Introduction
Gastric cancer (GC) is one of the most threatening
worldwide diseases. An estimated 951,600 new stomach cancer cases
and 723,100 deaths occurred in 2012 (1). GC is still the second most frequent
malignancy globally despite great improvement in the diagnosis and
treatment of GC. In China, which belongs to an area with the
highest incidence rate, a total of 679,000 new cases of GC and
498,000 deaths occurred in 2015 (2). The high mortality of GC is attributed
to the low rate of early diagnosis. Thus, the majority of patients
are diagnosed at an advanced stage with a poor patient prognosis.
Therefore, it is critical to improve the sensitivity and
specificity of diagnostic tools for the prevention and detection of
GC. Although the gold standard diagnostic methods for GC, endoscopy
and random biopsy endoscopy or image examination, could facilitate
the early diagnosis of GC, the invasive nature, potential sampling
errors and high expenditure impact their use only for patients at a
high-risk of GC.
MicroRNAs (miRNAs) are small non-coding RNAs
comprised of approximately 21 nucleotides in length that crucially
participate in regulating the translation and degradation of mRNAs
(3). miRNAs preferentially bind to
complementary sites at the 3′UTR of their target mRNAs, which
contribute to their pivotal regulation in a wide variety of
biological processes, including cell growth, development,
differentiation and apoptosis (4).
In the development of GC, aberrant expression of miRNAs has been
found to be correlated with the clinical features of GC, such as
occurrence, development and metastasis (5).
Recent studies have aimed to evaluate the microRNAs
present in serum/plasma as potential molecular biomarkers on
account of their high stability and convenience in biological
samples (e.g. miR-21, miR-148a and miR-124) (6,7).
MicroRNA expression profiling has been used to achieve this goal in
an effective way (8). An innovative
screening strategy which is called machine learning was carried out
in our study to select miRNAs from profiles and verify their
efficacy more efficiently. Machine learning is the programming of
computers to optimize a performance criterion using example data or
past experience. A mathematical model is built by the theory of
statistics, and learning is the execution of a computer program to
optimize the parameters of the model using the training data with
high speed and efficiency.
Herein, we excavated a profile of three combined
serum miRNAs using machine learning and confirmed its excellent
accuracy and reliability in the detection of GC.
Materials and methods
Ethics statements
The study was approved by the Human Research Review
Committee of Huashan Hospital, Fudan University and written
informed consents were obtained from all of the patients. The study
conformed to The Code of Ethics of the World Medical Association
(Declaration of Helsinki).
Study and microRNA selection
GEO (Gene Expression Omnibus) dataset search engine
was used for the GC microRNA expression profiling studies. The
following keywords: ‘miRNA’ OR ‘microRNA’ OR ‘miR’, ‘gastric’ OR
‘stomach’, ‘profiling’ OR ‘microarray’ were used to search for
potential studies. We selected a total of three GEO profiles
(GSE23739, GSE26595, GSE28700) as their sample sizes were
sufficiently large. The GSE23739 profile represented 723 human and
76 human viral miRNAs in 40 normal and 40 cancerous gastric
tissues. MicroRNAs identified from 60 primary GC tissues and 8
surrounding non-cancer tissues were used for microarray analysis in
GSE26595. A total of 22 paired GC and normal tissues were processed
in GSE28700. We eradicated any repetitive miRNAs and normalized
data of each profile.
Machine learning
Initially, we treated GSE23739 as a training set and
prepared to use GSE26595 and GSE28700 as validation sets. In order
to analyze the data from different profiles, total data were
standardized using a series of classifiers. These six classifiers
(9) were performed to screen
specific microRNAs to distinguish GC tissues from normal tissues:
Compound covariate classifier (10), Diagonal linear discriminant analysis
(DLDA) classifier (11), Bayesian
CCP classifier (12,13), 1/3-Nearest Neighbor classifier
(14), Nearest centroid classifier
(15), and Support vector machines
classifier (16). 1-Nearest
Neighbor classifier and 3-Nearest Neighbor classifier are different
types of this classifier to assess the stability of data. Leave one
out cross validation was run to ensure stability and accuracy of
the output.
Serum samples
All serum samples were collected from GC patients
and healthy individuals treated at Huashan Hospital (Shanghai,
China), affiliated to the Fudan University between 2014 and 2016.
All of the 24 patients were clinically and pathologically diagnosed
with GC. We evaluated the clinical and pathological features of the
patients and these data are summarized in Table I.
 | Table I.Clinical characteristics of the GC
patients vs. normal controls. |
Table I.
Clinical characteristics of the GC
patients vs. normal controls.
|
Characteristics | GC n=24 | Controls n=20 | P-value |
|---|
| Age (years), mean ±
SD | 57.6±14.0 | 53.2±12.5 | P=0.2823 |
| Sex |
|
| P=0.2929 |
|
Male | 13 | 11 |
|
|
Female | 11 | 9 |
|
| Tumor location |
|
|
|
|
Cardia | 10 |
|
|
|
Body | 9 |
|
|
|
Antrum | 3 |
|
|
|
Other | 2 |
|
|
| Histology |
|
|
|
|
Adenocarcinoma | 24 |
|
|
| Tumor size
(cm) |
|
|
|
| ≥5 | 13 |
|
|
|
<5 | 11 |
|
|
| TNM stage |
|
|
|
|
I+II | 5 |
|
|
|
III+IV | 19 |
|
|
| Metastatic
status |
|
|
|
|
Yes | 3 |
|
|
| No | 21 |
|
|
Serum preparation and microRNA
extraction
Venous blood was collected in EDTA anticoagulation
vacuum tubes and was centrifuged at 1000 × g for 15 min at 4ºC and
then the separated serum was transferred into 1.5 ml RNase-free
tubes stored at −80°C until RNA extraction. Small RNAs were
extracted from 200 µl of serum using the miRcute miRNA Isolation
kit (Tiangen Biotech Co., Beijing, China) according to the
manufacturer's instructions.
