Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies, and is the second leading cause of
cancer-related death worldwide (1).
HCC accounts for 91% of primary liver cancer cases and presents
poor prognosis with an overall 5-year survival rate of <5%
(2). Like other malignant tumors,
the metastasis and recurrence of HCC has become one of the major
obstacles to the therapeutic treatment of HCC.
Although blood vessels are considered as a main
route of HCC metastasis, vasculogenic mimicry (VM) has been
certified to be a potential bypass for metastasis. VM is a
microvascular channel which occurs de novo without the
presence of endothelial cells (3).
In VM, tumor cells arrange in lines to form vessel-like structure
effectively mimicking a true vascular endothelium, which provide
tumors with blood perfusion and promote tumor metastasis (4,5). VM
has been reported in HCC (6) and
other types of tumors including ovarian cancer (7), melanoma (8), osteosarcoma (9) and prostatic cancer (10).
VM is associated with poor patient prognosis and is
considered as a loophole for antiangiogenesis therapy. Currently,
antiangiogenic therapy mainly targets endothelium-dependent
angiogenesis. Several antiangiogenic agents such as endostatin (ES)
have been proved to be effective in inhibiting
endothelium-dependent angiogenesis in both mice and humans
(11,12). However, in the clinical trials, they
failed to decrease tumor metastasis, and instead, tended to
increase the risk of occurrence (13). Antiangiogenic therapy can lead to
hypoxia, which contributes to the formation of VM, thus,
facilitating tumor metastasis. Therefore, an effective treatment
strategy should target not only endothelium-dependent vessels but
also endothelium-independent vessels such as VM (14,15).
Recently, the effects of caspases, including caspase-3, −6 and −7,
were found to play a key role in apoptosis and may also play an
important role in tumorigenesis, particularly in VM (16,17).
However, the mechanism by which VM formation is promoted remains
elusive.
Overexpression of migration-inducing gene 7
(MIG7) is found in highly aggressive tumors with VM rather
than non-aggressive malignant cells without VM (18–20)
suggesting an important role in VM formation and cancer aggression.
Recently, it was reported that MIG7 was found to be related
to the formation of VM in gastric cancer, and to play a
complementary role in growth factors and COX-2/PGE2-related cancer
invasion and metastasis (20,21).
In the present study, we investigated the association of
MIG7 expression with VM formation in HCC and its effects on
the potential for HCC invasion and metastasis.
Materials and methods
Patient samples and cell lines
Forty matched pairs of HCC paraffin-embedded
specimens from 40 patients (male, n=28; female, n=12; mean age, 54)
and 10 normal liver paraffin-embedded specimens were purchased from
Shaanxi Chaoying Clinical Pathology Institute (Shaanxi, China). The
use of the specimens in the study was approved by the Ethics
Committee of the Affiliated Hospital of the Logistics University of
Chinese People's Armed Police Forces. Human HCC cell lines
(MHCC-97H, MHCC-97L, Huh-7) and human normal hepatocyte L-02 cells
were purchased from the Liver Cancer Institute of Fudan University
(Shanghai, China). Dulbeccos modified Eagles medium (DMEM) with 10%
fetal bovine serum (FBS) was used to culture all 4 types of cells
at 37°C and 5% CO2.
Primary antibodies and reagents
Rabbit anti-human MIG7 (cat. no. ab83494) was
purchased from Abcam (Cambridge, MA, USA). Mouse anti-human CD34
monoclonal antibody (cat. no. sc-19621), mouse anti-human β-actin
monoclonal antibody (cat. no. 130065), goat anti-rabbit IgG (cat.
no. sc-2004), goat anti-mouse IgG (cat. no. sc-2005) and laminin
rabbit anti-human polyclonal antibody (cat. no. sc-5582) were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Fluorescence marked (FITC) goat anti-mouse IgG (cat. no. 555988)
and fluorescence marked (TRITC) goat anti-mouse IgG (cat. no.
610055) were purchased from BD Biosciences (San Jose, CA, USA).
High Fidelity PrimeScript™ RT-PCR kit and PrimeSTAR® HS
DNA Polymerase were purchased from Takara Bio Group (Dalian,
China). Matrigel and cell culture plates were purchased from
Corning Inc. (Corning NY, USA). ES was purchased from Calbiochem
(San Diego, CA, USA) and 0.25% tryptase was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Immunostaining analysis
For MIG7 protein detection, formalin-fixed and
paraffin-embedded sections were deparaffinized and hydrated in a
graded ethanol series. After antigen retrieval, sections were
treated with 10% goat serum for 20 min and incubated with rabbit
anti-human MIG7 polyclonal antibody (1:100) at 4°C overnight, and
in turn biotinylated antibody and streptavidin-peroxidase at 37°C
for 30 min. Signaling was detected with DAB substrate for 5 min.
For VM formation detection, conventional treated sections were
incubated with rabbit anti-human laminin polyclonal antibody
(1:200) at 37°C for 30 min, and then treated with DAB for 5 min.
