Introduction
S100 proteins are associated with a multigenic
family of small proteins (10 kDa) that bind calcium via E-F hand
motifs. To date, at least 25 constituents of this protein family
have been identified in vertebrates. S100B is the first member of
the S100 protein family to be identified and the most active S100
protein in the brain. S100B consists primarily of S100 ββ
homodimers. This protein is highly abundant in astroglial and
oligodendroglial cells, and therefore has been considered as a
glial marker protein (1,2). The S100B protein provides a wide range
of biological activities and functions. This protein regulates cell
shape, energy metabolism, contraction, cell-to-cell communication,
intracellular signal transduction, and cell growth. Moreover, S100B
may play a crucial role in the pathogenesis of depression and its
treatment. High levels of S100B have been detected in various
clinical conditions, such as brain trauma and ischemia, as well as
neurodegenerative, inflammatory, and psychiatric disorders
(3). Cancers such as glioblastoma
in cell culture have also been shown to secrete S100B (4). Moreover, S100B is a well-established
prognostic marker for melanoma, and high serum concentration of
S100B correlates with poor prognosis (5,6).
At present, research on the function of S100B
protein is comprehensive, but the regulatory mechanisms,
particularly in tumorigenesis, require further studies. Therefore,
the possibility of generating the whole S100B protein by
recombinant techniques is significantly advantageous for such
applications. However, studies on the biochemical roles and
distribution of the S100B protein and its antibodies have been
hampered by technical problems, such as difficulty in preparation
and cross reactivity of available antibodies. In our previous
study, the human S100A4 protein was successfully expressed in
Escherichia coli, and an efficient method was developed to
produce biologically active S100A4 (7). We attempted to utilize the previously
described techniques to produce the human S100B protein. In the
present study, we describe the construction and expression of a
synthetic gene encoding S100B in E. coli. We also tested the
bioactivity by Transwell migration and invasion assays. We injected
soluble human S100B protein to mice as an antigen and produced four
hybridoma cell lines to generate antibodies against S100B. Three
monoclonal antibodies (mAbs) were generated against S100B, namely,
4E10F11, 3D2E5, and
4F3A5, and were selected for further research
because of their potential characteristics and functionality in
western blot analysis through an indirect enzyme-linked
immunosorbent assay (ELISA).
Materials and methods
Reagents
Cloning vector pMD18-T vector was purchased from
TransGen Biotech (Beijing, China). A375 cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA).
NdeI, XhoI, and T4 DNA ligases were purchased from
New England Biolabs, Ltd. (Beijing, China).
Isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Merck
& Co., Inc. (Darmstadt, Germany). QIA quick gel extraction and
nucleotide removal kits were obtained from Qiagen China Co., Ltd.
(Shanghai, China). Commercial recombinant human S100B protein and
S100B antibody were from Abcam (Cambridge, MA, USA). Unless
otherwise stated, other reagents and materials were performed as
described in the literature (7).
Animals were purchased from the Experimental Animal Room of the
Institute of Health and Environmental Medicine (Tianjin, China).
This study was approved by the Committee of the Institute of Health
and Environmental Medicine.
Construction of expression vector
pET32a-S100B
The gene fragment of human S100B was constructed by
overlapping polymerase chain reaction (PCR) after optimization.
NdeI and XhoI restriction sites were designed in
sense and antisense primers, respectively. Eight oligonucleotide
primers with mutual overlaps were synthesized based on the codon
preference in E. coli (Table
I). The NdeI restriction site (underlined) was contained
in the forward primer (5′-CGCCATATGTCTGAACTGGAAAAAGCC-3′),
whereas the XhoI restriction site (underlined) was contained
in the reverse primer (5′-CGGCTCGAGTCACTCATGTTCAAAGAAC-3′).
E. coli DH5α cells were transformed with the constructed
cloning vector pMD18-S100B plasmid. Standard PCR was used to screen
the positive plasmid. To identify the integrity of S100B,
pMD18-S100B plasmid was DNA sequenced (Invitrogen Life
Technologies, Shanghai, China). The expression vector pET32a-S100B
plasmid was constructed and then ligated with the coding region of
human S100B from the pMD18-S100B plasmid.
 | Table I.Oligonucleotide primers with mutual
overlaps. |
Table I.
Oligonucleotide primers with mutual
overlaps.
