Introduction
Breast cancer is the most frequently diagnosed
malignant tumor for female patients, which seriously threatens the
health of women. It accounts for 25% of all cancer cases and 15% of
all cancer deaths among women, with an estimated 1.7 million cases
and 521.9 thousand deaths in 2012 (1). Although comprehensive treatments based
on surgical operation have certain effects on breast cancer, the
majority of patients still inevitably die of tumor recurrence and
metastasis. The estimated number of breast cancer deaths in 2010 in
China was approximately 55,500 (2).
According to the statistics by the Ministry of Health in 2011,
approximately 37,000 people die of breast cancer recurrence and
metastasis in mainland China each year (data from 145
population-based cancer registries).
Nerve guidance factors (netrins) are the earliest
discovered soluble nerve guidance factor family, including three
secreted proteins (netrin-1, 3 and 4) and two anchored membrane
proteins (netrin-G1 and G2). Their secondary structures are
similar, all consist of an amino-terminal signal sequence, a
laminin-type globular domain, three laminin-type epidermal growth
factor repeats, and a carboxyl-terminal that is enriched in amino
acids (3).
NTN4, the new member of netrin family, is a kind of
secreted protein. Several studies have indicated that NTN4 is
widely detected in non-nervous systems and plays a vital role in
tissue morphogenesis, angiogenesis, apoptosis, tumorigenesis, cell
migration and invasion (4–9). Besides, emerging evidence have
testified that NTN4 expression is decreased in variety of
malignancies, including breast, pancreatic and colon cancers
(10–13). In vitro studies exhibited
that NTN4 was involved in the development of multiple types of
cancers by inhibiting proliferation in a concentration-dependent
manner (11,13–15).
Consequently, NTN4 is considered as a tumor suppressor.
Nevertheless, NTN4 promoted tumor cell proliferation at relatively
low concentrations (13,16,17).
In vivo studies demonstrated that NTN4 overexpression
induced inhibitory effect in metastasis and recurrence of
colorectal cancer (12), while
metastasis was elevated in NTN4-overexpressing breast cancer models
(18). Moreover, NTN4 is
upregulated in the effusions compared with corresponding solid
tumors (19), suggesting that NTN4
may be involved in tumor metastasis. However, the functions and
molecular mechanisms of NTN4 in breast cancer have not been
investigated thoroughly. In the present study, we firstly
investigated the effects of NTN4 on metastasis of breast cancer and
analyzed the underlying molecular mechanisms.
Materials and methods
Patient samples and tissue
microarrays
Breast cancer fresh frozen tissues were obtained
from the Pathology Department of Shaoxing People's Hospital
(Shaoxing, China). Briefly, samples were from 14 breast cancer
patients at initial diagnosis for breast cancer, and were rapidly
frozen using liquid nitrogen. The use of all samples for this study
was approved by the Ethics Committee of the hospital and informed
consents were obtained from all patients. Tissue microarrays were
purchased from Alenabio Biotechnolgy, Co., Ltd. (Xian, China).
Immunohistochemical staining
Immunohistochemical (IHC) staining was performed
using Polink-2 Plus® Polymer horseradish peroxidase
(HRP) detection system (ZSGB-BIO, Beijing, China). The tissue
slides were heated at 65°C for deparaffination, endogenous
peroxidase was blocked by immersion in 3% hydrogen peroxide for 10
min after antigen retrieval in a citrate buffer (pH 6.0) heated to
121°C for 90 sec. Then, slides were washed in phosphate-buffered
saline (PBS) and incubated with anti-NTN4 antibody (goat
anti-human, diluted 1:1800, AF1254; R&D Systems, Minneapolis,
MN, USA) at 37°C for 1.5 h. Then, the tissue sections were
incubated with polymer adjuvant and anti-goat IgG polymer labeled
with HRP both for 15 min. The staining was visualized using
3,3-diaminobenzidine (DAB) substrate-chromogen (ZLI-9017; ZSGB-BIO)
according to the manufacturers instructions and was counterstained
with hematoxylin. All sections were evaluated by two pathologists
who were unaware of the study contents. Three randomly selected
views were observed per case and 100 cells were observed per view
at ×400 magnification. NTN4 expression was divided into four groups
based on staining range: we consider <10% as -, 10–24% as +,
25–49% as ++, and ≥50% as +++.