The sections were then dehydrated and mounted with Permount and
viewed by bright-field microscopy. The results, statistically
analyzed according to the positive staining rate of the malignant
cells and staining intensity, were assessed by the independent film
reading of two professional physicians in a blinded manner. The
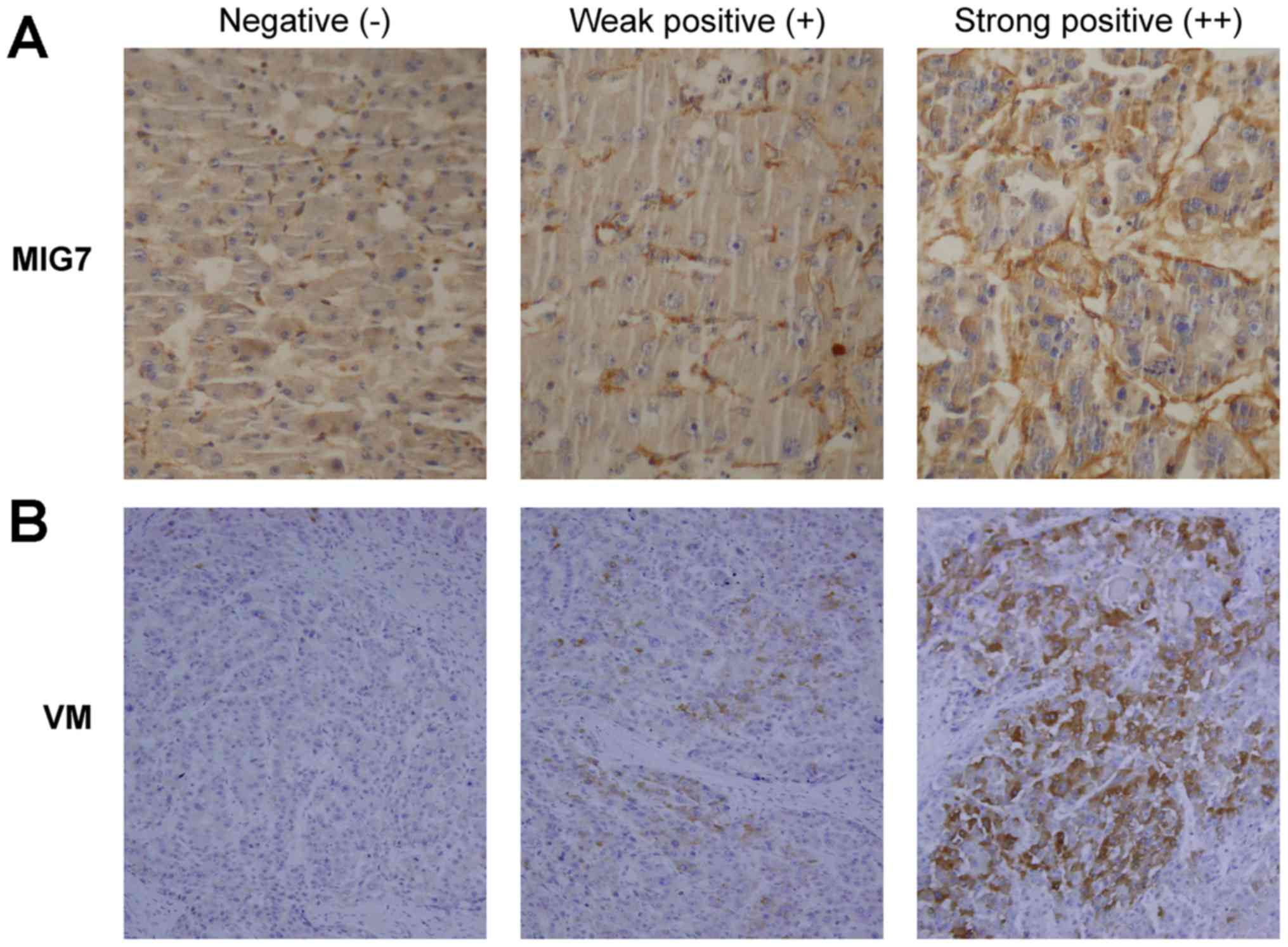
three levels of staining intensity were: negative (−, 0); weakly
positive (+, 1); intensely positive (++, 2).
3D cell culture
3D culture was employed to detect VM formation in
vitro. Six-wells of culture plates were coated with Matrigel
(50 µl/well). The cells were maintained in DMEM supplemented with
10% FBS and trypsinized, and then, suspended at 1×106/ml
in complete medium. Finally, the cells were seeded on gels and
incubated at 37°C in 5% CO2/9% air. The tube-like
connections were observed under an inverted microscope. The number
of tube-like connections per field (×200 magnification) was counted
(22). Five random fields were
analyzed in each sample.
Semi-quantitative PCR analysis
The semi-quantitative PCR was employed to detect
MIG7 mRNA in different cell groups and the β-actin RNA was
used as the internal standard control. According to the
instructions, TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was
used to harvest the total RNA. Subsequently, according to the
manufacturers protocol, the High Fidelity PrimeScript™ RT-PCR kit
(Takara Bio) was employed to transform to cDNA with 1 µg of total
RNA. Then, the resulting cDNA was used for semi-quantitative PCR
with SYBR-Green reagents (Fermentas, Waltham, MA, USA). The
sequences of the primers were as follows: MIG7 mRNA forward:
5-TCT CAG GCA GTC AGT GGG-3 and MIG7 mRNA reverse: 5-GTT GGA
TGG GAT GTC TCG-3; β-actin mRNA forward: 5-ATC GTG CGT GAC ATT AAG
GAG AAG-3 and β-actin mRNA reverse: 5-AGG AAG GAA GGC TGG AAG AGT
G-3. The cycling parameters were 94°C for 2 min, then 40 cycles of
94°C for 10 sec and 60°C for 30 sec, followed by a melting curve
analysis. Dissociation curve analysis was applied to confirm the
specificity of the PCR amplification. Relative expression levels
were determined using the housekeeping gene β-actin for
normalization.