| Primer name | Primer sequence | Length of primer
(bp) |
|---|
| 1 |
ATGTCTGAACTGGAAAAAGCCATGGTGGCCCTGATCGACGTTTTCCACCAGT | 52 |
| 2 |
CTTCAGCCTGTGCCTGTCGCCTTCGCGGCCAGAATACTGGTGGAAAACGTCGATC | 55 |
| 3 |
ACAGGCACAGGCTGAAGAAATCCGCACCGAAGGCGCTCATCAGCAGTGAGCTT | 53 |
| 4 |
AACCTCCTGCTCTTTGATTTCCTCTAAGAAATGGGAAAGCTCACTGCTGATGAGC | 55 |
| 5 |
ATCAAAGAGCAGGAGGTTGTGGACAAAGTCATGGAAACACTGGACAATGATGGAG | 55 |
| 6 |
CAAAGGCCATGAATTCCTGGAAGTCACATTCGCCGTCTCCATCATTGTCCAGTGT | 55 |
| 7 |
AGGAATTCATGGCCTTTGTTGCCATGGTTACTACTGCCTGCCACGAGTTC | 50 |
| 8 |
TCACTCATGTTCAAAGAACTCGTGGCAGGCAGT | 30 |
| BF |
CGCCATATGTCTGAACTGGAAAAAGCC | 27 |
| BR |
CGGCTCGAGTCACTCATGTTCAAAGAAC | 28 |
Expression, purification, and
identification of recombinant human S100B
The expression vector pET32a-S100B plasmids were
transformed into E. coli BL21 (DE3). Positive E. coli
was inoculated in 5 ml of Luria-Bertani (LB) media containing 50
µg/ml ampicillin. IPTG was used to induce the expression of human
S100B protein. Bacterial lysate was collected to isolate the
recombinant S100B. S100B was purified by ion-exchange
chromatography column (HiTrap™ DEAE FF; binding buffer: Tris-HCl
0.02 mol/l, pH 8.8; and elution buffer: 500 mmol/l NaCl Tris-HCl
0.02 mol/l, pH 8.8; GE Healthcare Life Sciences, Chalfont, UK) in
accordance with the manufacturer's instructions, desalted, and
freeze dried. Protein concentration was determined by bicinchoninic
acid (BCA) method (BCA Protein Assay kit; Tiangen Biotech Co. Ltd.,
Beijing, China) (7), and S100B
protein was characterized by western blot analysis.
Biological activity of human S100B
protein in promoting HeLa cell invasion and migration
Cell motility and invasion assays were performed in
a Transwell chamber to detect whether the recombinant S100B protein
can stimulate cell migration and invasion (8). In accordance with the manufacturer's
instructions, Transwell chambers (EMD Millipore) were coated with
Matrigel®, and assays were performed. HeLa cells
(1×105) without S100B protein were utilized as the
control group (C), whereas HeLa cells (1×105) with 50
µg/ml S100B protein were used as the experimental group (E). HeLa
cells (100 µl) of C or E were added to the top chambers of 24-well
Transwell plates. After 12 h of incubation at 37°C, the HeLa cells
at the bottom of each chamber were fixed with 0.1% v/v
glutaraldehyde for 30 min, rinsed with PBS, and then stained with
0.2% v/v crystal violet for 20 min, whereas the motile cells at the
top of each chamber were removed with cotton swabs. The number of
migrating cells or invasive cells was calculated under ×200
magnification (Olympus CKX31; Olympus, Tokyo, Japan). The number of
average cells per chamber was also determined. Duplicate experiment
was performed with each assay and repeated at least thrice. Data
were measured as the migration/invasion rates relative to the
parental control cells.
Monoclonal anti-human S100B antibody
preparation, purification, and identification
The immunization procedure was performed using a
previously described technique with slight modification (7,9,10).
Female BALB/c mice (weight, 18–25 g; age, 8–10 weeks old) were
provided by the Experimental Animal Room of the Tianjin Institute
of Health and Environmental Medicine. Four mice were bred per cage
at 22–25°C, ad libitum with tap water and standard diet,
under an alternating 12 h light/dark cycle. The purified
recombinant S100B (50 µg) was mixed with an equal volume of
Freund's complete adjuvant, which was multipoint-injected
subcutaneously on the back of the mice. Animals were immunized
thrice over the course of 45 days with purified recombinant S100B
protein. On the 10th day after the last injection, the mice were
sacrificed by cervical dislocation, and the splenocytes were
collected and fused with SP2/0 myeloma cells by using the PEG (PEG,
polyethylene) method. Hybridoma cells were selected in the
hypoxanthine, aminopterin, and thymidine media (purchased from
Gibco Life Technologies, Grand Island, NY, USA). Positive hybridoma
cell lines were obtained following three subcloning cycles and were
confirmed by indirect ELISA. Hybridoma cells were injected
intraperitoneally into liquid paraffin-primed female BALB/c mice
(8–10 weeks old) at ~1×106 cells/mouse to produce
anti-S100B mAbs. Purification of antibodies was performed using
HiTrap protein G HP (1 ml) (GE Healthcare Life Sciences) in
accordance with the manufacturer's instructions. The binding buffer
was 20 mmol/l Na3PO4 at pH 7.0, and the
elution buffer was 0.1 mol/l Gly-HCl at pH 2.7. The purity of the
antibody was determined by SDS-PAGE and western blot analysis. The
affinity constants of antibodies were identified by SPR (11,12).
Antibody titer determination
Antibody titers were measured by indirect ELISA
(11,12). The microtiter plates were coated
with ~100 µl of recombinant S100B (4 µg/ml) and then incubated at
4°C overnight. The plates were washed thrice with PBS containing
0.05% Tween-20 (PBST) and blocked with 3% BSA in PBS containing
Tween-20 for 1 h at 37°C. The plates were placed in different serum
dilutions from immunized mice, ascites, or cell culture
supernatants for 2 h at 37°C and then incubated with HRP-coupled
goat anti-mouse IgG for 1 h. The substrate used was
tetramethylbenzidine (TMB). The absorbance was determined at 450
and 630 nm wavelengths, respectively. The specification of the mAbs
were examined using competitive ELISA (11,12)
(Table II).
 | Table II.Features of S100B MAbs. |
Table II.