Cell culture and reagents
Human SK-BR-3, T-47D, BT-474, MCF-7, MDA-MB-231 and
Hs578T cell lines were obtained from the Cell Bank of Chinese
Academy of Medical Science (Shanghai, China). The cells were
cultured in Dulbeccos modified Eagles medium (DMEM, SH30243.01;
HyClone Laboratories, Logan, UT, USA) with 10% fetal bovine serum
(FBS, 10099-141; Life Technologies, Carlsbad, CA, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (15140122; Life Technologies)
at 37°C in a moist environment containing 5% CO2. Cells
were maintained in sterilized culture dishes and passaged every 3
days with 0.25% trypsin (SH30042.01; HyClone Laboratories).
Quantification of NTN4 in cell
supernatant by ELISA
NTN4 concentration of cell supernatant was measured
using an enzyme linked immunosorbent assay (ELISA) kit
(CSB-E11900h; CusaBio, Wuhan, China). Diluted cell supernatant and
standard samples (100 µl) were added to 96-well plates and
incubated at 37°C for 2 h, then biotin-conjugated antibody against
NTN4 was added and incubated at 37°C for 1 h. After incubated with
avidin-conjugated HRP for 1 h, 3,3′,5,5′-tetramethylbenzidine (TMB)
substrate reaction was performed and was stopped by adding stop
buffer. Absorbance was measured at a wavelength of 450 nm with
plate reader (Anthos Fluido 2010).
Construction and transfection of NTN4
expressive plasmid
The human NTN4 cDNA amplified by PCR (the primers:
F, 5-ATACTCGAGACCATGGGGAGCTGCGCGCGGCTG-3 and R,
5-CACGGATCCCTACTTGCACTCTCTTTTTAAA ATATCC-3) was cloned into the
pcDNA3.1 vector and the sequence of recombined plasmid was
confirmed by Platinum Biological Technology, Co., Ltd. (Shanghai,
China). The cells were seeded in 6-well plates and then transfected
with the recombined plasmid and negative control plasmid with
Lipofectamine 2000 (11668-019; Invitrogen) according to the
manufacturers instructions.
Transfection of siRNA
Cells were seeded in 6-well plates and transfected
with siRNA using Lipofectamine 2000 (11668-019; Invitrogen)
according to the manufacturers instructions. The sequences of
siRNAs are as follows: NTN4 siRNA-1: 5-GGCGCUAUUUGUACUUCUAdTdT-3;
NTN4 siRNA-2: 5-GUCCAUGGGAAGUGUAUGUdTdT-3; NTN4 siRNA-3:
5-CAGGCAAACUAAUUGUGAAdTdT-3. The siRNAs were chemically synthesized
by Biotend Bio-Technique, Co., Ltd. (Shanghai, China).
Quantification of NTN4 and EMT-related
biomarkers by RT-qPCR
Total RNA was extracted from breast cancer tissues
and cells using TRIzol (113702; Life Technologies). RT-PCR was
performed with the First-Strand cDNA Synthesis kit (RT0212-03;
Biomiga, San Diego, CA, USA) and the cDNA was amplified by
SYBR-Green PCR Master Mix (RT0411-02; Biomiga) according to the
manufacturers instructions in the LightCycler 480 PCR apparatus
(Hoffman-La Roche Ltd., Basel, Switzerland). PCR reactions were
performed under the following conditions: 95°C for 10 min, 35
cycles of 95°C for 15 sec, and 60°C for 1 min. Levels of gene
expression relative to β-actin were evaluated by the
2−∆∆CT method. PCR primers were used as follows: NTN4 F,
5-GTACTTTGCGACTAACT GCTCC-3 and R, 5-TCCAGTGCATGGAAAAGGACT-3;
N-cadherin F, 5-CTGCTTCAGGCGTCTGTAGA-3 and R,
5-AGGCTCACTGCTCTCATATTGT-3; vimentin F, 5-AT CTGGATTCACTCCCTCTG-3
and R, 5-AAGGTCATCGT GATGCTGAG-3; β-actin F, 5-ACCCACACTGTGCCCAT
CTAC-3 and R, 5-TCGGTGAGGATCTCATGAGGTA-3.
Wound healing assay
Transfected cells were seeded into 12-well plates,
when the monolayer confluence was 90–95%, cells were wounded using
a sterile 200 µl pipette tip and washed using sterile PBS 2 times.
Then, fresh medium with 0.5% FBS was added to the 12-well plates
and images were taken at 0–48 h using microscope (Leica DMI3000B).
The gap sizes were analyzed with the ImageJ program and measured as
the percentage of the original time-point (0 h).
