Introduction
Hepatocellular carcinoma (HCC) is one of the most
common causes of cancer-related death worldwide. Despite the
development of new therapeutic strategies and improved patient
care, the 5-year survival rate of HCC is still dismal at
approximately 15% (1–3). Although the available therapies,
including surgical resection and transplantation, have
significantly improved survival in patients with early stage
tumors, the prognosis of HCC for advanced-stage disease remains
very poor (4). HCC is known to be
associated with hepatitis B virus (HBV), hepatitis C virus, and
alcohol abuse. However, other risk factors that are genetic and
physiological also contribute to the etiology of HCC (5,6).
Therefore, understanding the molecular mechanisms of the complex
multistep process of HCC, and identify novel chemical entity with
activity against HCC, could facilitate the development of
preventive measures, early diagnostic methods, and better
treatments.
c-Met, originally identified as a TRP-Met fusion
gene from a human osteosarcoma cell line, encodes a prototype
member of a distinct subfamily of heterodimeric receptor tyrosine
kinases (7). Hepatocyte growth
factor (HGF) is the only known high affinity ligand for the c-Met
receptor. Binding of HGF to c-Met causes receptor multimerization,
phosphorylation, and catalytic activation. The activation of c-Met
leads to phosphorylation of multiple downstream effectors,
including RAS/MAPK, PI3K/AKT, FAK, and c-SRC, that are essential
for regulating cell growth, survival, motility, and metastasis
(8,9). Importantly, the overexpression or
activating mutations of c-Met has been frequently found in many
human solid tumors, such as liver (10), lung (11), colon (12), ovarian (13), breast (14) and gastric (15) cancers. Recently, Zhao et al
demonstrated that downregulation of the activation of c-Met via a
specific inhibitor, cabozantinib, leading to suppressed
epithelial-mesenchymal transition and tumor growth in breast tumors
(16). Clinical retrospective
analysis revealed that higher expression of c-Met in tumor tissue
had positive relationship with patients' poor prognosis (17).
Resveratrol, a natural product found in red grapes
and wine, is one of the most common dietary flavonoids. Except
antioxidant and anti-inflammatory effect as reported, recent
studies have demonstrated that resveratrol exerted antitumor
effects on a panel of human cancers, such as breast (18), colon (19), ovarian (20), lung (21) and prostate cancer (22). Mechanism investigation manifested
that multiple signaling pathways and mechanisms are involved in the
antitumor activity of resveratrol, including suppression of various
protein kinases, such as Akt, ERK and EGFR, induction of cell cycle
arrest or apoptosis, and inhibition of cell migration and
metastasis (23). Nonetheless, the
antitumor activity of resveratrol in HCC, as well as the effect on
c-Met signaling pathway, has not yet been fully investigated.
In this study, we first reported that the c-Met
signaling pathway was involved in resveratrol-induced tumor
suppression in hepatocellular carcinoma cells. Our results suggest
that targeting the activation of HGF-c-Met signaling pathway might
be an effective preventive and therapeutic pursuit in this kind of
tumor.
Materials and methods
Cell culture and reagents
The HCC cell line Hep3B was purchased from American
Type Culture Collection (ATCC). The MHCC97-L and MHCC97-H cells
were purchased from Cell Bank of Chinese Academy of Sciences,
Shanghai, China. Cells were cytogenetically tested and
authenticated before being frozen. Each vial of frozen cells was
thawed and maintained for 2 months (10 passages). Of note, Hep3B,
MHCC97-L and MHCC97-H were maintained with Dulbecco's modified
Eagle's medium (DMEM) containing 10% FBS and 1% antibiotics. All
cell lines were incubated at 37°C in a humidified atmosphere
containing 5% CO2. Resveratrol and chemical reagents,
including Tris, NaCl, SDS and DMSO, for molecular biology and
buffer preparation were purchased from Sigma-Aldrich (St. Louis,
MO, USA).
MTS assay
The MTS assay was performed as previously described
(24). Briefly, human HCC cells
were seeded (3×103/well/100 µl) into 96-well plates, and
treated with various doses of resveratrol for different time points
as indicated. The proliferation was assessed by MTS assay (Promega,
Madison, WI, USA) according to instructions provided.
Anchorage-independent cell growth
assay
The anchorage-independent cell growth was performed
as previously described (25).
Briefly, cells were suspended (8,000 cells/ml) in 1 ml of 0.3% agar
with Eagle's basal medium containing 10% FBS, 1% antibiotics, and
different concentrations of resveratrol overlaid into six-well
plates containing a 0.6% agar base. The cultures were maintained in
a 37°C, 5% CO2 incubator for 2 weeks, and then colonies
were counted under a microscope using the Image-Pro Plus software
program (Media Cybernetics, Silver Spring, MD, USA).
Invasion assay
An invasion assay was conducted using a modified
Boyden chamber and Matrigel-coated (BD Bioscience, San Jose, CA,
USA) polycarbonate nucleopore membranes (Corning, Inc., Corning,
NY, USA; 8-µm pore size). Serum-free medium containing HGF (10
ng/ml) was pipetted into the lower wells. MHCC97-H cells were
trypsinized and suspended at a density of 1×105
cells/100 µl in serum-free medium without HGF. Cells were then
pretreated with resveratrol for 30 min and 100 µl of the cell
suspension were loaded into the upper wells. The chamber was
incubated in a 5% CO2 incubator at 37°C. After 24 h of
incubation, the membrane was fixed and stained with crystal violet
solution. Invasiveness was determined by counting the cells that
passed through the filter.
Subcellular proteome
fractionation
The subcellular proteome fractions were prepared
using the ProteoExtract Subcellular Proteome Extraction kit
(539790, Millipore, Temecula, CA, USA) according to the
manufacturer's instructions.
Protein preparation and western
blotting
Protein samples were extracted with RIPA buffer (10
mM Tris-Cl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1%
sodium deoxycholate, 0.1% SDS, 140 mM NaCl). Protein concentration
was determined using the BCA Assay Reagent (Pierce, Rockford, IL,
USA). For immunoblotting, proteins (40 µg) were detected with
specific antibodies and an HRP-conjugated secondary antibody.
Primary antibodies were used for immunoblotting: p-c-Met, c-Met,
p-Akt, p-ERK1/2 and N-cadherin from Cell Signaling Technology;
β-actin from Sigma-Aldrich, Ki67 from Abcam. Secondary antibodies
anti-rabbit IgG HRP and anti-mouse IgG HRP were purchased from Cell
Signaling Technology. Antibody conjugates were visualized by
chemiluminescence (ECL, Thermo Scientific, Rockford, IL, USA).
Lentiviral infection
To generate knockdown c-Met cells,
pLKO.1-sh-GFP or pLKO.1-sh-c-Met lentivirus plasmids,
were co-transfected into 293T cells with PSPAX2 and
PMD2-G. Viral supernatant fractions were collected at 48 h
after transfection and filtered through a 0.45 µm filter followed
by infection into cells together with 10 µg/ml polybrene. The next
day, the medium was replaced with fresh medium containing 1 µg/ml
puromycin and cells were incubated for another 6 days.
