Introduction
MicroRNAs (miRNAs) are an endogenous group of small
non-coding single-stranded RNAs that play critical roles in
different biological processes through negatively regulating
various genes at posttranscriptional level, predominantly by
interacting with the 3′-untranslated regions (3′UTR) by targeting
corresponding messenger RNAs (mRNAs) (1,2).
Increasing evidence has demonstrated that aberrant miRNAs are
implicated in cellular processes, including cell cycle,
differentiation, proliferation, apoptosis and tumorigenesis
(3). Previous studies have
identified the pivotal role in human tumors and indicate that
abnormal miRNAs are involved in the initiation, progression,
migration and invasion of human cancers by modulating the target
mRNAs expression of oncogenes or suppressor genes, including
hepatocellular carcinoma (HCC) (4,5).
Hence, miRNAs have been regarded as a novel promising indicator and
attractive therapeutic strategy for HCC patients.
Recently, mounting studies demonstrated that miR-638
function as a critical regulator of tumorigenesis, development and
progression. miR-638 played an important role in a wide range of
human cancers (6–10). For instance, miR-638 functions as
suppressor to inhibit cell proliferation, invasion and regulate
cell cycle by targeting tetraspanin 1 in human colorectal carcinoma
(CRC) and a low expression of miR-638 is associated with poor
survival prognosis of CRC patients (11). miR-638 suppresses gastric cancer
cell proliferation by targeting specificity protein 2 (Sp2) with
influence on the expression of cyclin D1 (12). However, miR-638 supports melanoma
metastasis and progression and suppresses p53-mediated apoptosis
pathways and autophagy by targeting the TP53INP2 transcription
(13). miR-638 is overexpressed in
human vascular smooth muscle cells and inhibits PDGF-BB-induced
cell proliferation and migration through targeting orphan nuclear
receptor NOR1 (14). Therefore, the
functional significance of miR-638 in cancer progression seem to be
cancer-type specific. Recently, miR-638 was reported to be
downregulated in HCC specimens and to inhibit angiogenesis and
growth of HCC by targeting VEGF (15). However, the clinical significance of
miR-638 and the underlying mechanisms implicated in the initiation
and development of HCC remain to be investigated.
Epithelial to mesenchymal transition (EMT) has been
proposed to have a critical role in the invasion and metastasis of
different cancers through transformation of adherent and polarized
epithelial cells interacting with the basement membrane by its
basal surface, into invasive and motile mesenchymal cells (16,17).
It is characterized by loss of epithelial traits, such as
E-cadherin repression, accompanied by gain of mesenchymal features,
such as increased N-cadherin and vimentin (18). Accumulated evidence revealed that
EMT plays an important role in HCC invasion and metastasis. Recent
studies have demonstrated that miRNAs are involved in the EMT
process in HCC development (8,19).
However, the association between miR-638 and EMT in HCC has
remained elusive.
In this study, we investigated the critical role of
miR-638 in HCC progression. Our results revealed that miR-638 was
downregulated in the HCC and the reduced miR-638 was associated
with adverse prognostic characteristics and poor 5-year survival of
HCC patients. We identified that miR-638 could regulate the
migration and invasion of HCC by targeting SOX2 in vitro.
Furthermore, miR-638 induced EMT phenotype in vitro and
in vivo. These data identify the underlying mechanism by
which miR-638 inhibits migration and invasion of HCC, and miR-638
is regarded as a novel prognostic biomarker for HCC patients.
Materials and methods
Clinical tissues and cell
cultures
The HCC samples and matched adjacent tissues from
113 patients who underwent radical surgical resection at the Second
Affiliated Hospital of Xi'an Jiaotong University from March 2007 to
June 2009 were studied. Tissues were immediately snap-frozen and
stored at −80°C for RNA extraction. None of the patients had
received any perioperative chemo- or radiotherapy. The patients
signed an informed consent form, and this research was approved by
the Ethics Committee of Xi'an Jiaotong University.
The human HCC cell lines Hep3B, HepG2, MHCC-97L,
SMMC-7721, MHCC-97H and the normal human immortalized hepatocyte
LO2 cells were obtained from the Institute of Biochemistry and Cell
Biology (Chinese Academy of Sciences, Shanghai, China) and were
cultured in Dulbecco's modified Eagle's medium (DMEM, Hyclone,
Logan, UT, USA) containing 10% FBS (Invitrogen, Carlsbad, CA, USA),
1% penicillin-streptomycin (Sigma, St. Louis, MO, USA). All the
cells were incubated in a humidified atmosphere at 37°C with 5%
CO2.
RNA extraction and quantitative
real-time polymerase chain reaction (qRT-PCR)
Total RNA from HCC tissues and cells was extracted
using TRIzol reagent (Invitrogen) according to the manufacturer's
protocol. cDNA was reverse-transcribed from 1 µg total RNA using a
Reverse Transcription kit (Takara Biochemicals, Tokyo, Japan). cDNA
was then amplified with a SYBR® Premix Ex Taq™ II
(Perfect Real-Time) kit (Takara Biochemicals). The gene expression
levels were calculated using the ∆∆Ct method with U6 or GAPDH as an
internal control. Hsa-miR-638 primer (HmiRQP0748), snRNA U6 qPCR
Primer (HmiRQP9001), SOX2 (HQP017628) and GAPDH (HQP006940) were
purchased from Genecopoeia (Guangzhou, China).
Western blotting
The whole proteins from cultured cells and tissues
was quantified with BCA Protein Assay kit (Thermo Scientific,
Rockford, IL, USA), and an equal amount of 40 µg protein was
separated by 10% SDS-PAGE and then transferred onto PVDF membranes
(Millipore, Billerica, MA, USA). The membranes were blocked with 5%
nonfat milk in TBST for 2 h at room temperature and incubated
overnight with respective primary antibodies (1:1000, Cell
Signaling Technology, Inc.) at 4°C. Then the membranes were washed
three times by TBST and incubated with appropriate HRP-conjugated
secondary antibody for 2 h at room temperature (ZSGB-BIO, Beijing,
China). Detection was performed by enhanced chemiluminescence kit
(Amersham, Little Chalfont, UK).
Cell transfection
Hsa-miR-638 precursor was purchased from Songon Tech
(Beijing, China) and inserted into pcDNA6.2-GW/EmGFPmiR vector
(named miR-638) according to the manufacturer's instructions.
miR-638 inhibitor (HmiR-AN0748) and the negative control
(CmiR-AN0001-AM04) were obtained from Genecopoeia. The SOX2
overexpression plasmid and specific siRNA against SOX2 (sense,
5′-GGAAUGGACCUUGUAUAGAUC-3′; anti-sense,
5′-UCUAUACAAGGUCCAUUCCCC-3′) and a scramble siRNA were synthesized
by Sangon Biotech Co., Ltd. (Shanghai, China). Cells were
transfected with the above vectors using Lipofectamine 2000 Reagent
(Invitrogen Life Technologies) in accordance with the
manufacturer's protocol.
Immunohistochemical staining
Immunohistochemical staining was performed on 4 µm
sections of paraffin-embedded tissues to detect the protein
expression. The corresponding antibody (1:300, Cell Signaling
Technology, Inc.) was applied as the primary antibody by a
streptavidin peroxidase-conjugated (SP-IHC) method. The staining
results were semi-quantitatively evaluated by the multiplicity of
staining intensity and the percentage of positive staining cells.