Transwell migration and Matrigel
invasion assay
The invasion was evaluated in Transwell chambers
coated with 50 µl Matrigel (356234; BD Biosciences, San Jose, USA;
Leica DMI3000B) in serum-free DMEM at a dilution of 1:5, the upper
and lower chambers were separated by polycarbonate membranes with
8-µm pore size (3422; CoStar Group, Inc., Cambridge, MA, USA). For
the migration assay, uncoated Transwell chambers were used. The
lower chambers contained 600 µl of DMEM with 10% FBS as a
chemo-attractant. Transfected cells (6×104) were seeded
in the upper chambers with serum-free DMEM containing 0.1% albumin
from bovine serum (BSA). After 24 h (migration assay) or 48 h
(invasion assay) of cultivation, cells on the upper surface of the
membranes were removed with a cotton swab and cells migrated or
invaded to the bottom surface were fixed with 95% alcohol, stained
with 1% crystal violet, and counted in five random fields at ×400
magnification.
Western blotting
Cells transfected with recombination plasmids or
siRNAs for 48 h were washed by 4°C PBS, lysed with cell lysis
buffer (P0013B; Beyotime Institute of Biotechnology, Jiangsu,
China) with inhibitors of proteases (Po100; Solarbio, Beijing,
China), and protein concentrations were quantified with an Enhanced
BCA protein assay kit (Beyotime Institute of Biotechnology).
Protein electrophoresis was performed by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (P1200;
Solarbio) and immunoblotting was performed on polyvinylidene
fluoride (PVDF) membranes (IPVH00010; Millipore, Billerica, MA,
USA). After non-specific antigen blocking with 5% non-fat milk, the
PVDF membranes were incubated at 4°C overnight with the following
antibodies: β-actin (mouse anti-human, diluted 1:5000, BS6007M;
Bioward Technology, Bloomington, MN, USA), N-cadherin (rabbit
anti-human, diluted 1:2000, 22018-1-AP; Proteintech Group Inc.,
Chicago, IL, USA) and vimentin (rabbit anti-human, diluted 1:1000,
10366-1-AP; Proteintech Group Inc.). After 3 washes with
Tris-buffered saline with tween (TBST), the PVDF membranes were
incubated with secondary antibodies conjugated with HRP [diluted
1:5000, BS13278 (BS12478); Bioward Technology] and were visualized
using ECL-plus (32106; Thermo Fisher Scientific, Grand Island, NY,
USA). Then the blots were imaged with Tanon 5200 chemiluminescence
imaging system.
Statistical analysis
All data were expressed as mean ± standard deviation
and SPSS 20.0 was used for statistical analyses. The
independent-samples two-tailed Students t-test and Mann-Whitney U
test were used to analyze the significance between the groups. For
all the results, three levels of significance (P<0.05, P<0.01
and P<0.001) were used.
Results
NTN4 expression is decreased in breast
cancer
Through application of RNA sequencing (RNA-Seq)
technology and bioinformatic analyses for 5 paired breast cancer
and adjacent tissues, we achieved 871 differential genes. Then we
found that NTN4 expression is markedly varied in different stages
by combining differential genes with clinicopathologic data (data
not shown). In addition, Oncomine data analyses showed that NTN4
expression in breast cancer was reduced compared with normal breast
tissues (Fig. 1A), and NTN4
expression in grade 2 and grade 3 was significantly lower than
grade 1 (Fig. 1B).
Then, we detected NTN4 mRNA expression in 14 paired
fresh frozen breast cancer tissues, and the results manifested that
NTN4 expression was significantly decreased in majority of breast
cancer lesions compared with adjacent tissues (Fig. 2A). To further verify the results,
NTN4 protein expression in 52 paired paraffin sections (Table I) was measured by IHC, and the
difference between lesions and adjacent tissues (Fig. 2B and C and Table II) was consistent with that in
fresh frozen tissues. Thus, we speculated that NTN4 may be closely
associated with breast cancer metastasis.
 | Table I.The correlation between NTN4
expression and breast cancer clinicopathological features. |
Table I.
The correlation between NTN4
expression and breast cancer clinicopathological features.
| Clinicopathological
features | N | P-value |
|---|
| Age (years) |
|
|
|
<50 | 36 | 0.249 |
| ≥50 | 16 |
|
| TNM stage |
|
|
| I+II | 39 | 0.736 |
| III | 13 |
|
| Node metastasis |
|
|
|
Positive | 24 | 0.138 |
|
Negative | 28 |
|
 | Table II.NTN4 expression in cancer and
adjacent tissues. |
Table II.
NTN4 expression in cancer and
adjacent tissues.
| Results | Adjacent (n) | Cancer (n) | Total |
|---|
| − | 10 | 22 | 32 |
| + | 17 | 21 | 38 |
| ++ | 24 | 8 | 32 |
| +++ | 1 | 1 | 2 |
| Total | 52 | 52 | 104 |
Next, we tested the NTN4 mRNA expression in six
breast cancer cell lines. As the results showed, NTN4 expression in
Hs578T cells was significantly higher than other cell lines
(Fig. 2D). Based on the
characteristic and the NTN4 expression of breast cancer cell lines
(20), we selected the highly
metastatic MDA-MB-231 cells with relatively low NTN4 expression and
moderately metastatic Hs578T cells with high NTN4 expression to
conduct in vitro experiments.