In vivo tumor growth assay
All the experimentations for animals were approved
by the Ethics Committee of Central South University, Changsha,
Hunan, China. MHCC97-H cells (2×106) in 100 µl DMEM
medium were inoculated s.c. into the right flank of 6-week-old
female athymic nude mice. Nude mice (n=5) were randomly divided to
groups when tumor volume reached 50–100 mm3. The dosage
of resveratrol was 30 mg/kg and was administered every three days
by intraperitoneal injection, whereas control mice were
administered vehicle. The body weight of each mouse was recorded
and tumor volume was determined by vernier caliper twice a week.
Volume was calculated following the formula of A × B2 ×
0.5, wherein A is the longest diameter of tumor, B is the shortest
diameter and B2 is B squared. Mice were monitored until
day 34 and at that time mice were euthanized and tumors
extracted.
Immunohistochemical analysis
A Vectastain Elite ABC kit (Vector Laboratories;
Burlingame, CA, USA) was used for immunohistochemical staining
according to the recommended protocol. Briefly, the slide was baked
at 60°C for 2 h, deparaffinized, and rehydrated. To expose the
antigens, the slide was unmasked by submersion into boiling sodium
citrate buffer (10 mM, pH 6.0) for 10 min, and then treated with 3%
H2O2 for 10 min. The slide was blocked with
50% goat serum albumin in 1X PBS in a humidified chamber for 1 h at
room temperature and then with a first antibody (1:100 dilution in
50% goat serum with PBS) at 4°C in a humidified chamber overnight.
The slide was washed and hybridized with the secondary antibody
from Vector Laboratories (anti-rabbit 1:200) for 1 h at room
temperature. Slides were stained using the Vectastain Elite ABC
kit.
Statistical analysis
Standard statistical methods were performed using
Statistics Package for Social Science (SPSS) software (version
13.0; SPSS, Chicago, IL, USA). All data are presented as mean
values ± SD as indicated and analyzed using the Student's t-test or
ANOVA. A p-value <0.05 was considered statistically
significant.
Results
Resveratrol inhibits HCC cell growth
in vitro
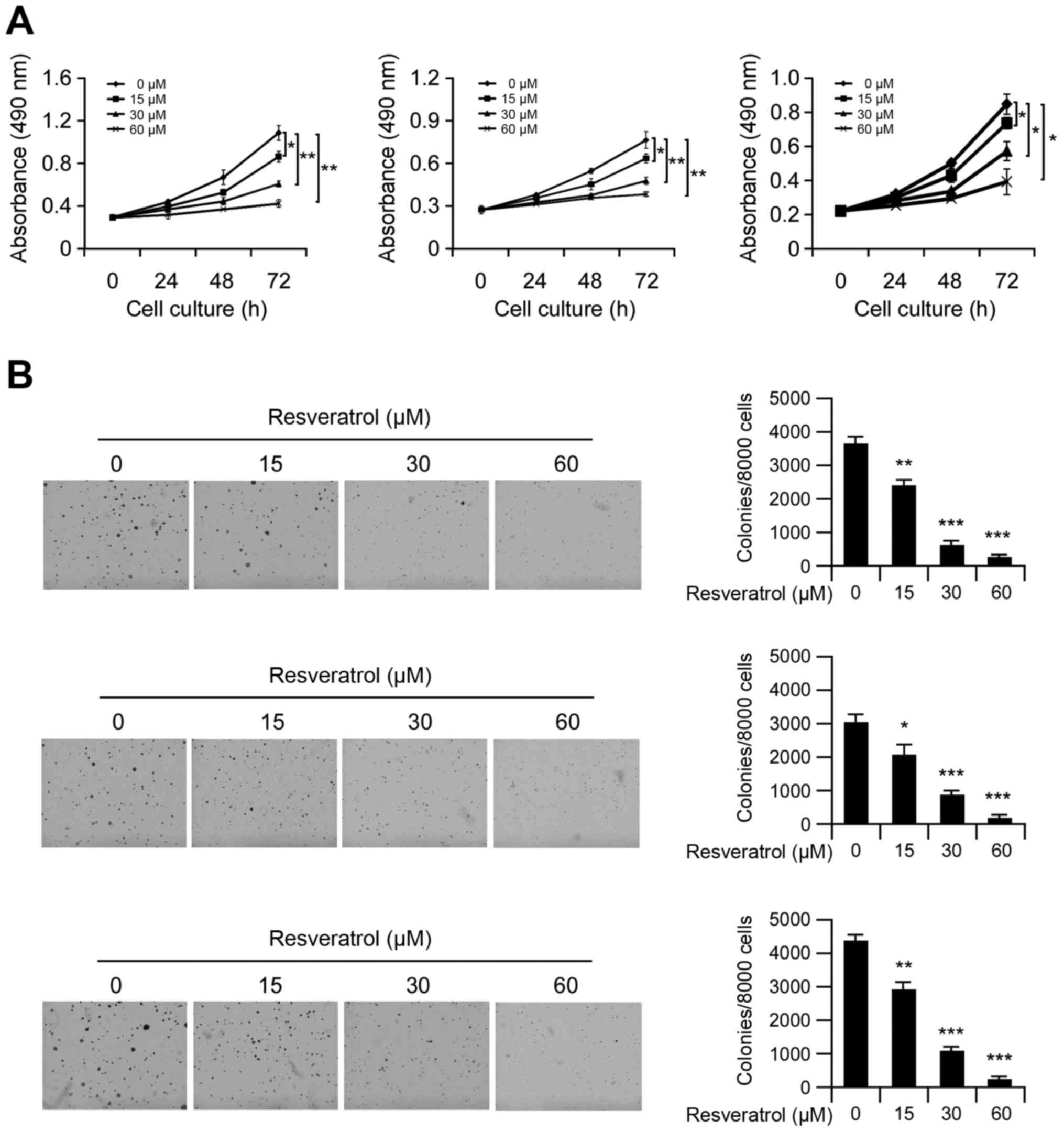
First, we investigated the effects of resveratrol on
cell viability in Hep3B, MHCC97-L and MHCC97-H cells. MTS data
showed that resveratrol significantly decreased anchorage-dependent
growth (Fig. 1A) of Hep3B, MHCC97-L
and MHCC97-H cells in a dose-dependent manner. Moreover, treatment
with resveratrol markedly inhibited anchorage-independent cell
growth in all test HCC cells (Fig.
1B), and treated with 60 µM resveratrol almost blocked the
colony formation in soft agar. These results indicated that
resveratrol inhibits HCC cell growth in vitro.
