Introduction
Ovarian cancer is the sixth most common cause of
cancer related death among Western women. Although it represents
30% of cancers of the female genital tract, ovarian cancers are
responsible for more than half of the deaths (1). Significant progress has been made in
surgery and the improvement of chemotherapeutic agents in ovarian
cancer which led to improved 5-year survival rate, however, ovarian
cancer remains the leading cause of death of all gynecological
cancers (1,2). The main challenge of current
chemotherapies for ovarian cancer treatment is due to drug
resistance or relapse. Thus, identification of novel anticancer
drugs and understanding the underlying molecular mechanisms will be
of great help in the treatment of ovarian cancer.
Natural products have attracted considerable
attention due to their potent anticancer effects in the past
decades (3,4). It is estimated that over 46% of the
new drugs and new drug candidates for cancer therapy approved by US
Food and Drug Administration (FDA) between 1989 and 1995 were
natural products or natural products derivatives (5). Notably, recent studies have
demonstrated that the β-carboline alkaloids, a large group of
natural and synthetic indole alkaloids that extensively exist in
extracts from the leaves, barks, roots and seeds of a variety of
plants, possess a broad range of psychopharmacological and
neuropharmacological effects (6).
Many alkaloids have been widely used in the clinical treatment of
cough, hypertension and inflammation. Among them, harmine
(7-methoxy-1-methyl-9Hpyrido [3,4-b] indole), which was originally
isolated from the seeds of Peganum harmala and
Banisteriopsis caapi and has been traditionally used as folk
medicine in the Middle East, Central Asia, and South America
(7), appears to exhibit various
pharmacological effects both in vitro and in vivo,
such as anti-Alzheimer, anti-inflammatory anxiolytic and
antidepressant effects (8).
Notably, recent studies also indicate that harmine shows to have
significant anticancer effect in several cancer cells including
hepatoblastoma HepG2 cells (9),
B16F-10 melanoma cells (10) and
gastric cancer MGC-803 cells (11).
However, the effect of harmine on the proliferation and migration
of ovarian cancer cells remains unknown.
To this end, the present study was aimed to
investigate the anticancer effect and the possible mechanisms of
harmine-mediated cell proliferation and migration in human SKOV-3
cells. Our results demonstrated that harmine significantly
suppressed the proliferation and migration of ovarian cancer cells
via inhibition of the ERK/CREB pathway. Moreover, the reduced
expression of vascular endothelial growth factor (VEGF), matrix
metalloproteinase (MMP)-2 and MMP-9 may be involved in
harmine-mediated SKOV-3 cell proliferation.
Materials and methods
Antibodies and reagents
Antibodies to phospho-ERK1/2
(Thr202/Tyr204) (cat. no. 9101), ERK1/2 (cat. no. 9102),
phospho-CREB (Ser133) (antibodies to phospho-ERK1/2
(Thr202/Tyr204) (cat. no. 9198), CREB (antibodies to
phospho-ERK1/2 (Thr202/Tyr204) (cat. no. 9197), β-actin
(antibodies to phospho-ERK1/2 (Thr202/Tyr204) (cat. no.
4970), goat anti-rabbit horseradish peroxidase (HRP)-linked
antibody (antibodies to phospho-ERK1/2 (Thr202/Tyr204)
(cat. no. 7074) as well as U0126 (specific ERK1/2
inhibitor) were purchased from Cell Signaling Technology (Beverly,
MA, USA). Harmine, epidermal growth factors (EGF), dimethyl
sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Culture medium and other solutions used for cell
culture were purchased from Invitrogen (Shanghai, China).
SYBR® Premix Ex Taq™ II was obtained from Takara Bio,
Inc. (Otsu, Japan).
Cell culture and treatment
Human SKOV-3 ovarian cancer cells were grown in
RPMI-1640 medium supplemented with 10% fetal bovine serum, 50 µg/ml
streptomycin, 50 IU/ml penicillin. Cultures were maintained at 37°C
in a humidified atmosphere containing 5% CO2. Cells were
regenerated every 3 days when they reached 70–90% confluence.
Harmine was dissolved in DMSO and diluted to appropriate
concentrations with culture medium. The final concentration of DMSO
in the culture medium did not exceed 0.1%.
Reverse transcription PCR
Total RNA was extracted using TRIzol reagent.
Reverse transcription was performed according to the manufacturer's
instructions from Invitrogen. PCR analysis was performed using the
following sense and antisense primers: The VEGF primers were
forward 5′-CTGGGCTGTTCTCGCTTCG-3′ and reverse
5′-CTCTCCTCTTCCTTCTCTTCTTCC-3′. The MMP-2 primers were
forward 5′-CCGTCGCCCATCATCAAGTTC-3′ and reverse
5′-GCAGCCATAGAAGGTGTTCAGG-3′. The MMP-9 primers were forward
5′-TGGTCCTGGTGCTCCTGGTG-3′ and reverse 5′-GCTGCCTGTCGGTGAGATTGG-3′.
The GAPDH primers were forward 5′-ACAACTTTGGTATCGTGGAAGG-3′
and reverse 5′-GCCATCACGCCACAGTTTC-3′. The amplification steps were
as follows: 94°C for 5 min, 30 cycles of denaturation for 45 sec at
95°C, 45 sec of annealing at 60°C, elongation at 72°C for 45 sec,
and extension at 72°C for 1 min. After amplification, the PCR
products were electrophoresed on a 1.5% agarose gel and visualized
by ethidium bromide staining.
Quantitative real-time PCR
(qRT-PCR)
cDNA was obtain as described above. PCR primers used
for the analysis are listed above. The 20 µl PCR system was
composed of 2 µl cDNA, 10 µl SYBR Premix Ex Taq II, 0.4 µl ROX
Reference Dye II, 0.8 µl 10 µM forward primer, 0.8 µl 10 µM reverse
primer and 6 µl ddH2O. The qRT-PCR amplification was
performed under the following conditions: 1 cycle of initial
denaturation for 30 sec at 95°C, 40 cycles of denaturation for 5
sec at 95°C, followed by DNA synthesis for 30 sec at 60°C. After
amplification, real-time data acquisition and analysis were
performed.