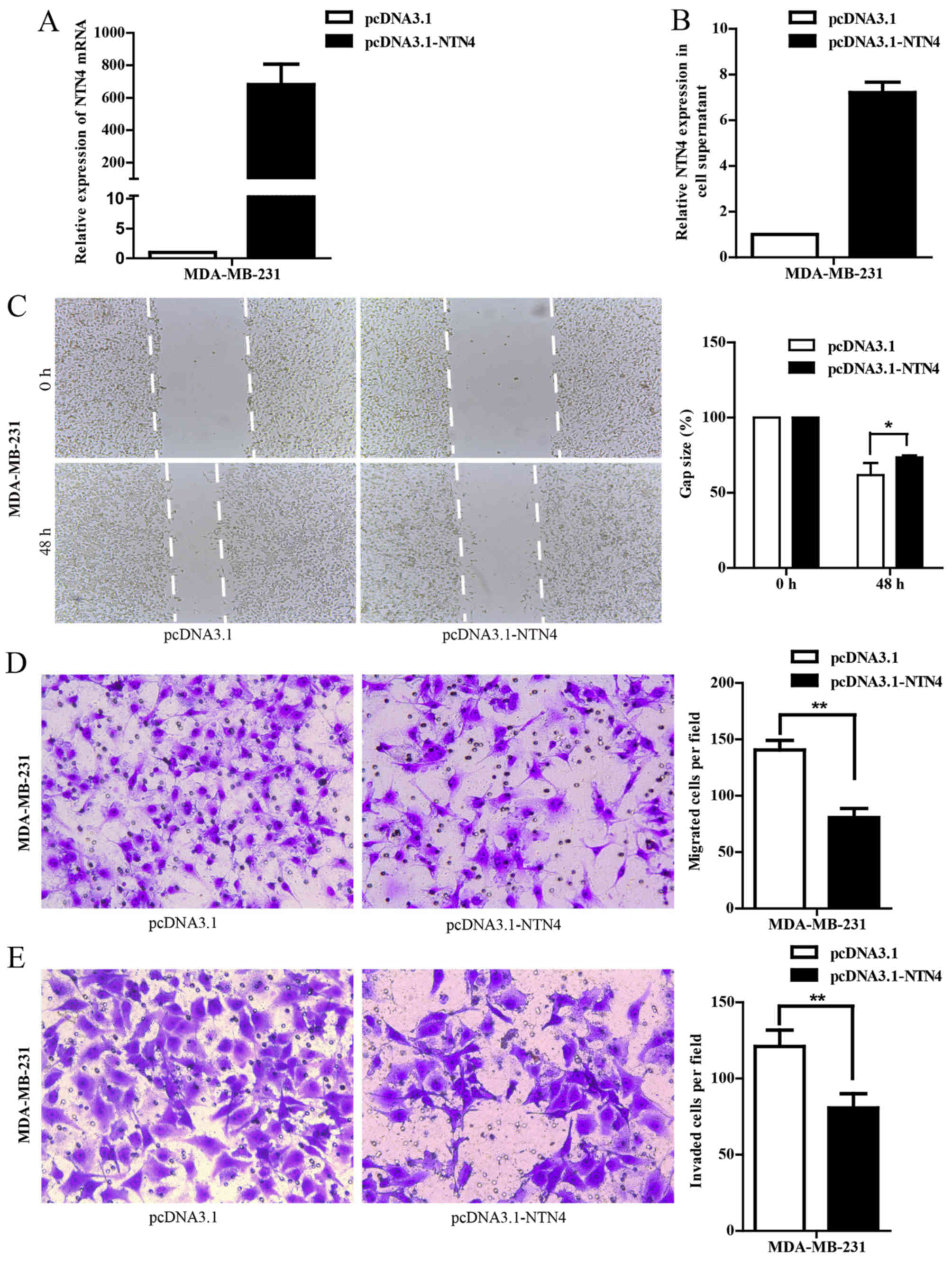
NTN4 overexpression inhibits cell
migration and invasion in MDA-MB-231 cells
To explore the role of NTN4 in breast cancer
metastasis, the migration and invasion abilities of MDA-MB-231
cells were investigated. NTN4 overexpression was achieved by
transiently transfecting with pcDNA3.1-NTN4. NTN4 overexpression at
the mRNA (Fig. 3A) and protein
level (Fig. 3B) were confirmed by
RT-qPCR and ELISA, respectively. The wound healing assay
demonstrated that the migration rate of cells with pcDNA3.1-NTN4
transfection was significantly reduced at 48 h compared with
control groups (Fig. 3C). The
results from the Transwell migration and the Matrigel invasion
assays showed that the cells in the pcDNA3.1-NTN4 groups had a
significant decrease in migration and invasion activity compared
with control cells (Fig. 3D and E).
