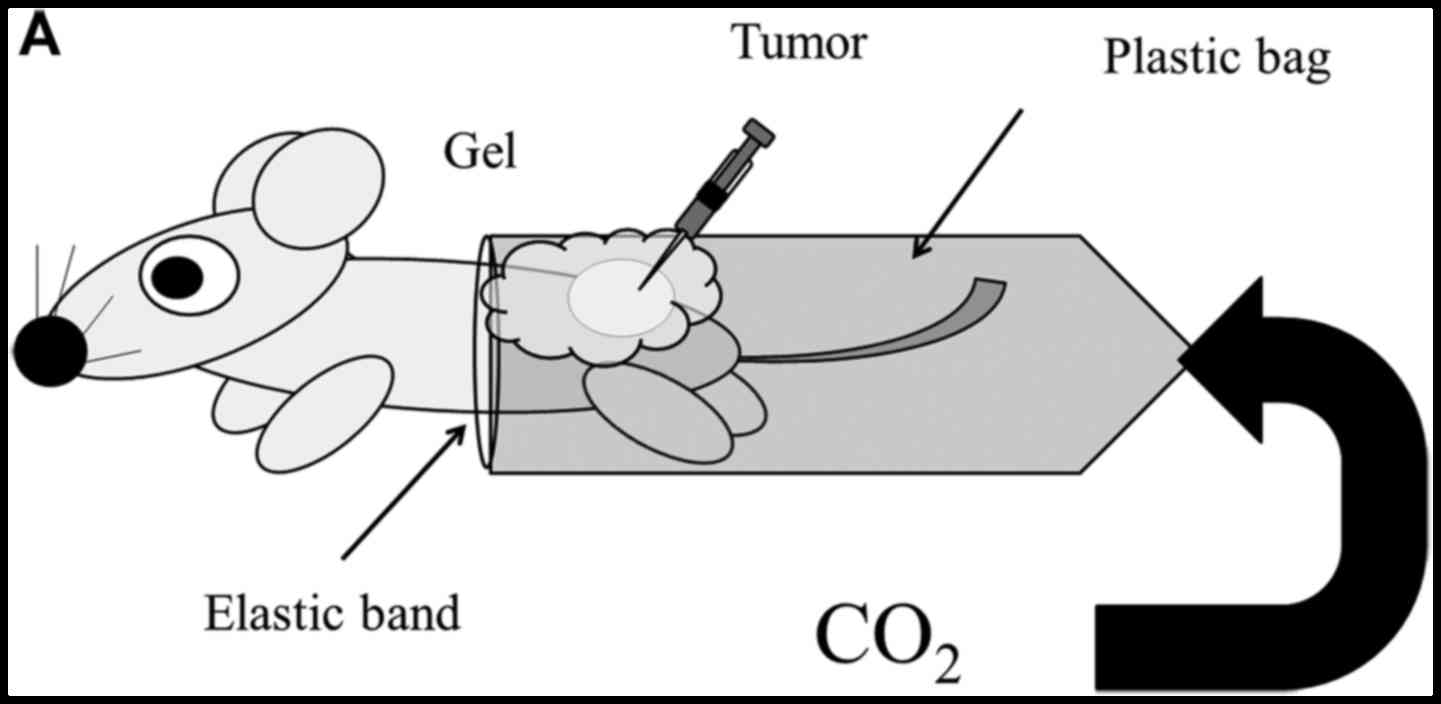
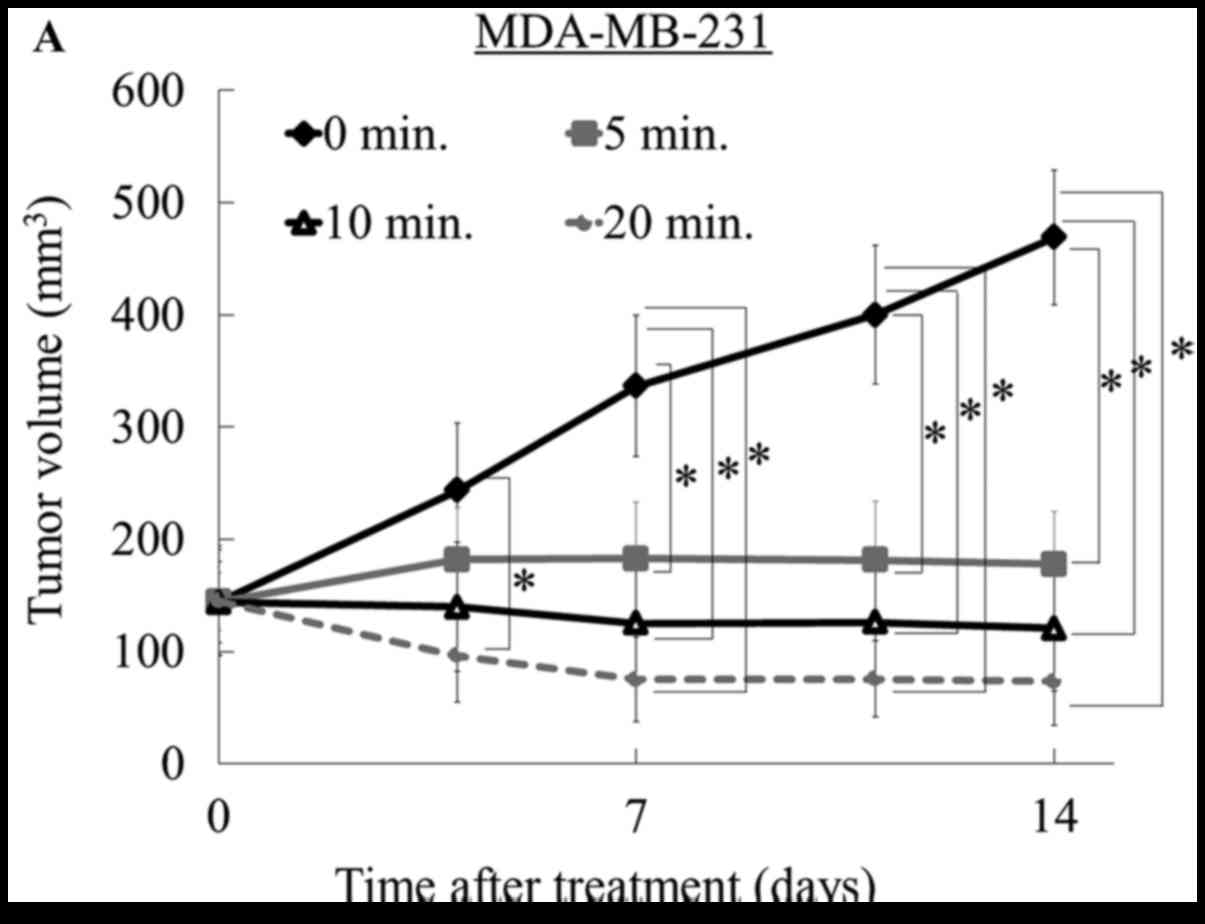
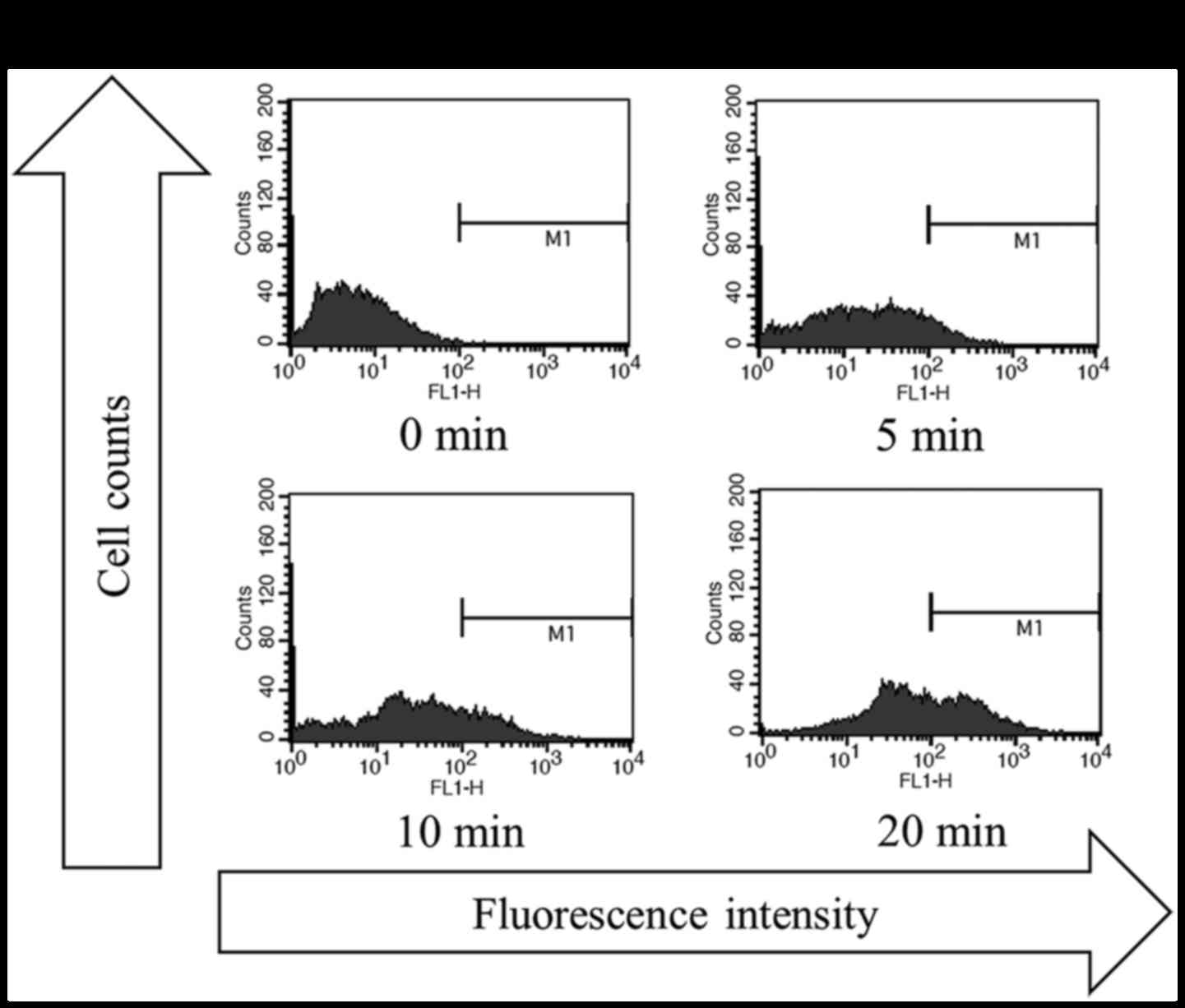
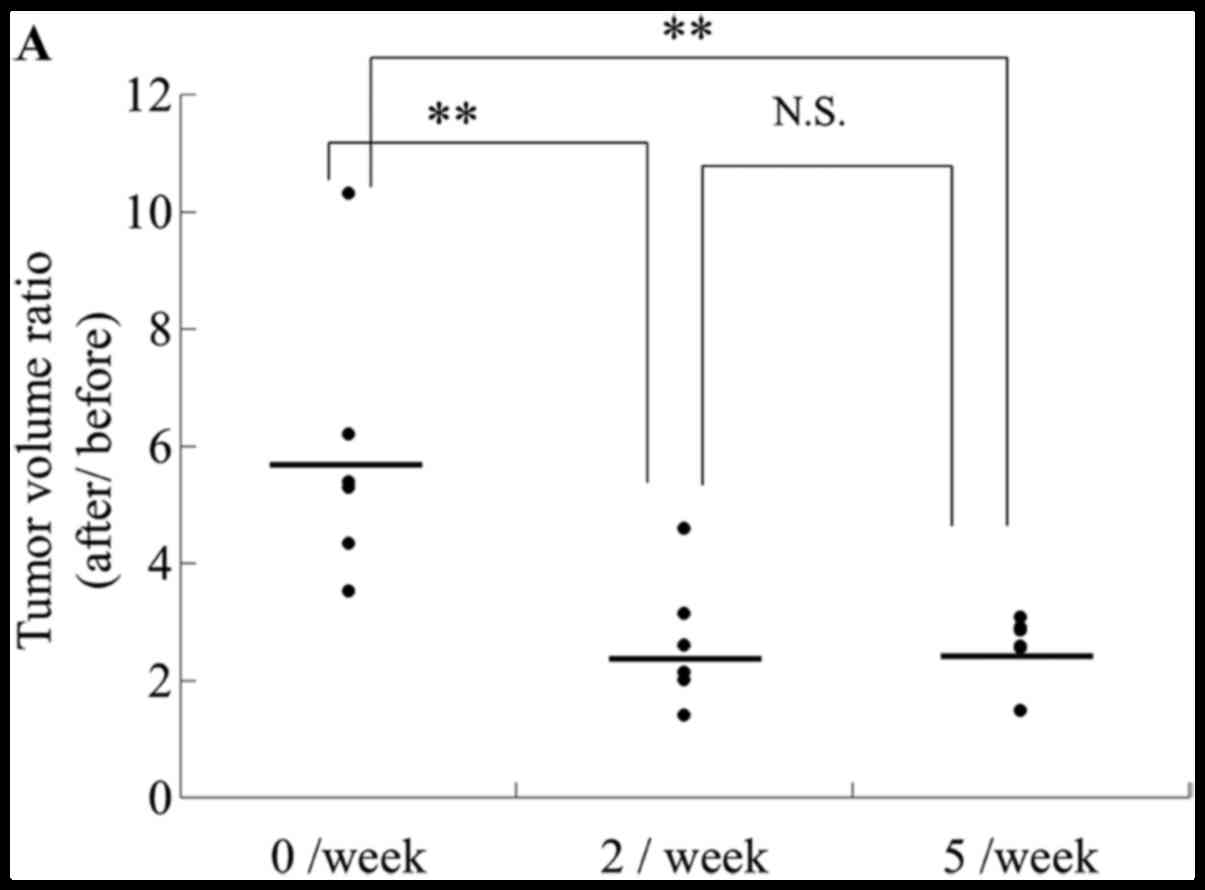
|
1
|
Bray F and Soerjomataram I: The changing
global burden of cancer: transitions in human development and
implications for cancer prevention and control, disease control
prioritiesCancer: Disease Control Priorities. 3. 3rd. Gelband H,
Jha P, Sankaranarayanan R and Horton S: The International Bank for
Reconstruction and Development/The World Bank; Washington, DC: pp.
23–44. 2015
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maurel J, López-Pousa A, de Las Peñas R,
Fra J, Martín J, Cruz J, Casado A, Poveda A, Martínez-Trufero J,
Balañá C, et al: Efficacy of sequential high-dose doxorubicin and
ifosfamide compared with standard-dose doxorubicin in patients with
advanced soft tissue sarcoma: An open-label randomized phase II
study of the Spanish group for research on sarcomas. J Clin Oncol.
27:1893–1898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dogliotti G, Galliera E, Iorio E, De