Features of S100B MAbs.
| Parameters |
4E10F11C11 |
3D2E5F7 |
4F3A5D5 |
|---|
| Titer (ascitic
fluid) | 1:204800 | 1:204800 | 1:204800 |
| Affinity constant
(l/mol) |
6.82×108 |
7.54×108 |
2.53×108 |
| Concentration
(mg/ml) | 7.5 | 6.8 | 6.6 |
SDS-PAGE and western blot analysis of
recombinant S100B
The SDS-PAGE procedure was performed as previously
described with slight modification (13). The concentrations of the resolving
and stacking gels were 15 and 5%, respectively. The purified
protein was transferred to a nitrocellulose membrane with a
semi-dry electrophoretic transfer device (Jim-X Biotechnology Co.,
Ltd., Dalian, China). The membrane was blocked with 0.5 ml of 10%
BSA in 4.5 ml of Tris-buffered saline/Tween-20 buffer (10 ml of 1
mol/l Tris+HCl at pH 7.5, 8.8 g of NaCl, 1000 ml of ultra-pure
water, and 1 ml of 20% Tween-20) and incubated with rabbit
polyclonal antibody against human S100B for 2 h at 25°C. Following
the HRP-coupled goat anti-rabbit IgG, a secondary antibody was
added and incubated for 45 min.
Cross reactivity
To identify the cross reactivity (CR) of the
recombinant S100B and its analogs, indirect ELISA was utilized as
previously described (12,13). As coated antigens, recombinant
S100B, recombinant rat S100A4, human S100A4, and S100A1 were all
dissolved in PBS. A diluted antibody protein of S100B was incubated
with these coated antigens for 2 h at 25°C. Afterward,
HRP-conjugated goat anti-mouse polyclonal antibody was added and
then incubated for 45 min at 25°C. Finally, the absorbance was
determined at 450 nm wavelength by using a microplate reader
(Multiskan MK3; Thermo Fisher Scientific, Waltham, MA, USA).
Affinity analysis
The mAb affinity against S100B was estimated by
surface plasmon resonance (SPR, AutoLab ESPRIT, Utrecht, The
Netherlands). In accordance with the manufacturer's instructions,
S100B was immobilized on a carboxylated sensor chip (Metrohm Auto
Laboratory). Extracted mAbs were added to the immobilized chip
surface. Data were analyzed using Kinetic Evaluation 5.0 software
(Metrohm Auto Laboratory) and Autolab ESPRIT Data Acquisition
4.3.
Effect of anti-human S100B mAbs on
cell proliferation of A375 cells
Cell proliferation assay was determined using Cell
Counting Kit-8 (CCK-8; Dojinoo Techno Research Park, Mashiki-machi,
Japan). A375 cells (5×104) of C or E were added to the
96-well plates and incubated for 24 h at 37°C. Different
concentrations (100 µg/ml, 10 µg/ml, 1 µg/ml, 100 ng/ml, 10 ng/ml,
and 1 ng/ml) of S100B mAbs (100 µL) were added to the plates as the
experimental group (E), whereas DMEM medium (100 µl) was used as
the control group (C). The absorbance was determined at 450 nm
wavelength by using a microplate reader (Multiskan MK3; Thermo
Fisher Scientific).
Effect of anti-human S100B mAbs on
cell apoptosis of A375 cells
Cell apoptosis assay was performed using a Histone
ELISA kit (from Biosoure™; Invitrogen, Carlsbad, CA, USA). A375
cells (1×105) were added to the 96-well plates.
Different concentrations (1 mg/ml, 100 µg/ml, 10 µg/ml, 1 µg/ml,
100 ng/ml, 10 ng/ml, and 1 ng/ml) of S100B mAbs (100 µl) were added
to the plates as the experimental group (E), whereas DMEM medium
(100 µl) was used as the control group (C). The absorbance of the
lysates of the cells was determined at 405/490 nm wavelength in
accordance with the manufacturer's instructions.
Effect of anti-human S100B mAbs on the
expression of S100B and p53 in A375 cells
To evaluate the effect of S100B mAbs on the
expression of S100B and p53 in A375 cells, western blot analysis
was performed. Anti-human S100B mAbs prepared in the present study
was added to A375 cells in a culture flask (25 cm). The
concentrations of S100B mAbs in the medium are 10 µg/ml and 100
ng/ml as the experimental group (E), whereas DMEM medium was used
as the control group (C). After incubation at 37°C for 24 h, A375
cells were collected and disrupted for 30 min. The entire set of
proteins was preserved at −70°C. Anti-human S100B mAbs (prepared
and purchased separately) were used as first antibodies in western
blot analysis.