Western blot assay
All cells (MHCC-97H, MHCC-97L, Huh-7 and L-02) were
washed with phosphate-buffered saline (PBS) and the lysates were
prepared using modified radioimmunoprecipitation assay buffer at
4°C for 15 min. The proteins were resolved by 0.1% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto nitrocellulose filter membranes. Subsequently,
blots were blocked by TBST and incubated overnight with primary
antibodies (MIG7 1:200, β-actin 1:500) at 4°C. Then, the blots were
washed in TBS containing 0.1% Tween-20 and labeled with goat
anti-rabbit IgG-HRP (1:1,000) and goat anti-mouse IgG-HRP
(1:1,000). MIG7/β-actin ratio was used for relative expression of
proteins.
MIG7 shRNA constructs
Two target sequences and one negative control (Neo)
sequence were synthesized based on the sequence of human
MIG7 (GenBank no. DQ080207.2) and cloned into pSIREN vector
to make pSIREN-M1 (MIG7 shRNA-1), pSIREN-M2 (MIG7
shRNA-2) shRNA constructs or MIG7 Neo construct pSIREN-MN
(MIG7 shRNA-N) construct. The target sequences were as
follows: Oligo #1: 5-GAT CCA AAG TTT CAT TCT TCG ACT TCA AGA GAG
TCG AAG AAA TGA AAC TTT TTT TTT G-3 and 3-GTT TCA AAG TAA GAA GCT
GAA GTT CTC TCA GCT TCT TTA CTT TGA AAA AAA AAC TTA A-5; Oligo #2:
5-GGA TCC CAC AGC TTG AGT GGA ATA CTT CAA GAG AGT ATT CCA CTC AAG
CTG TGT TTT TTG-3 and 3-GGT GTC GAA CTC ACC TTA TGA AGT TCT CTC ATA
AGG TGA GTT CGA CAC AAA AAA CTT AAG-5.
Transfection
MHCC-97H cells were cultured in DMEM high glucose
medium supplemented with 10% FBS (from Gibco), 80 U/ml penicillin,
and 100 µg/ml streptomycin at 37°C under 5% CO2.
MHCC-97H cells were transfected with MIG7 shRNA constructs
or control MIG7 Neo construct using Lipofectamine 2000
(Invitrogen) following the manufacturers instructions. The stable
knockdown cells were selected by 3 µg/ml puromycin for 2 weeks. The
stable cells in which MIG7 was efficiently knocked down were
named as MHCC-97H-1 cells derived from MHCC-97H+pSIREN-M1 and
MHCC-97H-2 cells derived from MHCC-97H+pSIREN-M2, and the stable
control cell line was named as MHCC-97H Neo. The clones were
characterized by semi-quantitative PCR and western blot analysis to
assess the expression of MIG7 mRNA and MIG7 protein,
respectively.
3D culture after transfection
Matrigel (50 µl/hole) was added to 6-well plates and
then the cells were incubated at 37°C for 20 min. The MHCC-97H
cells (1×106/ml), from the 6 groups, were maintained in
DMEM supplemented with 10% FBS, and were then seeded onto the gels
and incubated at 37°C in 5% CO2/95% air. The numbers of
tube-like connections were measured in a high-power field (Leica DM
ILM inverted microscope; Leica Microsystems GmbH, Wetzlar,
Germany).
Transwell invasion assay
Transwell assays were used to evaluate the invasion
of the cells in six groups. A total of 4×104 MHCC-97H
cells were seeded in the upper chamber (24-well plates, 8-µm pores)
coated with Matrigel (10 µg/hole) extracellular matrix (ECM) gel
and media containing 10% FBS was placed in the lower chamber. The
chambers were incubated at 37°C in 5% CO2 for 48 h.
Invasive cells at the bottom of the membrane were stained with 0.1%
crystal violet and were counted under a microscopic. Five random
fields were analyzed in each chamber.
Transwell migration assay
A total of 1×104 MHCC-97H cells were
seeded in the upper chamber (24-well plates, 8-µm pores) coated
with Matrigel (10 µg/hole) extracellular matrix (ECM) gel and media
containing 20% FBS was placed in the lower chamber. The chambers
were incubated at 37°C in 5% CO2 for 24 h. Cells that
migrated to the bottom of the insert were stained with 0.1% crystal
violet and were counted under a microscopic (Leica DM ILM inverted
microscope). Five random fields were analyzed in each chamber.
Cellular adhesion assay
MHCC-97H cells (1 ml cell suspension,
1×106/ml) were placed in an EP tube (1.5 ml) and
incubated at 37°C in 5% CO2 for 12 h. The number of
individual cells were counted at 2, 6 and 12 h, respectively. The
lower proportion of remining single cells, the stronger the
cell-to-cell adhesion.
Statistical methods
SPSS ver20.0 (IBM SPSS, Armonk, NY, USA) was used
for statistical analyses. Rank-sum test was used for ranked data.
P<0.05 was considered statistically significant.