Quantification of miRNA expression in
serum by qRT-PCR
Reverse transcription (RT) reactions were performed
using miRcute Plus miRNA First-Strand cDNA Synthesis kit (Tiangen
Biotech Co.). Singleplex reactions were conducted in a volume of 20
µl which consisted of 10 µl 2X miRNA RT reaction buffer, 2 µl miRNA
RT Enzyme Mix, and 8 µl miRNA template. Then, the RT reaction was
carried out in a thermocycler under the following conditions: 42°C
for 60 min, 95°C for 3 min, followed by a hold at 4°C. All the
operations were performed with caution to exclude RNase
contamination, and the total end products were preserved at −20°C
for further analysis.
Quantitative PCR (qPCR) was performed using 7500
Fast Real-Time PCR System (Applied Biosystems, Foster City, CA,
USA) with miRcute miRNA qPCR Detection kit (Tiangen Biotech Co.).
All forward primers were obtained from Tiangen Biotech Co. Their
catalog numbers are CD201-0092 (hsa-miR-21-5p), CD201-0404
(hsa-miR-29c-3p) and CD201-0305 (hsa-miR-22-3p). Then they were
diluted to 10 µM before being adding to the PCR reaction mixture.
qRT-PCR was performed in 3 duplicate reactions comprising 10 µl 2X
miRcute miRNA Premix (with SYBR and ROX), forward and reverse
primer, each with 0.4 µl, 2 µl miRNA First-Strand cDNA template,
and 7.2 µl RNase-free ddH2O. Mixtures were denatured at
94°C for 2 min and then run for 40 cycles (94°C for 20 sec, 60°C
for 34 sec). Melting curve analysis was run after all these
procedures were completed. The expression levels of miRNAs in serum
were normalized to U6 for the next quantification which was
calculated using the 2−∆∆CT method.
Target gene analysis
The union of predicted target genes was searched
using starBase v2.0 (http://starbase.sysu.edu.cn/index.php). The Gene
Ontology and Genome Pathway were processed and produced by
OmicsBean (http://www.omicsbean.cn/). Then we
created biological networks employing Cytoscape v3.2 open-source
software with CyTargetLinker App (17) and we treated miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/) as the tool
for selecting targets intersected by the results of three miRNAs in
this software. Gene-disease association data were retrieved from
the DisGeNET database (http://www.disgenet.org/). The term ‘gastric
adenocarcinoma’ (umls: C0278701) was used to identify GC-associated
genes. STRING (http://www.string-db.org/) was used to analysis the
interaction between different proteins.
Statistical analysis
The clinical characteristics among groups were
compared using the χ2 test and Fisher's exact test for
qualitative data, and t-test for quantitative data. A receiver
operating characteristic (ROC) curve was generated for the
specificity and sensitivity value calculated by classifiers, which
are represented by the area under the curve (AUC) value and 95%
confidence intervals (CI). Experimental data are presented as means
± SD. The results were considered to be statistically significant
at *P<0.05, **P<0.01, ***P<0.001, ****P<0.001.
Results
Training set marker selection
Firstly, treating GSE23739 as a training set after
normalization, six types of classifiers (see Machine learning in
Materials and method) were used to select markers. Since Compound
covariate classifier, Diagonal linear discriminant analysis (DLDA)
classifier and Support vector machine classifier are linear, we
achieved the linear discriminant and calculated the gene weight
value of diverse classifiers using maximum likelihood estimate
(MLE). Using the same method, the threshold values of the Compound
covariate classifier, DLDA classifier, and Support vector machine
classifier were determined as 1.469, 2.724 and 0.747, respectively.
We set each gene weight value as ωi and the expression
of gene as xi. If a sample's
∑iωixi > threshold value, then
it will be classified as cancerous. Following this principle, we
calculated the accuracy of single or a small cluster of miRNAs to
discriminate GC from normal tissue.
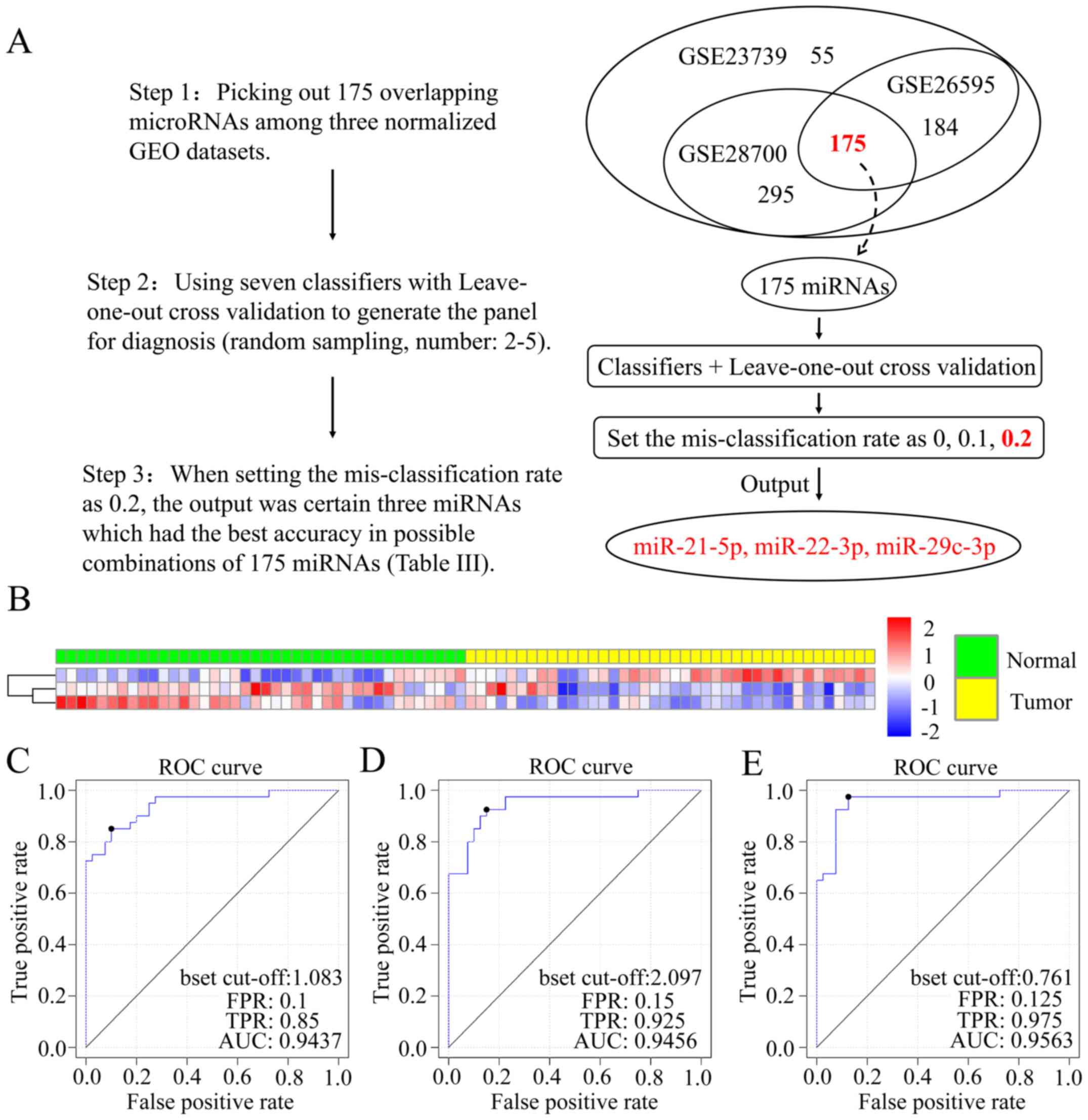
As elaborated in Fig.