The percentage of positive cells was graded: 0 for <5%; 1 for
6–25%; 2 for 26–50%; 3 for 51–75% and 4 for >75%. Staining
intensity was assessed by four degrees: 0, negative; 1, weak; 2,
moderate; and 3, strong. Each section was assayed in ten
independent high magnification (×400) fields to obtain the average
scores.
Cell migration and invasion
assays
Matrigel-uncoated and - coated Transwell inserts (8
µm pore size; Millipore) were used to evaluate cell migration and
invasion. Briefly, 5×104 transfected cells were
suspended in 200 µl serum free DMEM medium in the upper chamber,
and 750 µl DMEM medium containing 10% FBS was placed in the lower
chamber. After 24 h of incubation, cells were fixed in 4%
paraformaldehyde for 20 min and stained with 0.1% crystal violet
dye for 15 min. The cells on the inner layer were softly removed
with a cotton swab and counted at five randomly selected views, and
the average cell number per view was calculated.
Dual-luciferasee reporter gene
assay
The 3′-UTR sequence of SOX2 was predicted to
interact with miR-638, together with a corresponding mutated
sequence within the predicted target sites, were synthesized and
inserted into the pRL-TK control vector (Promega, Madison, WI, USA)
called wt-SOX2 3′-UTR and mt-SOX2 3′-UTR. Subsequently, MHCC-97H
cells that were plated into a 24-well plate and were transfected
with 120 ng miR-638 inhibitor or negative control. Cells were
co-transfected with 30 ng of the wild-type or mutant 3′-UTR of SOX2
vector using Lipofectamine 2000 reagent (Invitrogen). After 48 h,
cells were harvested and measured according to the manufacturer's
instructions (Dual-Luciferase Assay System; Promega). pRL-TK
expressing Renilla luciferase was co-transfected as an internal
control to correct the differences in both transfection and harvest
efficiencies.
In vivo metastasis assay
Female BALB/c nude mice (4–6 week-old) (Centre of
Laboratory Animals, The Medical College of Xi'an Jiaotong
University, Xi'an, China) were randomized into two groups (n=5),
and either MHCC-97H-miR-638 or MHCC-97H-miR-control cells
(1×106) were injected into the tail veins for the
establishment of a pulmonary metastatic model. Mice were sacrificed
10 weeks post-injection and examined microscopically by H&E
staining for the development of lung metastatic foci. Animals were
housed in cages under standard conditions. All in vivo
protocols were approved by the Institutional Animal Care and Use
Committee of Xi'an Jiaotong University.
Statistical analysis
Data are presented as the mean ± SD and performed at
least three independent replicates. SPSS software, 16.0 (SPSS, Inc,
Chicago, IL, USA) and Graphpad Prism 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) were used for a two-tailed Student's t-test,
Pearson's correlation analysis, Kaplan-Meier method and the
log-rank test to evaluate the statistical significance. Differences
were defined as P<0.05.
Results
Clinical significance of reduced
miR-638 in HCC samples
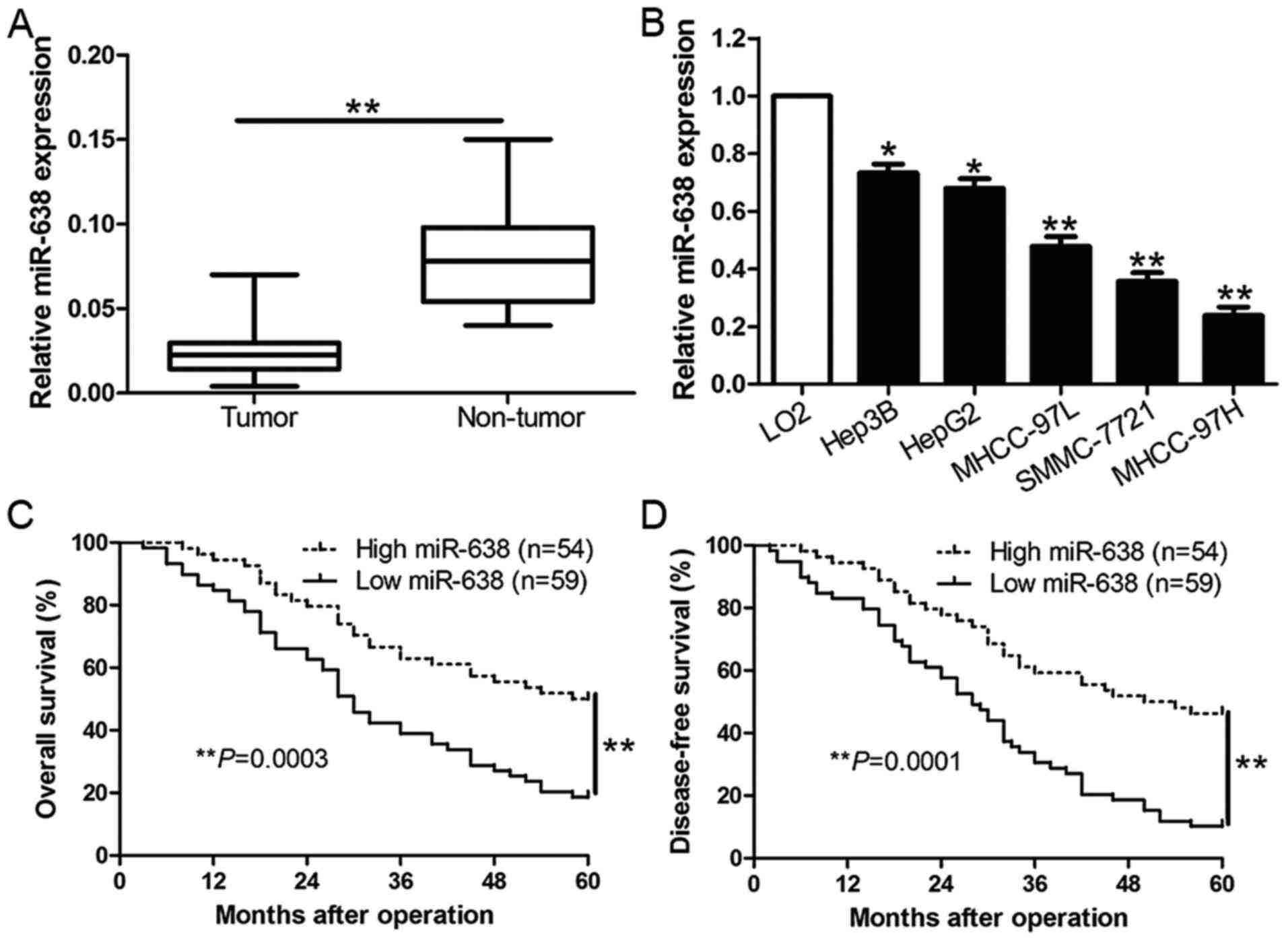
To assess the expression level of miR-638 in HCC, we
performed qRT-PCR to determine the expression of miR-638 in 113
pairs of HCC tissues and matched adjacent non-tumor tissues. As
shown in Fig. 1A, the mean level of
miR-638 expression in HCC tissues was obviously downregulated when
compared to the matched non-tumor tissues (P<0.01, Fig. 1A). Moreover, similar result was
found in HCC cell lines. The data revealed that miR-638 was
remarkably reduced in a panel of HCC cell lines than the normal
hepatocyte cell line LO2 (P<0.05, Fig. 1B). To further evaluate the role of
miR-638 in the progression of HCC, we analyzed the relationship
between miR-638 expression and the clinical characteristics and
prognosis of HCC patients. With the median level of miR-638 as the
cut-off, the low expression of miR-638 was prominently associated
with high Edmondson-Steiner grading (P=0.002), venous infiltration
(P=0.004) and tumor-node-metastasis (TNM) stage (P=0.007) (Table I). Furthermore, the low expression
of miR-638 was closely correlated with poor overall survival (OS)
(P=0.0003, Fig. 1C) and
disease-free survival (DFS) (P=0.0001, Fig. 1D) in HCC patients. In addition,
miR-638 expression was an independent factor for predicting both
5-year OS and DFS of HCC patients (P=0.016 and 0.010, respectively,
Table II). Taken together, these
data demonstrate that miR-638 showed reduced expression in HCC and
inversely associated with clinical characters and prognosis of HCC
patients, which suggest miR-638 was involved in the development of
HCC.