Statistical analysis
Data are shown as means ± SD. All results were
analyzed using SPSS version 17 (SPSS, Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Cloning, expression, and purification
of human recombinant protein S100B
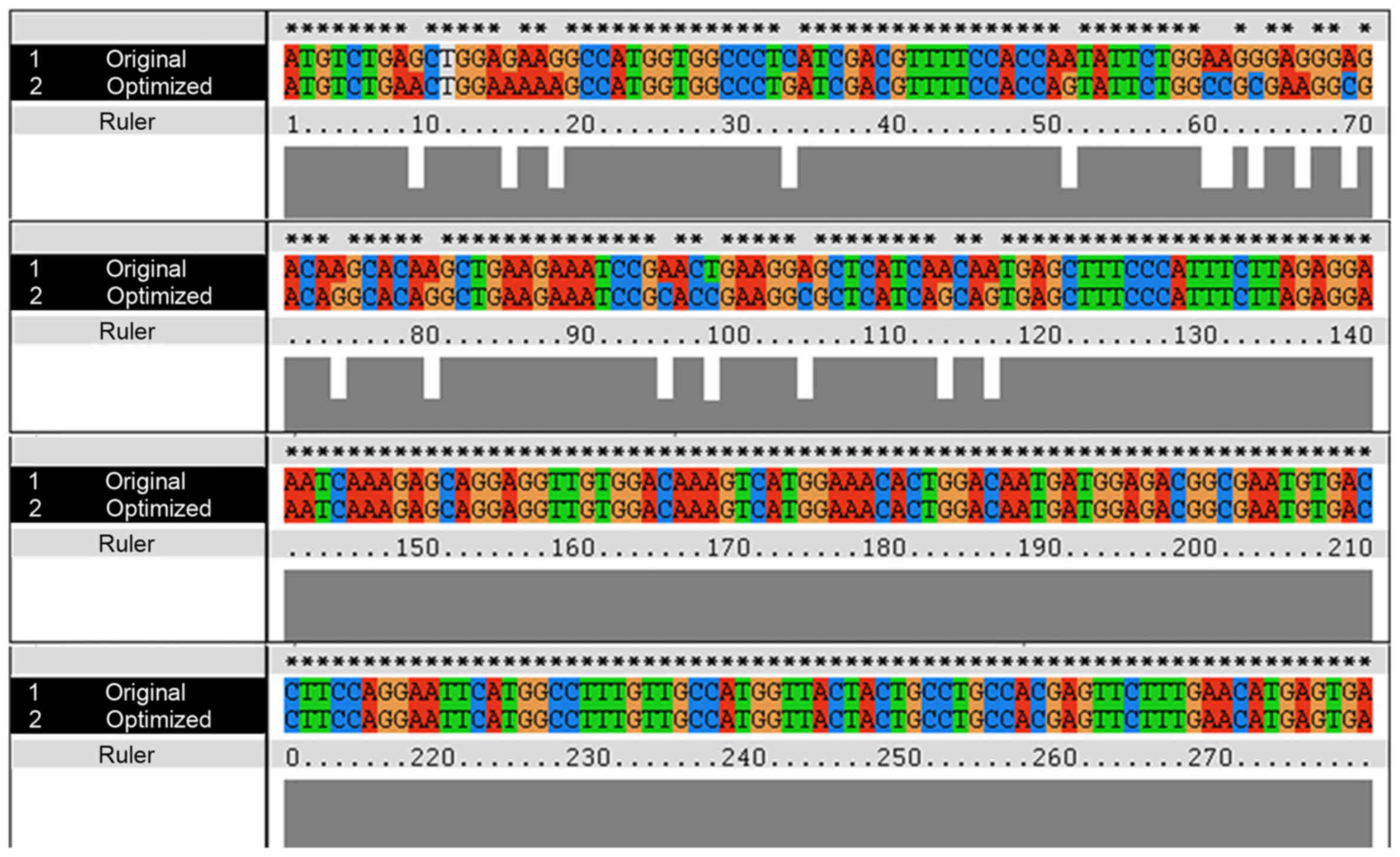
The human S100B protein natural gene sequence
(Genbank accession no. NM 006272.2) was optimized using the E.
coli codon. Sixteen rare codons of the S100B original gene were
superseded with synonymous high-frequency codons in an optimized
gene that encodes S100B (Fig.
1).
The amplified PCR products were
assessed by 1.5% agarose gel
The size of the PCR products was 250–300 bp, which
conforms to the expected size of 279 bp (Fig. 2A). By Ndel and Xhol digestion, the
S100B gene was successfully ligated into the pMD18-T vector.
Agarose gel analysis of the digestion of pMD18-S100B vector is
indicated in Fig. 2B. Through DNA
sequencing, the synthesized S100B gene was consistent with the
optimized S100B gene without point mutation and frameshift mutation
(Fig. 2D).
 | Figure 2.Recombinant expression and
purification of human S100B protein. (A) Agarose gel analysis of
human S100B DNA. Lanes 1–3, human S100B DNA; lane M, DNA marker.
