|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Sala M and Llovet JM:
Chemoembolization for hepatocellular carcinoma. Gastroenterology.
127:(Suppl 1). S179–S188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu AX: Development of sorafenib and other
molecularly targeted agents in hepatocellular carcinoma. Cancer.
112:250–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heiden MG Vander: Targeting cancer
metabolism: A therapeutic window opens. Nat Rev Drug Discov.
10:671–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Locasale JW: Serine, glycine and
one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer.
13:572–583. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tirado-Vélez JM, Joumady I, Sáez-Benito A,
Cózar-Castellano I and Perdomo G: Inhibition of fatty acid
metabolism reduces human myeloma cells proliferation. PLoS One.
7:e464842012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang YS, Tsai CT, Huangfu CA, Huang WY,
Lei HY, Lin CF, Su IJ, Chang WT, Wu PH, Chen YT, et al: ACSL3 and
GSK-3β are essential for lipid upregulation induced by endoplasmic
reticulum stress in liver cells. J Cell Biochem. 112:881–893. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang HL, Hsu HP, Shieh SC, Chang YS, Chen
WC, Cho CY, Teng CF, Su IJ, Hung WC and Lai MD: Attenuation of
argininosuccinate lyase inhibits cancer growth via cyclin A2 and
nitric oxide. Mol Cancer Ther. 12:2505–2516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Delage B, Fennell DA, Nicholson L, McNeish
I, Lemoine NR, Crook T and Szlosarek PW: Arginine deprivation and
argininosuccinate synthetase expression in the treatment of cancer.
Int J Cancer. 126:2762–2772. 2010.PubMed/NCBI
|
|
16
|
Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr
M, Spector S and Savaraj N: Arginine deprivation as a targeted
therapy for cancer. Curr Pharm Des. 14:1049–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ensor CM, Holtsberg FW, Bomalaski JS and
Clark MA: Pegylated arginine deiminase (ADI-SS PEG20,000 mw)
inhibits human melanomas and hepatocellular carcinomas in vitro and
in vivo. Cancer Res. 62:5443–5450. 2002.PubMed/NCBI