Resveratrol downregulates the c-Met
signaling pathway
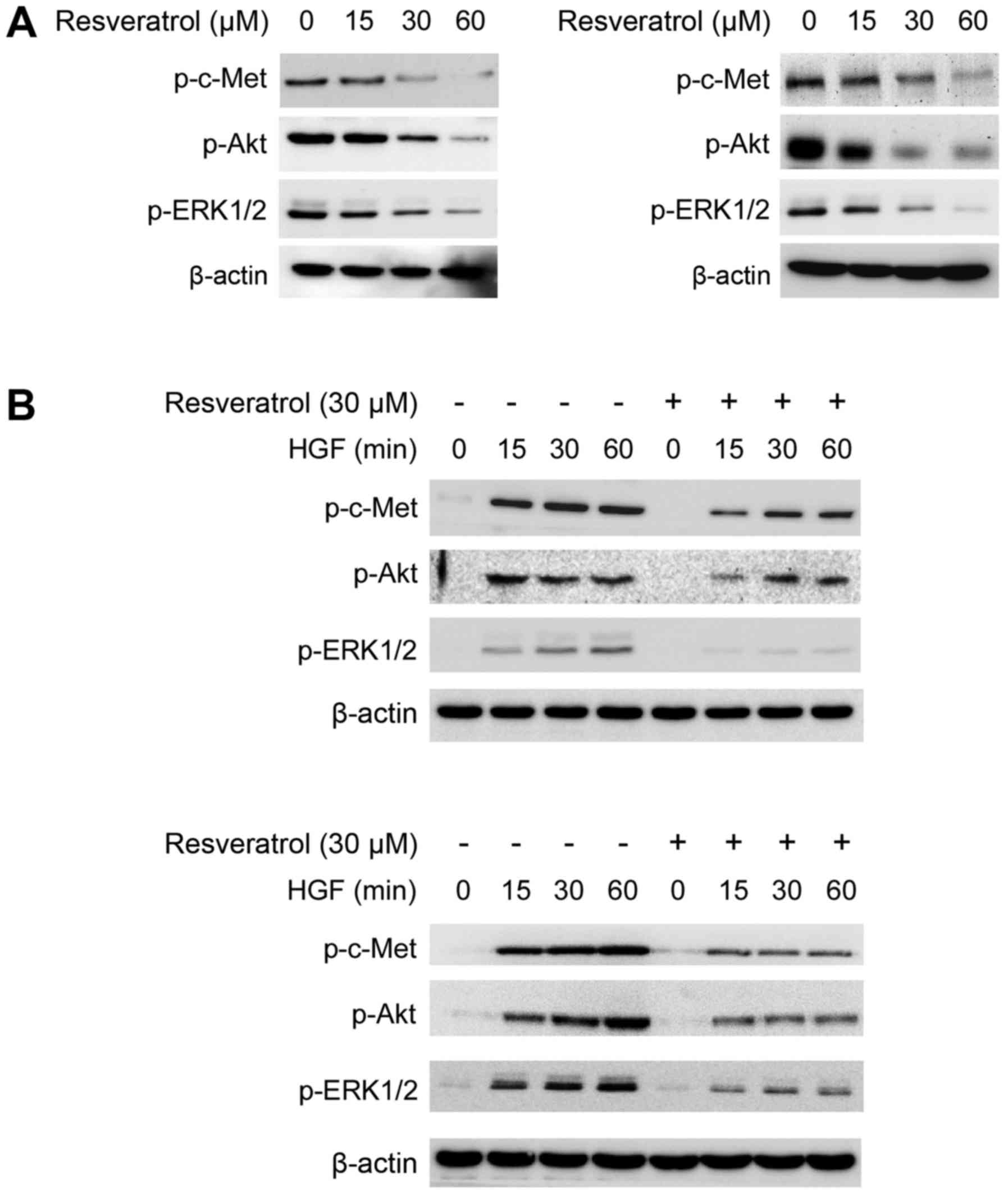
Previous studies have demonstrated that the c-Met
signaling pathway is dysregulated in human HCC. Hyperactivation of
c-Met, or its downstream target kinases Akt and ERK1/2 are always
involved in HCC tumorigenic regulation. Herein, we found that
resveratrol markedly impaired the phosphorylation of c-Met, Akt and
ERK1/2 in a dose-dependent manner in Hep3B (Fig. 2A, left) and MHCC97-H cells (Fig. 2A, right). Moreover, we found that
resveratrol treatment strikingly inhibited HGF-induced c-Met and
downstream target kinases Akt and ERK1/2 activation in Hep3B
(Fig. 2B, top) and MHCC97-H cells
(Fig. 2B, bottom). Our data implied
that resveratrol mediated growth inhibition may partly depend on
the downregulation of c-Met signaling pathway.
Resveratrol suppresses the c-Met
protein levels in HCC cells
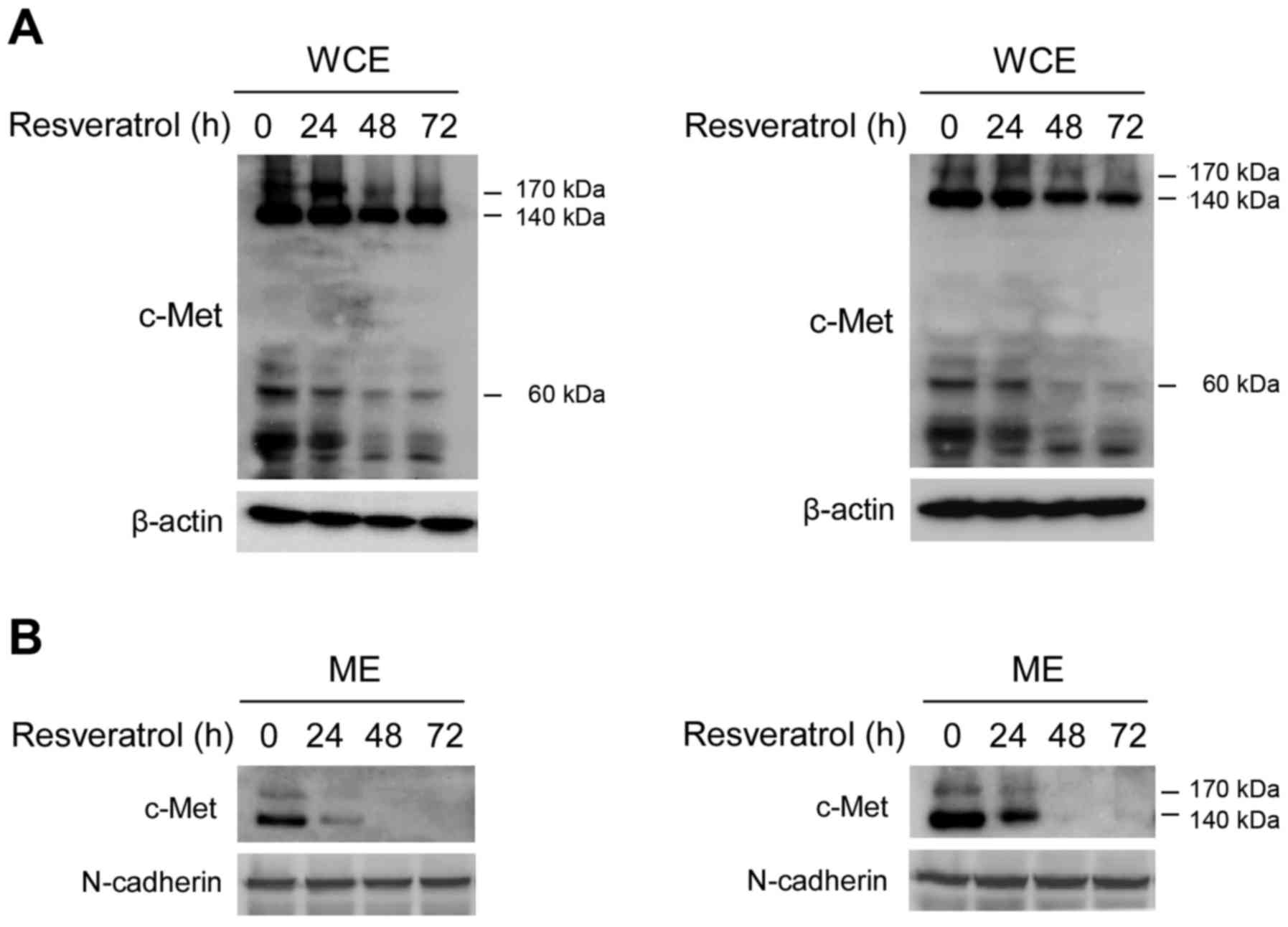
Our data demonstrated that the c-Met signaling
pathway was inhibited after short-term resveratrol treatment, which
indicated that resveratrol could dose-dependently suppress the
tyrosine kinase activity of c-Met. However, no published data have
demonstrated that resveratrol directly regulates c-Met expression
in human HCC. As shown in Fig. 3,
long-term exposure (24, 48 or 72 h) to resveratrol decreased the
protein levels of c-Met in a dose-dependent manner in Hep3B
(Fig. 3A, left) and MHCC97-H cells
