|
1
|
Linder P and Jankowsky E: From unwinding
to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell
Biol. 12:505–516. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linder P and Fuller-Pace F: Happy
birthday: 25 years of DEAD-box proteins. Methods Mol Biol.
1259:17–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tarn WY and Chang TH: The current
understanding of Ded1p/DDX3 homologs from yeast to human. RNA Biol.
6:17–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YS, Lee SG, Park SH and Song K: Gene
structure of the human DDX3 and chromosome mapping of its related
sequences. Mol Cells. 12:209–214. 2001.PubMed/NCBI
|
|
5
|
Kotov AA, Olenkina OM, Godneeva BK,
Adashev VE and Olenina LV: Progress in understanding the molecular
functions of DDX3Y (DBY) in male germ cell development and
maintenance. Biosci Trends. 11:46–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosner A and Rinkevich B: The DDX3
subfamily of the DEAD box helicases: Divergent roles as unveiled by
studying different organisms and in vitro assays. Curr Med Chem.
14:2517–2525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodamilans B and Montoya G: Expression,
purification, crystallization and preliminary X-ray diffraction
analysis of the DDX3 RNA helicase domain. Acta Crystallogr Sect F
Struct Biol Cryst Commun. 63:283–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
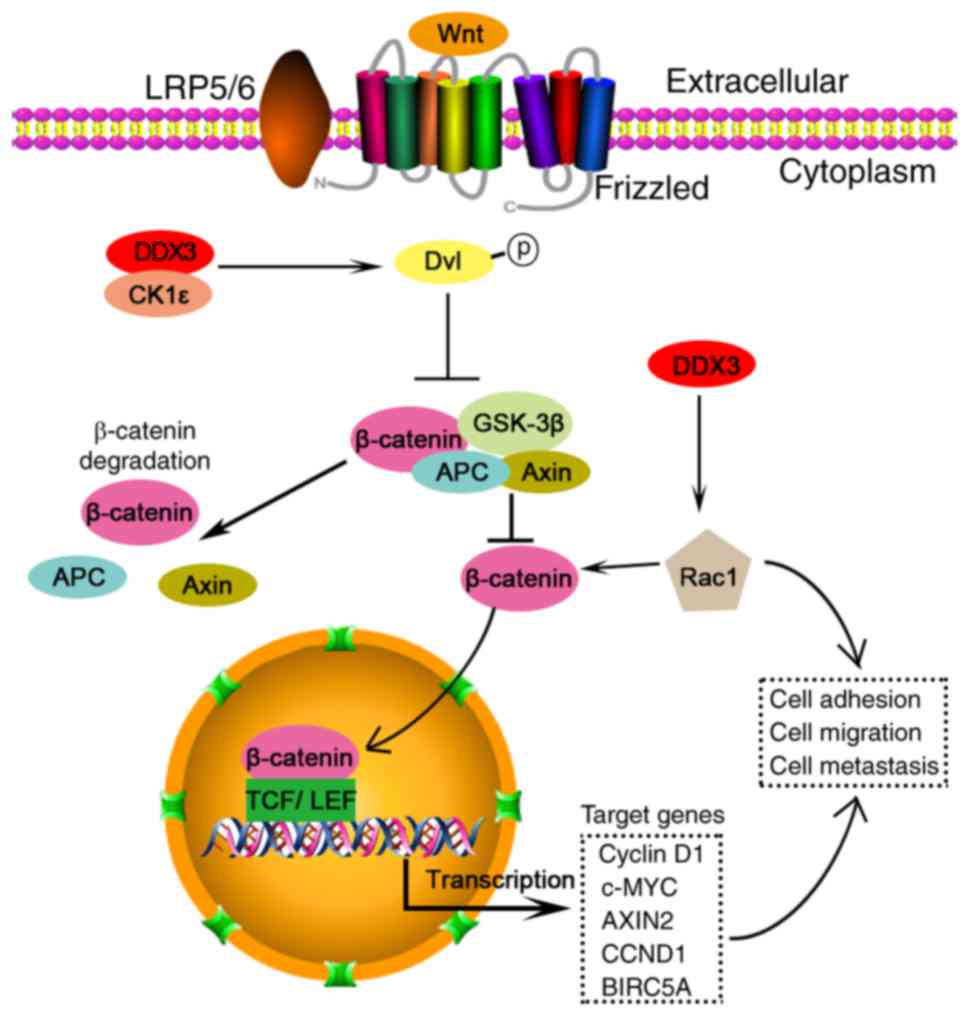
|
Högbom M, Collins R, Van den Berg S,
Jenvert RM, Karlberg T, Kotenyova T, Flores A, Karlsson Hedestam GB
and Schiavone LH: Crystal structure of conserved domains 1 and 2 of
the human DEAD box helicase DDX3X in complex with the
mononucleotide AMP. J Mol Biol. 372:150–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soto-Rifo R and Ohlmann T: The role of the
DEAD-box RNA helicase DDX3 in mRNA metabolism. Wiley Interdiscip
Rev RNA. 4:369–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rocak S and Linder P: DEAD-box proteins:
The driving forces behind RNA metabolism. Nat Rev Mol Cell Biol.