Results
Positive association of MIG7
expression with VM formation in HCC tissues
MIG7 expression and VM formation in 40
matched pairs of HCC specimens from 40 patients were detected by
immunostaining. The results (Table
I) showed that the expression of MIG7 (Fig. 1A) was negative in 20% of the cases
(8/40), weakly positive in 45% of the cases (18/40) and strongly
positive in 35% of the cases (14/40). Meanwhile, VM formation
(Fig. 1B) was negative in 15% of
the cases (6/40), weakly positive in 50% of the cases (20/40) and
strongly positive in 35% of the cases (14/40). The results showed
that there was a positive correlation between MIG7 expression and
VM formation (rs=0.554; P<0.001). There was no MIG7
expression and VM formation in normal liver tissues.
 | Table I.Expression of MIG7 protein and
vasculogenic mimicry (VM) formation in HCC tissues. |
Table I.
Expression of MIG7 protein and
vasculogenic mimicry (VM) formation in HCC tissues.
|
| MIG7 | VM |
|---|
|
| −, 0 | +, 1 | ++, 2 | −, 0 | +, 1 | ++, 2 |
|---|
| HCC specimens,
n/total (%) | 8/40 (20) | 18/40 (45) | 14/40 (35) | 6/40 (15) | 20/40 (50) | 14/40 (35) |
Positive correlation between VM
formation and the metastatic potential of the HCC cell lines in 3D
culture
After 6 h of incubation, the difference in
proliferation among the 4 types of cells (MHCC-97H, MHCC-97L, Huh-7
and L-02) was observed, and then it became more notable after 12
and 18 h. We found that (Fig. 2)
the density of VM formation in the MHCC-97H group (x¯=21.2) was higher than that of the
MHCC-97L group (x¯=6.8)
(P=0.000) and Huh-7 group (x¯=1.6) (P=0.000). VM formation in the
different HCC cell lines varied, and it was coincident with the
metastatic potential of the HCC cell lines. Moreover, there was no
VM formation observed in the normal liver L-02 cells after 6, 12
and 18 h. These results showed that there was a positive
correlation between VM formation and the metastatic potential of
the HCC cell lines.
Positive correlation between MIG7
expression and the metastatic potential of the HCC cell lines
The expression of MIG7 was detected in the
different cell groups. The results (Fig. 3A) showed that the HCC cell lines
with different metastatic potential had diverse expression of
MIG7 mRNA. Among them, the level of MIG7 mRNA
expression in MHCC-97H cells was higher than that of MHCC-97L
(P=0.000) and Huh-7 (P=0.000), and it was negative in L-02 cells.
Western blot analysis was used to detect the MIG7 protein in the 4
types of HCC cell lines. The results (Fig. 3B) indicated that the expression of
MIG7 protein in MHCC-97H cells was higher than that in the MHCC-97L
and Huh-7 cells. No MIG7 protein was detected in the normal liver
L-02 cells.
Decreased VM formation in MHCC-97H
cells with MIG7 knockdown
After 24 h from transfection with the constructs for
knockdown, the green fluorescent protein, expressed by the
constructed plasmid, was observed under a fluorescence inverted
microscope and the transfection efficiency was 10–15% (Fig. 4B). The transfected cells were
selected by puromycin and green fluorescent protein labeling the
positive cells increased gradually. Then, the cells were cultivated
and selected by limited dilution twice. Finally, the green
fluorescent protein-labeled positive cells accounted for >90%
(Fig. 4C). The knockdown efficiency
of MIG7 shRNA was detected by semi-quantitative PCR
(Fig. 5A) and western blot analysis
(Fig. 5B). The results showed that
MIG7 mRNA and protein was deceased significantly in both the
MIG7 shRNA-1 and MIG7 shRNA-2 groups, especially in
the MIG7 shRNA-1 group. The inhibitory efficiency of shRNA
on MIG7 expression in the MIG7 shRNA-1 group was ~70%
compared with MIG7 shRNA-N group. 3D culture was utilized to
detect the inhibitory effect of MIG7 shRNA on VM formation.
The results (Fig. 6) showed that VM
formation was significantly decreased in the MIG7 shRNA-1
(x¯=1.8) and MIG7
shRNA-2 groups (x¯=3.2)
compared with the control group (x¯=26.4; P=0.001, 0.001). MIG7
shRNA-N group (x¯=25.8), empty
vector group (x¯=23) and ES
group (x¯=25.6) did not have a
significant difference compared with the control group (P=0.842,
P=0.284 and P=0.819, respectively). The results indicated that
MIG7 plays an important role in VM formation.