 | Table I.The relationship between miR-638
expression and clinicopathological feature in HCC (n=113). |
Table I.
The relationship between miR-638
expression and clinicopathological feature in HCC (n=113).
|
|
| Expression level |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Cases (n) |
miR-638high (n=54) | miR-638low
(n=59) | P-value |
|---|
| Age (years) |
|
|
|
|
| <60
years | 35 | 16 | 19 | 0.768 |
| ≥60
years | 78 | 38 | 40 |
|
| Gender |
|
|
|
|
| Male | 89 | 44 | 45 | 0.499 |
|
Female | 24 | 10 | 14 |
|
| Tumor size
(cm) |
|
|
|
|
| <5
cm | 67 | 32 | 35 | 0.995 |
| ≥5
cm | 46 | 22 | 24 |
|
| Tumor number |
|
|
|
|
|
Solitary | 99 | 49 | 50 | 0.334 |
|
Multiple | 14 | 5 | 9 |
|
| Edmondson |
|
|
|
|
|
I+II | 42 | 28 | 14 | 0.002a |
|
III+IV | 71 | 26 | 45 |
|
| TNM stage |
|
|
|
|
|
I+II | 96 | 51 | 45 | 0.007a |
|
III+IV | 17 | 3 | 14 |
|
| Venous
infiltration |
|
|
|
|
|
Present | 15 | 2 | 13 | 0.004a |
|
Absent | 98 | 52 | 46 |
|
| AFP |
|
|
|
|
| <400
ng/ml | 30 | 15 | 15 | 0.777 |
| ≥400
ng/ml | 83 | 39 | 44 |
|
| HBsAg |
|
|
|
|
|
Positive | 101 | 49 | 52 | 0.653 |
|
Negative | 12 | 5 | 7 |
|
 | Table II.Multivariate Cox regression analysis
of 5-year OS and DFS of 113 HCC patients. |
Table II.
Multivariate Cox regression analysis
of 5-year OS and DFS of 113 HCC patients.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| miR-638
expression | 0.254 | 0.076–0.812 | 0.016a | 0.238 | 0.072–0.667 | 0.010a |
| Edmondson
grade | 1.196 | 0.679–3.385 | 0.219 | 1.106 | 0.658–1.069 | 0.284 |
| TNM stage | 2.475 | 1.342–5.917 | 0.006a | 2.217 | 1.128–4.864 | 0.008a |
| Venous
infiltration | 2.894 | 1.776–6.049 | 0.003a | 3.012 | 1.893–6.347 | 0.001a |
miR-638 represses migration and
invasion of HCC cells in vitro
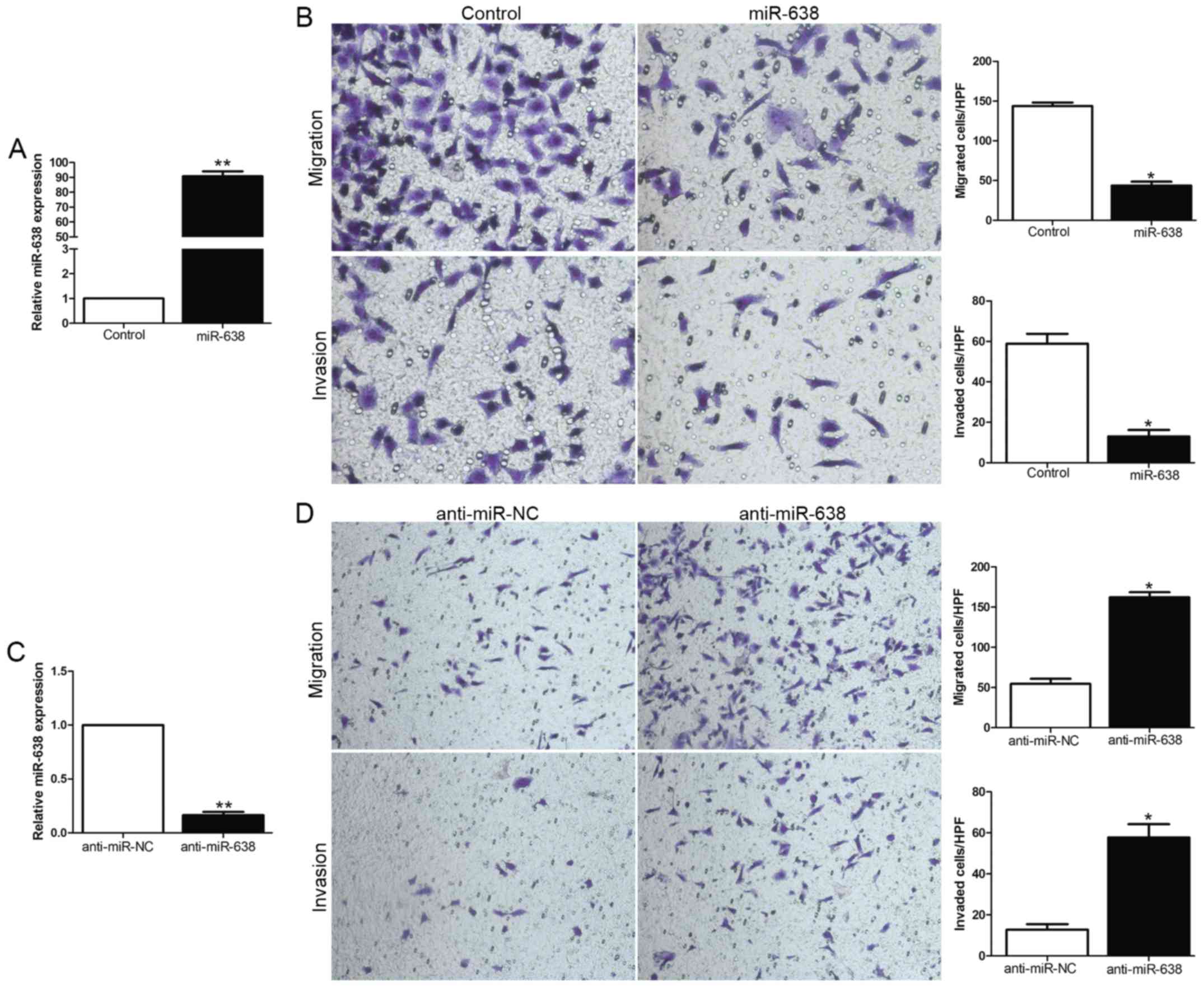
We focused on the functional effects of miR-638 in
HCC cells, gain- and loss-of-function assays were performed through
transfection of miR-638 or anti-miR-638 expression vector into HCC
cell lines with moderate miR-638 expression level. The transfection
efficiency was confirmed by using qRT-PCR (Fig. 2A and C). As measured by
Matrigel-coated (for invasion) and -uncoated (for migration)
Transwell assays, ectopic expression of miR-638 significantly
inhibited the migration and invasion of MHCC-97H cells (P<0.05,
Fig. 2B), whereas the silencing of
miR-638 expression obviously enhanced the number of migrated and
invaded Hep3B cells (P<0.05, Fig.