(B) Agarose gel analysis of the pMD18-S100B vector following
restriction enzyme treatment by utilizing Ndel and
Xhol. Lane 1, pMD18-S100B plasmid; lane 2, pMD18-S100B
digested by Ndel and Xhol; lane M, DNA marker. (C)
Agarose gel analysis of the pET32a-S100B expression vector
following restriction enzyme treatment by utilizing Ndel and
Xhol. Lane 1, pET32a-S100B digested by Ndel and
Xhol; lane 2, pET32a-S100B plasmid; lane 3, positive
control; lane M, DNA marker. (D) DNA sequencing result. (E) Western
blot analysis of purified S100B mAbs with antigen. Lanes 1,
commercially purchased S100B mAbs; lanes 2, purified ascites of
BALB/c mice injected hybridoma cell lines. (F) SDS-PAGE analysis of
recombinant protein. Lane 1, induced products of pET32a-S100B; lane
2, induced products of pET32a; (G) Western blot analysis of the
recombinant protein. Lane 1, induced products of pET32a-S100B; lane
2, induced products of pET32a; (H) Purification of the recombinant
protein. Lane 1, liquid of penetration; lanes 2–9, eluting peak of
different concentrations of NaCl; lane 3, human S1004 protein as a
positive control. |
Recombinant S100B protein expression
and purification
S100B gene was synthesized, and the recombinant
expression plasmid pET32a-S100B was constructed successfully
(Fig. 2C). The results of the
restriction enzyme digestion showed that the S100B gene plasmid
contained the full coding sequence, and the open reading frame was
correct. The recombinant plasmid pET32a-S100B was transformed into
expression strain E. coli BL21 (DE3) and was induced by
IPTG. The molecular weight of the S100B fusion protein was 11.5
kDa. The expressed recombinant S100B was identified by SDS-PAGE and
western blotting using a rabbit anti-human S100B antibody. The
recombinant S100B protein was successfully expressed in E.
coli BL21 (DE3) because of the reaction with the antibody
(Fig. 2F and G) and was effectively
purified by ion-exchange chromatography (Fig. 2H).
Recombinant S100B protein promotes
invasion and migration of HeLa cells
The function of human recombinant S100B was detected
by Transwell chamber test. The results showed that the recombinant
S100B protein increased the invasion of HeLa cells 2.8 times
(Fig. 3A-C) and the migration 3.5
times (Fig. 3D-F), indicating that
S100B protein can promote HeLa cell invasion and migration.