(Fig. 3A, right). We further
performed sub-cellular separation to investigate the effect of
resveratrol on c-Met sub-cellular expression. As shown in Fig. 3B, membrane-associated c-Met was
remarkably decreased after resveratrol treatment for 24 h in both
Hep3B (Fig. 3B, left) and MHCC97-H
cells (Fig. 3B, right), and at the
time points of 48 and 72 h, resveratrol almost blocked the membrane
expression of c-Met. These results suggested that after the
long-term treatment of resveratrol, the expression of c-Met on cell
membrane was suppressed.
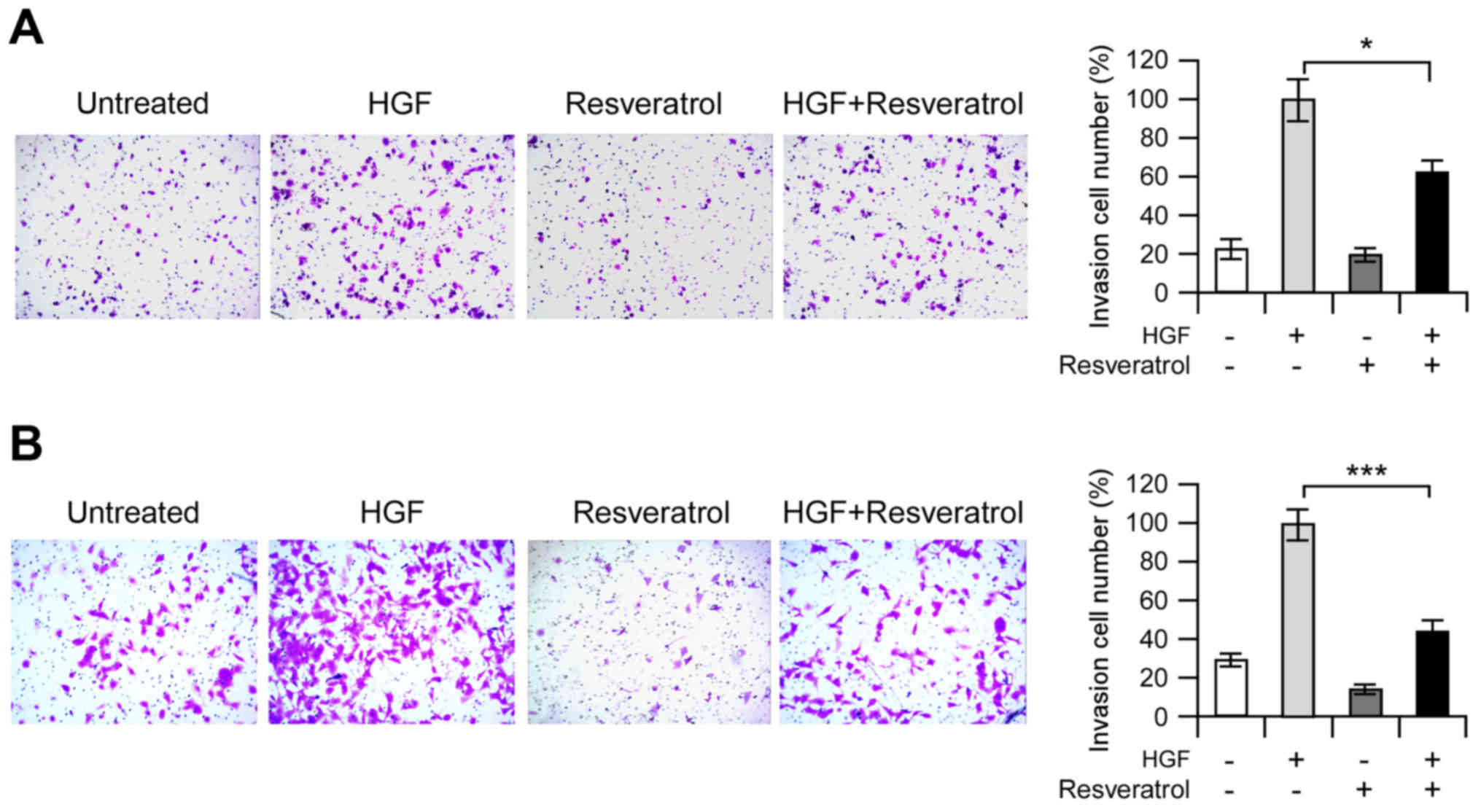
Resveratrol suppresses HGF-induced
invasion in vitro
The HGF-c-Met signaling pathway has a crucial role
in cancer cell invasion and metastasis. Based on previous data, to
explore the potential anti-invasive effects of resveratrol,
invasion assays were performed on Hep3B and MHCC97-H cells. As
shown in Fig. 3A, HGF substantially
induced Hep3B cell's invasion, and resveratrol markedly inhibited
the HGF-induced invasive capacity of malignant Hep3B cells
(Fig. 4A). We also found that the
MHCC97-H cells possessed much higher invasion capacity than Hep3B
cell, even without HGF treatment. As expected, resveratrol showed
very similar inhibitory effect against the invasion capacity of
MHCC97-H cell (Fig. 4B). These
results suggested that resveratrol might be an effective inhibitor
for HGF-induced invasion of Hep3B and MHCC97-H cells.