Bernardi Di Valserra M, Solimene U and Corsi MM: Effect of
immersion in CO2-enriched water on free radical release
and total antioxidant status in peripheral arterial occlusive
disease. Int Angiol. 30:12–17. 2011.PubMed/NCBI
|
|
6
|
Hartmann BR, Bassenge E, Pittler M and
Hartmann BR: Effect of carbon dioxide-enriched water and fresh
water on the cutaneous microcirculation and oxygen tension in the
skin of the foot. Angiology. 48:337–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joly F, Galoppin L, Bordat P, Cousse H and
Neuzil E: Calcium and bicarbonate ions mediate the inhibition of
mast cell histamine release by Avène spa water. Fundam Clin
Pharmacol. 14:611–613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toriyama T, Kumada Y, Matsubara T, Murata
A, Ogino A, Hayashi H, Nakashima H, Takahashi H, Matsuo H and
Kawahara H: Effect of artificial carbon dioxide foot bathing on
critical limb ischemia (Fontaine IV) in peripheral arterial disease
patients. Int Angiol. 21:367–373. 2002.PubMed/NCBI
|
|
9
|
Irie H, Tatsumi T, Takamiya M, Zen K,
Takahashi T, Azuma A, Tateishi K, Nomura T, Hayashi H, Nakajima N,
et al: Carbon dioxide-rich water bathing enhances collateral blood
flow in ischemic hindlimb via mobilization of endothelial
progenitor cells and activation of NO-cGMP system. Circulation.
111:1523–1529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bohr C, Hasselbach K and Krogh A: Ueber
emen in biologischen Bezuehung wichtigen Einfluss, den die Kohlen
saurespannung des Blutes anf dessen Samerstoffbinding ubt (In
German). Arch Physiol. 16:402–412. 1904. View Article : Google Scholar
|
|
11
|
Sakai Y, Miwa M, Oe K, Ueha T, Koh A,
Niikura T, Iwakura T, Lee SY, Tanaka M and Kurosaka M: A novel
system for transcutaneous application of carbon dioxide causing an
‘artificial Bohr effect’ in the human body. PLoS One. 6:e241372011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oe K, Ueha T, Sakai Y, Niikura T, Lee SY,
Koh A, Hasegawa T, Tanaka M, Miwa M and Kurosaka M: The effect of
transcutaneous application of carbon dioxide (CO2) on
skeletal muscle. Biochem Biophys Res Commun. 407:148–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onishi Y, Kawamoto T, Ueha T, Kishimoto K,
Hara H, Fukase N, Toda M, Harada R, Minoda M, Sakai Y, et al:
Transcutaneous application of carbon dioxide (CO2)
induces mitochondrial apoptosis in human malignant fibrous
histiocytoma in vivo. PLoS One. 7:e491892012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda D, Hasegawa T, Ueha T, Imai Y,
Sakakibara A, Minoda M, Kawamoto T, Minamikawa T, Shibuya Y, Akisue
T, et al: Transcutaneous carbon dioxide induces mitochondrial
apoptosis and suppresses metastasis of oral squamous cell carcinoma
in vivo. PLoS One. 9:e1005302014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harada R, Kawamoto T, Ueha T, Minoda M,
Toda M, Onishi Y, Fukase N, Hara H, Sakai Y, Miwa M, et al:
Reoxygenation using a novel CO2 therapy decreases the
metastatic potential of osteosarcoma cells. Exp Cell Res.