The purified S100B proteins demonstrate a potential biological
activity and can improve the ability of tumor cell invasion and
migration.
Preparation and characterization of
anti-human S100B mAbs
Murine mAbs against S100B were prepared to further
explore the function of human S100B. Three hybridoma cell lines,
namely, 4E10F11C11,
3D2E5F7, and
4F3A5D5, were subsequently formed
from the immunization of mice with soluble S100B as the antigen.
Highly concentrated S100B mAbs were prepared from BALB/c mouse
ascites and purified by protein G affinity chromatography. These
cell lines stably produced anti-S100B mAbs (Table II). The specificity of the S100B
mAbs was determined by western blot analysis (Fig. 2E).
CR is a key parameter used to assess the specificity
of an antibody because the human S100B exhibits similar structures
and functions of the following proteins: Human S100A4, Mouse
S100A4, Human S100A1, and Human S100A1. The data summarized in
Table III indicate that this
antibody achieved little CRs to mouse S100A4, human S100A1, and
S100B.
 | Table III.Cross-reactivity of S100B
antibody. |
Table III.
Cross-reactivity of S100B
antibody.
| Protein | S100B antibody
3D2E5F7 (450 nm wavelength) |
|---|
| Human S100B | 1.275 |
| Human S100A4 | 0.123 |
| Mouse S100A4 | 0.101 |
| Human S100A1 | 0.116 |
| Negative
control | 0.092 |
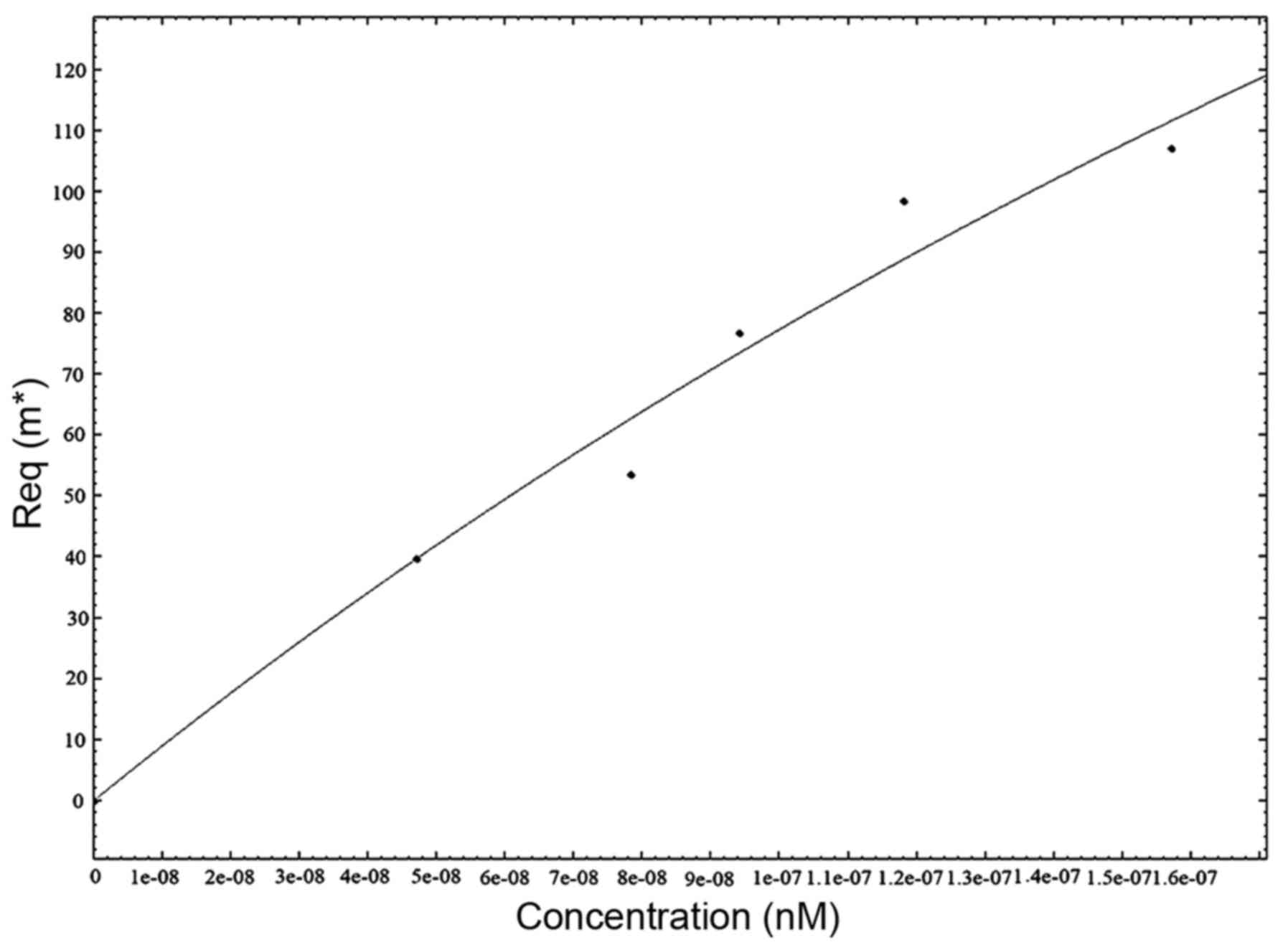
To determine the association rate constant,
3D2E5F7 mAb was properly diluted
in PBS and analyzed by SPR at different concentrations (Fig. 4). The equilibrium dissociation
constant (KD) for the 3D2E5F7
clone was determined independently by Kinetic Evaluation 5.0
software. The KD of 3D2E5F7 was
~4.72×10−8 mol/l.
Effect of anti-human S100B mAbs on
cell proliferation of A375 cells
We examined the effect of S100B mAbs on cell
proliferation of A375 cells. The results showed that cell
proliferation of A375 cells was inhibited by anti-human S100B mAbs
to some extent. Different concentrations of mAbs exert various
effects on cell proliferation. The effect of mAbs on cell
proliferation of A375 cells increased from low to high
concentration of anti-human S100B mAbs (Fig. 5A).
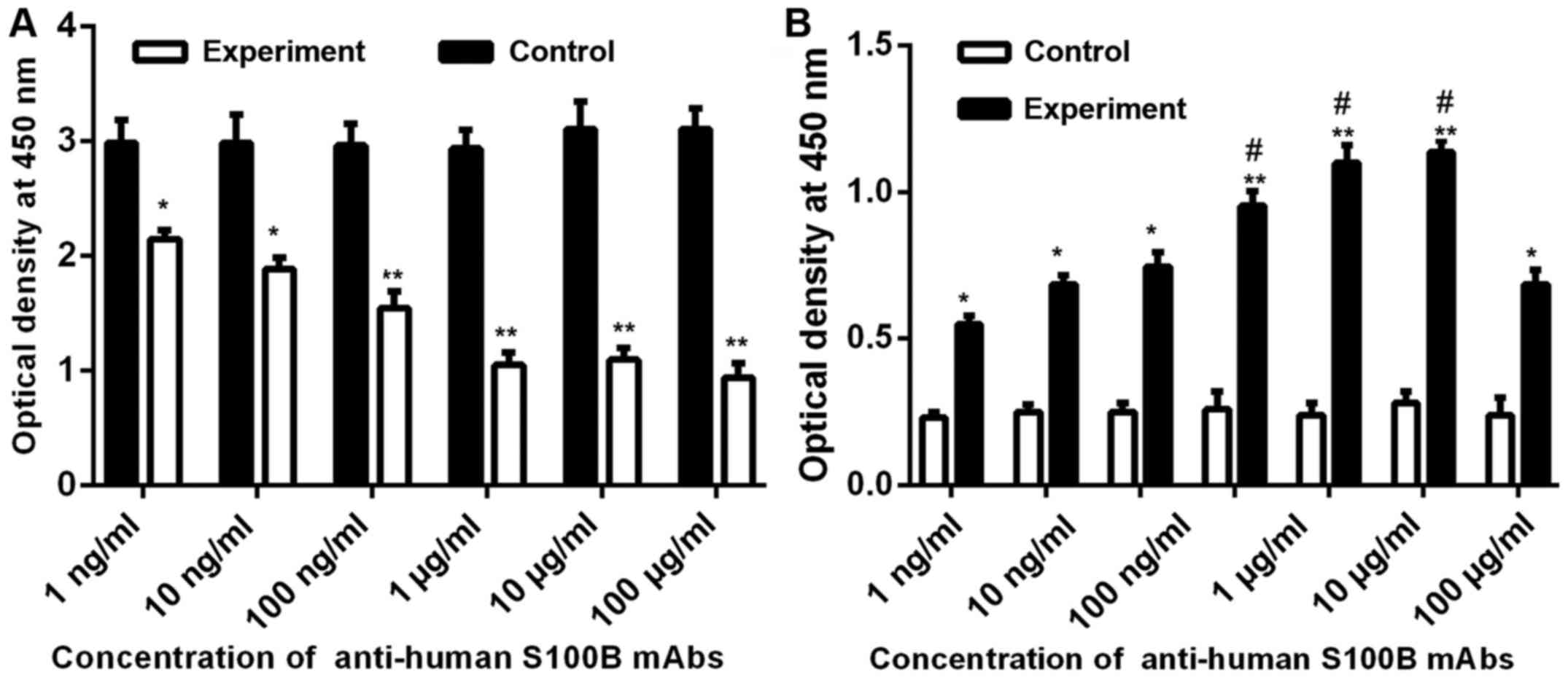 | Figure 5.Effect of anti-human S100B mAbs on
cell proliferation and apoptosis of A375 cells. (A) Effect of
different concentration of S100B mAbs on cell proliferation.