5:232–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Z, Licklider LJ, Gygi SP and Reed R:
Comprehensive proteomic analysis of the human spliceosome. Nature.
419:182–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fröhlich A, Rojas-Araya B,
Pereira-Montecinos C, Dellarossa A, Toro-Ascuy D, Prades-Pérez Y,
García-de-Gracia F, Garcés-Alday A, Rubilar PS, Valiente-Echeverría
F, et al: DEAD-box RNA helicase DDX3 connects CRM1-dependent
nuclear export and translation of the HIV-1 unspliced mRNA through
its N-terminal domain. Biochim Biophys Acta. 1859:719–730. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yedavalli VS, Neuveut C, Chi YH, Kleiman L
and Jeang KT: Requirement of DDX3 DEAD box RNA helicase for HIV-1
Rev-RRE export function. Cell. 119:381–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai MC, Lee YH and Tarn WY: The DEAD-box
RNA helicase DDX3 associates with export mRNPs as well as TAP and
participates in translational control. Mol Biol Cell. 19:3847–3858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao CH, Chen CM, Cheng PL, Shih JW, Tsou
AP and Lee YH: DDX3, a DEAD box RNA helicase with tumor
growth-suppressive property and transcriptional regulation activity
of the p21waf1/cip1 promoter, is a candidate tumor suppressor.
Cancer Res. 66:6579–6588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CS, Dias AP, Jedrychowski M, Patel AH,
Hsu JL and Reed R: Human DDX3 functions in translation and
interacts with the translation initiation factor eIF3. Nucleic
Acids Res. 36:4708–4718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shih JW, Tsai TY, Chao CH and Wu Lee YH:
Candidate tumor suppressor DDX3 RNA helicase specifically represses
cap-dependent translation by acting as an eIF4E inhibitory protein.
Oncogene. 27:700–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh S, Flynn RA, Floor SN, Purzner J,
Martin L, Do BT, Schubert S, Vaka D, Morrissy S, Li Y, et al:
Medulloblastoma-associated DDX3 variant selectively alters the
translational response to stress. Oncotarget. 7:28169–28182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun M, Zhou T, Jonasch E and Jope RS: DDX3
regulates DNA damage-induced apoptosis and p53 stabilization.
Biochim Biophys Acta. 1833:1489–1497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang PC, Chi CW, Chau GY, Li FY, Tsai YH,
Wu JC and Wu Lee YH: DDX3, a DEAD box RNA helicase, is deregulated
in hepatitis virus-associated hepatocellular carcinoma and is
involved in cell growth control. Oncogene. 25:1991–2003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CY, Chan CH, Chen CM, Tsai YS, Tsai
TY, Wu Lee YH and You LR: Targeted inactivation of murine Ddx3×:
Essential roles of Ddx3× in placentation and embryogenesis. Hum Mol
Genet. 25:2905–2922. 2016.PubMed/NCBI
|
|
22
|
Bol GM, Xie M and Raman V: DDX3, a
potential target for cancer treatment. Mol Cancer. 14:1882015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H and Ryu WS: Hepatitis B virus
polymerase blocks pattern recognition receptor signaling via
interaction with DDX3: Implications for immune evasion. PLoS
Pathog. 6:e10009862010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angus AG, Dalrymple D, Boulant S, McGivern
DR, Clayton RF, Scott MJ, Adair R, Graham S, Owsianka AM,
Targett-Adams P, et al: Requirement of cellular DDX3 for hepatitis
C virus replication is unrelated to its interaction with the viral
core protein. J Gen Virol. 9:122–132. 2010. View Article : Google Scholar
|
|
25
|
Huang JS, Chao CC, Su TL, Yeh SH, Chen DS,
Chen CT, Chen PJ and Jou YS: Diverse cellular transformation
capability of overexpressed genes in human hepatocellular
carcinoma. Biochem Biophys Res Commun. 315:950–958. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HK, Mai RT, Huang HD, Chou CH, Chang
YA, Chang YW, You LR, Chen CM and Lee YH: DDX3 Represses stemness
by epigenetically modulating tumor-suppressive miRNAs in
hepatocellular carcinoma. Sci Rep. 6:286372016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Botlagunta M, Vesuna F, Mironchik Y, Raman
A, Lisok A, Winnard P Jr, Mukadam S, Van Diest P, Chen JH,
Farabaugh P, et al: Oncogenic role of DDX3 in breast cancer
biogenesis. Oncogene. 27:3912–3922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Botlagunta M, Krishnamachary B, Vesuna F,
Winnard PT Jr, Bol GM, Patel AH and Raman V: Expression of DDX3 is
directly modulated by hypoxia inducible factor-1 alpha in breast
epithelial cells. PLoS One. 6:e175632011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bol GM, Raman V, van der Groep P,
Vermeulen JF, Patel AH, van der Wall E and van Diest PJ: Expression
of the RNA helicase DDX3 and the hypoxia response in breast cancer.
