siRNA silencing EZH2 reverses cisplatin-resistance of human
non-small cell lung and gastric cancer cells. Asian Pac J Cancer
Prev. 16:2425–2430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viré E, Brenner C, Deplus R, Blanchon L,
Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden
JM, et al: The Polycomb group protein EZH2 directly controls DNA
methylation. Nature. 439:871–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hall IM, Shankaranarayana GD, Noma K,
Ayoub N, Cohen A and Grewal SI: Establishment and maintenance of a
heterochromatin domain. Science. 297:2232–2237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volpe TA, Kidner C, Hall IM, Teng G,
Grewal SI and Martienssen RA: Regulation of heterochromatic
silencing and histone H3 lysine-9 methylation by RNAi. Science.
297:1833–1837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen S, He H and Deng X: Allele-specific
DNA methylation analyses associated with siRNAs in Arabidopsis
hybrids. Sci China Life Sci. 57:519–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zilberman D, Cao X and Jacobsen SE:
ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and
histone methylation. Science. 299:716–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maison C, Bailly D, Peters AH, Quivy JP,
Roche D, Taddei A, Lachner M, Jenuwein T and Almouzni G:
Higher-order structure in pericentric heterochromatin involves a
distinct pattern of histone modification and an RNA component. Nat
Genet. 30:329–334. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lippman Z, Gendrel AV, Black M, Vaughn MW,
Dedhia N, McCombie WR, Lavine K, Mittal V, May B, Kasschau KD, et
al: Role of transposable elements in heterochromatin and epigenetic
control. Nature. 430:471–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruvkun G: Molecular biology. Glimpses of a
tiny RNA world. Science. 294:797–799. 2001.
|
|
38
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo M, Hao L, Hu F, Dong Y, Gou L, Zhang
W, Wang X, Zhao Y, Jia M, Hu S, et al: MicroRNA profiles and
potential regulatory pattern during the early stage of
spermatogenesis in mice. Sci China Life Sci. 58:442–450. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42(D1): D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rajewsky N: microRNA target predictions in
animals. Nat Genet. 38:(Suppl). S8–S13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Denis H, Ndlovu MN and Fuks F: Regulation
of mammalian DNA methyltransferases: A route to new mechanisms.
EMBO Rep. 12:647–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan JH, Yang F, Chen BF, Lu Z, Huo XS,
Zhou WP, Wang F and Sun SH: The histone deacetylase
4/SP1/microRNA-200a regulatory network contributes to aberrant
histone acetylation in hepatocellular carcinoma. Hepatology.
54:2025–2035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tuddenham L, Wheeler G, Ntounia-Fousara S,
Waters J, Hajihosseini MK, Clark I and Dalmay T: The cartilage
specific microRNA-140 targets histone deacetylase 4 in mouse cells.
FEBS Lett. 580:4214–4217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim DH, Saetrom P, Snøve O Jr and Rossi
JJ: MicroRNA-directed transcriptional gene silencing in mammalian
cells. Proc Natl Acad Sci USA. 105:16230–16235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Khraiwesh B, Arif MA, Seumel GI, Ossowski
S, Weigel D, Reski R and Frank W: Transcriptional control of gene
expression by microRNAs. Cell. 140:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu
C and Qi Y: DNA methylation mediated by a microRNA pathway. Mol
Cell. 38:465–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fabbri M, Garzon R, Cimmino A, Liu Z,
Zanesi N, Callegari E, Liu S, Alder H, Costinean S,
Fernandez-Cymering C, et al: MicroRNA-29 family reverts aberrant
methylation in lung cancer by targeting DNA methyltransferases 3A
and 3B. Proc Natl Acad Sci USA. 104:15805–15810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Benetti R, Gonzalo S, Jaco I, Muñoz P,
Gonzalez S, Schoeftner S, Murchison E, Andl T, Chen T, Klatt P, et
al: A mammalian microRNA cluster controls DNA methylation and
telomere recombination via Rbl2-dependent regulation of DNA
methyltransferases. Nat Struct Mol Biol. 15:9982008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sinkkonen L, Hugenschmidt T, Berninger P,
Gaidatzis D, Mohn F, Artus-Revel CG, Zavolan M, Svoboda P and
Filipowicz W: MicroRNAs control de novo DNA methylation through
regulation of transcriptional repressors in mouse embryonic stem
cells. Nat Struct Mol Biol. 15:259–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gonzalez S, Pisano DG and Serrano M:
Mechanistic principles of chromatin remodeling guided by siRNAs and
miRNAs. Cell Cycle. 7:2601–2608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa
S, Nakano T, Bartel DP and Kingston RE: Characterization of the
piRNA complex from rat testes. Science. 313:363–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grivna ST, Beyret E, Wang Z and Lin H: A
novel class of small RNAs in mouse spermatogenic cells. Genes Dev.