Control, proliferation of A375 cells (5×104) added to
basic medium; experiment, proliferation of A375 cells
(5×104) added to different concentrations (100 µg/ml, 10
µg/ml, 1 µg/ml, 100 ng/ml, 10 ng/ml, and 1 ng/ml) of S100B mAbs
(100 µl). (B) Effects of different concentrations of S100B mAbs on
cell apoptosis. Control, apoptosis of A375 cells (1×105)
added to basic medium; experiment, apoptosis of A375 cells
(1×105) added to different concentrations (1 mg/ml, 100
µg/ml, 10 µg/ml, 1 µg/ml, 100 ng/ml, 10 ng/ml, and 1 ng/ml) of
S100B mAbs (100 µl). OD value of HeLa cells in the invasion
experiment group compared with the control group. OD, optical
density. *P<0.05, compared with control; **P<0.01, compared
with control. #P<0.05, compared with 1 ng/ml. |
Effect of anti-human S100B mAbs on
cell apoptosis of A375 cells
To demonstrate the effect of anti-human S100B mAbs
on cell apoptosis of A375 cells, cell apoptosis assay was examined
in accordance with the manufacturer's instructions in the ELISA
kit. The results in Fig. 5B suggest
that with increasing anti-human S100B mAb concentration added to
the cell culture medium, the amount of apoptosis in A375 cells was
significantly increases compared with that of the control
group.
Effect of anti-human S100B mAbs on the
expression of S100B and p53 in A375 cells
The results indicated that with increasing
anti-human S100B mAb concentration added to the cell culture
medium, the expression of S100B in A375 cells decreased, whereas
the expression of p53 in A375 cells increased significantly
(P<0.05). In this study, the use of commercially purchased S100B
mAb was compared with that of self-prepared S100B mAb. The results
presented a similar trend. The above data illustrated that
anti-human S100B mAb plays an important role in decreasing the
expression of S100B and increasing the expression of P53 protein in
A375 (Fig. 6).
Discussion
The S100 protein family is a group of low molecular
weight, acidic, EF-hand Ca2+-binding proteins,
consisting of more than 20 subfamily members. The S100 protein was
first identified by Moor in 1965 and was named as such because it
is 100% saturated in ammonium sulfate solution (14). S100 proteins are only expressed in
vertebrates, showing cell-specific expression patterns and playing
an important role in both intracellular and extracellular functions
in the regulation of motility and differentiation, cell cycle,
cytoskeletal dynamics, and Ca2+ homeostasis. In recent
years, research showed that S100 proteins are involved in
cardiomyopathy, neurological diseases, inflammatory, and neoplasia
diseases.
S100B protein appertains to the S100 protein family
and is mainly expressed in astrocytes and Schwann cells of the
central nervous system. S100B acts as a Ca2+ sensor
protein in cells. The human gene encoding S100B maps to chromosome
21q22.3 (15) with consequent
overexpression of the protein in Down syndrome (16). Recently, S100B has been identified
as a novel dyslexia candidate gene (17). S100B is closely related to the
pathophysiological mechanism in traumatic injury (TBI) and neonatal
hypoxic ischemic encephalopathy, supposing that neonatal hypoxic
ischemic encephalopathy, TBI, and intracellular S100B from the
injury or apoptosis of nerve cells can be released into the blood,
urine, or cerebrospinal fluid. Therefore, serum, urine, or
cerebrospinal fluid levels of S100B are of prognostic and
predictive values in patients with related diseases.