These results indicate that NTN4 overexpression inhibits cell
migration and invasion of breast cancer cells.
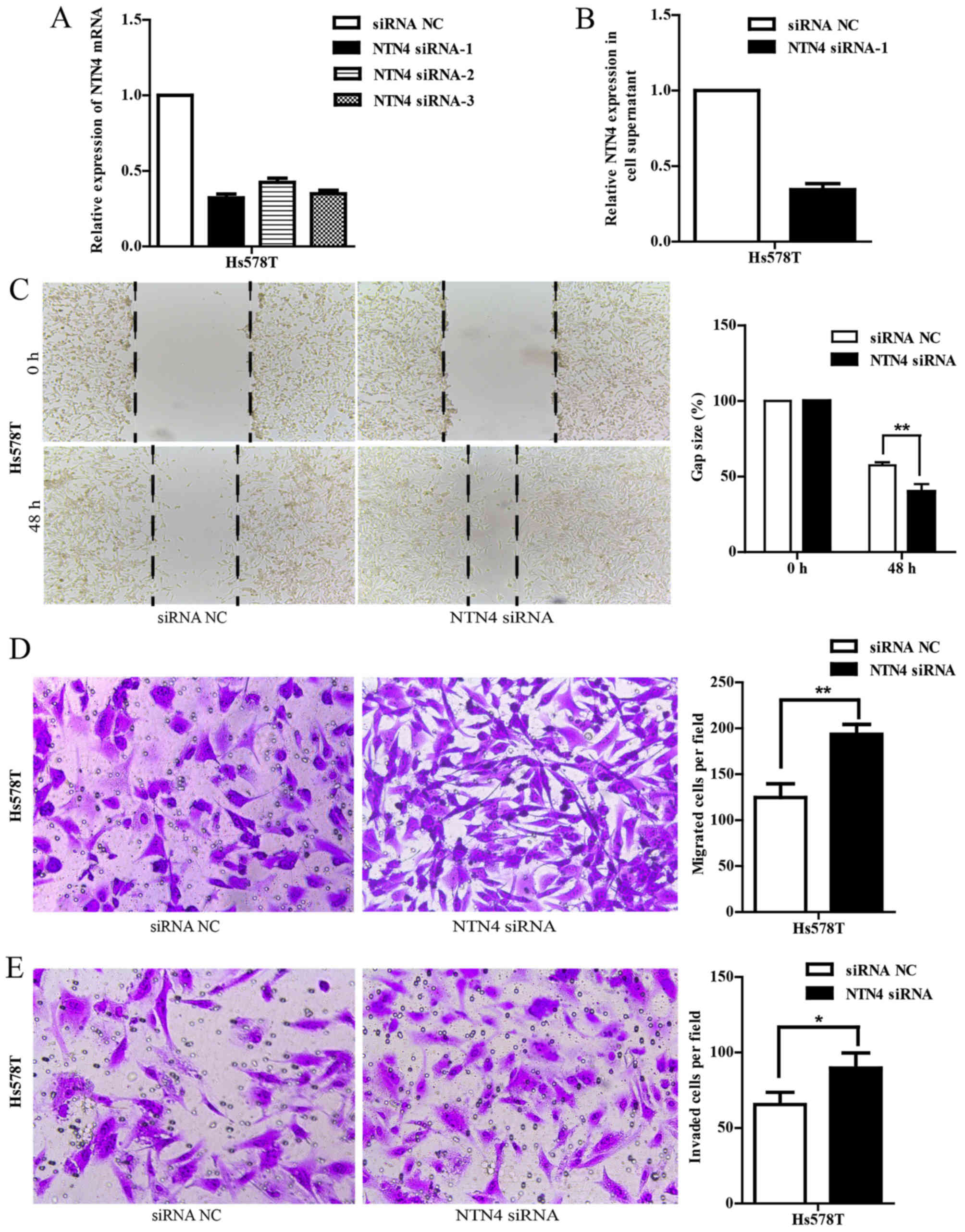
NTN4 silencing promotes cell migration
and invasion in Hs578T cells
To further validate the function of NTN4, we knocked
down NTN4 expression in Hs578T cells using NTN4-specific siRNA
targeting 5-GGCGCTATT TGTACTTCTA-3 (NTN4 siRNA-1). As shown in
Fig. 4A and B, NTN4 expression was
efficiently silenced in Hs578T cells at both mRNA and protein
levels compared with the controls. We also evaluated whether NTN4
knockdown could influence cell migration and invasion. Wound
healing assay showed that siRNA-mediated silencing of NTN4
significantly promoted migration at 48 h, as shown by an
accelerated wound closure (Fig.