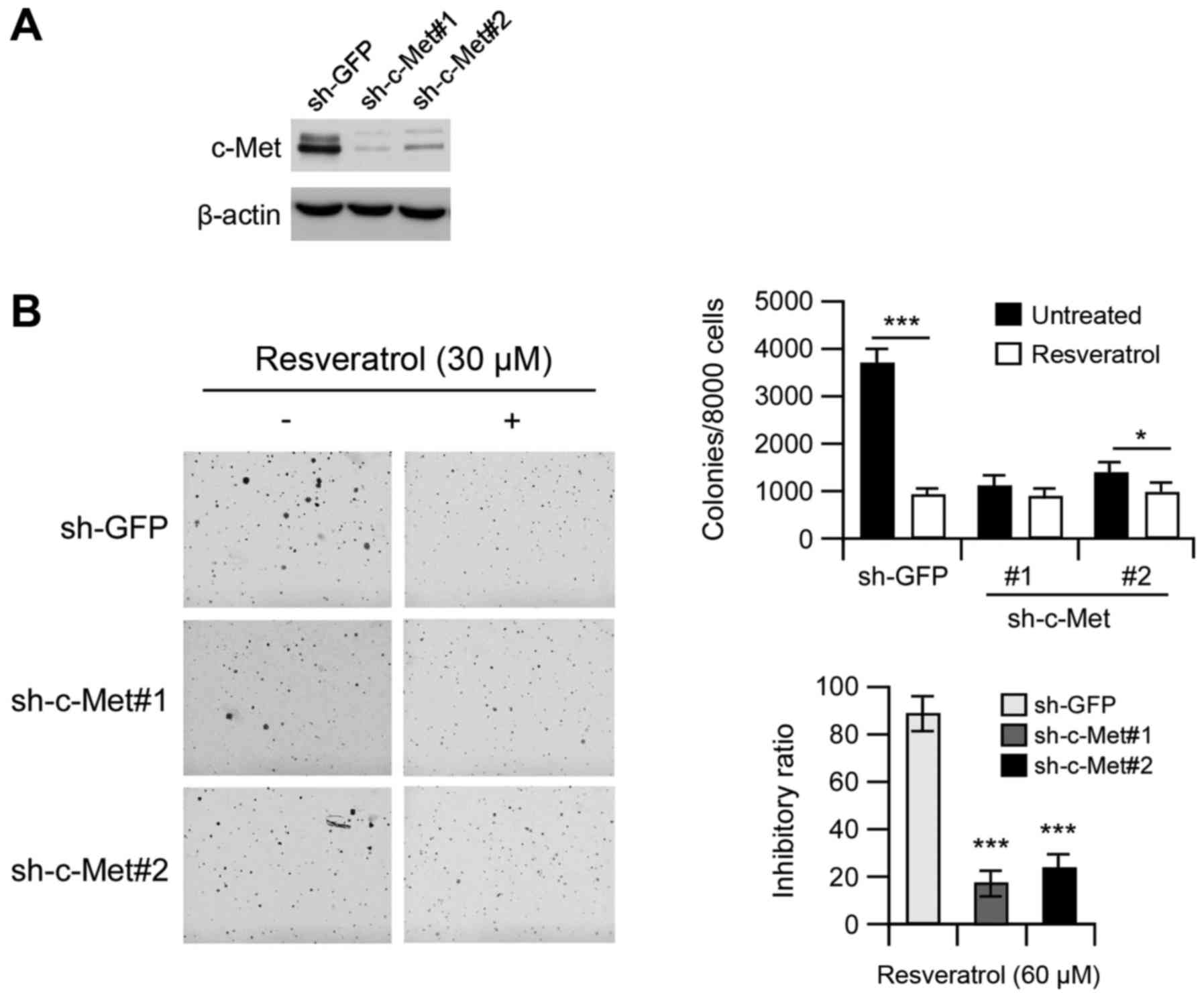
Knockdown of c-Met decreases the
sensitivity of MHCC97-H cells to resveratrol
We then examined whether knocking down c-Met
expression influences the sensitivity of MHCC97-H cells to
resveratrol. The efficiency of short hairpin RNA (shRNA) knockdown
was examined, and the expression of C-Met was obviously decreased
after shRNA transfection (Fig. 5A).
Moreover, the growth of cells in soft agar also decreased after
transfection compared with the GFP group (Fig. 5B, left). Resveratrol (30 µM)
inhibited anchorage-independent growth of MHCC97-H cells
transfected with GFP shRNA by ~80%. In contrast, the inhibition was
<50% in MHCC97-H cells transfected with c-Met shRNA, indicating
that MHCC97-H cells transfected with c-Met shRNAs were more
resistant to resveratrol treatment (Fig. 5B, right). These results suggested
that c-Met plays an important role in the sensitivity of MHCC97-H
cells to the anti-proliferative effects of resveratrol.
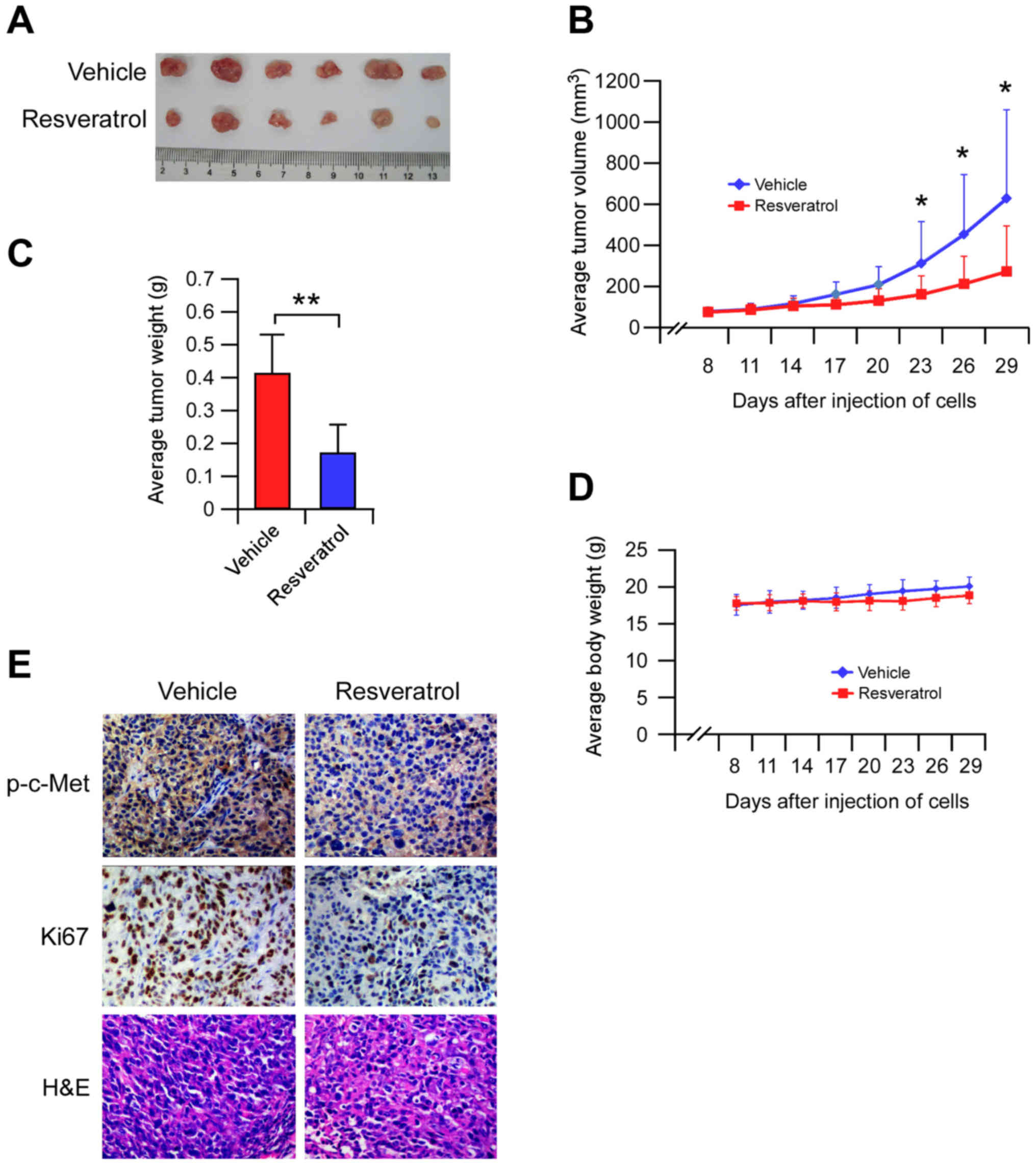
Resveratrol inhibits tumor growth and
c-Met phosphorylation in a xenograft mouse model
We examined the effects of resveratrol on tumor
growth and c-Met activation using a human HCC xenograft. MHCC97-H
cells were injected (s.c.) into the right flank of six-week-old
female athymic nude mice. Data showed that resveratrol markedly
reduced tumor size in MHCC97-H xenograft model (Fig. 6A-C). Weights of all animals
increased normally after treatment with resveratrol or vehicle
control (Fig. 6D). Additionally,
immunohistochemical analysis of resveratrol treated MHCC97-H
xenograft tumors was conducted to evaluate the expression level of
p-c-Met and Ki-67. Our results showed that Ki-67 and p-c-Met were
significantly suppressed in the resveratrol treated group compared
with the vehicle treated group (Fig.
6E). These results clearly indicated that resveratrol inhibits
tumor cell proliferation in vivo. In addition, these
findings showed that resveratrol significantly inhibited tumor cell
c-Met activation.
Discussion
Previous studies have demonstrated that resveratrol
displayed potent antitumor effect in various types of tumors,
including lung, breast, prostate and colon cancer (23,26).
Herein, we found that resveratrol inhibited hepatocellular
carcinoma cell anchorage-dependent and -independent growth, as well
as HGF-induced c-Met signaling pathway activation and tumor cell
invasion. Importantly, for the first time, we also uncovered that
exposure to resveratrol directly downregulated the membrane
expression of c-Met. Knockdown of c-Met decreased the sensitivity
of HCC cells to resveratrol treatment. Our in vivo data
further demonstrated that resveratrol suppressed the HCC tumor
growth in a xenograft mouse model.
Hepatocellular carcinoma, which accounts for 70–85%
of the cases, is the most common primary liver cancer (27). Despite improvements in local
therapies, including surgical resection, liver transplantation, and
transarterial embolization, the prognosis remains poor for the
majority of patients who develop recurrence, metastasis or present
with advanced disease (28). HCC is
a complex and heterogeneous tumor with several genomic alterations,
the dysregulation of several signaling cascades such as PI3K/Akt,