Western blot analysis
After treated with different compounds, cells were
harvested at indicated time, washed, and lysed in ice-cold lysis
buffer. The cell lysates were sonicated and centrifuged at 12,000 ×
g at 4°C for 5 min. The protein concentration of extracts was
determined by using the Bradford reagent from Bio-Rad Laboratories.
Equal amounts of protein (20 µg/lane) were resolved with sodium
dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to nitrocellulose membranes (Millipore, Bedford, MA,
USA). Next, the membrane was blocked in 5% non-fat dry milk in
Tris-buffered saline with Tween-20 for 2 h at room temperature and
then incubated with different primary antibodies overnight at 4°C.
HRP-conjugated secondary antibodies (1:20,000) were used
subsequently for 2 h at room temperature. Immunoreactive bands were
visualized by enhanced chemiluminescence (Pierce, Rockford, IL,
USA) and quantified by using ImageJ software (National Institutes
of Health, Bethesda, MD, USA).
MTT assay
Cell viability was assessed by MTT assay. Briefly,
cells were seeded in a 48-well flat-bottomed plate
(3×104 cells per well) and cultured overnight. Then, the
cells were starved overnight and treated with different drugs as
indicated later. For dose course experiment, the cells were treated
with concentrations of harmine and incubated 48 h. For time course
experiment, the cells were incubated with 10 µM harmine for 12, 24,
36, and 48 h. After incubation, 20 µl MTT (5 mg/ml) was added to
each well, and the cells were allowed to grow in complete media at
37°C for 3 h. The supernatant was removed, then 500 µl DMSO was
added to each well and swung for 10 min to dissolve the crystal.
Subsequently, and absorbance was determined by using a microplate
spectrophotometer assay reader at 570 nm.
Wound healing assay
SKOV-3 cells were seeded in 6-well plate and
cultured to a confluent monolayer. The cells were pretreated with
hydroxyurea (5 mmol/l) to prevent cell proliferation. A pipette tip
(200 µl) was used to scratch a wound on the midline of each well
and the cells were then washed twice with PBS. Following 0, 12, 24,
36 h of culture in RPMI-1640 supplemented with 10% serum (control)
or harmine stimulants, the migration of the cells was evaluated by
measuring the difference in the area of the wounds with a Leica
DM2500 image analysis system (Leica, Mannheim, Germany).
Colony formation assay
SKOV-3 cells in the logarithmic growth phase were
seeded in a 6-well plate and cultured in RPMI-1640 medium
supplemented with 10% FBS overnight. Then, the cells were treated
with the indicated concentrations of harmine for another two weeks.
Then, the supernatants were discarded, and the cells were carefully
washed three times with phosphate-buffered saline (PBS).
Subsequently, cells were fixed with a fixative composed of
methanol-glacial acetic acid (3:1) for 10 min. Finally, the cells
were stained in crystal violet for 20 min. Images of the colonies
were captured by a digital camera.
Statistical analysis
Data are indicated as mean ± SEM from at least 3
independent experiments. Statistical analysis was performed using
the Student's t-test (comparison of two groups) or one-way analysis
of variance (ANOVA) followed by the Dunnett's or Tukey's test
(comparison of more than two groups). A value of p<0.05 was
considered statistically different. All statistical analyses were
conducted using Prism version 6.0 (GraphPad Software).
Results
Harmine inhibits the basal cell
proliferation level in SKOV-3 cells
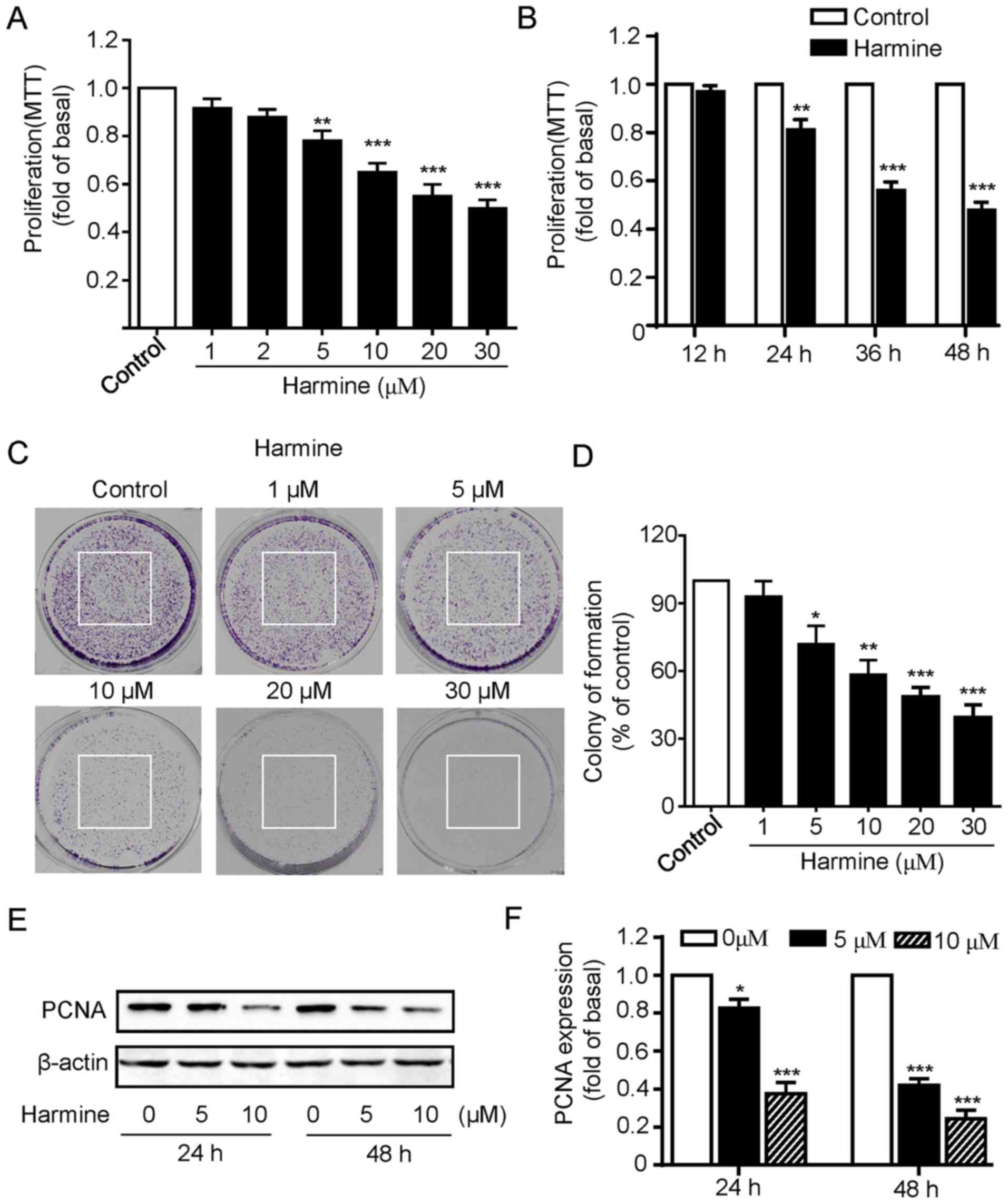
We first investigated the influence of harmine on
the proliferation of SKOV-3 cells. The SKOV-3 cells were incubated
with various concentration of harmine (0, 1, 2, 5, 10, 20 and 30
µM) for 48 h and then cell viability was measured by MTT assay. As
shown in Fig. 1A, harmine markedly
inhibited the growth of SKOV-3 cells in a dose-dependent manner as
concentration of less than 2 µM had little effect while
concentrations of more than 5 µM progressively inhibited the growth
of SKOV-3 cells (Fig. 1A). We next
explored the effects of harmine at different treatment times on
SKOV-3 cell viability, the cells were incubated with 10 µM harmine
for 12, 24, 36 or 48 h. Cell viability assays revealed that harmine
significantly inhibited SKOV-3 cell growth except 12 h incubation,
with the strongest effect at 48 h (Fig.