4C). Furthermore, the Transwell migration results also
determined that NTN4 siRNA-transfected cells had a significant
increase of migrated cells compared with control scramble RNA
transfected cells (Fig. 4D).
Similar results of invasion assay were obtained (Fig. 4E). These findings demonstrate that
NTN4 silencing promotes the migration and invasion of breast cancer
cells.
NTN4 upregulation or downregulation
influences expression of EMT-related biomarkers
Gene-set enrichment analysis demonstrated that the
downregulated genes, NTN4 included, participated in the focal
adhesion kinase (FAK), mitogen-activated protein kinase (MAPK) and
transforming growth factor-β (TGF-β) signaling pathways (data not
shown). In addition, recent studies have reported that these
signaling pathways are involved in EMT and tumor metastasis
(21–24).
To obtain insight into the molecular mechanisms of
NTN4 on breast cancer cells metastasis, the expression of
EMT-related biomarkers (vimentin and N-cadherin) in mRNA and
protein levels were examined. We found that vimentin and N-cadherin
were downregulated at both mRNA and protein levels in MDA-MB-231
cells with transfection of NTN4 expressive plasmid (Fig. 5A and B). Conversely, NTN4 silencing
increased the expression of vimentin and N-cadherin in Hs578T cells
(Fig. 5C and D). These results
manifest that NTN4 overexpression or silencing is associated with
breast cancer migration and invasion via regulation of EMT-related
biomarkers.
Discussion
Metastasis is the major cause of deaths for breast
cancer patients. It is necessary to discover effective factors to
hinder the metastasis of breast cancer. NTN4, a nerve guidance
factor, exerts an influence on physiological functions such as
neurocyte growth and migration through binding to its various
receptors (25,26). Recent studies have revealed that
NTN4 is involved in tumor proliferation, as well as metastasis
(12,18).
In the present study, we found that NTN4 was
decreased in breast cancer lesion tissues compared with matched
adjacent tissues, which was in accordance with the results observed
by others (10). In addition,
previous studies have demonstrated that NTN4 inhibited tumor growth
and angiogenesis (11,12,27),
and promoted tumor cell proliferation at relatively low
concentrations (13,16,17).
Similarly, we found that NTN4 overexpression reduced the migration
and invasion activity in MDA-MB-231 cells, in contrast, NTN4
silencing enhanced migration and invasion in Hs578T cells.
Therefore, we conclude that NTN4 is associated with breast cancer
metastasis. However, a study in gastric cancer demonstrated that
NTN4 overexpression accelerated cell proliferation and invasion,
and NTN4 knockdown had the opposite effects. Investigations have
manifested that high concentration NTN4 inhibited proliferation and
migration through combining with Unc5B, while low concentration
NTN4 promoted proliferation and migration through binding to
integrin β4, that is why NTN4 exerted different functions on tumor
proliferation and metastasis (17).
Hence, NTN4 may have diverse functions at different concentrations
in different tumors.
Besides, studies have reported that NTN4 knockout
suppressed proliferation and motility through reducing the
phosphorylation of signal transducer and activator of transcription
3 (Stat3), extracellular regulated protein kinases (ERK), Akt and
p38, and inhibited invasion through decreasing matrix
metalloproteinase 2 (MMP2) expression and increasing tissue
inhibitor of metalloproteinase 1 (TIP1) expression in gastric
cancer cells, while overexpression or adding exogenous NTN4 can
reverse the effects (8). In
addition, proliferation and migration was hindered with decreased
expression of p-Akt-1, p-Jnk-2 and p-c-Jun when pancreatic cancer
cells were treated with NTN4 (13).
However, the molecular mechanisms involved in NTN4-mediated
metastasis in breast cancer are less understood.
NTN4 participates in the FAK, MAPK and TGF-β
signaling pathways. Moreover, these signaling pathways are involved
in EMT and tumor metastasis (21–24).
FAK activation led to numerous cell processes including cell
adhesion, migration, invasion and proliferation (28), and activation of TGF-β promoted the
occurrence of EMT (29). EMT is a
major mechanism to explain metastatic events in breast cancer.