1A, we found that the combination (miR-21-5p, miR-22-3p and
miR-29c-3p) of these three markers had the greatest accuracy of the
total 175 markers following three steps. The weight values of three
miRNAs in the linear classifiers are listed in Table II. The accuracy predicted using 7
markers is documented in Table
III. The heat map in Fig. 1B
represents the expression data clustering analysis of the three
markers in all 80 samples of GSE23739. By employing leave-one-out
cross validation, we found the results for sensitivity and
specificity in diverse classifiers. Then, we drew 3 ROC curves
corresponding to Compound covariate classifier (Fig. 1C), DLDA classifier (Fig. 1D) and Support vector machine
classifier (Fig. 1E). Thus, we
determined the AUC values for the three curves, 0.9437, 0.9456 and
0.9563, which were high and reliable confirming these markers as
having potential diagnostic criteria.
 | Table II.Weight values of three miRNAs. |
Table II.
Weight values of three miRNAs.
| Genes | Compound
covariate | Diagonal linear
discriminant analysis | Support vector
machines |
|---|
| hsa-miR-21-5p | −6.6501 | −0.5599 | −0.469 |
| hsa-miR-22-3p | 4.8583 | 0.815 | 0.2505 |
| hsa-miR-29c-3p | 6.9725 | 0.9744 | 0.9594 |
 | Table III.The accuracy of miR-21-5p, miR-22-3p
and miR-29c-3p in GSE23739 using 7 classifiers (shown as
percentages). |
Table III.
The accuracy of miR-21-5p, miR-22-3p
and miR-29c-3p in GSE23739 using 7 classifiers (shown as
percentages).
| Classifier | Compound
covariate | Diagonal linear
discriminant analysis | 1-Nearest
neighbor | 3-Nearest
neighbor | Nearest
centroid | Support vector
machines | Bayesian CCP |
|---|
| Accuracy | 84 | 88 | 86 | 85 | 80 | 88 | 88 |
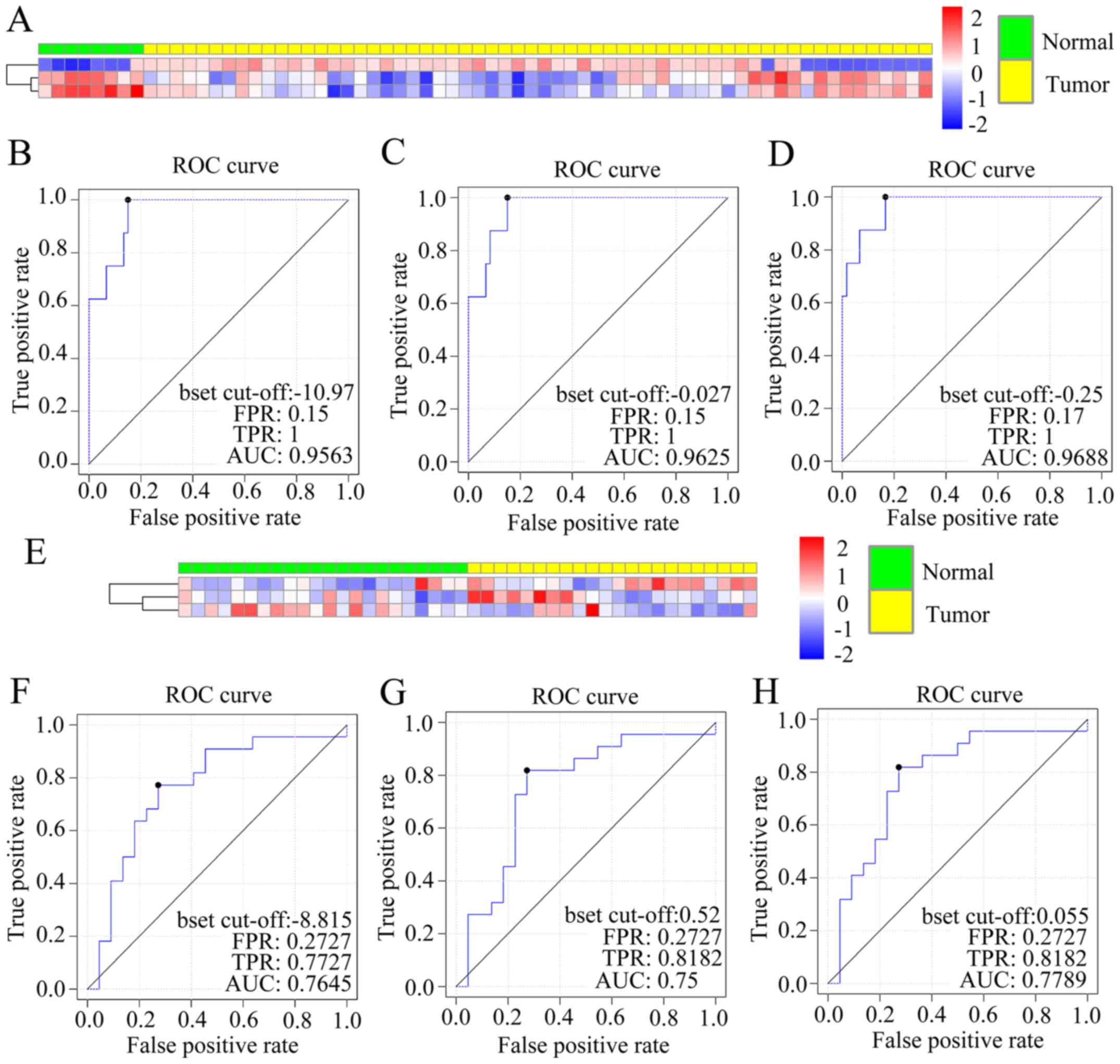
Marker validation