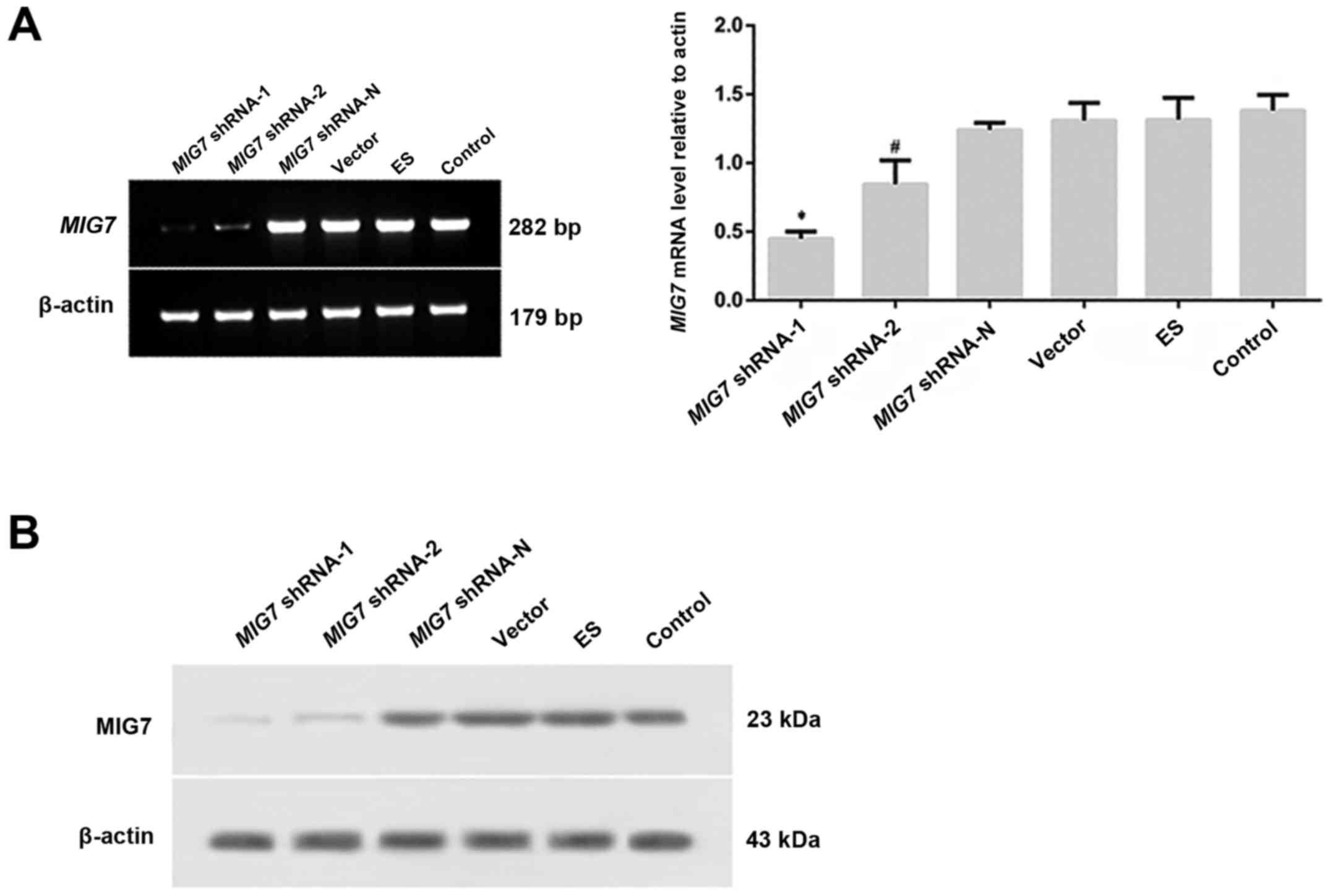 | Figure 5.After transfection, MIG7 mRNA,
MIG7 protein and vasculogenic mimicry (VM) formation were detected
by semi-quantitative PCR, western blot assay and 3D culture,
respectively. (A) Expression of MIG7 mRNA was detected with
semi-quantitative PCR. *P<0.01, compared with MIG7
shRNA-2 (P=0.005), MIG7 shRNA-N (P=0.000), vector (P=0.000),
ES (P=0.000) and control groups (P=0.000); #P<0.01,
compared with MIG7 shRNA-N (P=0.005), vector (P=0.005), ES
(P=0.007) and control groups (P=0.002). (B) The expression of MIG7
protein was detected by western blot assay. The results showed that
MIG7 protein was deceased significantly in both MIG7 shRNA-1
and MIG7 shRNA-2 groups, especially in the MIG7
shRNA-1 group. |
 | Figure 6.After transfection, the results of 3D
culture showed that the quantity of VM in the MIG7 shRNA-1
and MIG7 shRNA-2 groups was significantly lower than that in
the MIG7 shRNA-N, ES, empty vector, and the control group
(P=0.000). MIG7 shRNA-N group, ES group and empty vector
group did not have significant difference compared with the
MHCC-97H cell group (P>0.05). *P<0.01, compared with
MIG7 shRNA-N, vector, ES and control groups;
#P<0.01, compared with MIG7 shRNA-N, vector,
ES and control groups. |
Suppressed invasive properties and
increased cellular adhesion in MHCC-97H cells with MIG7
knockdown
To evaluate the invasive properties of the MHCC-97H
cells with MIG7 knockdown, we performed Transwell invasion
assay (Fig. 7A) and Transwell
migration assay (Fig. 7B). The
result of the Transwell invasion assay (48 h) showed that the
number of cells that invaded to the lower portion of the chamber in
the MIG7 shRNA-1 (x¯=112) and MIG7 shRNA-2 groups
(x¯=146) was significantly
lower than that of in MIG7 shRNA-N group (x¯=360; P=0.000, 0.000), empty vector group
(x¯=358; P=0.000, 0.000), ES
group (x¯=365.2; P=0.000,
0.000) and MHCC-97H cell group (x¯=367; P=0.000, 0.000). The results also
indicated that there were no significant differences between the
MIG7 shRNA-N group, empty vector group, ES group and
MHCC-97H cell group (P=0.549). The Transwell migration assay showed
that the cell migration in the MIG7 shRNA-1 (x¯=51.25) and MIG7 shRNA-2 groups
(x¯=74.25) was significantly
lower than that of the MIG7 shRNA-N (x¯=231; P=0.000, 0.000), empty vector
(x¯=229.25; P=0.000, 0.000), ES
(x¯=225.25; P=0.000, 0.000) and
MHCC-97H cell group (x¯=229.75;
P=0.000, 0.000) after 24 h from transfection. We also assessed the
cellular adherent ability using cellular adhesion assay. The
results (Fig. 7C) showed that after
6 h of incubation, the number of single cells in the MIG7
shRNA-1 (x¯=51.2) and
MIG7 shRNA-2 groups (x¯=65.2) was significantly lower than that
of in MIG7 shRNA-N group (x¯=88.2; P=0.000, 0.000), empty vector
group (x¯=89.8; P=0.000,
0.000), ES group (x¯=90.6;
P=0.000, 0.000) and MHCC-97H cell group (x¯=91.4; P=0.000, 0.000). Moreover, the
number of single cells in the MIG7 shRNA-1 group was
significantly lower than that of the MIG7 shRNA-2 group
(P=0.000); there were no significant differences among the
MIG7 shRNA-N group, empty vector group, ES group and
MHCC-97H cell group (P=0.431). These results suggest that
MIG7 plays an important role in regulating HCC cellular
adhesion and invasive properties.