2D). In conclusion, these data suggested that miR-638 could
inhibit HCC cell migration and invasion.
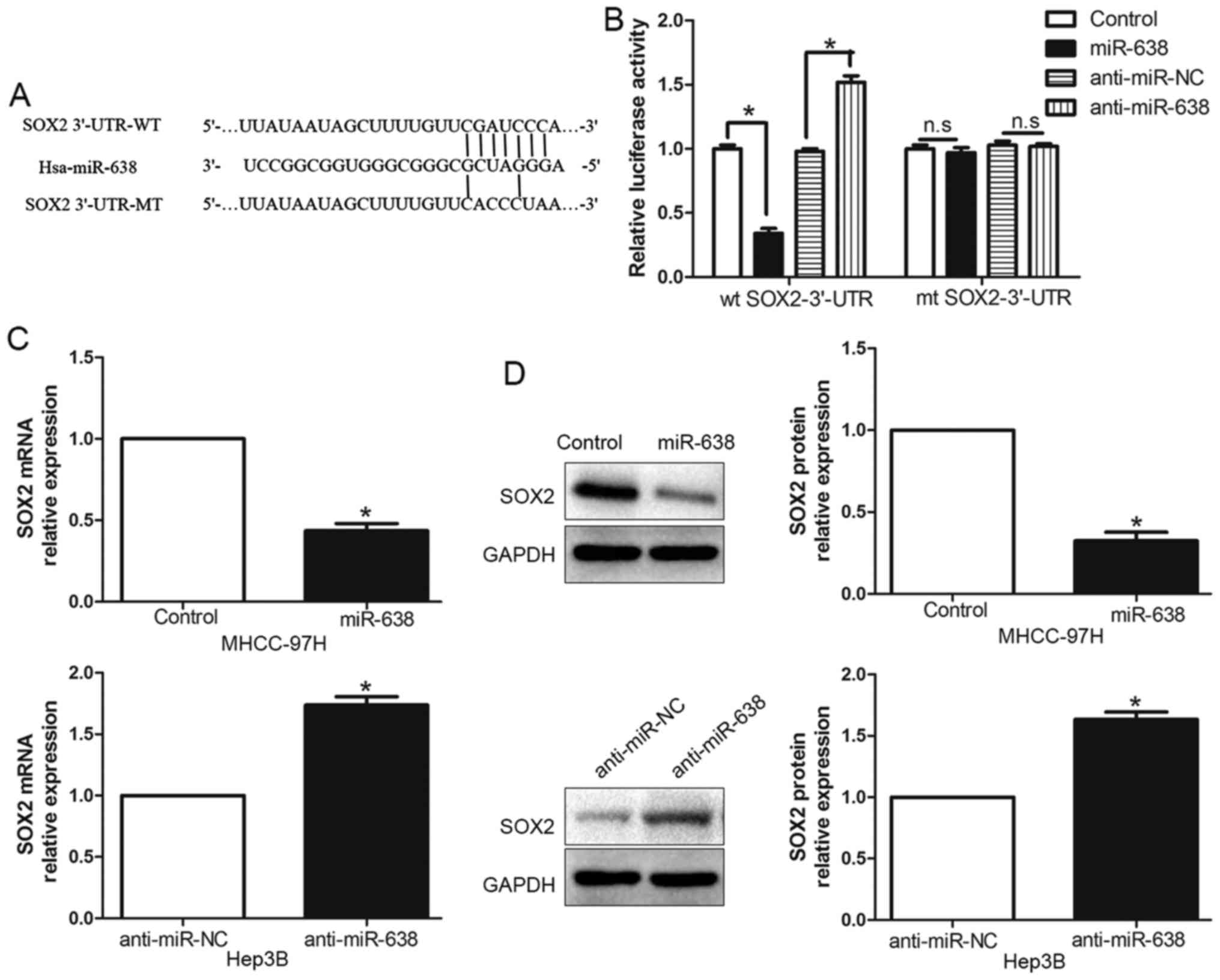
miR-638 directly inhibits SOX2
expression by interacting with its 3′-UTR
To elucidate the underlying mechanism of
miR-638-mediated suppression of cell migration and invasion, we
used TargetScan and miRanda algorithms to search for putative
protein-coding gene targets of miR-638. Bioinformatics software
indicated that SOX2 3′-UTR binds to miR-638 with high score
(Fig. 3A). Moreover, previous
studies have confirmed that miR-638 could modulate SOX2 expression
by directly binding its 3′-UTR (8,19). To
verify this, we generated a luciferase reporter plasmid which
carried the mutated binding site of miR-638 in the SOX2 3′-UTR and
found that miR-638 overexpression significantly inhibited the
luciferase activity of SOX2 containing a wild-type (wt) 3′-UTR but
did not suppress the activity of SOX2 with a mutant (mt) 3′-UTR
(P<0.05, Fig. 3B). On the
contrary, miR-638 suppression increased the luciferase activity of
wt SOX2 3′-UTR (P<0.05, Fig. 3B)
but had no effect on mt SOX2 3′-UTR constructs. Subsequently, we
examined the response to the alteration of miR-638 expression in
vitro. Our data showed the negative regulatory effect of
miR-638 on SOX2 both in MHCC-97H and Hep3B cell lines.
Overexpression of miR-638 inhibited SOX2 expression, moreover,
downregulated miR-638 could increase SOX2 expression both in mRNA
(P<0.05, Fig. 3C) and protein
(P<0.05, Fig. 3D) level. These
data indicated that miR-638 could modulate SOX2 expression by
directly binding its 3′-UTR in HCC cells.