1B).
To further confirm the inhibitory effect of cell
proliferation of harmine, colony formation assays were performed.
As shown in Fig. 1C, consistent
with the MTT assay, harmine significantly suppressed the colony
formation of SKOV-3 cells in a dose-dependent manner (Fig. 1C and D). To provide additional
evidence, we then detected the expression of proliferating cell
nuclear antigen (PCNA), a protein marker that has been widely used
for indicating cell proliferation. We found that the expression of
PCNA was significantly reduced by harmine treatment for either 24
or 48 h (Fig. 1E and F). Taken
together, our above results strongly indicated that harmine showed
significant inhibitory effect toward the cell growth in SKOV-3
cells.
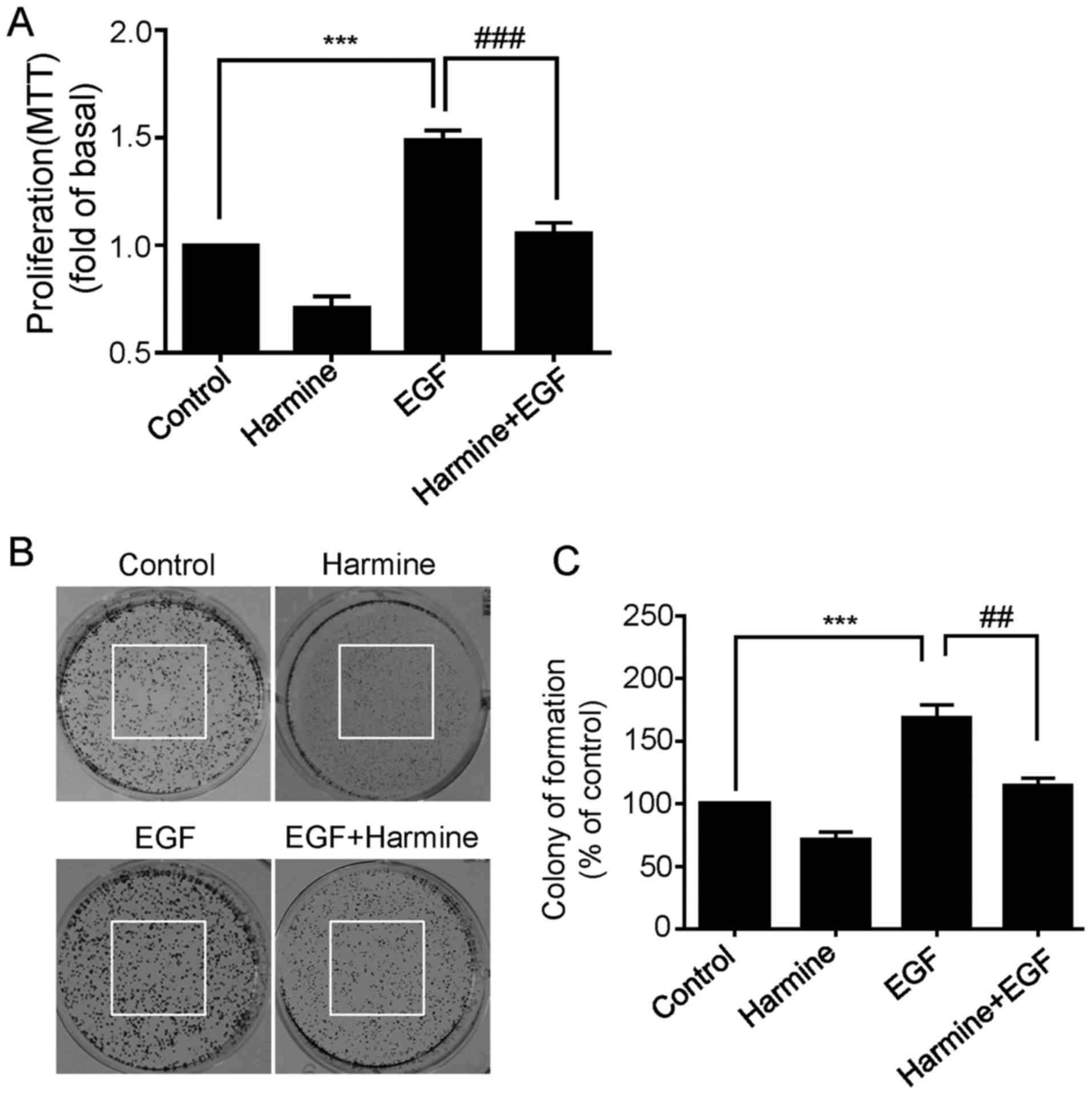
Harmine inhibits EGF-induced
proliferation of SKOV-3 cells
It is widely known that epidermal growth factor
receptor (EGFR) is often overexpressed in numerous human cancers
including ovarian cancer, and activation of EGFR is known to be
involved in the growth and progression of a variety of malignancies
(12,13). We next asked whether harmine was
able to inhibit EGF-induced cell proliferation of SKOV-3 cells. To
do this, the cells were treated with harmine in the absence or
presence of EGF (50 ng/ml) for 48 h and then cell viability was
measured by MTT assay. As shown in Fig.