In this study, we have successfully constructed the
recombinant plasmid pET32a-S100B, which was expressed in E.
coli, and purified soluble recombinant S100B protein with
biological activity. We ultimately obtained human monoclonal
antibodies against S100B through immunization of mice with the
purified S100B protein. S100B proteins in the human melanoma cell
line A375 were detected with the monoclonal antibodies against
S100B proteins. The results were all positive, and the results
detected with the antibodies were the same as those with the
commercial monoclonal antibody. When the monoclonal antibodies were
added to A375, cell proliferation decreased and the apoptotic ratio
increased, which may increase the expression of wild-type P53
protein. Thus, antibodies can play the role of targeted therapy for
diseases.
In this study, the DNA sequence of human S100B was
optimized and synthesized in accordance with the codon usage bias
of E. coli, which may be improved for the soluble expression
and biological activity. The following results demonstrated that
the recombinant S100B protein was functionally expressed in E.
coli BL21 (DE3) at a high level and showed high biological
activity in the immunization procedure, antibody preparation,
western blot analysis, and A375 cell model.
This study provides a favorable means for conducting
qualitative and quantitative detection of S100B and is beneficial
for diagnosis and treatment of related diseases. Concurrently, this
study provides theoretical and technical basis for further research
on standardized commercial kit diagnosis of malignant tumors and
for studies on other human antibodies.
Acknowledgements
The authors gratefully acknowledge the financial
support of the National Natural Science Foundation of China (grant
nos. 81373108 and 30971421).
References
|
1
|
Donato R: S100: A multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracellular functional roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiras A, Bhosale A, Shepal V, Shukla R,
Baburao VS, Prabhakara K and Shastry P: A unique model system for
tumor progression in GBM comprising two developed human
neuro-epithelial cell lines with differential transforming
potential and coexpressing neuronal and glial markers. Neoplasia.
6:520–532. 2003. View Article : Google Scholar
|
|
3
|
Nash DL, Bellolio MF and Stead LG: S100 as
a marker of acute brain ischemia: A systematic review. Neurocrit
Care. 8:301–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dagdan E, Morris DW, Campbell M, Hill M,
Rothermundt M, Kästner F, Hohoff C, von Eiff C, Krakowitzky P, Gill
M, et al: Functional assessment of a promoter polymorphism in
S100B, a putative risk variant for bipolar disorder. Am J Med Genet
B Neuropsychiatr Genet. 156B:691–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egberts F, Pollex A, Egberts JH, Kaehler
KC, Weichenthal M and Hauschild A: Long-term survival analysis in
metastatic melanoma: Serum S100B is an independent prognostic
marker and superior to LDH. Onkologie. 31:380–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weide B, Richter S, Büttner P, Leiter U,
Forschner A, Bauer J, Held L, Eigentler TK, Meier F and Garbe C:
Serum S100B, lactate dehydrogenase and brain metastasis are
prognostic factors in patients with distant melanoma metastasis and
systemic therapy. PLoS One. 8:e816242013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang XG, Meng Q, Qi FM and Yang QF:
Blocking TGF-β inhibits breast cancer cell invasiveness via
ERK/S100A4 signal. Eur Rev Med Pharmacol Sci. 18:3844–3853.
2014.PubMed/NCBI
|
|
9
|
Davydov DM, Lobanov AV, Morozov SG,
Gribova IE and Murashev AN: Neurodevelopment and
phenotype-modulating functions of S100B protein: A pilot study.
Physiol Behav. 140:188–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang XX, Li F, Hu WG, Xia HC and Zhang ZC:
Preparation and preliminary application of monoclonal antibodies
against Trichokirin-S1, a small ribosome-inactivating peptide from
the seeds of Trichosanthes kirilowii. Acta Biochim Biophys
Sin (Shanghai). 37:447–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vasconcellos FA, Aleixo PB, Stone SC,
Conceição FR, Dellagostin OA and Aleixo JA: Generation and
characterization of new HER2 monoclonal antibodies. Acta Histochem.
115:240–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuasa N, Koyama T and Fujita-Yamaguchi Y:
Purification and refolding of anti-T-antigen single chain
antibodies (scFvs) expressed in Escherichia coli as
inclusion bodies. Biosci Trends. 8:24–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang D, Zhang J, Liu Z, Chen Y, Xu C,
Zhang Z, Liu X, Wu L, Zhou X, Meng X, et al: Functional expression,
characterization and application of the human S100A4 protein. Mol
Med Rep. 11:175–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allore R, O'Hanlon D, Price R, Neilson K,
Willard HF, Cox DR, Marks A and Dunn RJ: Gene encoding the beta
subunit of S100 protein is on chromosome 21: Implications for Down
syndrome. Science. 239:1311–1313. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Esposito G, Scuderi C, Steardo L,
Delli-Bovi LC, Hecht JL, Dickinson BC, Chang CJ, Mori T and Sheen
V: S100B and APP promote a gliocentric shift and impaired
neurogenesis in Down syndrome neural progenitors. PLoS One.
6:e221262011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poelmans G, Engelen JJ, Van Lent-Albrechts
J, Smeets HJ, Schoenmakers E, Franke B, Buitelaar JK,
Wuisman-Frerker M, Erens W, Steyaert J, et al: Identification of
novel dyslexia candidate genes through the analysis of a
chromosomal deletion. Am J Med Genet B Neuropsychiatr Genet.
150B:140–147. 2009. View Article : Google Scholar : PubMed/NCBI
|