We performed 2 validation sets to validate the three
markers. As mentioned above, we investigated the accuracy of
prediction in GSE26595 and GES28700 by 7 classifiers (Table IV). The heat map of GSE26595 is
shown in Fig. 2A. After calculating
the sensitivity and specificity of the data in GSE26595, we
determined the AUC of three curves by three linear classifiers
separately (0.9563, 0.9625, 0.9688), which confirmed the
feasibility of the diagnostic criteria (Fig. 2B-D). The three markers were
validated again in another GEO microRNA profile (GSE28700). The
heat map is presented in Fig. 2E
and the AUC of ROC curves are 0.7645, 0.75 and 0.7789 (Fig. 2F-H).
 | Table IV.The accuracy of three miRNAs in
validation sets (GSE26595 and GSE28700) using 7 classifiers (shown
as percentages). |
Table IV.
The accuracy of three miRNAs in
validation sets (GSE26595 and GSE28700) using 7 classifiers (shown
as percentages).
| Classifier | Compound
covariate | Diagonal linear
discriminant analysis | 1-Nearest
neighbor | 3-Nearest
neighbor | Nearest
centroid | Support vector
machines | Bayesian CCP |
|---|
| GSE26595 | 88 | 88 | 88 | 88 | 87 | 93 | 93 |
| GSE28700 | 64 | 56 | 56 | 60 | 73 | 69 | 71 |
Confirmation of the selected miRNAs in
serum of GC patients
There were 20 samples from healthy volunteers
regarded as control subjects and 24 serum samples from GC patients
in this study. No significant differences in sex or age (Table I)were noted between the GC patients
and the healthy volunteers (P=0.2823, P=0.2929, Student's t-test,
respectively).
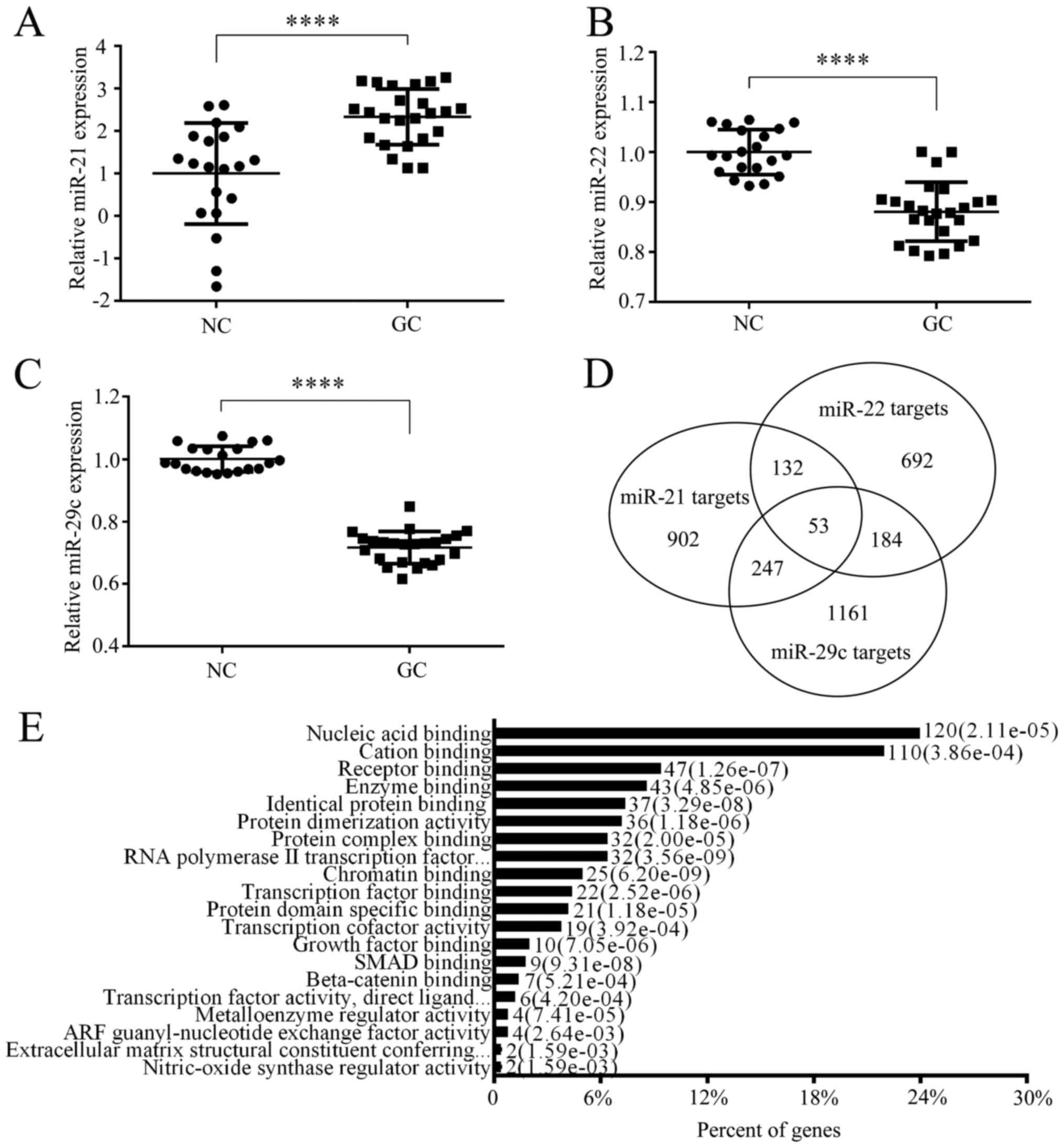
The expression of three candidate miRNAs (miR-21-5p,
miR-22-3p and miR-29c-3p) was assessed by qRT-PCR in individual
serum samples. The level of miR-21 was significantly upregulated in
GC (P<0.0001) (Fig. 3A).