2A, EGF significantly increased the proliferation of SKOV-3
cells, while this EGF-enhanced proliferation was remarkably
suppressed by harmine treatment (Fig.
2A). Consistent with the previously results, harmine not only
inhibited EGF-enhanced proliferation, but also inhibited basal
proliferation level of the SKOV-3 cells in the absence of EGF
(Fig. 2A). Similar results were
observed by colony formation assay. As expected, harmine
significantly inhibited EGF-induced colony formation capacity
(Fig. 2B and C). These results
further confirmed that harmine could obviously inhibit ovarian
cancer cell proliferation.
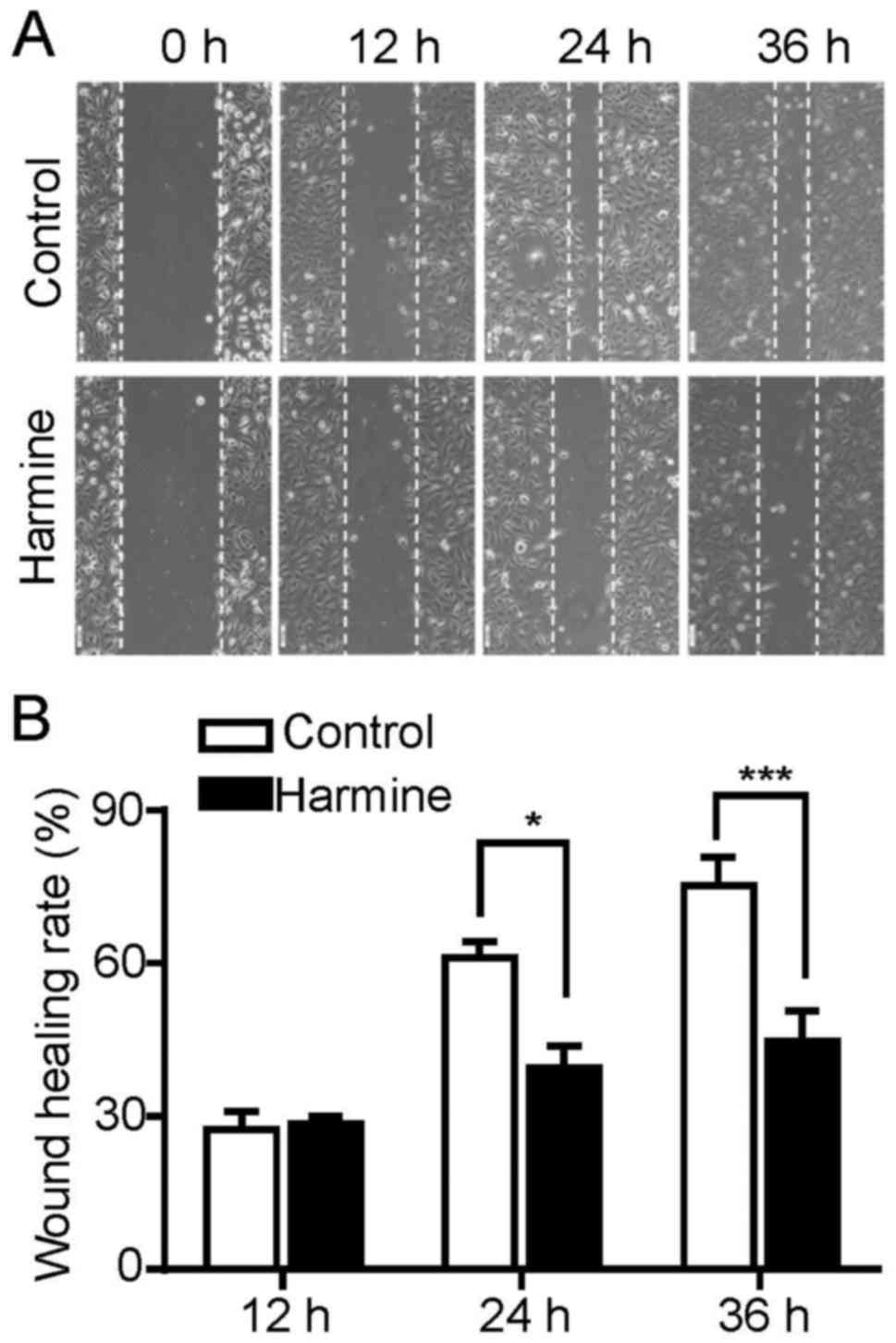
Harmine significantly suppresses the
migration of SKOV-3 cells
Cell migration plays an important role during cancer
progression. To explore the effect of harmine on the migration of
SKOV-3 cells, a typical scratch wound healing assay was performed
to measure the migration ratio. As our above results indicated that
harmine was able to inhibit the proliferation of SKOV-3 cells, this
may interfere with the analysis of cell migration results. Thus, to
exclude the proliferation inhibitory effect of harmine on
migration, the wound healing assay were performed in the presence
of hydroxyurea which prevents cell proliferation by inhibiting the
DNA synthesis. As shown in Fig. 3,
harmine significantly inhibited the migration of SKOV-3 cells at 24
and 36 h after treatment while had little effect at 12 h (Fig. 3A and B).