|
|
18
|
Cheng PNM, Lam TL, Lam WM, Tsui SM, Cheng
AW, Lo WH and Leung YC: Pegylated recombinant human arginase
(rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation
of human hepatocellular carcinoma through arginine depletion.
Cancer Res. 67:309–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YY, Wu C, Chen SM, Shah SS,
Wangpaichitr M, Feun LG, Kuo MT, Suarez M, Prince J and Savaraj N:
BRAF inhibitor resistance enhances vulnerability to arginine
deprivation in melanoma. Oncotarget. 7:17665–17680. 2016.PubMed/NCBI
|
|
20
|
Bobak Y, Kurlishchuk Y,
Vynnytska-Myronovska B, Grydzuk O, Shuvayeva G, Redowicz MJ,
Kunz-Schughart LA and Stasyk O: Arginine deprivation induces
endoplasmic reticulum stress in human solid cancer cells. Int J
Biochem Cell Biol. 70:29–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miraki-Moud F, Ghazaly E, Ariza-McNaughton
L, Hodby KA, Clear A, Anjos-Afonso F, Liapis K, Grantham M, Sohrabi
F, Cavenagh J, et al: Arginine deprivation using pegylated arginine
deiminase has activity against primary acute myeloid leukemia cells
in vivo. Blood. 125:4060–4068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
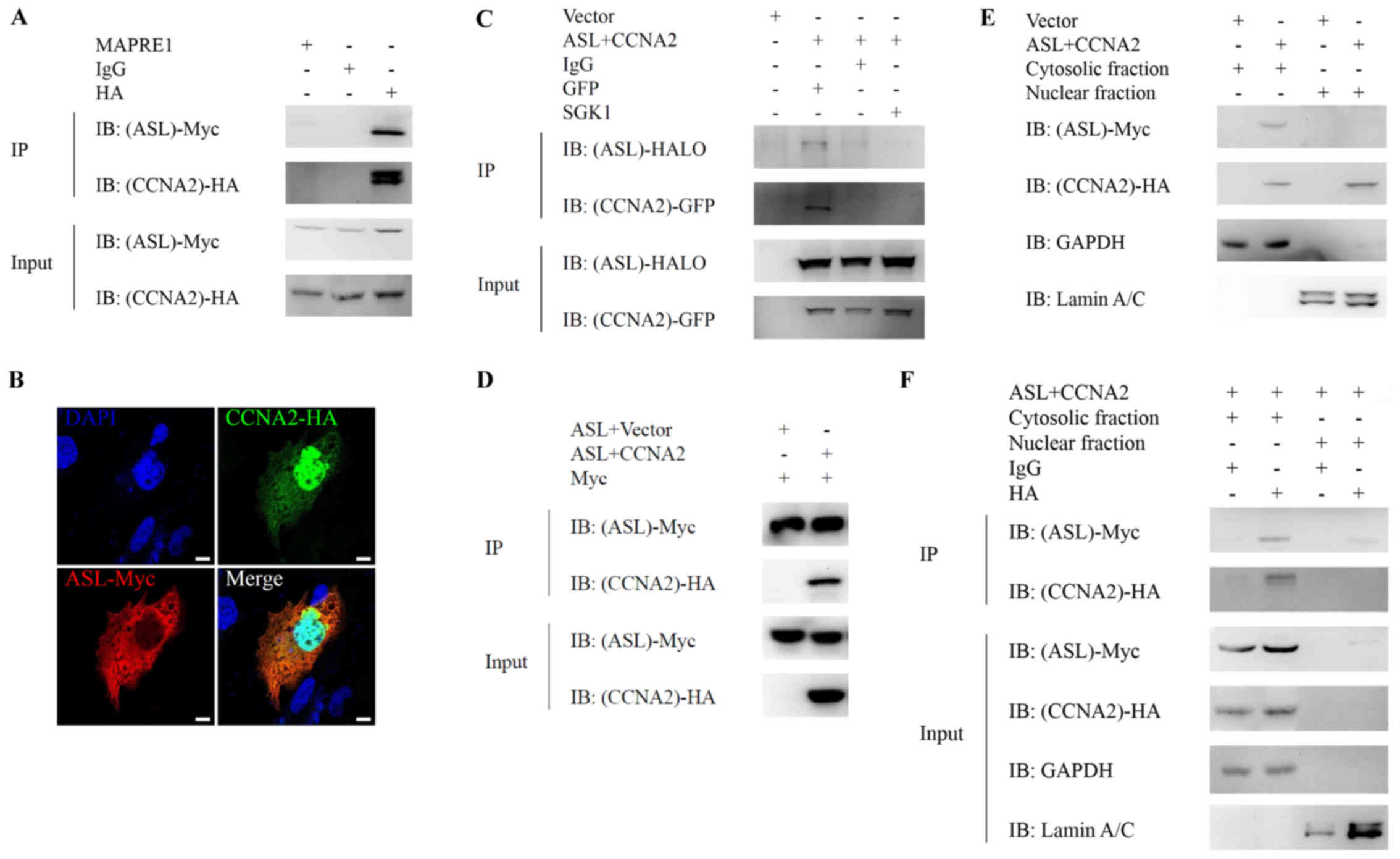
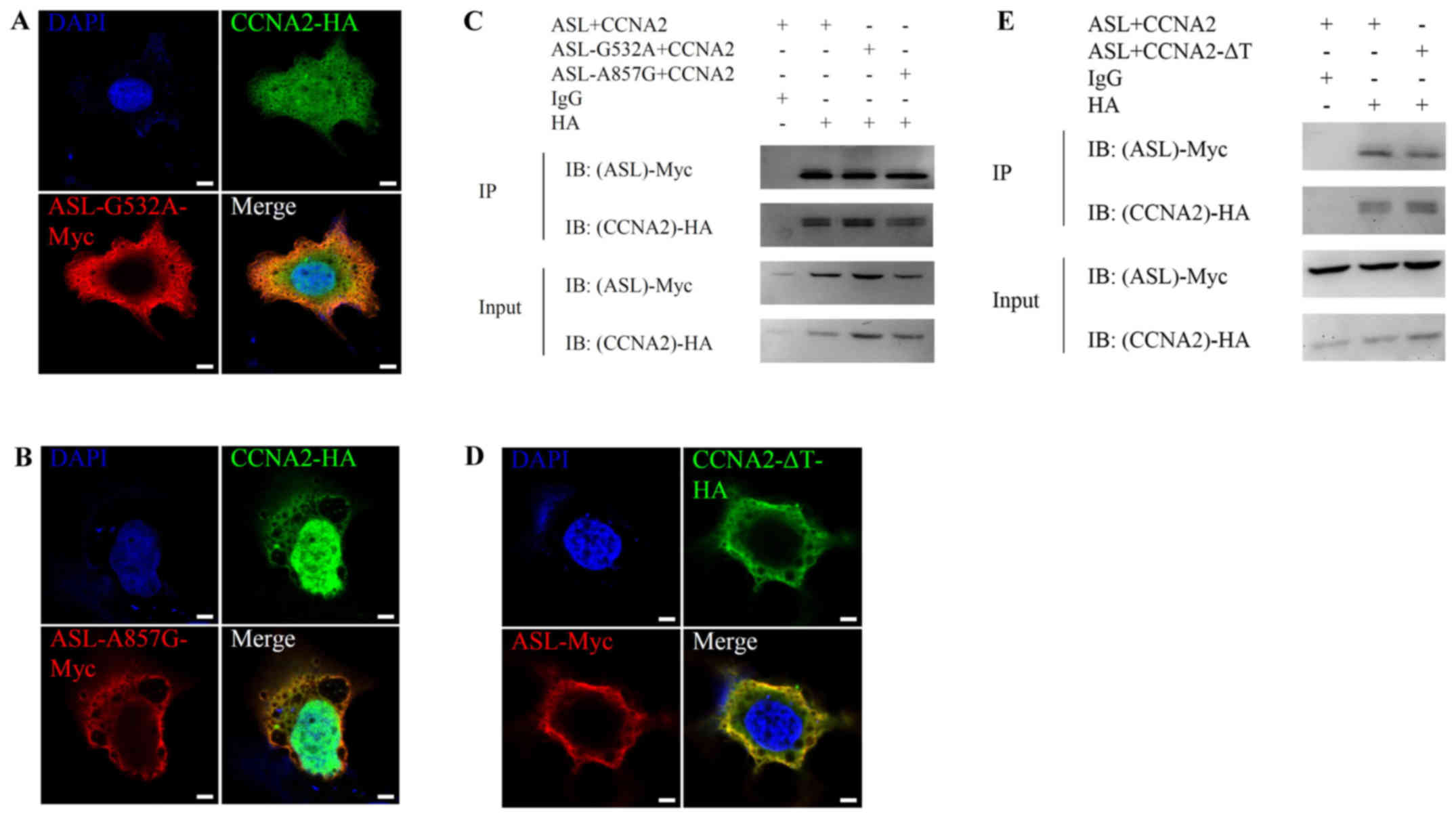
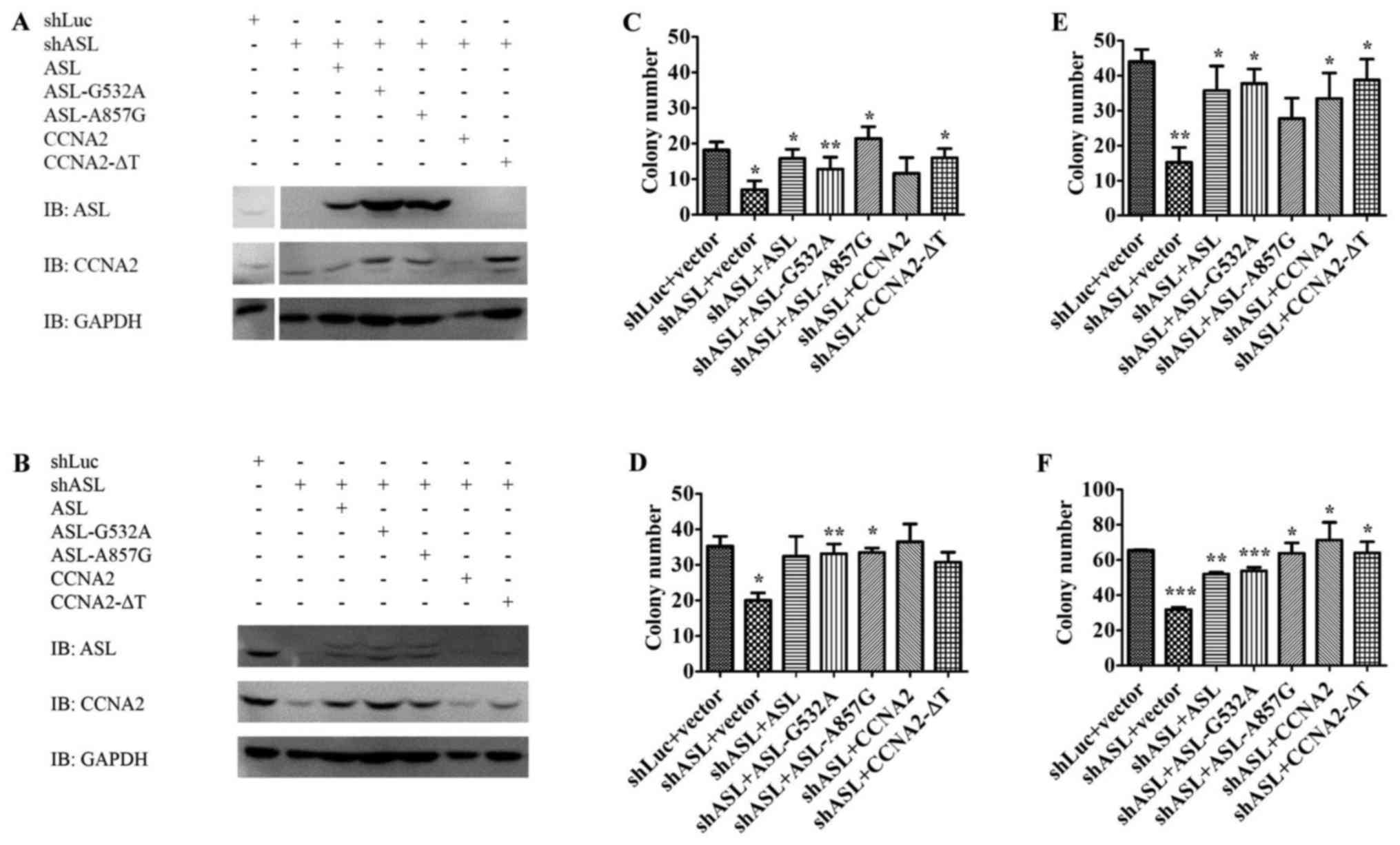
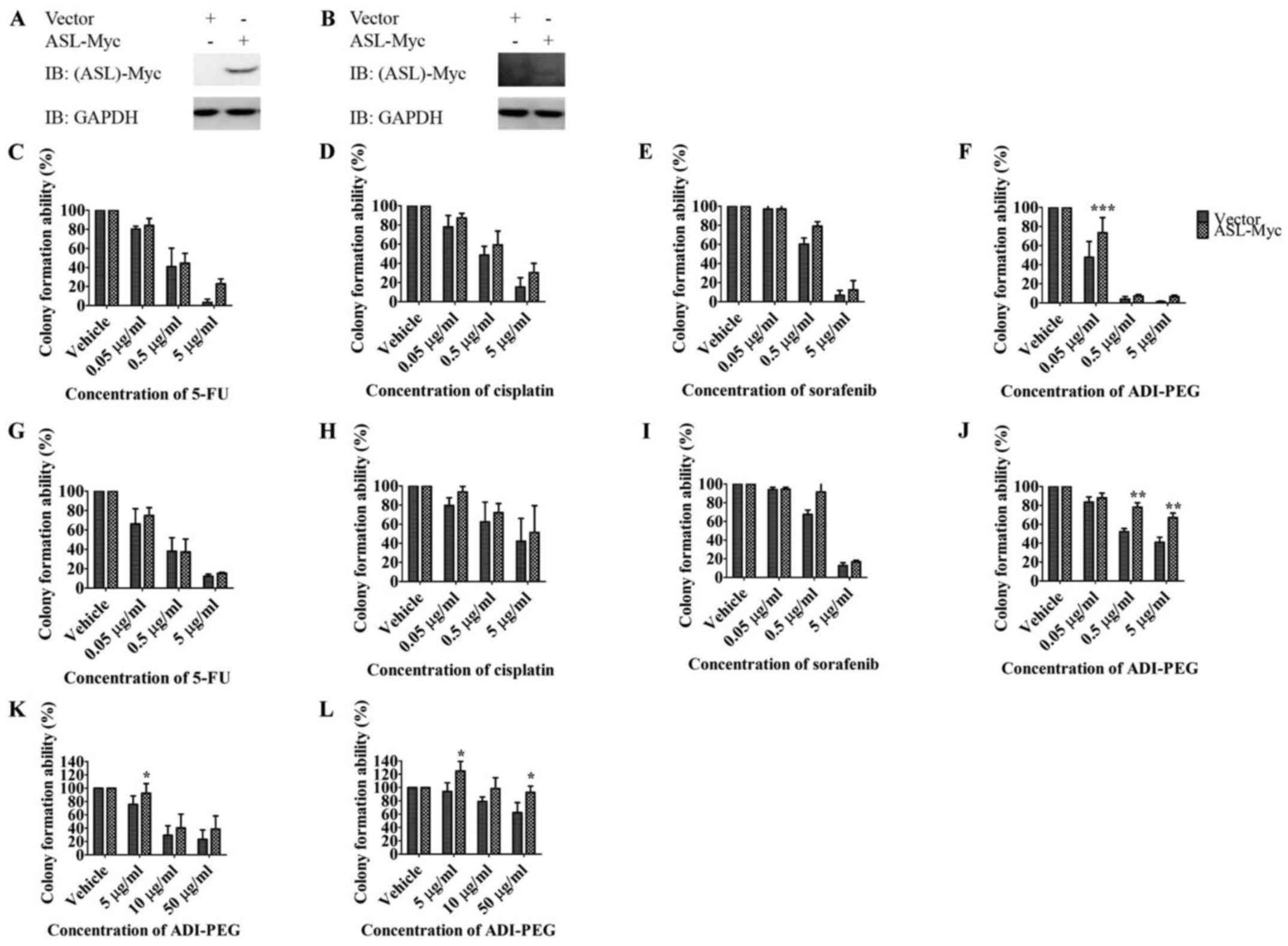
Huang H-L, Chen W-C, Hsu H-P, Cho CY, Hung
YH, Wang CY and Lai MD: Argininosuccinate lyase is a potential
therapeutic target in breast cancer. Oncol Rep. 34:3131–3139.
2015.PubMed/NCBI
|
|
23
|
Khoury O, Ghazale N, Stone E, El-Sibai M,
Frankel AE and Abi-Habib RJ: Human recombinant arginase I
(Co)-PEG5000 [HuArgI (Co)-PEG5000]-induced arginine depletion is
selectively cytotoxic to human glioblastoma cells. J Neurooncol.
122:75–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shan Y-S, Hsu H-P, Lai M-D, Yen MC, Chen
WC, Fang JH, Weng TY and Chen YL: Argininosuccinate synthetase 1
suppression and arginine restriction inhibit cell migration in
gastric cancer cell lines. Sci Rep. 5:97832015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ott PA, Carvajal RD, Pandit-Taskar N,
Jungbluth AA, Hoffman EW, Wu BW, Bomalaski JS, Venhaus R, Pan L,
Old LJ, et al: Phase I/II study of pegylated arginine deiminase
(ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs.
31:425–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang TS, Lu SN, Chao Y, Sheen IS, Lin CC,
Wang TE, Chen SC, Wang JH, Liao LY, Thomson JA, et al: A randomised
phase II study of pegylated arginine deiminase (ADI-PEG 20) in
Asian advanced hepatocellular carcinoma patients. Br J Cancer.
103:954–960. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glazer ES, Piccirillo M, Albino V, Di
Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V,
Petrillo A, et al: Phase II study of pegylated arginine deiminase
for nonresectable and metastatic hepatocellular carcinoma. J Clin
Oncol. 28:2220–2226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomlinson BK, Thomson JA, Bomalaski JS,
Diaz M, Akande T, Mahaffey N, Li T, Dutia MP, Kelly K, Gong IY, et
al: Phase I trial of arginine deprivation therapy with ADI-PEG 20
plus docetaxel in patients with advanced malignant solid tumors.