20:1709–1714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lin H: piRNAs in the germ line. Science.
316:3972007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Aravin AA, Sachidanandam R, Bourc'his D,
Schaefer C, Pezic D, Toth KF, Bestor T and Hannon GJ: A piRNA
pathway primed by individual transposons is linked to de novo DNA
methylation in mice. Mol Cell. 31:785–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aravin AA, Hannon GJ and Brennecke J: The
Piwi-piRNA pathway provides an adaptive defense in the transposon
arms race. Science. 318:761–764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Houwing S, Kamminga LM, Berezikov E,
Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz
E, Moens CB, et al: A role for Piwi and piRNAs in germ cell
maintenance and transposon silencing in Zebrafish. Cell. 129:69–82.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yin H and Lin H: An epigenetic activation
role of Piwi and a Piwi-associated piRNA in Drosophila
melanogaster. Nature. 450:304–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang XA, Yin H, Sweeney S, Raha D, Snyder
M and Lin H: A major epigenetic programming mechanism guided by
piRNAs. Dev Cell. 24:502–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bourc'his D and Bestor TH: Meiotic
catastrophe and retrotransposon reactivation in male germ cells
lacking Dnmt3L. Nature. 431:96–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kuramochi-Miyagawa S, Watanabe T, Gotoh K,
Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri
TW, et al: DNA methylation of retrotransposon genes is regulated by
Piwi family members MILI and MIWI2 in murine fetal testes. Genes
Dev. 22:908–917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cao X, Yeo G, Muotri AR, Kuwabara T and
Gage FH: Noncoding RNAs in the mammalian central nervous system.
Annu Rev Neurosci. 29:77–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang PK and Kuroda MI: Noncoding RNAs and
intranuclear positioning in monoallelic gene expression. Cell.
128:777–786. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cai X and Cullen BR: The imprinted H19
noncoding RNA is a primary microRNA precursor. RNA. 13:313–316.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu HA and Bernstein E: Partners in
imprinting: Noncoding RNA and polycomb group proteins. Dev Cell.
15:637–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pandey RR, Mondal T, Mohammad F, Enroth S,
Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D and Kanduri C:
Kcnq1ot1 antisense noncoding RNA mediates lineage-specific
transcriptional silencing through chromatin-level regulation. Mol
Cell. 32:232–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Regha K, Sloane MA, Huang R, Pauler FM,
Warczok KE, Melikant B, Radolf M, Martens JH, Schotta G, Jenuwein
T, et al: Active and repressive chromatin are interspersed without
spreading in an imprinted gene cluster in the mammalian genome. Mol
Cell. 27:353–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Clemson CM, McNeil JA, Willard HF and
Lawrence JB: XIST RNA paints the inactive X chromosome at
interphase: Evidence for a novel RNA involved in nuclear/chromosome
structure. J Cell Biol. 132:259–275. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao J, Sun BK, Erwin JA, Song JJ and Lee
JT: Polycomb proteins targeted by a short repeat RNA to the mouse X
chromosome. Science. 322:750–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou X, Ren Y, Zhang J, Zhang C, Zhang K,
Han L, Kong L, Wei J, Chen L, Yang J, et al: HOTAIR is a
therapeutic target in glioblastoma. Oncotarget. 6:8353–8365. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang K, Sun X, Zhou X, Han L, Chen L, Shi
Z, Zhang A, Ye M, Wang Q, Liu C, et al: Long non-coding RNA HOTAIR
promotes glioblastoma cell cycle progression in an EZH2 dependent
manner. Oncotarget. 6:537–546. 2015.PubMed/NCBI
|
|
77
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et
al: Functional demarcation of active and silent chromatin domains
in human HOX loci by noncoding RNAs. Cell. 129:1311–1323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ogawa Y, Sun BK and Lee JT: Intersection
of the RNA interference and X-inactivation pathways. Science.
320:1336–1341. 2008. View Article : Google Scholar : PubMed/NCBI
|