Ras/ERK, EGF/EGFR and HGF/c-Met signaling pathways are involved in
the initiation and progression of this kind of disease (29–32).
Especially, ongoing efforts to study hepato-carcinogenesis have
identified an important role for c-Met signaling in the promotion
of tumor growth, angiogenesis, and metastasis. The transcription of
c-Met is increased in 30–100% of tumors compared with surrounding
liver tissue. Similarly, c-Met is overexpressed at the protein
level in 25–100% of HCCs compared with normal liver (4,33).
Importantly, recent studies suggest that c-Met overexpression is
significantly associated with clinicopathological features of HCC,
such as tumor grade, vascular invasion or thrombosis, tumor
recurrence, metastases, and worse prognosis with impaired 5-year
survival (34–36). This evidence suggests a potential
tumor-promoting role of c-Met and the HGF-c-Met signaling pathway
in HCC.
In the canonical HGF/c-Met signaling pathway, the
activation of c-Met and downstream kinases dependent on the binding
of HGF on c-Met receptor leads to c-Met homodimerization,
autophosphorylation, and signal transduction. Moreover, c-Met binds
with EGF receptor (EGFR) and some other receptor tyrosine kinases
on cell membrane to activate downstream kinases, even in the
absence of HGF (4,10,37).
Regardless of the mode of activation, c-Met dimerization on cell
membrane and autophosphorylation seem to be necessary for
activation of downstream kinases in human malignant tumors. In the
present study, we firstly demonstrated that resveratrol not only
decreased HGF-induced c-Met signaling activation, but also
downregulated c-Met protein on cell membrane directly. Given
resveratrol is a kind of flavonoid which is abundantly distributed
in fruits and vegetables, our results suggested that a daily diet
of high intake of resveratrol would be beneficial for HCC patients,
especially for those c-Met-positive, or the tumors which are
addicted to c-Met signaling pathways.
Our studies also found the antitumor effect of
resveratrol was correlated with its effect on cancer cell motility
and invasiveness. It is well known that the invasion and metastasis
are the cause of >90% of human cancer deaths, and c-Met plays a
pivotal role in the process of tumor invasion and metastasis
(9,38–40).
c-Met signaling pathway regulates epithelial-mesenchymal transition
in cancer cells, overexpression or hyperactivation of c-Met were
involved in regulation of metastasis in multiple different types of
human cancer, including lung (41),
liver (42) and breast (14) cancer. Additionally, transgenic mice
overexpressing HGF exhibited increased angiogenesis and VEGF
transcription in chemically-induced hepatic adenomas and HCC
(43). Ogunwobi et al
reported that epigenetic upregulation of HGF and c-Met drives
metastasis in hepatocellular carcinoma (44), which indicated that the promotion of
metastasis in HCC, at least partly, depend on the HGF-c-Met
pathway.
Our in vitro data also demonstrated that
HGF-induced invasion of HCC cells was significantly decreased after
resveratrol treatment. Our results implied that combination of
resveratrol with other anti-angiogenetic agents may have
synergistic effect on invasion or metastasis suppression.