 | Figure 7.Transwell invasion, migration and
cellular adhesion assay. (A) The Transwell invasion assay showed
that the MIG7 shRNA inhibited cell invasive ability.
*P<0.01, compared with MIG7 shRNA-2 (P=0.008),
MIG7 shRNA-N (P=0.000), vector (P=0.000), ES (P=0.000) and
control groups (P=0.000); **P<0.01, compared with MIG7
shRNA-N (P=0.000), vector (P=0.000), ES (P=0.000) and control
groups (P=0.000). There was no significant difference between
MIG7 shRNA-N, vector, ES and control groups (P>0.05). (B)
The Transwell migration assay showed that the cell migration
ability was suppressed by MIG7 shRNA. *P>0.05, compared
with MIG7 shRNA-2, MIG7 shRNA-N, vector, ES and
control groups; **P<0.05, compared with MIG7 shRNA-N
(P=0.031), vector (P=0.000), ES (P=0.000) and control groups
(P=0.000); ∆P>0.05, compared with MIG7
shRNA-N, vector, ES and control groups; ∆∆P<0.01,
compared with MIG7 shRNA-N (P=0.000), vector (P=0.000), ES
(P=0.000) and control groups (P=0.000). There was no significantly
difference between MIG7 shRNA-N, vector, ES and control
groups (P>0.05). (C) The results of cellular adhesion assay
showed that after 6 h from incubation, MIG7 shRNA increased
cellular adhesion. *P>0.05, compared with MIG7 shRNA-2,
MIG7 shRNA-N, vector, ES and control groups; **P<0.01,
compared with MIG7 shRNA-2 (P=0.003), MIG7 shRNA-N
(P=0.000), vector (P=0.000), ES (P=0.000) and control groups
(P=0.000); ∆P<0.01, compared with MIG7
shRNA-N, vector, ES and control groups. There was no significantly
difference between MIG7 shRNA-N, vector, ES and control
groups (P>0.05). |
Discussion
The metastasis of HCC is one of the major reasons
responsible for the failure of HCC treatment and the death of
patients. Therefore, more attention should be focused on the
mechanism of HCC metastasis. In the present study, we found a
positive correlation between MIG7 and VM in both clinical
specimens and in vitro experiments. MIG7 knockdown in
3D cultured MHCC-97H cells reduced the VM formation and weakened
the invasive properties accompanied by enhanced cellular adhesion.
Thus, this study provides evidence for a causal association of
MIG7 with VM formation in HCC, which suggests that
MIG7 could be a potential treatment target for cancer
invasion and metastasis.
Previously, VM has been described in HCC and was
associated with advanced tumor grade, invasion, metastasis and poor
patient prognosis (23,24). More studies investigated the
relevant mechanisms and signaling pathways of VM formation, such as
hypoxia inducible factor 1-α (HIF-1α), (25) matrix metalloproteinases (MMPs),
phosphoinositide 3-kinase (PI3K), laminin 5 (Ln-5) γ2
chain (26,27), focal adhesion kinase (FAK) (28) vascular endothelial-cadherin
(VE-cadherin) (29) and epithelial
cell kinase (EphA2) (30). However,
the exact and detailed mechanisms underlying VM remain unclear. In
the present study, we found that MIG7 and VM were highly
expressed in HCC tissues and that MIG7 has a significant
positive correlation with VM formation. We also found that
MIG7 expression in different HCC cell lines was coincident
with VM formation, invasion and metastasis. The result was
consistent with previously reported studies which showed a positive
correlation of MIG7 with VM in human lung cancer and gastric
carcinoma (20,31).
Based on the analyses above, we proposed that
MIG7 may induce the invasion and metastasis of HCC by
regulating VM formation. Furthermore, the metastasis of HCC may be
inhibited if the expression of MIG7 is downregulated.
Downregulation of MIG7 may be an effective and safe
therapeutic strategy. Based on the data above, the overexpression
of MIG7 generally leads to a poor prognosis and there is no
MIG7 expression and VM formation in normal liver tissues.