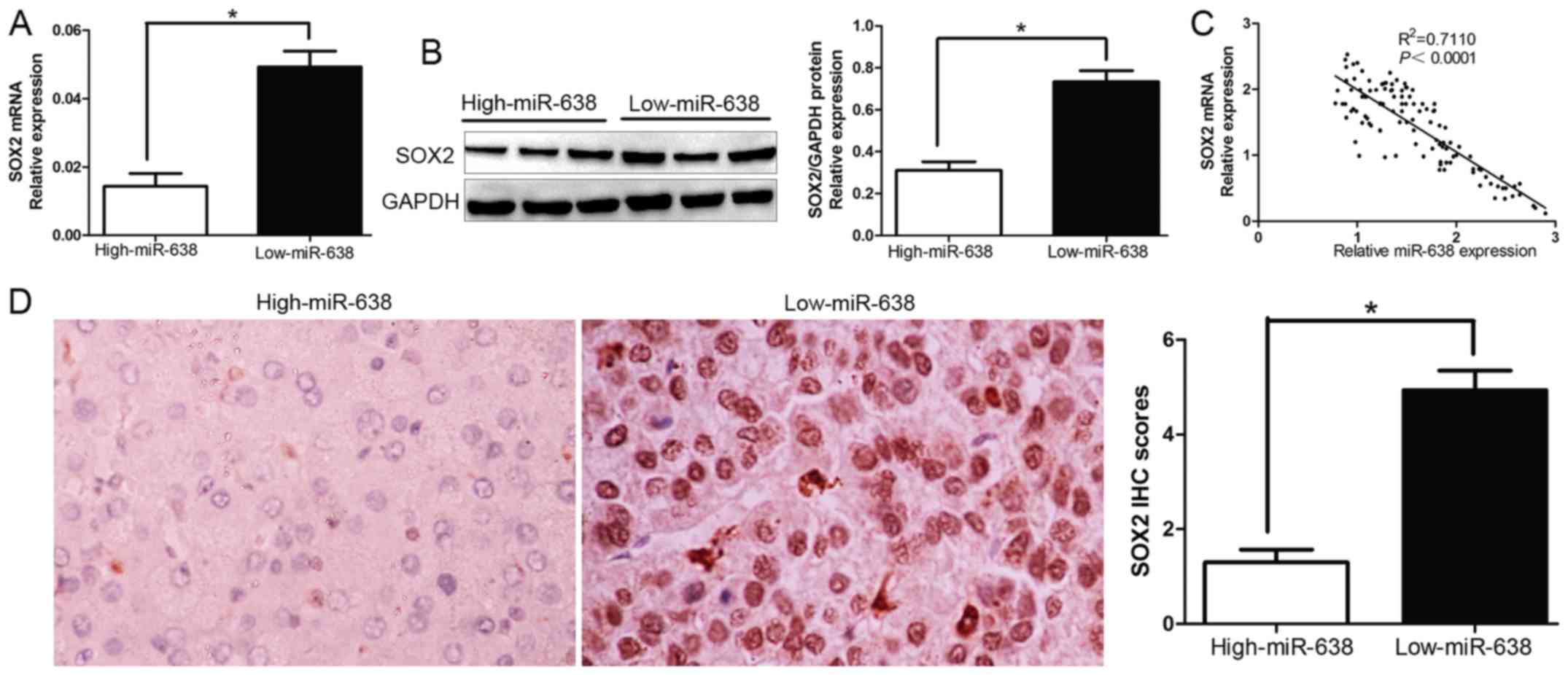
SOX2 levels are negatively correlated
with miR-638 expression
To further evaluate the relationship between SOX2
and miR-638 in HCC tissues, we investigated the SOX2 mRNA and
protein expression in different miR-638 levels. As expected, we
demonstrated that both SOX2 mRNA and protein expression level in
high miR-638 group were significantly lower compared with those in
low miR-638 group in HCC (P<0.05, Fig. 4A and B). Furthermore, the results
showed that the mRNA level of SOX2 in the HCC tissues was inversely
correlated with miR-638 expression (R2=0.711,
P<0.0001, Fig. 4C).
Consistently, as assessed by IHC assay, SOX2 protein expression in
miR-638 high-expressing tumors was obviously lower than miR-638
low-expressing tumors (P<0.05, Fig.
4D), which was similar with previous studies. The data
suggested that the increased SOX2 expression in HCC was caused by
miR-638 downregulation. Taken together, these results indicated
that SOX2 was a target gene of miR-638 in HCC.
Alterations of SOX2 expression levels
influences the effects of miR-638 on HCC cells
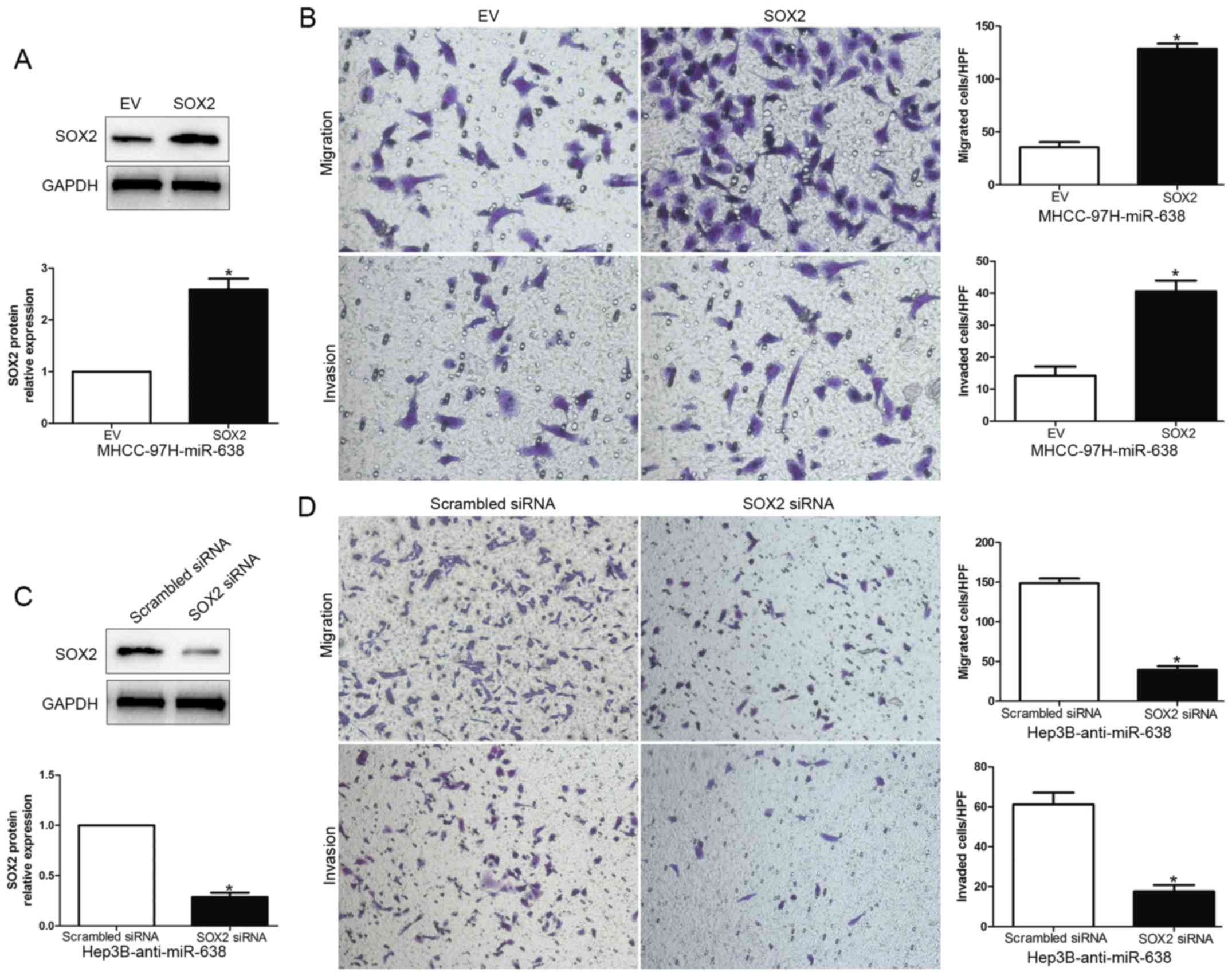
To further determine that SOX2 is a functional
target of miR-638, we overexpressed SOX2 by the transfecting SOX2
expression plasmid in MHCC-97H-miR-638 cells, or silenced SOX2 by
SOX2 siRNA in Hep3B-anti-miR-638 cells (P<0.05, Fig. 5A and C). As expected, restoration of
SOX2 expression partially abrogated the migration and invasion
suppressive effect of miR-638 on MHCC-97H cells (P<0.05,
Fig. 5B). Additionally, SOX2 siRNA
reversed the effects of miR-638 inhibition on Hep3B cells
(P<0.05, Fig. 5D). Taken
together, these experimental data suggest that the migration and
invasion effect of HCC are regulated by miR-638, and these miR-638
functions at least partially rely on the suppression of SOX2
expression.