Reversely, in Fig. 3B and C, levels
of miR-22 and miR-29c were downregulated in the tumor group (both
P<0.0001). These changes were consistent with the results in the
training and validation set. Furthermore, we explored the
relationship between the expression of these miRNAs with the
clinical and pathological features of GC (Table V). There was a higher expression of
miR-21 in GC patients with larger tumor sizes (≥5 cm) as previous
reported (18).
 | Table V.Association between the three
selected microRNAs and clinicopathological features of the GC
patients. |
Table V.
Association between the three
selected microRNAs and clinicopathological features of the GC
patients.
|
| miR-21 | miR-22 | miR-29c |
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | ∆Ct | P-value | ∆Ct | P-value | ∆Ct | P-value |
|---|
| Tumor location |
| 0.07 |
| 0.5 |
| 0.35 |
|
Cardia |
3.07±0.93 |
|
4.87±0.83 |
|
0.19±0.86 |
|
| Not in
cardia |
3.78±0.86 |
|
6.02±1.07 |
|
0.83±0.96 |
|
| Tumor size
(cm) |
| 0.05 |
| 0.25 |
| 0.99 |
| ≥5 |
3.14±1.05 |
|
5.19±1.20 |
|
0.23±0.91 |
|
|
<5 |
3.89±0.62 |
|
5.95±0.89 |
|
0.96±0.89 |
|
| TNM stage |
| 0.38 |
| 0.29 |
| 0.27 |
| Early
(I+II) |
3.14±0.49 |
|
4.77±0.69 |
|
−0.26±0.52 |
|
| Later
(III+IV) |
3.58±1.03 |
|
5.74±1.14 |
|
0.78±0.95 |
|
| Metastatic
status |
| 0.38 |
| 0.85 |
| 0.74 |
|
Yes |
3.04±1.25 |
|
4.80±1.02 |
|
−0.14±0.81 |
|
| No |
3.55±0.89 |
|
5.65±1.11 |
|
0.66±0.95 |
|
Target prediction and analysis
Initially, StarBase v2.0 was used to search for the
targets of miR-21, miR-22, and miR-29c. All predicted targets of
the deregulated miRNAs are illustrated in Fig. 3D. An overview of the Gene Ontology
(GO) analysis indicated that the binding attribute of molecular
function was high by OmicsBean website (data not shown). Fig. 3E further shows that the highest
percentage of genes are involved in the nucleic acid binding
activity of enriched processes of level 4. The top 20 highly
enriched KEGG pathways are listed in Table VI. Noteworthy, the tumor-suppressor
gene, PTEN, was discovered in the class of enriched KEGG pathways,
such as: focal adhesion and PI3K-Akt signaling pathway.
 | Table VI.KEGG pathway analysis of shared
target genes of the three miRNAs. |
Table VI.
KEGG pathway analysis of shared
target genes of the three miRNAs.
| Pathway name | Pathway ID | P-value | Genes |
|---|
| Protein digestion
and absorption | hsa04974 | 1.30E-10 | COL7A1; COL3A1 |
| ECM-receptor
interaction | hsa04512 | 1.38E-06 | COL4A5; COL4A4 |
| Focal adhesion | hsa04510 | 6.21E-06 | PTEN; COL4A5;
COL4A4 |
| PI3K-Akt signaling
pathway | hsa04151 | 3.19E-05 | PTEN; COL4A5;
COL4A4 |
| Small cell lung
cancer | hsa05222 | 8.28E-05 | PTEN; COL4A5;
COL4A4 |
| Amoebiasis | hsa05146 | 2.94E-04 | COL3A1; COL4A5;
COL4A4 |
| Insulin
resistance | hsa04931 | 5.89E-04 | PTEN; PPARA;
RPS6KA3 |
| Proteoglycans in
cancer | hsa05205 | 1.04E-03 | ESR1; CBL; FLNA;
HGF |
|
Phosphatidylinositol signaling system | hsa04070 | 4.31E-03 | PTEN; PIKFYVE;
CALM1 |
| MAPK signaling
pathway | hsa04010 | 7.61E-03 | RASGRP1; RPS6KA3;
FLNA |
| Pathways in
cancer | hsa05200 | 8.69E-03 | PTEN; RASGRP1;
COL4A5 |
| N-Glycan
biosynthesis | hsa00510 | 9.19E-03 | ALG9; ALG1;