Therefore, MIG7 can be considered as a potential safe and
specific molecular target for HCC gene therapy. The invasion and
metastasis of HCC could be suppressed if we transport MIG7
antagonist into HCC cells in order to inhibit VM formation. This
therapeutic strategy, targeting MIG7, for HCC has great
application potential. Moreover, MIG7-targeting therapy
combined with various treatment methods at present, especially
endothelial-targeting angiogenesis inhibitors (such as ES), may
show better efficacy than any single use of angiogenesis inhibitors
alone.
Subsequently, in order to verify the hypothesis,
RNAi technique was employed to construct the recombinant retrovirus
MIG7 shRNA expression vector plasmid. Additionally, because
of the high expression of MIG7 in the MHCC-97H cell line,
MHCC-97H cells were chosen as effector cells to explore the role of
MIG7 in VM formation and HCC metastatic regulation. After
the transfection of MIG7 shRNA into MHCC-97H cells, we found
that MIG7 mRNA and protein were significantly decreased
according to semi-quantitative PCR and western blot assay.
Moreover, VM formation, invasion, migration of MHCC-97H cells were
inhibited significantly by 3D culture, Transwell invasion and
migration assay, respectively (P<0.05), while the adhesion
capability of MHCC-97H cells was increased significantly
(P<0.05). However, there was no significant effect of ES on MIG7
expression and intercellular adhesion, invasion and metastasis.
A recent study reported that intra-tumor
heterogeneity does exist in patients with HCC (32). The authors performed genome
sequencing on 43 lesions from 10 patients with hepatitis B virus
(HBV)-associated HCC and compared the genetic features of different
lesions from each patient. They found that the mutations which were
shared by all the lesions in each patient varied from 8 to 97%,
indicating the indetermination existing in intra-tumor
heterogeneity. Thus, the genomic features of HCC in patients might
not be characterized by sequence analysis of one single lesion, and
this would be a challenge for precision medicine in patients with
HCC. Therefore, more specific targets and the key genes that link
to them are still needed to be identified in order to facilitate
effective and specific precision medicine for tumor treatment.
In closing, MIG7 expression in HCC tissue is
correlated positively with VM formation and MIG7 expression
in different HCC cell lines is coincident with their VM formation,
invasion and metastasis. Moreover, MIG7 shRNA inhibits
MIG7 expression, VM formation and HCC invasion and
metastasis stably and effectively. There was no significant effect
of ES on MIG7 expression, VM formation and intercellular
adhesion, invasion and metastasis. However, relevant in vivo
experiments are still necessary for investigation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81272547) and Tianjin Science and
Technology Project (15ZXLCSY00040).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
European Association for Study of Liver, ;
European Organisation for Research and Treatment of Cancer, .
EASL-EORTC clinical practice guidelines: management of
hepatocellular carcinoma. Eur J Cancer. 48:599–641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folberg R, Hendrix MJC and Maniotis AJ:
Vasculogenic mimicry and tumor angiogenesis. Am J Pathol.
156:361–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, He Y, Sun S, Sun B and Tang X:
Vasculogenic mimicry is a major feature and novel predictor of poor
prognosis in patients with orbital rhabdomyosarcoma. Oncol Lett.
10:1635–1641. 2015.PubMed/NCBI
|
|
5
|
Yang JP, Liao YD, Mai DM, Xie P, Qiang YY,
Zheng LS, Wang MY, Mei Y, Meng DF, Xu L, et al: Tumor vasculogenic
mimicry predicts poor prognosis in cancer patients: A
meta-analysis. Angiogenesis. 19:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao N, Sun BC, Zhao XL, Wang Y, Meng J,
Che N, Dong XY and Gu Q: Role of Bcl-2 and its associated miRNAs in
vasculogenic mimicry of hepatocellular carcinoma. Int J Clin Exp
Pathol. 8:15759–15768. 2015.PubMed/NCBI
|
|
7
|
Tang J, Wang J, Fan L, Li X, Liu N, Luo W,
Wang J and Wang Y and Wang Y: cRGD inhibits vasculogenic mimicry
formation by down-regulating uPA expression and reducing EMT in
ovarian cancer. Oncotarget. 7:24050–24062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hendrix MJ, Seftor EA, Seftor RE, Chao JT,
Chien DS and Chu YW: Tumor cell vascular mimicry: Novel targeting
opportunity in melanoma. Pharmacol Ther. 159:83–92. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren K, Yao N, Wang G, Tian L, Ma J, Shi X,
Zhang L, Zhang J, Zhou X, Zhou G, et al: Vasculogenic mimicry: A
new prognostic sign of human osteosarcoma. Hum Pathol.
45:2120–2129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo F, Yang K, Liu RL, Meng C, Dang RF and
Xu Y: Formation of vasculogenic mimicry in bone metastasis of
prostate cancer: Correlation with cell apoptosis and senescence
regulation pathways. Pathol Res Pract. 210:291–295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folkman J: Antiangiogenesis in cancer
therapy - endostatin and its mechanisms of action. Exp Cell Res.