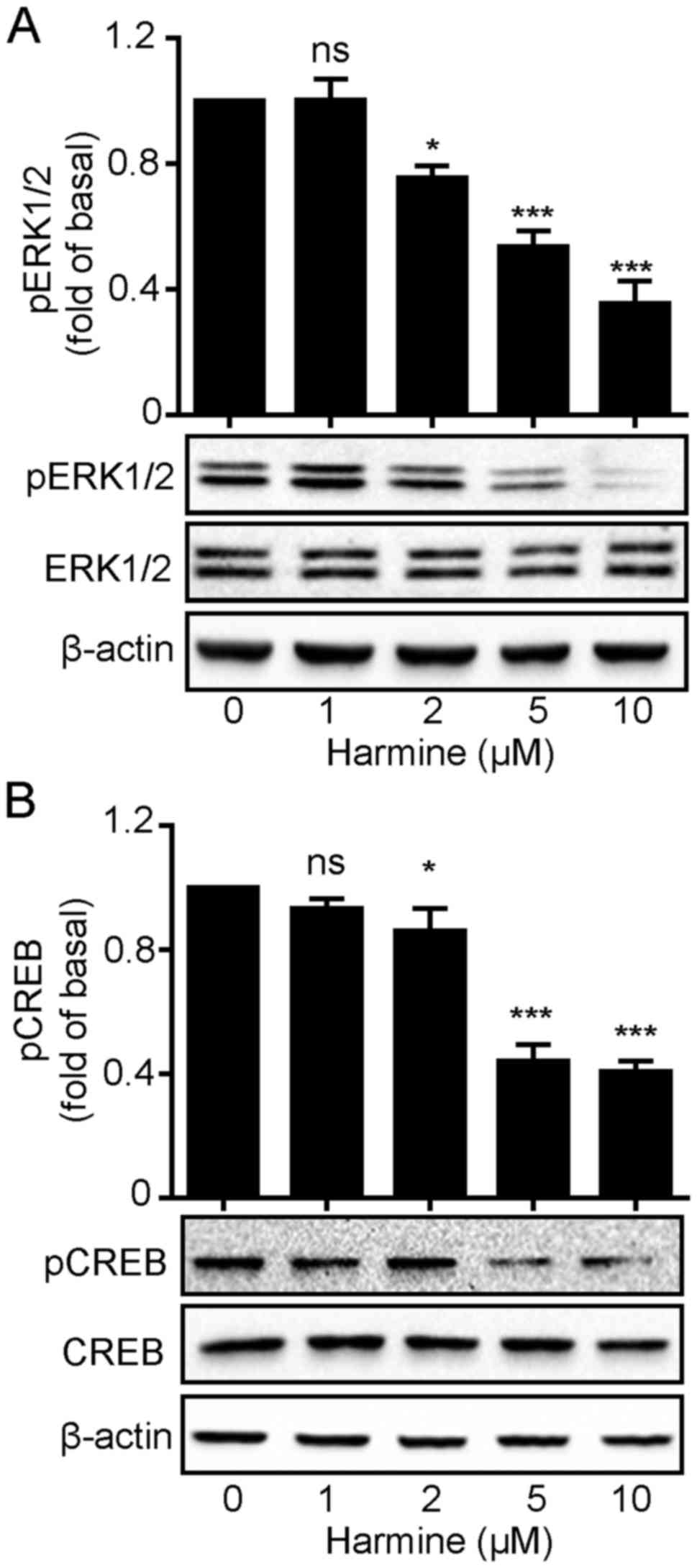
Harmine blocks ERK1/2 and
CREB phosphorylation in SKOV-3 cells
We demonstrated that harmine significantly
suppressed SKOV-3 cell growth. This raised the question how harmine
acts to inhibit SKOV-3 cell growth. Mitogen-activated protein
kinase (MAPK) pathways constitute a large modular network that
regulates a variety of physiological processes including cell
growth and differentiation. Thus, we firstly monitored
ERK1/2 phosphorylation levels following harmine
treatment. As shown in Fig. 4,
harmine caused a rapid decrease in the phosphorylation level of
ERK1/2 with no changes in total ERK1/2
expression levels (Fig. 4A).
Similarly, the phosphorylation level of CREB, an important
transcription factor that plays important roles in cell
proliferation, was also decreased in the same manner as
ERK1/2 (Fig. 4B), which
might suggest that harmine inhibited ERK1/2
phosphorylation which in turn suppressed CREB activation.
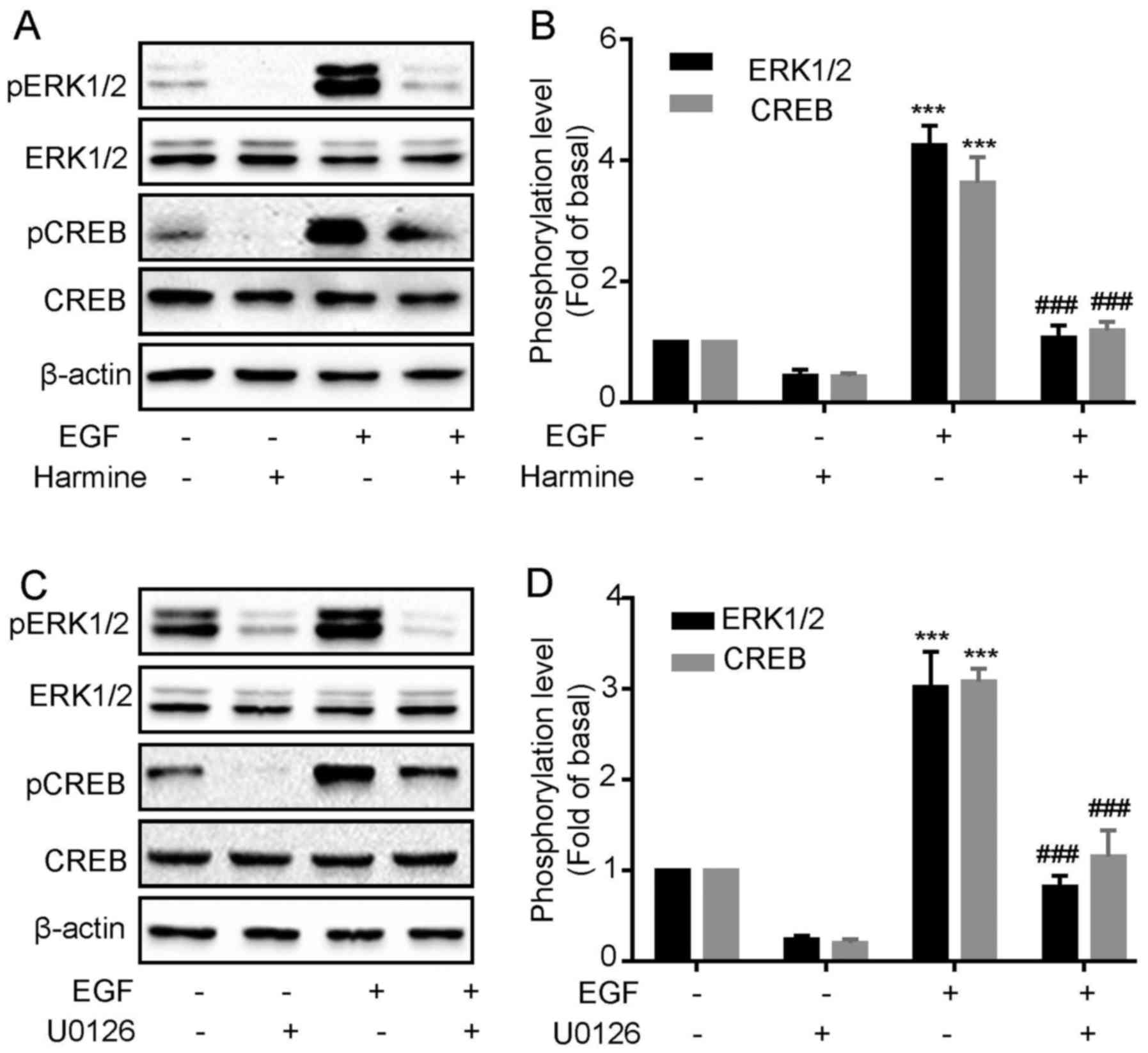
Harmine inhibits EGF-induced
ERK1/2 and CREB phosphorylation in SKOV-3 cells
Harmine significantly inhibited the cell
proliferation and ERK1/2 and CREB phosphorylation in
SKOV-3 cells, however, we do not know whether the inhibitory effect
of harmine on cell proliferation was mediated by
ERK1/2/CREB pathway. To verify this, we next
investigated whether harmine could block EGF-induced
ERK1/2 and CREB phosphorylation. As shown in Fig. 5, we first confirmed that EGF