MAN1A2 |
| Inositol phosphate
metabolism | hsa00562 | 1.10E-02 | PTEN; PIKFYVE;
MTMR2 |
| Melanoma | hsa05218 | 1.10E-02 | PTEN; HGF; PTEN;
CDK6 |
| Neurotrophin
signaling pathway | hsa04722 | 1.41E-02 | CAMK4; CALM1;
RPS6KA3 |
| AGE-RAGE signaling
pathway in diabetic complications | hsa04933 | 1.74E-02 | COL3A1; COL4A5;
COL4A4 |
| Glioma | hsa05214 | 2.84E-02 | PTEN; CALM1; PTEN;
CDK6 |
| Long-term
potentiation | hsa04720 | 3.01E-02 | CAMK4; GRM5;
CALM1 |
| Prostate
cancer | hsa05215 | 3.03E-02 | PTEN; CREB1; PTEN;
CREB5 |
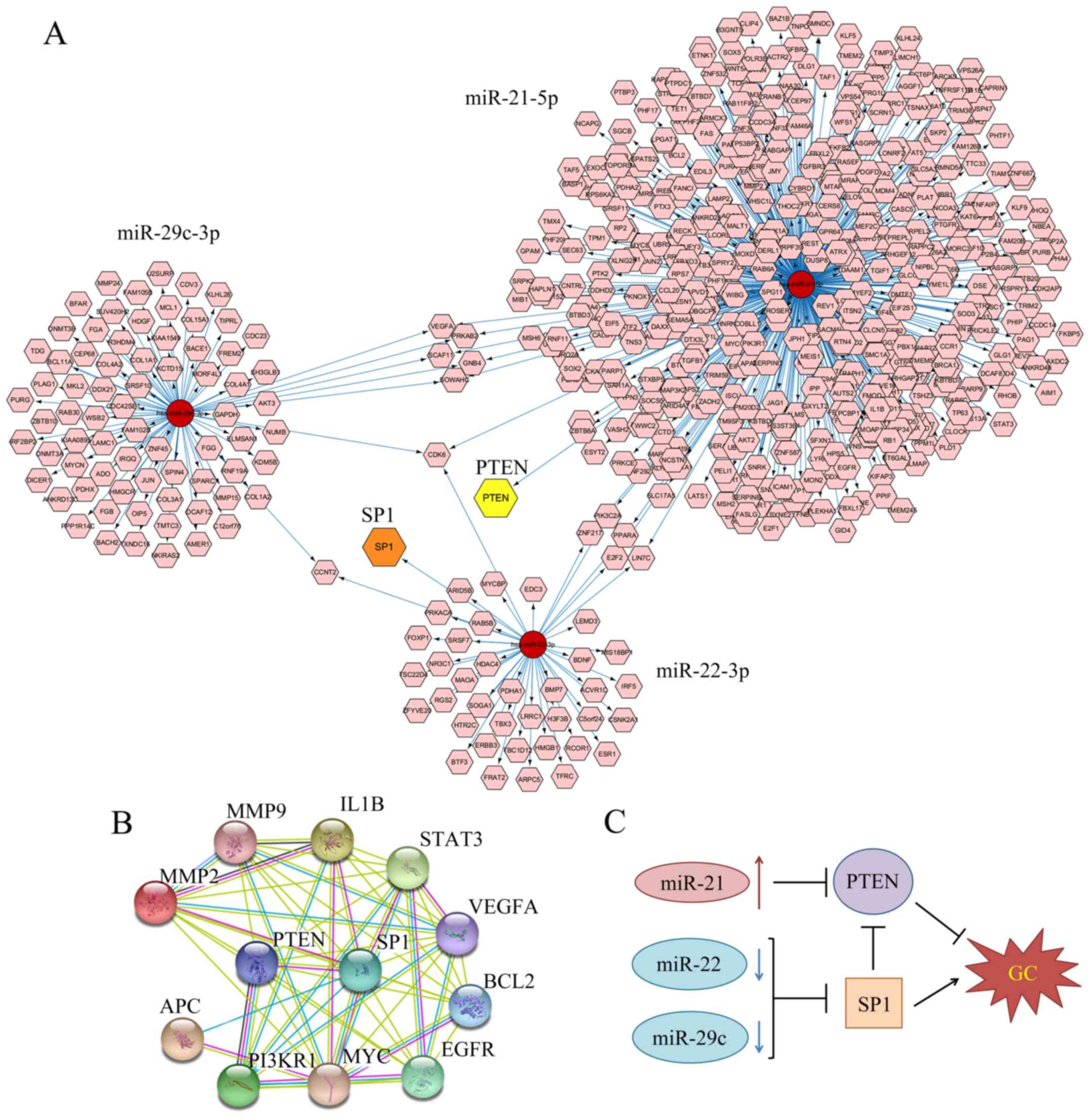
Furthermore, Cytoscape v3.2 software was applied to
focus on the shared genes by both miR-22 and miR-29c. Their
decreased level may contribute to GC by upregulating various
oncogenes. By applying miRTarBase database in CyTargetLinker App to
search for targets, we created a biological network with numerous
nodes which stand for target genes and gave attention to the shared
targets between two miRNAs (Fig.
4A). However, there may have been several missing targets as
this database has not been included. Sp1, which is a transcription
factor and may be associated with poor prognosis of GC patients,
was selected from the targets of miR-22 and its node is indicated
in orange (19). Recent research
also reveals that miR-29c could target Sp1 in lung cancer (20). Therefore, SP1 has been confirmed to
be a shared target between two downregulated miRNAs (miR-22 and
miR-29c).
STRING was used to screen genes between Sp1 and 476
targets of miR-21 predicted by miRTarBase. A total of 38 relevant
genes were selected out. Next, we search for the gene list related
to gastric adenocarcinoma in DisGeNet database (umls: C0149826).
Then we took the intersection between 38 genes related to Sp1 and
284 GC-related genes. Twelve genes were selected out and STRING was
used to predict the interaction between them (Fig. 4B). Finally, we gave attention to
PTEN, which has been reported to be targeted by miR-21 in GC and
was transcriptionally inhibited by Sp1 (21–24).
Sp1 and PTEN were consistent with the results we found in GO
analysis and KEGG pathway. In conclusion, Fig. 4C illustrates that the higher level
of SP1 and lower level of PTEN may contribute to the progression of
GC.
Discussion
It is crucial to identify practical biomarkers for
the detection of gastric cancer (GC) in order to improve patient
outcomes. In comparison to tissue biopsy, using serum miRNAs as
biomarkers is simple, has a lower cost and is non-invasive, which
has benefit for the screening and monitoring of tumors (25). A combination of miR-21, miR-22 and
miR-29c was identified using machine learning. Their ROC analyses
in the training set revealed marked AUC (0.9437, 0.9456 and 0.9563)
with more than 80% positive predictive value (PPV)and negative
predictive value (NPV) in three linear classifiers. Then we
validated them in two training sets and the serum of patients for
further confirmation. It should be noted that the number of samples
was few and we required further proof to validate these three
biomarkers in GC. In the final step, we used tools to identify
their targets and elucidate the possible mechanisms.