312:594–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skovseth DK, Veuger MJ, Sorensen DR, De
Angelis PM and Haraldsen G: Endostatin dramatically inhibits
endothelial cell migration, vascular morphogenesis, and
perivascular cell recruitment in vivo. Blood. 105:1044–1051. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alahuhta I, Aikio M, Väyrynen O,
Nurmenniemi S, Suojanen J, Teppo S, Pihlajaniemi T, Heljasvaara R,
Salo T and Nyberg P: Endostatin induces proliferation of oral
carcinoma cells but its effect on invasion is modified by the tumor
microenvironment. Exp Cell Res. 336:130–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu B, Guo L, Ma J and Lv Y:
Antiangiogenesis therapy might have the unintended effect of
promoting tumor metastasis by increasing an alternative circulatory
system. Med Hypotheses. 74:360–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eikesdal HP and Kalluri R: Drug resistance
associated with antiangiogenesis therapy. Semin Cancer Biol.
19:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu YR, Sun B, Zhao XL, Gu Q, Liu ZY, Dong
XY, Che N and Mo J: Basal caspase-3 activity promotes migration,
invasion, and vasculogenic mimicry formation of melanoma cells.
Melanoma Res. 23:243–253. 2013.PubMed/NCBI
|
|
17
|
Linder M and Tschernig T: Vasculogenic
mimicry: Possible role of effector caspase-3, caspase-6 and
caspase-7. Ann Anat. 204:114–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petty AP, Garman KL, Winn VD, Spidel CM
and Lindsey JS: Overexpression of carcinoma and embryonic
cytotrophoblast cell-specific Mig-7 induces invasion and
vessel-like structure formation. Am J Pathol. 170:1763–1780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng X, Yao J, Gao X, Jing Y, Kang T,
Jiang D, Jiang T, Feng J, Zhu Q, Jiang X, et al: Multi-targeting
peptide-functionalized nanoparticles recognized vasculogenic
mimicry, tumor neovasculature and glioma cells for enhanced
anti-glioma therapy. ACS Appl Mater Interfaces. 7:27885–27899.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho MY, Liang CM and Liang SM: MIG-7 and
phosphorylated prohibitin coordinately regulate lung cancer
invasion/metastasis. Oncotarget. 6:381–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li WL and Gao Q: Mig-7 enhances
vasculogenic mimicry in gastric cancer cells. Xi Bao Yu Fen Zi Mian
Yi Xue Za Zhi. 28:1142–1145. 2012.(In Chinese). PubMed/NCBI
|
|
22
|
Lissitzky JC, Parriaux D, Ristorcelli E,
Vérine A, Lombardo D and Verrando P: Cyclic AMP signaling as a
mediator of vasculogenic mimicry in aggressive human melanoma cells
in vitro. Cancer Res. 69:802–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu WB, Xu GL, Jia WD, Li JS, Ma JL, Chen
K, Wang ZH, Ge YS, Ren WH, Yu JH, et al: Prognostic significance
and mechanisms of patterned matrix vasculogenic mimicry in
hepatocellular carcinoma. Med Oncol. 28 Suppl 1:S228–S238. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Sun B, Zhao X, Shao B, An J, Gu Q,
Wang Y, Dong X, Zhang Y and Qiu Z: Erythropoietin and
erythropoietin receptor in hepatocellular carcinoma: Correlation
with vasculogenic mimicry and poor prognosis. Int J Clin Exp
Pathol. 8:4033–4043. 2015.PubMed/NCBI
|
|
25
|
Li S, Meng W, Guan Z, Guo Y and Han X: The
hypoxia-related signaling pathways of vasculogenic mimicry in tumor
treatment. Biomed Pharmacother. 80:127–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Wang JH, Li S, Li LL, Huang M,
Zhang YH, Liu Y, Yang YT, Ding R and Ke YQ: Histone deacetylase 3
expression correlates with vasculogenic mimicry through the
phosphoinositide3-kinase/ERK-MMP-laminin5γ2 signaling pathway.
Cancer Sci. 106:857–866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin H, Pan JC, Zhang FM, Huang B, Chen X,
Zhuang JT, Wang H, Mo CQ, Wang DH and Qiu SP: Matrix
metalloproteinase-9 is required for vasculogenic mimicry by clear
cell renal carcinoma cells. Urol Oncol. 33:168.e9–168.e16. 2015.
View Article : Google Scholar
|
|
28
|
Zang M, Zhang Y, Zhang B, Hu L, Li J, Fan
Z, Wang H, Su L, Zhu Z, Li C, et al: CEACAM6 promotes tumor
angiogenesis and vasculogenic mimicry in gastric cancer via FAK
signaling. Biochim Biophys Acta. 1852:1020–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang
Y, Gu Q, Dong X, Liu F, Zhang Y, et al: miR-27a-3p suppresses tumor
metastasis and VM by down-regulating VE-cadherin expression and
inhibiting EMT: An essential role for Twist-1 in HCC. Sci Rep.
6:230912016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vukoja V, Brandenbusch T, Tura A, Nassar
K, Rohrbach DJ, Lüke M, Grisanti S and Lüke J: Expression of EphA2
in metastatic and non-metastatic primary uveal melanoma. Klin
Monatsbl Augenheilkd. 232:290–297. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liao S and Gao Q: Expressions and clinical
significance of vasculogenic mimicry and related protein Mig-7 and
MMP-2 in gastric carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
29:194–196. 2013.(In Chinese). PubMed/NCBI
|
|
32
|
Xue R, Li R, Guo H, Guo L, Su Z, Ni X, Qi
L, Zhang T, Li Q, Zhang Z, et al: Variable intra-tumor genomic
heterogeneity of multiple lesions in patients with hepatocellular
carcinoma. Gastroenterology. 150:998–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|