treatment significantly increased the phosphorylation of
ERK1/2 and CREB (Fig. 5A and
B). However, both EGF-induced ERK1/2 and CREB
phosphorylation were remarkably inhibited by harmine treatment
(Fig. 5A and B). To demonstrate
that CREB phosphorylation was downstream of ERK1/2, we
evaluated the effect of the EKR1/2 selective inhibitor
on EGF-induced CREB phosphorylation in SKOV-3 cells. Cells were
pretreated for 30 min with U0126 (10 µM) and then stimulated with
EGF. We found that U0126 blocked EGF-induced ERK1/2 and
CREB (Fig. 5C and D)
phosphorylation, suggesting CREB acted as a downstream factor of
ERK1/2.
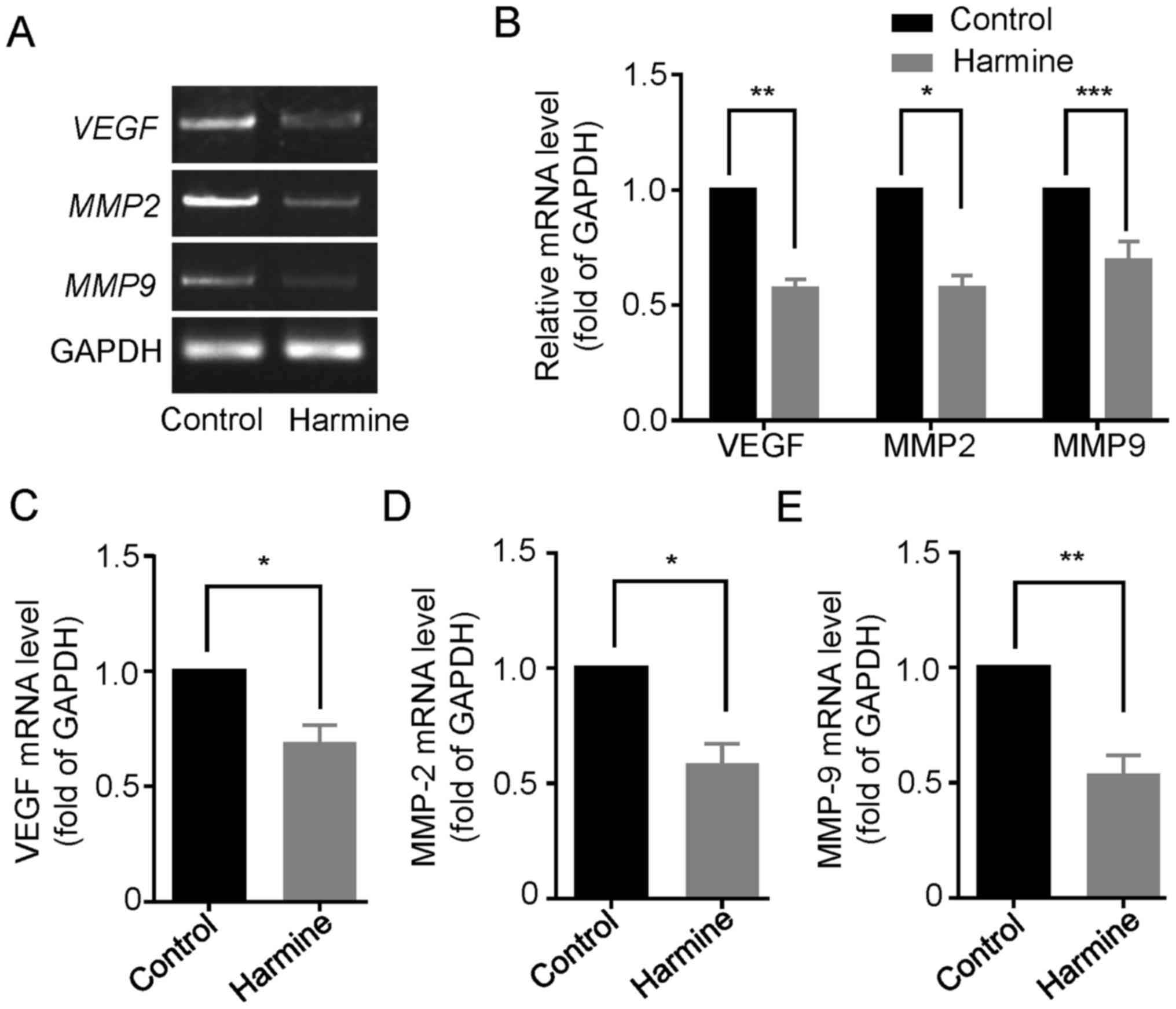
Harmine reduces the expression of
VEGF, MMP-2 and MMP-9 in SKOV-3 cells
Since vascular endothelial growth factor (VEGF) and
matrix metalloproteinases (MMPs), particularly the gelatinases
MMP-2 and MMP-9, have been shown to play important roles in the
proliferation, migration and metastasis of many primary epithelial
tumors (14,15), we then examined the effect of
harmine on the expression of VEGF, MMP-2 and MMP-9 in
SKOV-3 cells. As shown in Fig. 6A,
reverse transcription-PCR analysis showed that the gene
transcription of VEGF, MMP-2 and MMP-9 were
significant downregulated in the presence of harmine (10 µM) for 48
h (Fig. 6A and B). Consistently,
quantitative real-time PCR analysis also indicated that harmine
markedly suppressed the mRNA level of VEGF (Fig. 6C), MMP-2 (Fig. 6D) and MMP-9 (Fig. 6E).