Crosstalk between c-Met and other RTKs on cell
membrane has also been studied in great depth because of its
potential importance in the development of resistance to cancer
therapeutics (17). For instance,
the amplification of c-Met is one of the main reasons for lung
cancer cells gaining acquired resistance to gefitinib treatment
(45). Importantly, c-Met has also
been shown by multiple studies to interact directly with the
epidermal growth factor receptor (EGFR) on membrane, allowing
activation of c-Met after stimulation with the EGFR ligands EGF or
transforming growth factor (TGF-α) (46). Additionally, treatment with EGF in
cells expressing both c-Met and EGFR directly induced
phosphorylation of c-Met (47). In
cancer cells, c-Met interacts with other ERBB family members, such
as ERBB2 and ERBB3, causing transactivation of both receptors
(48). Recently, the
ligand-independent activation of c-Met through the interaction with
IGF-1R, was also demonstrated in prostate cancer in an IGF-1/IGF-1R
signaling pathway-dependent manner (49). Unexpectedly, our results uncovered
for the first time that exposure to resveratrol directly
downregulated the expression of c-Met on the cell membrane. The
data suggested that resveratrol not only inhibited HGF-induced
c-Met receptor tyrosine kinase activity but also directly decreased
the expression of c-Met on the cell membrane.
Overall, for the first time, the antitumor activity
of resveratrol against HCC via suppression of c-Met signaling
pathway was investigated both in vitro and in
vivo.
Similar to other natural compounds, such as EGCG
(50) and quercetin (51), resveratrol has also been
demonstrated as a multiple kinase inhibitor in human cancers.
Herein, the downregulation of HGF-c-Met signaling might not be
specific, other signaling pathways and mechanisms also involved in
resveratrol-mediated HCC suppression will need further study in the
nearly future. Despite the large number of pre-clinical studies
dealing with different aspects of the biological effects of
resveratrol, its translation to clinics for cancer therapies is far
from reality due to a variety of challenges. Different from the
mechanism reported by previous studies, we demonstrated that the
c-Met signaling pathway was one of the underlying mechanisms for
resveratrol to exert its tumor fighting effect. Our study implied
that c-Met is a potent antitumor target to be applied to prevention
and clinical treatment of human hepatocellular carcinoma.
Acknowledgements
This work was supported by National Natural Science
Foundation of China (no. 81371690), Research Project of Health and
Family Planning Commission of Hunan Province (B2016125), and
Science and Technology Program Project Funds of Changsha
(K1403054-31, K15ZD052-33).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HGF
|
hepatocyte growth factor
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seton-Rogers S: Hepatocellular carcinoma:
Gender differences. Nat Rev Cancer. 14:578–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goyal L, Muzumdar MD and Zhu AX: Targeting
the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res.
19:2310–2318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Villanueva A, Lachenmayer A and
Finn RS: Advances in targeted therapies for hepatocellular
carcinoma in the genomic era. Nat Rev Clin Oncol. 12:408–424. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forner A, Gilabert M, Bruix J and Raoul
JL: Treatment of intermediate-stage hepatocellular carcinoma. Nat
Rev Clin Oncol. 11:525–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Wang Q, Yang G, Marando C, Koblish
HK, Hall LM, Fridman JS, Behshad E, Wynn R, Li Y, et al: A novel
kinase inhibitor, INCB28060, blocks c-MET-dependent signaling,
neoplastic activities, and cross-talk with EGFR and HER-3. Clin
Cancer Res. 17:7127–7138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peters S and Adjei AA: MET: A promising
anticancer therapeutic target. Nat Rev Clin Oncol. 9:314–326. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gherardi E, Birchmeier W, Birchmeier C and
Woude G Vande: Targeting MET in cancer: Rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
You H, Ding W, Dang H, Jiang Y and
Rountree CB: c-Met represents a potential therapeutic target for
personalized treatment in hepatocellular carcinoma. Hepatology.
54:879–889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim IH, Lee IH, Lee JE, Hong SH, Kim TJ,
Lee KY, Kim YK, Kim SJ, Sung SW, Park JK, et al: Prognostic impact
of multiple clinicopathologic risk factors and c-Met overexpression
in patients who have undergone resection of stage IB non-small-cell
lung cancer. Clin Lung Cancer. 17:e31–e43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burggraaf J, Kamerling IM, Gordon PB,
Schrier L, de Kam ML, Kales AJ, Bendiksen R, Indrevoll B, Bjerke
RM, Moestue SA, et al: Detection of colorectal polyps in humans
using an intravenously administered fluorescent peptide targeted
against c-Met. Nat Med. 21:955–961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Otte A, Rauprich F, von der Ohe J, Yang Y,
Kommoss F, Feuerhake F, Hillemanns P and Hass R: c-Met inhibitors
attenuate tumor growth of small cell hypercalcemic ovarian
carcinoma (SCCOHT) populations. Oncotarget. 6:31640–31658.
2015.PubMed/NCBI
|
|
14
|
Xing F, Liu Y, Sharma S, Wu K, Chan MD, Lo
HW, Carpenter RL, Metheny-Barlow LJ, Zhou X, Qasem SA, et al:
Activation of the c-Met pathway mobilizes an inflammatory network
in the brain microenvironment to promote brain metastasis of breast
cancer. Cancer Res. 76:4970–4980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marano L, Chiari R, Fabozzi A, De Vita F,
Boccardi V, Roviello G, Petrioli R, Marrelli D, Roviello F and
Patriti A: c-Met targeting in advanced gastric cancer: An open
challenge. Cancer Lett. 365:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Z, Zhu X, Cui K, Mancuso J, Federley
R, Fischer K, Teng GJ, Mittal V, Gao D, Zhao H, et al: In vivo
visualization and characterization of epithelial-mesenchymal
transition in breast tumors. Cancer Res. 76:2094–2104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Organ SL and Tsao MS: An overview of the
c-MET signaling pathway. Ther Adv Med Oncol. 3:(Suppl). S7–S19.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medina-Aguilar R, Pérez-Plasencia C,
Marchat LA, Gariglio P, García Mena J, Rodríguez Cuevas S,
Ruíz-García E, Astudillo-de la Vega H, Hernández Juárez J,
Flores-Pérez A, et al: Methylation landscape of human breast cancer
cells in response to dietary compound resveratrol. PLoS One.
11:e01578662016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buhrmann C, Shayan P, Popper B, Goel A and
Shakibaei M: Sirt1 is required for resveratrol-mediated
chemopreventive effects in colorectal cancer cells. Nutrients.
8:1452016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gwak H, Kim S, Dhanasekaran DN and Song
YS: Resveratrol triggers ER stress-mediated apoptosis by disrupting
N-linked glycosylation of proteins in ovarian cancer cells. Cancer
Lett. 371:347–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sahin E, Baycu C, Koparal AT, Donmez D
Burukoglu and Bektur E: Resveratrol reduces IL-6 and VEGF secretion
from co-cultured A549 lung cancer cells and adipose-derived
mesenchymal stem cells. Tumour Biol. 37:7573–7582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taniguchi T, Iizumi Y, Watanabe M, Masuda
M, Morita M, Aono Y, Toriyama S, Oishi M, Goi W and Sakai T:
Resveratrol directly targets DDX5 resulting in suppression of the
mTORC1 pathway in prostate cancer. Cell Death Dis. 7:e22112016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh CK, Ndiaye MA and Ahmad N:
Resveratrol and cancer: Challenges for clinical translation.