Clin Cancer Res. 21:2480–2486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hegedüs C, Truta-Feles K, Antalffy G,
Várady G, Német K, Ozvegy-Laczka C, Kéri G, Orfi L, Szakács G,
Settleman J, et al: Interaction of the EGFR inhibitors gefitinib,
vandetanib, pelitinib and neratinib with the ABCG2 multidrug
transporter: Implications for the emergence and reversal of cancer
drug resistance. Biochem Pharmacol. 84:260–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ekins S, Bugrim A, Brovold L, Kirillov E,
Nikolsky Y, Rakhmatulin E, Sorokina S, Ryabov A, Serebryiskaya T,
Melnikov A, et al: Algorithms for network analysis in
systems-ADME/Tox using the MetaCore and MetaDrug platforms.
Xenobiotica. 36:877–901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lamb J: The Connectivity Map: A new tool
for biomedical research. Nat Rev Cancer. 7:54–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lamb J, Crawford ED, Peck D, Modell JW,
Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, et
al: The Connectivity Map: Using gene-expression signatures to
connect small molecules, genes, and disease. Science.
313:1929–1935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Linnebank M, Tschiedel E, Häberle J,
Linnebank A, Willenbring H, Kleijer WJ and Koch HG:
Argininosuccinate lyase (ASL) deficiency: Mutation analysis in 27
patients and a completed structure of the human ASL gene. Hum
Genet. 111:350–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arsic N, Bendris N, Peter M, Begon-Pescia
C, Rebouissou C, Gadéa G, Bouquier N, Bibeau F, Lemmers B and
Blanchard JM: A novel function for Cyclin A2: Control of cell
invasion via RhoA signaling. J Cell Biol. 196:147–162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bendris N, Lemmers B, Blanchard J-M and
Arsic N: Cyclin A2 mutagenesis analysis: A new insight into CDK
activation and cellular localization requirements. PLoS One.
6:e228792011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jackman M, Kubota Y, den Elzen N, Hagting
A and Pines J: Cyclin A- and cyclin E-Cdk complexes shuttle between
the nucleus and the cytoplasm. Mol Biol Cell. 13:1030–1045. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fux L, Ilan N, Sanderson RD and Vlodavsky
I: Heparanase: Busy at the cell surface. Trends Biochem Sci.
34:511–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ilan N, Elkin M and Vlodavsky I:
Regulation, function and clinical significance of heparanase in
cancer metastasis and angiogenesis. Int J Biochem Cell Biol.
38:2018–2039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheppard N Gustafsson, Jarl L, Mahadessian
D, Strittmatter L, Schmidt A, Madhusudan N, Tegnér J, Lundberg EK,
Asplund A, Jain M, et al: The folate-coupled enzyme MTHFD2 is a
nuclear protein and promotes cell proliferation. Sci Rep.
5:150292015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JH, Jang H, Lee SM, Lee JE, Choi J,
Kim TW, Cho EJ and Youn HD: ATP-citrate lyase regulates cellular
senescence via an AMPK- and p53-dependent pathway. FEBS J.
282:361–371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang W, Xia Y, Ji H, Zheng Y, Liang J,
Huang W, Gao X, Aldape K and Lu Z: Nuclear PKM2 regulates β-catenin
transactivation upon EGFR activation. Nature. 480:118–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li B, Qiu B, Lee DS, Walton ZE, Ochocki
JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, et al:
Fructose-1,6-bisphosphatase opposes renal carcinoma progression.
Nature. 513:251–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jeffery CJ: Why study moonlighting
proteins? Front Genet. 6:2112015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cole SW and Sood AK: Molecular pathways:
Beta-adrenergic signaling in cancer. Clin Cancer Res. 18:1201–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harris RE, Alshafie GA, Abou-Issa H and
Seibert K: Chemoprevention of breast cancer in rats by celecoxib, a
cyclooxygenase 2 inhibitor. Cancer Res. 60:2101–2103.
2000.PubMed/NCBI
|
|
47
|
Jendrossek V: Targeting apoptosis pathways
by Celecoxib in cancer. Cancer Lett. 332:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao C-M, Hayakawa Y, Kodama Y,
Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen
H, Friedman RA, Renz BW, et al: Denervation suppresses gastric
tumorigenesis. Sci Transl Med. 6:250ra115. 2014. View Article : Google Scholar
|