Discussion
In the present study, we investigated the antitumor
effect and potential mechanism of harmine on human ovarian cancer
cells. Our results showed that harmine not only significantly
inhibited the proliferation and migration of SKOV-3 cells, but also
suppressed the EGF-induced proliferation of SKOV-3 cells. Moreover,
this antitumor effect of harmine might be through inhibiting the
EKR1/2/CREB pathway and downregulation the expression of
VEGF and MMPs.
Many natural alkaloids such as camptothecin,
vincristine and ellipticine are found to be potent anticancer
agents in recent years (16). These
alkaloids kill tumor cells by different biochemical modes of
action, such as inhibition of apoptosis, inhibition of
topoisomerase I and II and inhibition of microtubule formation.
However, the molecular mechanisms for the antitumor activity of
β-carbolines are not fully defined. Of note, it is reported that
some β-carbolines and their related compounds appear to have
cytotoxic effects on several cell lines including cancer cells and
cerebellar granule neurons. Though cytotoxic agents which result in
cell death and eventually tumor shrinkage are conventional
anticancer drugs and still the most widely used around the world
(17), the non-cytotoxic,
molecularly targeted drugs that inhibit tumor growth without direct
cytotoxicity is of greatest concern (18). In our study, the inhibitory effect
of cell growth in the presence of harmine may result from two
possible reasons, from the unspecific cytotoxic effect of harmine
that killed the cancer cells acutely, or that harmine may suppress
the proliferation of SKOV-3 cells without inducing acute cellular
damage. To discern the later from the former, we measured the cell
viability of SKOV-3 cells treated with or without 10 µM harmine for
12 h. Our results showed that there was no significant difference
between the cell viability of control cells and harmine-treated
cells. On the other hand, a recent study indicated that treatment
with harmine increased proliferation of cultured neural progenitor
cells without DNA damage or cell death (19). Moreover, harmine was found to
promote cell proliferation as exposure of chick embryos to harmine
markedly increased BrdU incorporation and number of mitotic cells
in the spinal cord (20). Together
with our results, one can speculate that the inhibition of the
proliferation of SKOV-3 cells by harmine may not be due to its
unspecific cytotoxic effect.
Our results showed that harmine inhibited the basal
proliferation level of SKOV-3 cells. Noteworthy, it also
significantly suppressed EGF-induced proliferation of SKOV-3 cells,
suggesting an ideal antitumor effect of harmine. Aberrant
expression and signaling of the EGFR-mediated pathway are
associated with various human cancers including breast, colon, head
and neck, pancreatic, lung and ovarian cancers. Undoubtedly, EGFR
and its family members have emerged as the most useful biomarkers
and rational target for cancer therapy (21). It is worth noting that EGFR has been
reported to be overexpressed in up to 60% of ovarian cancers
(22), while increased expression
and activation of EGFR is related to high cell proliferation index,
high migration and stromal invasion rate (23,24).
This may explain, to some extent, why harmine inhibits the basal
proliferation level of SKOV-3 cells. However, how harmine affect
EGFR signaling pathway needs to be further investigated.
Mitogen-activated protein kinase (MAPK) pathways
such as ERK1/2 play an important role in the regulation
of proliferation and migration of cancer cells. Consistent with the
results showing harmine inhibited the proliferation of SKOV-3
cells, the phosphorylation of ERK1/2 was profoundly
blocked by harmine treatment. Moreover, harmine also blocked the
CREB phosphorylation which was reported to suppress apoptosis,
induce cell proliferation, migration and mediate tumor metastasis
(25). Since the inhibition manner
of CREB phosphorylation was quite in accordance with
ERK1/2, suggesting that CREB acts downstream of
ERK1/2. This hypothesis was confirmed by the following
data. First, we observed that the phosphorylation of
ERK1/2 and CREB mediated by EGF were markedly inhibited
in the presence of harmine. Second, the EGF-induced CREB
phosphorylation can be significantly blocked by U0126, an inhibitor
widely used to block ERK1/2 activity, which indicates
EGF-induced CREB phosphorylation is mediated by ERK1/2.
Thus, we conclude that inhibition of MEK/ERK pathway which in turn
blocked CREB activity is involved in harmine's suppressive effect
on EGF-induced cell proliferation in human ovarian cancer
cells.
Degradation of extracellular matrix (ECM) and
basement membranes by the tumor cells is a critical step in the
processes of tumor invasion and metastasis (26). Matrix metalloproteinases (MMPs) are
a family of proteolytic enzymes that regulate various cell
behaviors with relevance to cancer biology through degradation of
extracellular matrix surrounding tumors (14). In our study, we found that harmine
can inhibit the expression of MMP-2 and MMP-9. Early
adhesion and metastasis of ovarian cancer cells are mediated by the
MMP family of proteins, the expression of which is upregulated
during ovarian cancer progression (27). Though MMPs have been considered to
be closely correlated with tumor invasion and metastasis (27), studies also show that inhibition of
the activities of MMP-2 and MMP-9 can result in
reduced ECM remodeling and suppression of endothelial cell
proliferation and migration (28).