Biochim Biophys Acta. 1852:1178–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu X, Deng Q, Li W, Xiao L, Luo X, Liu X,
Yang L, Peng S, Ding Z, Feng T, et al: Neoalbaconol induces cell
death through necroptosis by regulating RIPK-dependent autocrine
TNFα and ROS production. Oncotarget. 6:1995–2008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li W, Peng C, Lee MH, Lim D, Zhu F, Fu Y,
Yang G, Sheng Y, Xiao L, Dong X, et al: TRAF4 is a critical
molecule for Akt activation in lung cancer. Cancer Res.
73:6938–6950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kulkarni SS and Cantó C: The molecular
targets of resveratrol. Biochim Biophys Acta. 1852:1114–1123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davis GL, Dempster J, Meler JD, Orr DW,
Walberg MW, Brown B, Berger BD, O'Connor JK and Goldstein RM:
Hepatocellular carcinoma: Management of an increasingly common
problem. Proc (Bayl Univ Med Cent). 21:266–280. 2008.PubMed/NCBI
|
|
28
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui SX, Shi WN, Song ZY, Wang SQ, Yu XF,
Gao ZH and Qu XJ: Des-gamma-carboxy prothrombin antagonizes the
effects of Sorafenib on human hepatocellular carcinoma through
activation of the Raf/MEK/ERK and PI3K/Akt/mTOR signaling pathways.
Oncotarget. 7:36767–36782. 2016.PubMed/NCBI
|
|
30
|
Li K, Shen B, Cheng X, Ma D, Jing X, Liu
X, Yang W, Peng C and Qiu W: Phenotypic and signaling consequences
of a novel aberrantly spliced transcript FGF receptor-3 in
hepatocellular carcinoma. Cancer Res. 76:4205–4215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lanaya H, Natarajan A, Komposch K, Li L,
Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic
M, et al: EGFR has a tumour-promoting role in liver macrophages
during hepatocellular carcinoma formation. Nat Cell Biol.
16:972–981, 971–977. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, Ng
JK, Chai S, Lin CH, Tsang SY, Ma S, et al: Cancer-associated
fibroblasts regulate tumor-initiating cell plasticity in
hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell
Rep. 15:1175–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu F, Wu L, Zheng S, Ding W, Teng L, Wang
Z, Ma Z and Zhao W: The clinical value of hepatocyte growth factor
and its receptor - c-met for liver cancer patients with
hepatectomy. Dig Liver Dis. 38:490–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki K, Hayashi N, Yamada Y, Yoshihara
H, Miyamoto Y, Ito Y, Ito T, Katayama K, Sasaki Y, Ito A, et al:
Expression of the c-met protooncogene in human hepatocellular
carcinoma. Hepatology. 20:1231–1236. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi KJ, Baik IH, Ye SK and Lee YH:
Molecular targeted therapy for hepatocellular carcinoma: Present
status and future directions. Biol Pharm Bull. 38:986–991. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Torrecilla S and Llovet JM: New molecular
therapies for hepatocellular carcinoma. Clin Res Hepatol
Gastroenterol. 39:(Suppl 1). S80–S85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki M, Shiraha H, Fujikawa T, Takaoka
N, Ueda N, Nakanishi Y, Koike K, Takaki A and Shiratori Y:
Des-gamma-carboxy prothrombin is a potential autologous growth
factor for hepatocellular carcinoma. J Biol Chem. 280:6409–6415.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Spano D, Heck C, De Antonellis P,
Christofori G and Zollo M: Molecular networks that regulate cancer
metastasis. Semin Cancer Biol. 22:234–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng Y, Thiagarajan PS and Ma PC: MET
signaling: Novel targeted inhibition and its clinical development
in lung cancer. J Thorac Oncol. 7:459–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen QY, Jiao DM, Wu YQ, Chen J, Wang J,
Tang XL, Mou H, Hu HZ, Song J, Yan J, et al: MiR-206 inhibits
HGF-induced epithelial-mesenchymal transition and angiogenesis in
non-small cell lung cancer via c-Met/PI3k/Akt/mTOR pathway.
Oncotarget. 7:18247–18261. 2016.PubMed/NCBI
|
|
42
|
Wang Y, Zhan Z, Jiang X, Peng X, Shen Y,
Chen F, Ji Y, Liu W, Shi Y, Duan W, et al: Simm530, a novel and
highly selective c-Met inhibitor, blocks c-Met-stimulated signaling
and neoplastic activities. Oncotarget. 7:38091–38104.
2016.PubMed/NCBI
|
|
43
|
Horiguchi N, Takayama H, Toyoda M, Otsuka
T, Fukusato T, Merlino G, Takagi H and Mori M: Hepatocyte growth
factor promotes hepatocarcinogenesis through c-Met autocrine
activation and enhanced angiogenesis in transgenic mice treated
with diethylnitrosamine. Oncogene. 21:1791–1799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ogunwobi OO, Puszyk W, Dong HJ and Liu C:
Epigenetic upregulation of HGF and c-Met drives metastasis in
hepatocellular carcinoma. PLoS One. 8:e637652013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jo M, Stolz DB, Esplen JE, Dorko K,
Michalopoulos GK and Strom SC: Cross-talk between epidermal growth
factor receptor and c-Met signal pathways in transformed cells. J
Biol Chem. 275:8806–8811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Puri N and Salgia R: Synergism of EGFR and
c-Met pathways, cross-talk and inhibition, in non-small cell lung
cancer. J Carcinog. 7:92008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bachleitner-Hofmann T, Sun MY, Chen CT,
Tang L, Song L, Zeng Z, Shah M, Christensen JG, Rosen N, Solit DB,
et al: HER kinase activation confers resistance to MET tyrosine
kinase inhibition in MET oncogene-addicted gastric cancer cells.
Mol Cancer Ther. 7:3499–3508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Varkaris A, Gaur S, Parikh NU, Song JH,
Dayyani F, Jin JK, Logothetis CJ and Gallick GE: Ligand-independent
activation of MET through IGF-1/IGF-1R signaling. Int J Cancer.
133:1536–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peter B, Bosze S and Horvath R:
Biophysical characteristics of proteins and living cells exposed to
the green tea polyphenol epigallocatechin-3-gallate (EGCg): Review
of recent advances from molecular mechanisms to nanomedicine and
clinical trials. Eur Biophys J. Jun 16–2016.(Epub ahead of print).
PubMed/NCBI
|
|
51
|
Khan F, Niaz K, Maqbool F, Hassan F
Ismail, Abdollahi M, Venkata KC Nagulapalli, Nabavi SM and Bishayee
A: Molecular targets underlying the anticancer effects of
quercetin: An update. Nutrients. 8(pii): E5292016.doi:
10.3390/nu8090529. View Article : Google Scholar : PubMed/NCBI
|