During EMT, epithelial cells display reduced expression of
epithelial markers (E-cadherin) and enhance mesenchymal traits
(upregulation of vimentin and N-cadherin). Expression of N-cadherin
and vimentin was significantly higher in metastases than in the
related primary breast tumors (30–32).
In order to further understand the molecular mechanisms that NTN4
influences the migration and invasion in breast cancer cells, we
investigated the effects of NTN4 on vimentin and N-cadherin
expression. In the study, NTN4 overexpression reduced the
expression of vimentin and N-cadherin, while NTN4 knockdown
facilitated vimentin and N-cadherin expression. Studies have
demonstrated that expression of N-cadherin would confer on breast
cancer cells the capacity to invade (33,34),
and elevated vimentin expression was correlated with increased
migration and invasion of breast cancer cells (35). Given that upregulation of N-cadherin
and vimentin are mesenchymal traits during EMT, we consider that
NTN4 is associated with breast cancer migration and invasion via
regulation of EMT-related biomarkers.
In conclusion, this study demonstrates that NTN4 is
decreased in breast cancer tissues and that NTN4 is associated with
breast cancer migration and invasion via regulation of EMT-related
biomarkers.
Acknowledgements
The present study was supported by the National
Science Foundation of Zhejiang Province (LY14H200001), the
Medicines Health Platform Plan Project of Zhejiang Province
(2015DTA018) and the Medicines Health Platform Key Project of
Zhejiang Province (2013ZDA024).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
3
|
Yin Y, Sanes JR and Miner JH:
Identification and expression of mouse netrin-4. Mech Dev.
96:115–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Stein E, Oliver T, Li Y, Brunken
WJ, Koch M, Tessier-Lavigne M and Hogan BL: Novel role for Netrins
in regulating epithelial behavior during lung branching
morphogenesis. Curr Biol. 14:897–905. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoang S, Liauw J, Choi M, Choi M, Guzman
RG and Steinberg GK: Netrin-4 enhances angiogenesis and neurologic
outcome after cerebral ischemia. J Cereb Blood Flow Metab.
29:385–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han Y, Shao Y, Liu T, Qu YL, Li W and Liu
Z: Therapeutic effects of topical netrin-4 inhibits corneal
neovascularization in alkali-burn rats. PLoS One. 10:e01229512015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YH, Szabat M, Bragagnini C, Kott K,
Helgason CD, Hoffman BG and Johnson JD: Paracrine signalling loops
in adult human and mouse pancreatic islets: Netrins modulate beta
cell apoptosis signalling via dependence receptors. Diabetologia.
54:828–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv B, Song C, Wu L, Zhang Q, Hou D, Chen
P, Yu S, Wang Z, Chu Y, Zhang J, et al: Netrin-4 as a biomarker
promotes cell proliferation and invasion in gastric cancer.
Oncotarget. 6:9794–9806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lambert E, Coissieux MM, Laudet V and
Mehlen P: Netrin-4 acts as a pro-angiogenic factor during zebrafish
development. J Biol Chem. 287:3987–3999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esseghir S, Kennedy A, Seedhar P, Nerurkar
A, Poulsom R, Reis-Filho JS and Isacke CM: Identification of NTN4,
TRA1, and STC2 as prognostic markers in breast cancer in a screen
for signal sequence encoding proteins. Clin Cancer Res.
13:3164–3173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eveno C, Broqueres-You D, Feron JG,
Rampanou A, Tijeras-Raballand A, Ropert S, Leconte L, Levy BI and
Pocard M: Netrin-4 delays colorectal cancer carcinomatosis by
inhibiting tumor angiogenesis. Am J Pathol. 178:1861–1869. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eveno C, Contreres JO, Hainaud P, Nemeth
J, Dupuy E and Pocard M: Netrin-4 overexpression suppresses primary
and metastatic colorectal tumor progression. Oncol Rep. 29:73–78.
2013.PubMed/NCBI
|
|
13
|
Nacht M, St Martin TB, Byrne A, Klinger
KW, Teicher BA, Madden SL and Jiang Y: Netrin-4 regulates
angiogenic responses and tumor cell growth. Exp Cell Res.
315:784–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujikane T, Nishikawa N, Toyota M, Suzuki
H, Nojima M, Maruyama R, Ashida M, Ohe-Toyota M, Kai M, Nishidate
T, et al: Genomic screening for genes upregulated by demethylation
revealed novel targets of epigenetic silencing in breast cancer.