Moreover, consistent with our results, a recent study indicates
that activation of ERK1/2/CREB pathway promotes the
expression of MMP-2 and MMP-9 which may account for
lung cancer cell proliferation (29). On the other hand, our results also
showed that harmine can inhibit the expression of VEGF.
Since VEGF is the most important mitogenic and survival factors
involved in angiogenesis while angiogenesis is pre-requisite for
the growth of solid tumors, vascular targeting has been explored as
a potential strategy to suppress tumor growth and metastasis. Thus,
this result further confirmed the antitumor effect of harmine.
However, whether harmine is able to inhibit angiogenesis in the
development of a solid tumor needs further study.
Acknowledgements
The present study was supported by grants from the
Traditional Chinese Medicine Research (no. 2016B032), Science and
Technology Planning Project (no. 20175032) of Jiangxi Provincial
Health and Family Planning Commission, the Guiding Science and
Technology Program of Nanchang (no. 2016-ZDXXM-039).
Glossary
Abbreviations
Abbreviations:
|
CREB
|
cyclic adenosine monophosphate
response element-binding protein
|
|
ECM
|
extracellular matrix
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
epithelial growth factor receptor
|
|
ERK1/2
|
extracellular signal regulated kinase
1/2
|
|
MAPK
|
mitogen activated protein kinase
|
|
MMP
|
matrix metalloproteinase
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukherjee AK, Basu S, Sarkar N and Ghosh
AC: Advances in cancer therapy with plant based natural products.
Curr Med Chem. 8:1467–1486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McChesney JD: Natural products in drug
discovery - organizing for success. P R Health Sci J. 21:91–95.
2002.PubMed/NCBI
|
|
5
|
Cragg GM, Newman DJ and Snader KM: Natural
products in drug discovery and development. J Nat Prod. 60:52–60.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao R, Peng W, Wang Z and Xu A:
beta-Carboline alkaloids: Biochemical and pharmacological
functions. Curr Med Chem. 14:479–500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel K, Gadewar M, Tripathi R, Prasad SK
and Patel DK: A review on medicinal importance, pharmacological
activity and bioanalytical aspects of beta-carboline alkaloid
‘Harmine’. Asian Pac J Trop Biomed. 2:660–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farzin D and Mansouri N:
Antidepressant-like effect of harmane and other beta-carbolines in
the mouse forced swim test. Eur Neuropsychopharmacol. 16:324–328.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao MR, Li Q, Liu ZL, Liu HH, Wang W, Liao
XL, Pan YL and Jiang JW: Harmine induces apoptosis in HepG2 cells
via mitochondrial signaling pathway. Hepatobiliary Pancreat Dis
Int. 10:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamsa TP and Kuttan G: Harmine activates
intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma.
Chin Med. 6:112011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Huang CR, Wang W, Zhang XK, Chen
JJ, Wang JJ, Lin C and Jiang JW: Harmine hydrochloride triggers G2
phase arrest and apoptosis in MGC-803 cells and SMMC-7721 cells by
upregulating p21, activating caspase-8/Bid, and downregulating
ERK/Bad pathway. Phytother Res. 30:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lafky JM, Wilken JA, Baron AT and Maihle
NJ: Clinical implications of the ErbB/epidermal growth factor (EGF)
receptor family and its ligands in ovarian cancer. Biochim Biophys
Acta. 1785:232–265. 2008.PubMed/NCBI
|
|
13
|
Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X,
Fang J and Jiang BH: Reactive oxygen species regulate epidermal
growth factor-induced vascular endothelial growth factor and
hypoxia-inducible factor-1alpha expression through activation of
AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med.
41:1521–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Newby AC: Matrix metalloproteinases
regulate migration, proliferation, and death of vascular smooth
muscle cells by degrading matrix and non-matrix substrates.
Cardiovasc Res. 69:614–624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bernatchez PN, Soker S and Sirois MG:
Vascular endothelial growth factor effect on endothelial cell
proliferation, migration, and platelet-activating factor synthesis
is Flk-1-dependent. J Biol Chem. 274:31047–31054. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kummar S, Gutierrez M, Doroshow JH and
Murgo AJ: Drug development in oncology: Classical cytotoxics and
molecularly targeted agents. Br J Clin Pharmacol. 62:15–26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fox E, Curt GA and Balis FM: Clinical
trial design for target-based therapy. Oncologist. 7:401–409. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dakic V, Maciel RM, Drummond H, Nascimento
JM, Trindade P and Rehen SK: Harmine stimulates proliferation of
human neural progenitors. PeerJ. 4:e27272016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hämmerle B, Ulin E, Guimera J, Becker W,
Guillemot F and Tejedor FJ: Transient expression of Mnb/Dyrk1a
couples cell cycle exit and differentiation of neuronal precursors
by inducing p27KIP1 expression and suppressing NOTCH signaling.
Development. 138:2543–2554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimova I, Zaharieva B, Raitcheva S,
Dimitrov R, Doganov N and Toncheva D: Tissue microarray analysis of
EGFR and erbB2 copy number changes in ovarian tumors. Int J Gynecol
Cancer. 16:145–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dancey JE and Freidlin B: Targeting
epidermal growth factor receptor - are we missing the mark? Lancet.
362:62–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lassus H, Sihto H, Leminen A, Joensuu H,
Isola J, Nupponen NN and Butzow R: Gene amplification, mutation,
and protein expression of EGFR and mutations of ERBB2 in serous
ovarian carcinoma. J Mol Med (Berl). 84:671–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal S, Kim SW, Ryu SH, Chung WC and
Koo JS: Growth suppression of lung cancer cells by targeting cyclic
AMP response element-binding protein. Cancer Res. 68:981–988. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomez DE, Alonso DF, Yoshiji H and
Thorgeirsson UP: Tissue inhibitors of metalloproteinases:
Structure, regulation and biological functions. Eur J Cell Biol.
74:111–122. 1997.PubMed/NCBI
|
|
29
|
Hu P, He J, Liu S, Wang M, Pan B and Zhang
W: β2-adrenergic receptor activation promotes the proliferation of
A549 lung cancer cells via the ERK1/2/CREB pathway. Oncol Rep.
36:1757–1763. 2016. View Article : Google Scholar : PubMed/NCBI
|