Application of machine learning to large databases
is also called data excavation which means that a large volume of
raw data are processed into a small amount of precious material
using classifier models. During recent years, machine learning has
been widely utilized as a method to predict the progression,
susceptibility and recurrence of cancerous conditions (26). For example, machine learning models,
including Support vector machine (SVM) classifier, was used to
predict childhood acute lymphoblastic leukemia (ALL) relapse based
on medical data (27). In the field
of diagnostics, Chen et al (28) applied four classical machine
learning-based classifications to estimate the stage of hepatic
fibrosis. Radiomic machine-learning classifiers were applied for
prognostic biomarkers of advanced nasopharyngeal carcinoma
(29). In this study, we fully
utilized the predominance of machine learning and used it for the
screening of biological biomarkers in GC. The panel of miR-21,
miR-22, and miR-29c was found to have the highest accuracy in
predicting GC tissues (Fig. 1A).
Machine learning is a novel method that is worthy to be popularized
in identifying biomarkers in different types of disease.
Among the three miRNAs identified in this study,
miR-21-5p was upregulated in the serum of GC patients, which was
consistent with previous research (6,30). As
early as 2008, Zhang et al (31) found that miR-21 could regulate GC
cell invasion and migration. Concurrently, Chan et al
(32) verified that miR-21 was
overexpressed in 92% (34/37) of GC samples and PTEN may be a target
gene of miR-21 (21). H.
pylori infection was found to induce miR-21 and the level of
miR-21 was upregulated in gastric juice of GC patients (33). Accumulating evidence indicates that
miR-21 can serve as a diagnostic candidate for GC. In contrast, the
levels of miR-22 and miR-29c were decreased in our serum samples,
which was in accordance with results in other research (34). miR-29 family plays a vital role in
tumor-related changes including cell proliferation, cell cycle,
cell differentiation, apoptosis and metastasis (35). Han et al (36) showed that miR-29c suppressed the
initiation of gastric carcinogenesis in transgenic mouse models.
Sufficient evidence revealed that the level of miR-22 was
downregulated in GC, which was related to lymph node metastasis,
poor prognosis in patients (37),
and acted as a metastasis suppressor by directly targeting Sp1
(38). Therefore, the combination
of these three miRNAs would achieve more specificity than separate
miRNAs in the prediction of GC, achieving high accuracy.
Next, we aimed to explain how these three miRNAs
work together. Sp1, which functions as a transcription factor, is a
ubiquitously expressed, zinc finger-containing DNA binding protein
that can activate or repress transcription in a variety of diseases
(39). It is overexpressed in GC
and is closely correlated with poor outcome (40). miR-22 targets Sp1 and represses GC
(38), while miR-29c may function
in the same way (41). PTEN is one
of the well-known tumor suppressor gene that plays a crucial role
in various types of tumors including GC (42) and was validated to be targeted by
miR-21 (21–24). Sp1 can inhibit PTEN promoter
activity through a specific Sp1-binding site at the PTEN core
promote (43). The mechanism is
summarized in Fig. 4C. miR-22 and
miR-29c both suppress the level of Sp1 and miR-21 suppresses the
expression of PTEN inhibited by Sp1, which contributes to the
development of GC.
In summary, our study revealed that miRNAs or other
biomarkers could be excavated effectively by machine learning.
Three miRNAs were screened: miR-21, miR-22 and miR-29c. Their
diagnostic potential was evaluated by various classifiers and AUC
curves. We then verified their differential expression in the serum
of patients and explained this phenomenon by predicting their
targets. Further studies will aid in confirming this serum miRNA
panel for the diagnosis of GC.
Acknowledgements
This research was supported by a grant from the
Shanghai Municipal Commission of Health and Family Planning (no.
20134132).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song JH and Meltzer SJ: MicroRNAs in
pathogenesis, diagnosis, and treatment of gastroesophageal cancers.
Gastroenterology. 143:35–47.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo
X, Mao XH, Zou QM, Yu PW, Zuo QF, et al: Plasma microRNAs, miR-223,
miR-21 and miR-218, as novel potential biomarkers for gastric
cancer detection. PLoS One. 7:e416292012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shrestha S, Hsu SD, Huang WY, Huang HY,
Chen W, Weng SL and Huang HD: A systematic review of microRNA
expression profiling studies in human gastric cancer. Cancer Med.
3:878–888. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berrar DP, Dubitzky W and Granzow M: A
practical approach to microarray data analysis. Springer; New York:
pp. 3682003
|
|
10
|
Radmacher MD, McShane LM and Simon R: A
paradigm for class prediction using gene expression profiles. J
Comput Biol. 9:505–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dudoit S, Fridlyand J and Speed TP:
Comparison of discrimination methods for the classification of
tumors using gene expression data. J Am Stat Assoc. 97:77–87. 2002.
View Article : Google Scholar
|
|
12
|
Efron B, Tibshirani R, Storey JD and
Tusher V: Empirical Bayes analysis of a microarray experiment. J Am
Stat Assoc. 96:1151–1160. 2001. View Article : Google Scholar
|
|
13
|
Wright G, Tan B, Rosenwald A, Hurt EH,
Wiestner A and Staudt LM: A gene expression-based method to
diagnose clinically distinct subgroups of diffuse large B cell
lymphoma. Proc Natl Acad Sci USA. 100:pp. 9991–9996. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Darden TA, Weinberg CR, Levine AJ
and Pedersen LG: Gene assessment and sample classification for gene
expression data using a genetic algorithm/k-nearest neighbor
method. Comb Chem High Throughput Screen. 4:727–739. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pal M: Modified nearest neighbour
classifier for hyperspectral data classification. Int J Remote
Sens. 32:9207–9217. 2011. View Article : Google Scholar
|
|
16
|
Furey TS, Cristianini N, Duffy N,
Bednarski DW, Schummer M and Haussler D: Support vector machine
classification and validation of cancer tissue samples using
microarray expression data. Bioinformatics. 16:906–914. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kutmon M, Kelder T, Mandaviya P, Evelo CT
and Coort SL: CyTargetLinker: A cytoscape app to integrate
regulatory interactions in network analysis. PLoS One.