Breast Cancer Res Treat. 122:699–710. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Latil A, Chêne L, Cochant-Priollet B,
Mangin P, Fournier G, Berthon P and Cussenot O: Quantification of
expression of netrins, slits and their receptors in human prostate
tumors. Int J Cancer. 103:306–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Hu Y, Ylivinkka I, Li H, Chen P,
Keski-Oja J and Hyytiäinen M: NETRIN-4 protects glioblastoma cells
FROM temozolomide induced senescence. PLoS One. 8:e803632013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Y, Ylivinkka I, Chen P, Li L,
Hautaniemi S, Nyman TA, Keski-Oja J and Hyytiäinen M: Netrin-4
promotes glioblastoma cell proliferation through integrin β4
signaling. Neoplasia. 14:219–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Larrieu-Lahargue F, Welm AL, Thomas KR and
Li DY: Netrin-4 induces lymphangiogenesis in vivo. Blood.
115:5418–5426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan Y, Leszczynska M, Konstantinovsky S,
Tropé CG, Reich R and Davidson B: Netrin-4 is upregulated in breast
carcinoma effusions compared to corresponding solid tumors. Diagn
Cytopathol. 39:562–566. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amith SR, Wilkinson JM and Fliegel L:
Na+/H+ exchanger NHE1 regulation modulates
metastatic potential and epithelial-mesenchymal transition of
triple-negative breast cancer cells. Oncotarget. 7:21091–21113.
2016.PubMed/NCBI
|
|
21
|
Ho JN, Jun W, Choue R and Lee J: I3C and
ICZ inhibit migration by suppressing the EMT process and FAK
expression in breast cancer cells. Mol Med Rep. 7:384–388.
2013.PubMed/NCBI
|
|
22
|
Cheng HL, Lin CW, Yang JS, Hsieh MJ, Yang
SF and Lu KH: Zoledronate blocks geranylgeranylation not
farnesylation to suppress human osteosarcoma U2OS cells metastasis
by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways.
Oncotarget. 7:9742–9758. 2016.PubMed/NCBI
|
|
23
|
Taliaferro-Smith L, Oberlick E, Liu T,
McGlothen T, Alcaide T, Tobin R, Donnelly S, Commander R, Kline E,
Nagaraju GP, et al: FAK activation is required for IGF1R-mediated
regulation of EMT, migration, and invasion in mesenchymal triple
negative breast cancer cells. Oncotarget. 6:4757–4772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W
and Peng Y: AEG-1 participates in TGF-beta1-induced EMT through p38
MAPK activation. Cell Biol Int. 37:1016–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koch M, Murrell JR, Hunter DD, Olson PF,
Jin W, Keene DR, Brunken WJ and Burgeson RE: A novel member of the
netrin family, beta-netrin, shares homology with the beta chain of
laminin: Identification, expression, and functional
characterization. J Cell Biol. 151:221–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rajasekharan S and Kennedy TE: The netrin
protein family. Genome Biol. 10:2392009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lejmi E, Leconte L, Pédron-Mazoyer S,
Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M,
Assayag F, et al: Netrin-4 inhibits angiogenesis via binding to
neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA.
105:12491–12496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Micalizzi DS, Christensen KL, Jedlicka P,
Coletta RD, Barón AE, Harrell JC, Horwitz KB, Billheimer D,
Heichman KA, Welm AL, et al: The Six1 homeoprotein induces human
mammary carcinoma cells to undergo epithelial-mesenchymal
transition and metastasis in mice through increasing TGF-beta
signaling. J Clin Invest. 119:2678–2690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bock C, Kuhn C, Ditsch N, Krebold R,
Heublein S, Mayr D, Doisneau-Sixou S and Jeschke U: Strong
correlation between N-cadherin and CD133 in breast cancer: Role of
both markers in metastatic events. J Cancer Res Clin Oncol.
140:1873–1881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakagawa M, Bando Y, Nagao T, Morimoto M,
Takai C, Ohnishi T, Honda J, Moriya T, Izumi K, Takahashi M, et al:
Expression of p53, Ki-67, E-cadherin, N-cadherin and TOP2A in
triple-negative breast cancer. Anticancer Res. 31:2389–2394.
2011.PubMed/NCBI
|
|
32
|
Whipple RA, Balzer EM, Cho EH, Matrone MA,
Yoon JR and Martin SS: Vimentin filaments support extension of
tubulin-based microtentacles in detached breast tumor cells. Cancer
Res. 68:5678–5688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nieman MT, Prudoff RS, Johnson KR and
Wheelock MJ: N-cadherin promotes motility in human breast cancer
cells regardless of their E-cadherin expression. J Cell Biol.
147:631–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zajchowski DA, Bartholdi MF, Gong Y,
Webster L, Liu HL, Munishkin A, Beauheim C, Harvey S, Ethier SP and
Johnson PH: Identification of gene expression profiles that predict
the aggressive. Cancer Res. 61:5168–5178. 2001.PubMed/NCBI
|