Loss of miR-638 promotes a
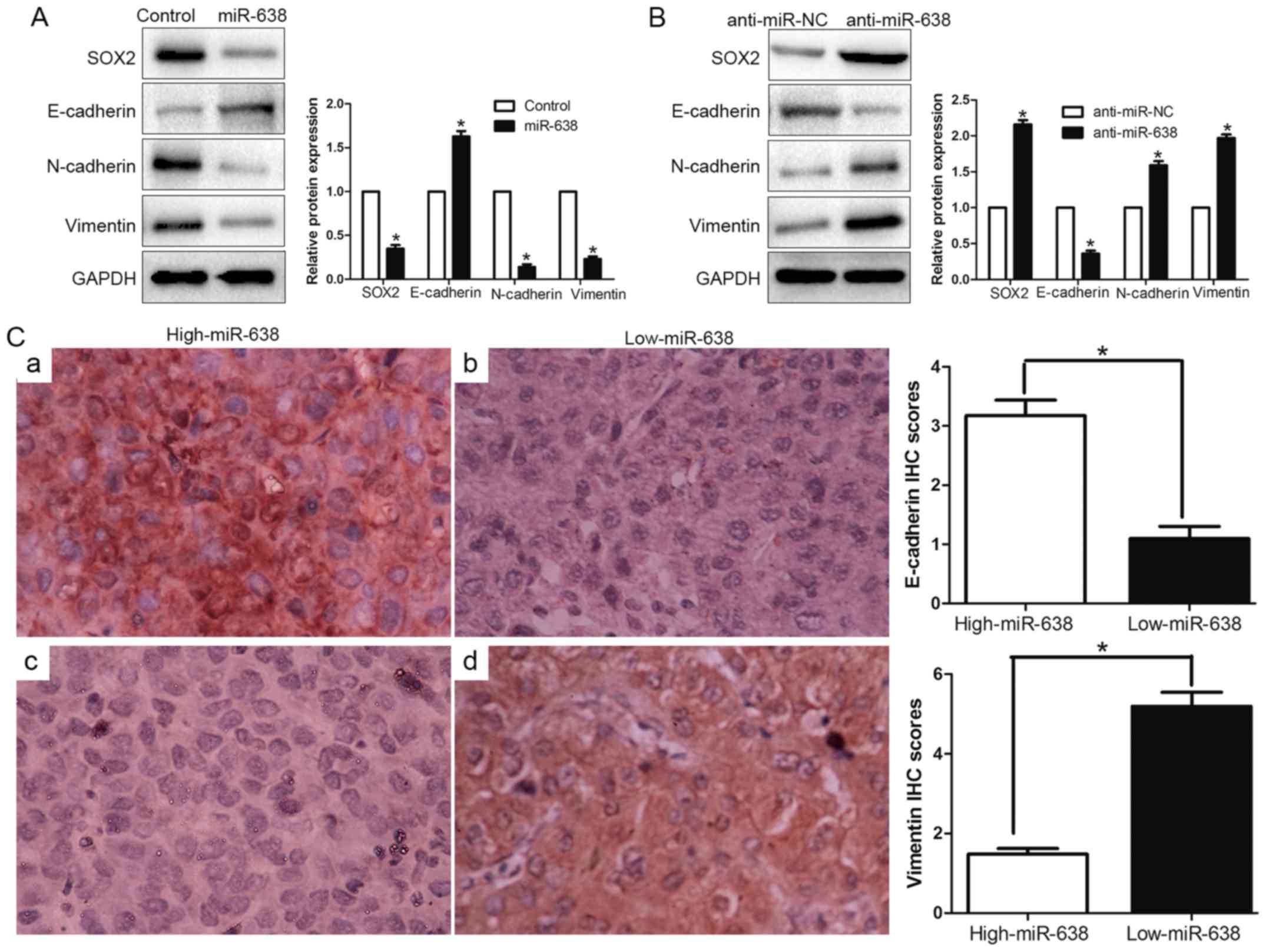
mesenchymal-like transition in HCC cells
EMT has been identified as a pivotal role in the
invasion of diverse cancer cells by the transformation of polarized
and adherent epithelial cells into invasive mesenchymal cells. To
confirm whether miR-638 was implicated in the EMT process, we
examined the epithelial marker (E-cadherin) and mesenchymal marker
(N-cadherin and vimentin) through western blotting. We found
upregulated miR-638 increased the epithelial marker E-cadherin and
decreased N-cadherin and vimentin expression (P<0.05, Fig. 6A). In contrast, inhibition of
miR-638 suppressed E-cadherin expression and induced N-cadherin and
vimentin expression (P<0.05, Fig.
6B). Furthermore, we determined the correlation between miR-638
expression and E-cadherin expression and vimentin expression in HCC
tissues. We found that the E-cadherin expression in high miR-638
group was higher than that in low miR-638 group. Conversely, the
expression level of vimentin in the high miR-638 group was
significantly lower than that in low miR-638 group (P<0.05,
Fig. 6C). Taken together, these
data indicated that miR-638 might contribute to regulation of EMT
in HCC.
