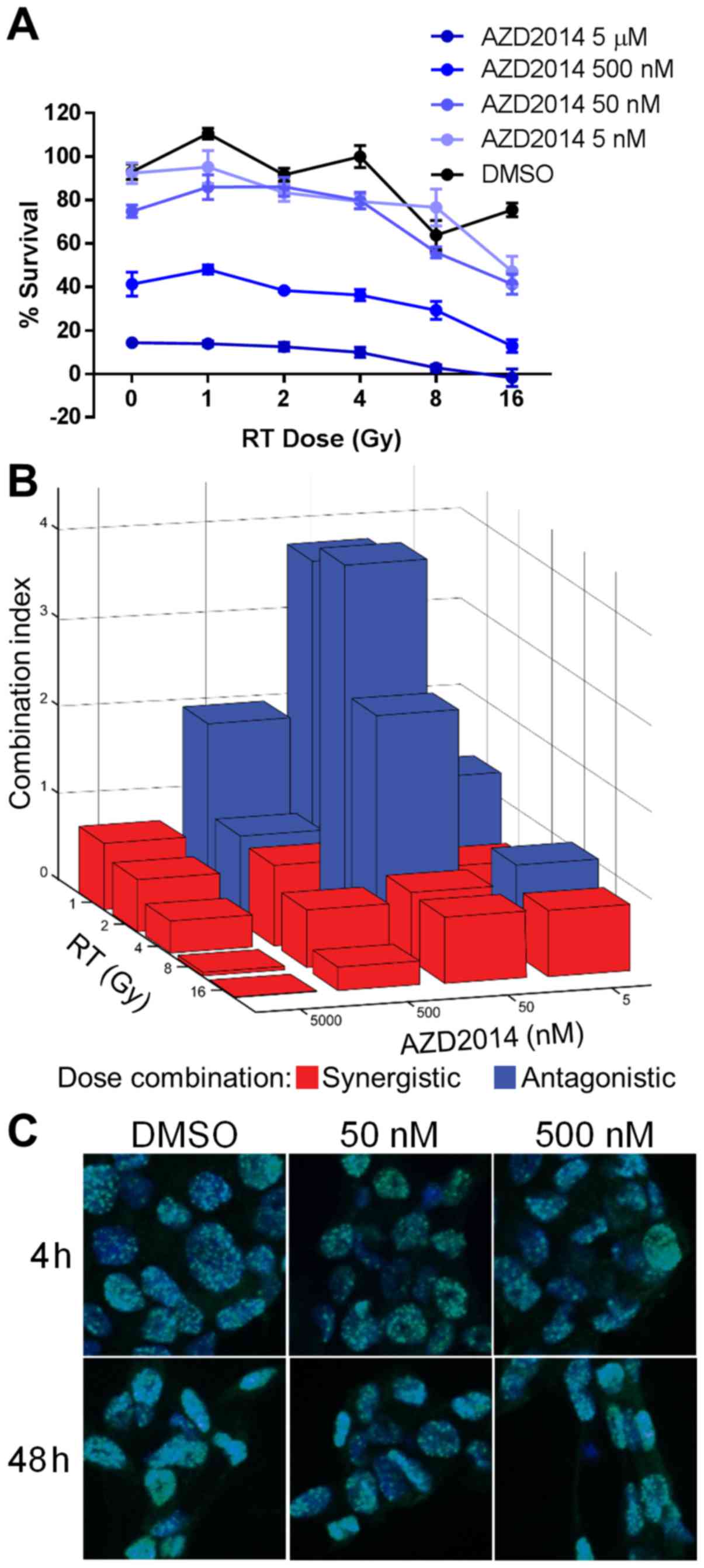
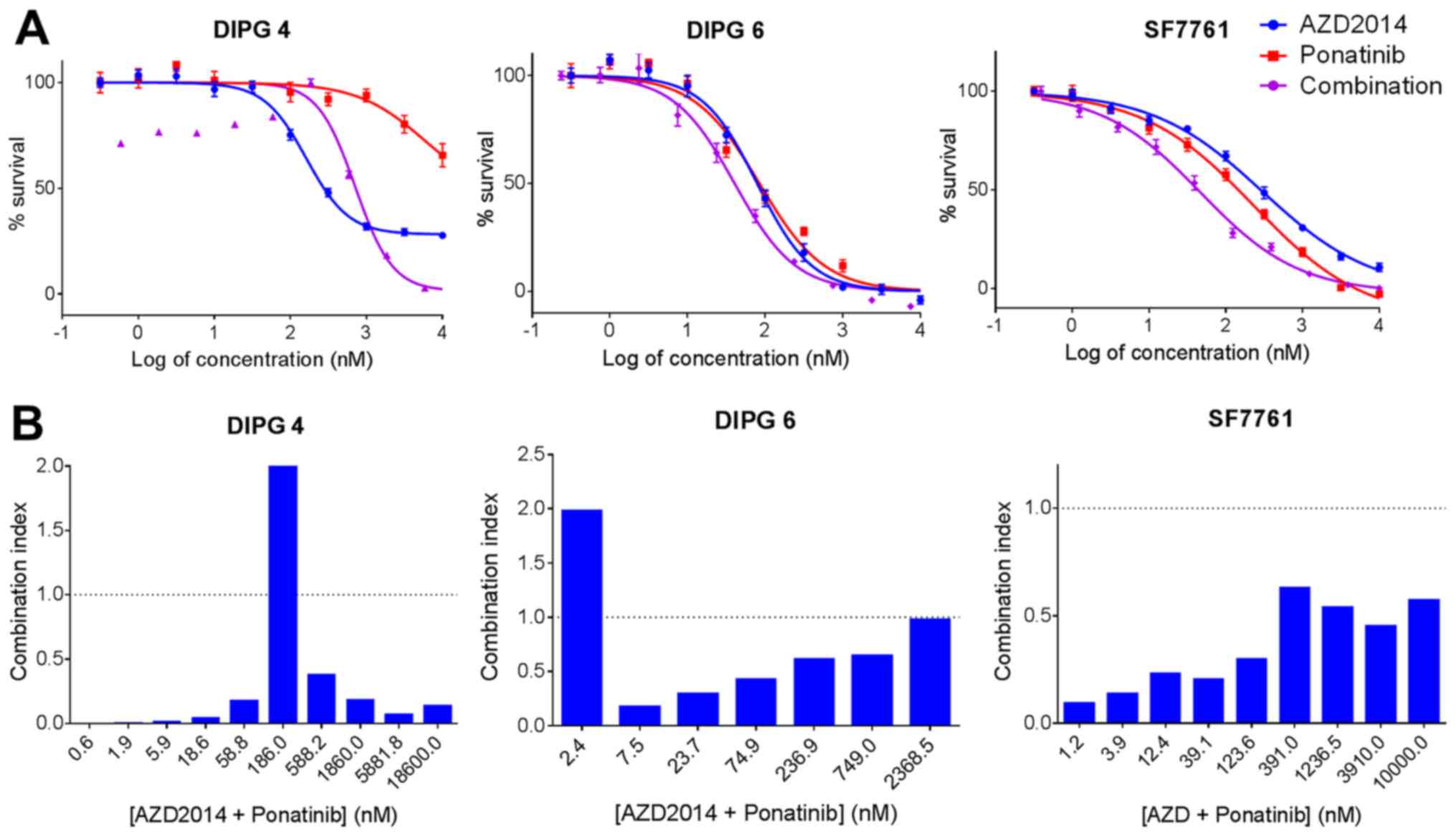
|
1
|
Fangusaro J: Pediatric high-grade gliomas
and diffuse intrinsic pontine gliomas. J Child Neurol.
24:1409–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massimino M, Spreafico F, Biassoni V,
Simonetti F, Riva D, Trecate G, Giombini S, Poggi G, Pecori E,
Pignoli E, et al: Diffuse pontine gliomas in children: Changing
strategies, changing results? A mono-institutional 20-year
experience. J Neurooncol. 87:355–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green AL and Kieran MW: Pediatric
brainstem gliomas: New understanding leads to potential new
treatments for two very different tumors. Curr Oncol Rep.
17:4362015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashizume R, Ozawa T, Gryaznov SM, Bollen
AW, Lamborn KR, Frey WH II and Deen DF: New therapeutic approach
for brain tumors: Intranasal delivery of telomerase inhibitor
GRN163. Neuro-oncol. 10:112–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodwin CR, Xu R, Iyer R, Sankey EW, Liu
A, Abu-Bonsrah N, Sarabia-Estrada R, Frazier JL, Sciubba DM and
Jallo GI: Local delivery methods of therapeutic agents in the
treatment of diffuse intrinsic brainstem gliomas. Clin Neurol
Neurosurg. 142:120–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barua NU, Lowis SP, Woolley M, O'Sullivan
S, Harrison R and Gill SS: Robot-guided convection-enhanced
delivery of carboplatin for advanced brainstem glioma. Acta
Neurochir (Wien). 155:1459–1465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen K, Jones A, Raabe E and Pearl M:
Highly selective intra-arterial chemotherapy for the treatment of
progressive diffuse intrinsic pontine gliomas (DIPG). In: 20th
International Conference on Brain Tumor Research and Therapy
Neuro-Oncology, Lake Tahoe, CA. Neurooncology. 16:iii292014.
|
|
8
|
Zeng Z, Sarbassov D, Samudio IJ, Yee KW,
Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M and
Konopleva M: Rapamycin derivatives reduce mTORC2 signaling and
inhibit AKT activation in AML. Blood. 109:3509–3512. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guertin DA, Stevens DM, Thoreen CC, Burds
AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ and Sabatini DM:
Ablation in mice of the mTORC components raptor, rictor, or mlST8
reveals that mTORC2 is required for signaling to Akt-FOXO and
PKCalpha, but not S6K1. Dev Cell. 11:859–871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akhavan D, Cloughesy TF and Mischel PS:
mTOR signaling in glioblastoma: Lessons learned from bench to
bedside. Neuro-oncol. 12:882–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galanis E, Buckner JC, Maurer MJ,
Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR,
Morton RF, et al North Central Cancer Treatment Group, : Phase II
trial of temsirolimus (CCI-779) in recurrent glioblastoma
multiforme: A North Central Cancer Treatment Group Study. J Clin
Oncol. 23:5294–5304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kahn J, Hayman TJ, Jamal M, Rath BH, Kramp
T, Camphausen K and Tofilon PJ: The mTORC1/mTORC2 inhibitor AZD2014
enhances the radiosensitivity of glioblastoma stem-like cells.