8:e821602013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JL, Hu Y, Kong X, Wang ZH, Chen HY,
Xu J and Fang JY: Candidate microRNA biomarkers in human gastric
cancer: A systematic review and validation study. PLoS One.
8:e736832013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang W, Jin Z, Zhou F, Cui J and Wang L
and Wang L: High co-expression of Sp1 and HER-2 is correlated with
poor prognosis of gastric cancer patients. Surg Oncol. 24:220–225.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HW, Wang EW, Li LX, Yi SH, Li LC, Xu
FL, Wang DL, Wu YZ and Nian WQ: A regulatory loop involving miR-29c
and Sp1 elevates the TGF-β1 mediated epithelial-to-mesenchymal
transition in lung cancer. Oncotarget. 7:85905–85916.
2016.PubMed/NCBI
|
|
21
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eto K, Iwatsuki M, Watanabe M, Ida S,
Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N,
et al: The microRNA-21/PTEN pathway regulates the sensitivity of
HER2-positive gastric cancer cells to trastuzumab. Ann Surg Oncol.
21:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai H, Yuan Y, Hao YF, Guo TK, Wei X and
Zhang YM: Plasma microRNAs serve as novel potential biomarkers for
early detection of gastric cancer. Med Oncol. 30:4522013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kourou K, Exarchos TP, Exarchos KP,
Karamouzis MV and Fotiadis DI: Machine learning applications in
cancer prognosis and prediction. Comput Struct Biotechnol J.
13:8–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan L, Liu G, Lin F, Zhong S, Xia H, Sun X
and Liang H: Machine learning applications for prediction of
relapse in childhood acute lymphoblastic leukemia. Sci Rep.
7:74022017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Luo Y, Huang W, Hu D, Zheng RQ,
Cong SZ, Meng FK, Yang H, Lin HJ, Sun Y, et al:
Machine-learning-based classification of real-time tissue
elastography for hepatic fibrosis in patients with chronic
hepatitis B. Comput Biol Med. 89:18–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang B, He X, Ouyang F, Gu D, Dong Y,
Zhang L, Mo X, Huang W, Tian J and Zhang S: Radiomic
machine-learning classifiers for prognostic biomarkers of advanced
nasopharyngeal carcinoma. Cancer Lett. 403:21–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sekar D, Krishnan R, Thirugnanasambantham
K, Rajasekaran B, Islam VIH and Sekar P: Significance of microRNA
21 in gastric cancer. Clin Res Hepatol Gastroenterol. 40:538–545.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu
W, Xiao S and Lu H: miR-21 plays a pivotal role in gastric cancer
pathogenesis and progression. Lab Invest. 88:1358–1366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan SH, Wu CW, Li AF, Chi CW and Lin WC:
miR-21 microRNA expression in human gastric carcinomas and its
clinical association. Anticancer Res. 28:907–911. 2008.PubMed/NCBI
|
|
33
|
Karimi Kurdistani Z, Saberi S, Tsai KW and
Mohammadi M: MicroRNA-21: Mechanisms of Oncogenesis and its
Application in Diagnosis and Prognosis of Gastric Cancer. Arch Iran
Med. 18:524–536. 2015.PubMed/NCBI
|
|
34
|
Wang D, Fan Z, Liu F and Zuo J: Hsa-miR-21
and Hsa-miR-29 in tissue as potential diagnostic and prognostic
biomarkers for gastric cancer. Cell Physiol Biochem. 37:1454–1462.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Zhang X, Li H, Yu J and Ren X: The
role of miRNA-29 family in cancer. Eur J Cell Biol. 92:123–128.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han TS, Hur K, Xu G, Choi B, Okugawa Y,
Toiyama Y, Oshima H, Oshima M, Lee HJ, Kim VN, et al: MicroRNA-29c
mediates initiation of gastric carcinogenesis by directly targeting
ITGB1. Gut. 64:203–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Li F, Zhang Y, Tu Y, Yang Q and
Gao X: Reduced expression of miR-22 in gastric cancer is related to
clinicopathologic characteristics or patient prognosis. Diagn
Pathol. 8:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo MM, Hu LH, Wang YQ, Chen P, Huang JG,
Lu N, He JH and Liao CG: miR-22 is down-regulated in gastric
cancer, and its overexpression inhibits cell migration and invasion
via targeting transcription factor Sp1. Med Oncol. 30:5422013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tan NY and Khachigian LM: Sp1
phosphorylation and its regulation of gene transcription. Mol Cell
Biol. 29:2483–2488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L, Wei D, Huang S, Peng Z, Le X, Wu
TT, Yao J, Ajani J and Xie K: Transcription factor Sp1 expression
is a significant predictor of survival in human gastric cancer.
Clin Cancer Res. 9:6371–6380. 2003.PubMed/NCBI
|
|
41
|
Xiao S, Yang Z, Qiu X, Lv R, Liu J, Wu M,
Liao Y and Liu Q: miR-29c contribute to glioma cells temozolomide
sensitivity by targeting O6-methylguanine-DNA methyltransferases
indirectely. Oncotarget. 7:50229–50238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li C, Song L, Zhang Z, Bai XX, Cui MF and
Ma LJ: MicroRNA-21 promotes TGF-β1-induced epithelial-mesenchymal
transition in gastric cancer through up-regulating PTEN expression.
Oncotarget. 7:66989–67003. 2016.PubMed/NCBI
|
|
43
|
Kou XX, Hao T, Meng Z, Zhou YH and Gan YH:
Acetylated Sp1 inhibits PTEN expression through binding to PTEN
core promoter and recruitment of HDAC1 and promotes cancer cell
migration and invasion. Carcinogenesis. 34:58–67. 2013. View Article : Google Scholar : PubMed/NCBI
|