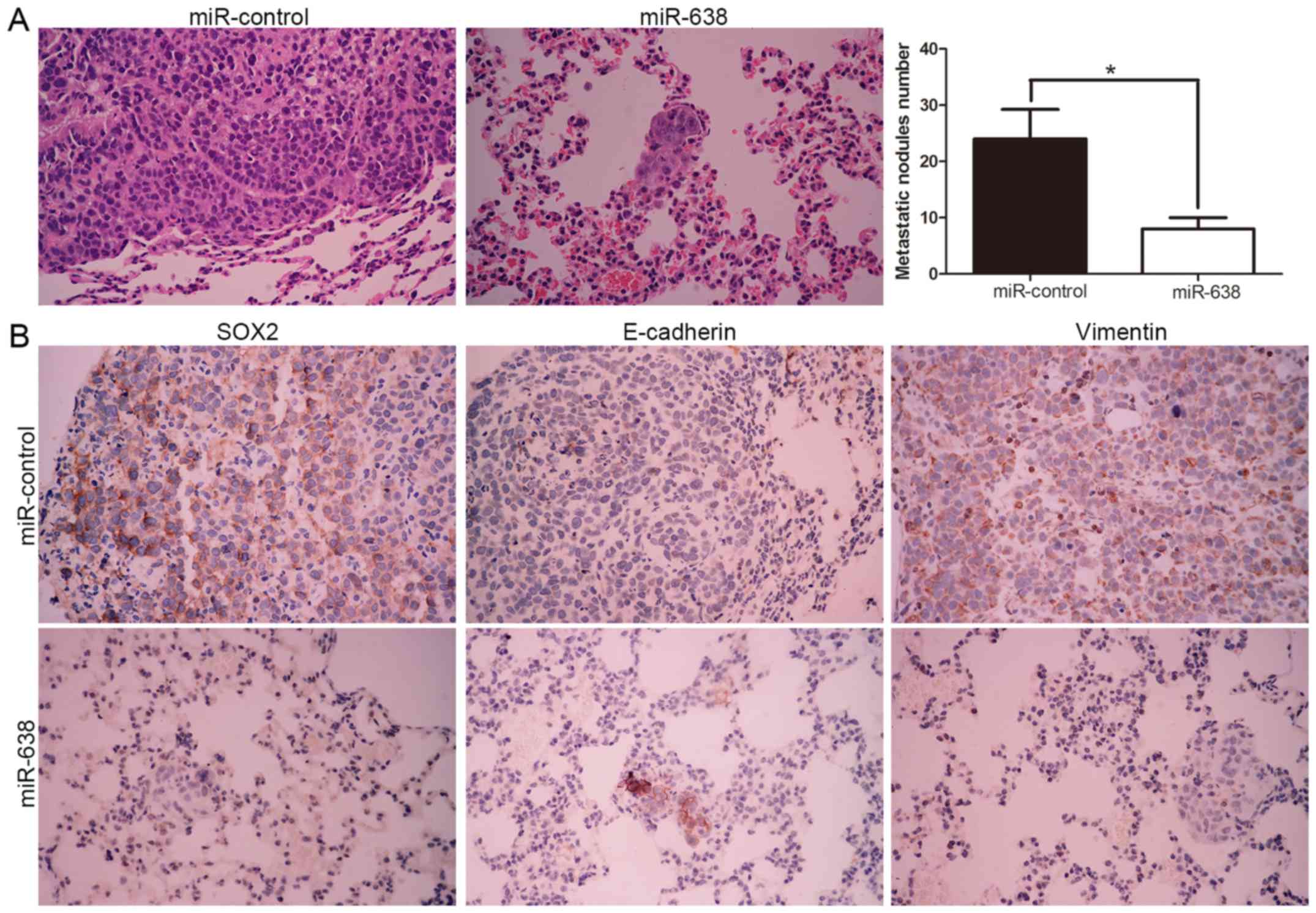
miR-638 mediated repression of SOX2
ameliorates the metastatic potential of HCC cells in vivo
To further verify the molecular mechanism and
biological function of miR-638, we subsequently injected
MHCC-97H-miR-638 and MHCC-97H-control cells into the lateral veins
of the nude mice. We found that miR-638 overexpression showed fewer
and smaller foci in the lungs of the nude mice after injection
through microscopic evaluation (8 vs. 24 nodules per lung in
MHCC-97H-miR-638 and miR-control cells, respectively; P<0.01,
Fig. 7A). Moreover, we also
demonstrated that lung sections of overexpressed miR-638 in fact
showed decreased SOX2 and vimentin expression and conversely
increased E-cadherin expression (Fig.
7B). Collectively, these results indicated that miR-638 is
capable of manipulating invasive biological function and EMT
phenotype of HCC by targeting SOX2 both in vitro and in
vivo.
Discussion
HCC is one of the most frequent malignancies and the
third leading cause of cancer-related death worldwide (20,21).
Recent investigations demonstrated that miRNAs played a pivotal
regulatory role in cancer initiation, proliferation, migration,
invasion and other various cellular processes. Therefore, miRNAs
are increasingly identified as a promising and potential diagnostic
and therapeutic target of HCC. In previous studies, Manfred et
al showed that the overexpression of miR-638 promoted the
proliferation, migration and colony formation properties of
melanoma cells both in vitro and metastatic capacities in
vivo. miR-638 has an oncogenic role in protecting melanoma
cells from apoptosis and autophagy. However, on the contrary,
miR-638 was downregulated in colorectal carcinoma cells and loss of
miR-638 in vitro promotes cell invasion and a
mesenchymal-like transition by targeting SOX2 expression in CRC
cells. Moreover, miR-638 inhibited cell proliferation by targeting
phospholipase D1 in human gastric carcinoma (22). In addition, downregulation of
miR-638 enhanced invasion and proliferation by regulating SOX2 and
induced EMT in NSCLC. miR-638-mediated regulation of BRCA1 affected
DNA repair and sensitivity to UV and cisplatin in triple-negative
breast cancer (6).
In the present study, we initially confirmed that
the mean expression level of miR-638 was significantly
downregulated in HCC tissues compared to matched tumor-adjacent
tissues, and similar result was obtained in HCC cells. Reduced
miR-638 expression conferred a significant correlation with
malignant clinicopathological characteristics of HCC patients,
including high histological grade, venous infiltration and advanced
TNM stage. Moreover, we found that high miR-638 group had a
significantly better 5-year OS and DFS in HCC patients.
Multivariate Cox repression analysis indicated that miR-638 was an
independent prognostic factor for predicting survival of HCC
patients. Taken together, these results suggest that miR-638 is
critical for prognosis outcome of HCC patients. Mechanistically,
gain- and loss-of-function experiment confirmed miR-638 inhibited
HCC cell migration and invasion in vitro, which suggested
that miR-638 was a tumor suppressor and invasion-related miRNA in
HCC. Importantly, we identified SOX2 as a direct target of miR-638
by luciferase report activity. Our data also showed miR-638
overexpression diminished while miR-638 knockdown promoted SOX2
mRNA and protein expression in HCC cell lines by directly binding
the 3′-UTR of SOX2. Similarly, we tried to elucidate the reverse
correlation of miR-638 and SOX2 expression in HCC tissues. The
effects of miR-638 alteration on migration and invasion of HCC
cells were also abolished by SOX2 modulation, which indicated that
miR-638 suppressed migration and invasion, at least in part, by
targeting SOX2. These data were consistent with previous studies
(8,19). Collectively, these results suggested
miR-638 may play critical role of a negative regulator or tumor
suppressor for the cell migration and invasion by targeting SOX2
expression.
Recently, increasing evidence has demonstrated that
SOX2 participated in oncogenesis and progression of various cancers
by regulation of multiple cell signaling pathways, including HCC
(23–28). SOX2 expression predicts poor
survival of HCC patients and it promotes liver cancer cell invasion
by activating Slug (24). In
pancreatic ductal adenocarcinoma cells, SOX2 functions as a
molecular rheostat to control the growth, tumorigenicity and drug
response (29). Moreover, Li et
al demonstrated that SOX2 could promote tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
Wnt/β-catenin signal pathway (30).
Here, we also found that miR-638 inhibited tumor migration and
invasion and EMT phenotype through targeting SOX2 in vitro
and in vivo. In addition, we found the miR-638 expression
was inversely correlated with the EMT marker (E-cadherin and
vimentin) expression, which reinforce the biological function of
miR-638 on EMT. Moreover, we discovered that the overexpression of
miR-638 suppressed the lung metastasis through targeting SOX2, and
increased E-cadherin expression and decreased vimentin expression.
These data confirmed that the functional effect of miR-638 on HCC
in vitro and in vivo was dependent on SOX2.