Neuro-oncol. 16:29–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwartzentruber J, Korshunov A, Liu XY,
Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA,
Tönjes M, et al: Driver mutations in histone H3.3 and chromatin
remodelling genes in paediatric glioblastoma. Nature. 482:226–231.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buczkowicz P, Hoeman C, Rakopoulos P,
Pajovic S, Letourneau L, Dzamba M, Morrison A, Lewis P, Bouffet E,
Bartels U, et al: Genomic analysis of diffuse intrinsic pontine
gliomas identifies three molecular subgroups and recurrent
activating ACVR1 mutations. Nat Genet. 46:451–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehta S, Huillard E, Kesari S, Maire CL,
Golebiowski D, Harrington EP, Alberta JA, Kane MF, Theisen M, Ligon
KL, et al: The central nervous system-restricted transcription
factor Olig2 opposes p53 responses to genotoxic damage in neural
progenitors and malignant glioma. Cancer Cell. 19:359–371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagaraja S, Vitanza NA, Woo PJ, Taylor KR,
Liu F, Zhang L, Li M, Meng W, Ponnuswami A, Sun W, et al:
Transcriptional dependencies in diffuse intrinsic pontine glioma.
Cancer Cell. 31:635–652 e636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodrik-Outmezguine VS, Chandarlapaty S,
Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J,
Guichard S and Rosen N: mTOR kinase inhibition causes
feedback-dependent biphasic regulation of AKT signaling. Cancer
Discov. 1:248–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bahena-Ocampo I, Espinosa M,
Ceballos-Cancino G, Lizarraga F, Campos-Arroyo D, Schwarz A,
Garcia-Lopez P, Maldonado V and Melendez-Zajgla J: miR-10b
expression in breast cancer stem cells supports self-renewal
through negative PTEN regulation and sustained AKT activation. EMBO
Rep. 17:10812016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X,
Zhou L, Liu C, Chen C, He J, et al: Reciprocal regulation of Akt
and Oct4 promotes the self-renewal and survival of embryonal
carcinoma cells. Mol Cell. 48:627–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paugh BS, Broniscer A, Qu C, Miller CP,
Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS,
et al: Genome-wide analyses identify recurrent amplifications of
receptor tyrosine kinases and cell-cycle regulatory genes in
diffuse intrinsic pontine glioma. J Clin Oncol. 29:3999–4006. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Halvorson KG, Barton KL, Schroeder K,
Misuraca KL, Hoeman C, Chung A, Crabtree DM, Cordero FJ, Singh R,
Spasojevic I, et al: A high-throughput in vitro drug screen in a
genetically engineered mouse model of diffuse intrinsic pontine
glioma identifies BMS-754807 as a promising therapeutic agent. PLoS
One. 10:e01189262015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fontanilla HP, Pinnix CC, Ketonen LM, Woo
SY, Vats TS, Rytting ME, Wolff JE and Mahajan A: Palliative
reirradiation for progressive diffuse intrinsic pontine glioma. Am
J Clin Oncol. 35:51–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolff JE, Rytting ME, Vats TS, Zage PE,
Ater JL, Woo S, Kuttesch J, Ketonen L and Mahajan A: Treatment of
recurrent diffuse intrinsic pontine glioma: The MD Anderson Cancer
Center experience. J Neurooncol. 106:391–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frankfurt O and Licht JD: Ponatinib - a
step forward in overcoming resistance in chronic myeloid leukemia.
Clin Cancer Res. 19:5828–5834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jansen MH, van Vuurden DG, Vandertop WP
and Kaspers GJ: Diffuse intrinsic pontine gliomas: A systematic
update on clinical trials and biology. Cancer Treat Rev. 38:27–35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim DH, Kwak Y, Kim ND and Sim T:
Antitumor effects and molecular mechanisms of ponatinib on
endometrial cancer cells harboring activating FGFR2 mutations.
Cancer Biol Ther. 17:65–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyahara H, Yadavilli S, Natsumeda M,
Rubens JA, Rodgers L, Kambhampati M, Taylor IC, Kaur H, Asnaghi L,
Eberhart CG, et al: The dual mTOR kinase inhibitor TAK228 inhibits
tumorigenicity and enhances radiosensitization in diffuse intrinsic
pontine glioma. Cancer Lett. 400:110–116. 2017. View Article : Google Scholar : PubMed/NCBI
|