319:1988–1997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng Q, Banaszak L, Fracci S, Basali D,
Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger
NA, et al: Leptin receptor maintains cancer stem-like properties in
triple negative breast cancer cells. Endocr Relat Cancer.
20:797–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muñoz M, Berger M, Rosso M,
Gonzalez-Ortega A, Carranza A and Coveñas R: Antitumor activity of
neurokinin-1 receptor antagonists in MG-63 human osteosarcoma
xenografts. Int J Oncol. 44:137–146. 2014.PubMed/NCBI
|
|
18
|
Minoda M, Kawamoto T, Ueha T, Kamata E,
Morishita M, Harada R, Toda M, Onishi Y, Hara H, Kurosaka M, et al:
Antitumor effect of YM155, a novel small-molecule survivin
suppressant, via mitochondrial apoptosis in human MFH/UPS. Int J
Oncol. 47:891–899. 2015.PubMed/NCBI
|
|
19
|
Foote SO and Coleman JR: Medication
administration: The implementation process of bar-coding for
medication administration to enhance medication safety. Nurs Econ.
26:207–210. 2008.PubMed/NCBI
|
|
20
|
Ueshima E, Yamaguchi M, Ueha T, Muradi A,
Okada T, Idoguchi K, Sofue K, Akisue T, Miwa M, Fujii M, et al:
Inhibition of growth in a rabbit VX2 thigh tumor model with
intraarterial infusion of carbon dioxide-saturated solution. J Vasc
Interv Radiol. 25:469–476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moding EJ, Kastan MB and Kirsch DG:
Strategies for optimizing the response of cancer and normal tissues
to radiation. Nat Rev Drug Discov. 12:526–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: European Organisation and Treatment of
Cancer Soft Tissue and Bone Sarcoma Group: Doxorubicin alone versus
intensified doxorubicin plus ifosfamide for first-line treatment of
advanced or metastatic soft-tissue sarcoma: A randomised controlled
phase 3 trial. Lancet Oncol. 15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roila F, Herrstedt J, Aapro M, Gralla RJ,
Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer
P, et al: ESMO/MASCC Guidelines Working Group: Guideline update for
MASCC and ESMO in the prevention of chemotherapy- and
radiotherapy-induced nausea and vomiting: Results of the Perugia
consensus conference. Ann Oncol. 21 Suppl 5:v232–v243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S, Juresic E, Barton M and Shafiq J:
Management of skin toxicity during radiation therapy: A review of
the evidence. J Med Imaging Radiat Oncol. 54:264–279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stubbe CE and Valero M: Complementary
strategies for the management of radiation therapy side effects. J
Adv Pract Oncol. 4:219–231. 2013.PubMed/NCBI
|
|
26
|
Onishi Y, Kawamoto T, Ueha T, Hara H,
Fukase N, Toda M, Harada R, Sakai Y, Miwa M, Nishida K, et al:
Transcutaneous application of carbon dioxide (CO2)
enhances chemosensitivity by reducing hypoxic conditions in human
malignant fibrous histiocytoma. J Cancer Sci Ther. 4:174–181. 2012.
View Article : Google Scholar
|
|
27
|
Onishi Y, Akisue T, Kawamoto T, Ueha T,
Hara H, Toda M, Harada R, Minoda M, Morishita M, Sasaki R, et al:
Transcutaneous application of CO2 enhances the antitumor
effect of radiation therapy in human malignant fibrous
histiocytoma. Int J Oncol. 45:732–738. 2014.PubMed/NCBI
|