In summary, we demonstrated that miR-638 was
downregulated in HCC tissues and cell lines, and its expression was
correlated with malignant clinicopathological features.
Furthermore, we confirmed miR-638 inhibited cell migration and
invasion in vitro and in vivo by inhibiting SOX2
mediated EMT signaling pathway. These results suggest that miR-638
is a potential invasion-associated tumor suppressor in HCC. In
future, therapeutic interventions concentrating on miR-638-SOX2 may
help repress the development and metastasis of HCC.
References
|
1
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosa A and Brivanlou AH: MicroRNAs in
early vertebrate development. Cell Cycle. 8:3513–3520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: microRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Dou C, Yao B, Xu M, Ding L, Wang Y,
Jia Y, Li Q, Zhang H, Tu K, et al: Ftx non coding RNA-derived
miR-545 promotes cell proliferation by targeting RIG-I in
hepatocellular carcinoma. Oncotarget. 7:25350–25365.
2016.PubMed/NCBI
|
|
5
|
Tu K, Zheng X, Dou C, Li C, Yang W, Yao Y
and Liu Q: MicroRNA-130b promotes cell aggressiveness by inhibiting
peroxisome proliferator-activated receptor gamma in human
hepatocellular carcinoma. Int J Mol Sci. 15:20486–20499. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan X, Peng J, Fu Y, An S, Rezaei K,
Tabbara S, Teal CB, Man YG, Brem RF and Fu SW: miR-638 mediated
regulation of BRCA1 affects DNA repair and sensitivity to UV and
cisplatin in triple-negative breast cancer. Breast Cancer Res.
16:4352014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Lou JF, Cao Y, Shi XH, Wang P, Xu
J, Xie EF, Xu T, Sun RH, Rao JY, et al: miR-638 is a new biomarker
for outcome prediction of non-small cell lung cancer patients
receiving chemotherapy. Exp Mol Med. 47:e1622015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Y, Wu Y, Liu B, Wang P and Chen Y:
Downregulation of miR-638 promotes invasion and proliferation by
regulating SOX2 and induces EMT in NSCLC. FEBS Lett. 588:2238–2245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Y, Li D, Liang Q, Liu S, Zuo X, Li L,
Sun X, Li W, Guo M and Huang Z: miR-638 regulates differentiation
and proliferation in leukemic cells by targeting cyclin-dependent
kinase 2. J Biol Chem. 290:1818–1828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sand M, Skrygan M, Sand D, Georgas D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Expression of
microRNAs in basal cell carcinoma. Br J Dermatol. 167:847–855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Fei B, Wang Q, Song M, Yin Y,
Zhang B, Ni S, Guo W, Bian Z, Quan C, et al: MicroRNA-638 inhibits
cell proliferation, invasion and regulates cell cycle by targeting
tetraspanin 1 in human colorectal carcinoma. Oncotarget.
5:12083–12096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao LY, Yao Y, Han J, Yang J, Wang XF,
Tong DD, Song TS, Huang C and Shao Y: miR-638 suppresses cell
proliferation in gastric cancer by targeting Sp2. Dig Dis Sci.
59:1743–1753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhattacharya A, Schmitz U, Raatz Y,
Schönherr M, Kottek T, Schauer M, Franz S, Saalbach A, Anderegg U,
Wolkenhauer O, et al: miR-638 promotes melanoma metastasis and
protects melanoma cells from apoptosis and autophagy. Oncotarget.
6:2966–2980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li P, Liu Y, Yi B, Wang G, You X, Zhao X,
Summer R, Qin Y and Sun J: MicroRNA-638 is highly expressed in
human vascular smooth muscle cells and inhibits PDGF-BB-induced
cell proliferation and migration through targeting orphan nuclear
receptor NOR1. Cardiovasc Res. 99:185–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng J, Chen Y, Zhao P, Liu X, Dong J, Li
J, Huang C, Wu R and Lv Y: Downregulation of miRNA-638 promotes
angiogenesis and growth of hepatocellular carcinoma by targeting
VEGF. Oncotarget. 7:30702–30711. 2016.PubMed/NCBI
|
|
16
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spaderna S, Schmalhofer O, Hlubek F, Berx
G, Eger A, Merkel S, Jung A, Kirchner T and Brabletz T: A
transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye J, Wu D, Shen J, Wu P, Ni C, Chen J,
Zhao J, Zhang T, Wang X and Huang J: Enrichment of colorectal
cancer stem cells through epithelial-mesenchymal transition via
CDH1 knockdown. Mol Med Rep. 6:507–512. 2012.PubMed/NCBI
|
|
19
|
Ma K, Pan X, Fan P, He Y, Gu J, Wang W,
Zhang T, Li Z and Luo X: Loss of miR-638 in vitro promotes cell
invasion and a mesenchymal-like transition by influencing SOX2
expression in colorectal carcinoma cells. Mol Cancer. 13:1182014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127:(Suppl 1). S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Bian Z, Zhou J, Song M, Liu Z,
Feng Y, Zhe L, Zhang B, Yin Y and Huang Z: MicroRNA-638 inhibits
cell proliferation by targeting phospholipase D1 in human gastric
carcinoma. Protein Cell. 6:680–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang P, Qiu J, Li B, Hong J, Lu C, Wang
L, Wang J, Hu Y, Jia W and Yuan Y: Role of Sox2 and Oct4 in
predicting survival of hepatocellular carcinoma patients after
hepatectomy. Clin Biochem. 44:582–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun C, Sun L, Li Y, Kang X, Zhang S and
Liu Y: Sox2 expression predicts poor survival of hepatocellular
carcinoma patients and it promotes liver cancer cell invasion by
activating Slug. Med Oncol. 30:5032013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X, Sun B, Sun D, Liu T, Che N, Gu Q,
Dong X, Li R, Liu Y and Li J: Slug promotes hepatocellular cancer
cell progression by increasing sox2 and nanog expression. Oncol
Rep. 33:149–156. 2015.PubMed/NCBI
|
|
26
|
Wen W, Han T, Chen C, Huang L, Sun W, Wang
X, Chen SZ, Xiang DM, Tang L, Cao D, et al: Cyclin G1 expands liver
tumor-initiating cells by Sox2 induction via Akt/mTOR signaling.
Mol Cancer Ther. 12:1796–1804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao C, Li Y, Zhang M, Yang Y and Chang L:
miR-126 inhibits cell proliferation and induces cell apoptosis of
hepatocellular carcinoma cells partially by targeting Sox2. Hum
Cell. 28:91–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Velpula KK, Dasari VR, Tsung AJ, Dinh DH
and Rao JS: Cord blood stem cells revert glioma stem cell EMT by
down regulating transcriptional activation of Sox2 and Twist1.
Oncotarget. 2:1028–1042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wuebben EL, Wilder PJ, Cox JL, Grunkemeyer
JA, Caffrey T, Hollingsworth MA and Rizzino A: SOX2 functions as a
molecular rheostat to control the growth, tumorigenicity and drug
responses of pancreatic ductal adenocarcinoma cells. Oncotarget.
7:34890–34906. 2016.PubMed/NCBI
|
|
30
|
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T,
Liu Y, Li X, Xiang R and Li N: SOX2 promotes tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
WNT/β-catenin signal network. Cancer Lett. 336:379–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|