PLoS One. 8:e635482013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heerma van Voss MR, Schrijver WA, Ter
Hoeve ND, Hoefnagel LD, Manson QF, van der Wall E, Raman V and van
Diest PJ; Dutch distant breast cancer metastases consortium, : The
prognostic effect of DDX3 upregulation in distant breast cancer
metastases. Clin Exp Metastasis. 34:85–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie M, Vesuna F, Botlagunta M, Bol GM,
Irving A, Bergman Y, Hosmane RS, Kato Y, Winnard PT Jr and Raman V:
NZ51, a ring-expanded nucleoside analog, inhibits motility and
viability of breast cancer cells by targeting the RNA helicase
DDX3. Oncotarget. 6:29901–29913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heerma van Voss MR, Brilliant JD, Vesuna
F, Bol GM, van der Wall E, van Diest PJ and Raman V: Combination
treatment using DDX3 and PARP inhibitors induces synthetic
lethality in BRCA1-proficient breast cancer. Med Oncol. 34:332017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu DW, Liu WS, Wang J, Chen CY, Cheng YW
and Lee H: Reduced p21WAF1/CIP1 via alteration of
p53-DDX3 pathway is associated with poor relapse-free survival in
early-stage human papillomavirus-associated lung cancer. Clin
Cancer Res. 17:1895–1905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
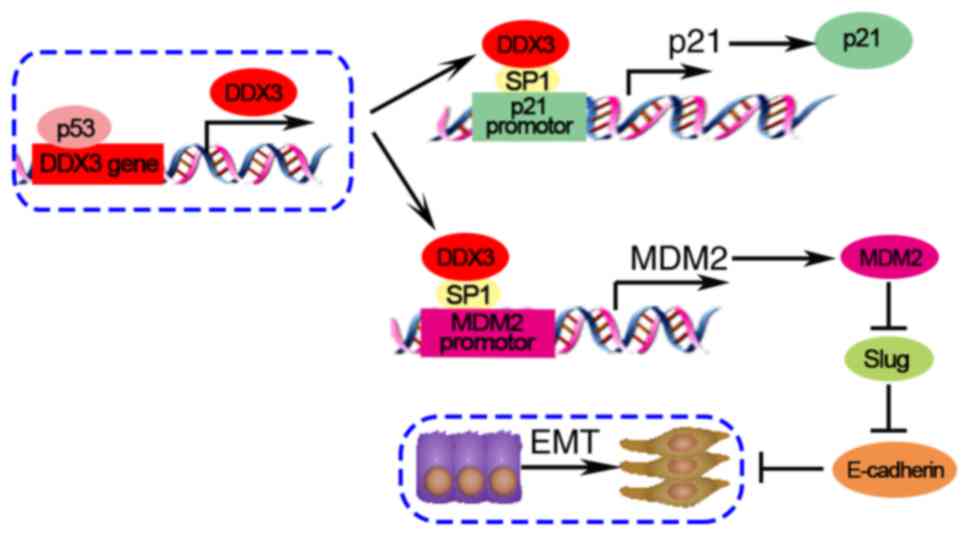
Wu DW, Lee MC, Wang J, Chen CY, Cheng YW
and Lee H: DDX3 loss by p53 inactivation promotes tumor malignancy
via the MDM2/Slug/E-cadherin pathway and poor patient outcome in
non-small-cell lung cancer. Oncogene. 33:1515–1526. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bol GM, Vesuna F, Xie M, Zeng J, Aziz K,
Gandhi N, Levine A, Irving A, Korz D, Tantravedi S, et al:
Targeting DDX3 with a small molecule inhibitor for lung cancer
therapy. EMBO Mol Med. 7:648–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su CY, Lin TC, Lin YF, Chen MH, Lee CH,
Wang HY, Lee YC, Liu YP, Chen CL and Hsiao M: DDX3 as a strongest
prognosis marker and its downregulation promotes metastasis in
colorectal cancer. Oncotarget. 6:18602–18612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He TY, Wu DW, Lin PL, Wang L, Huang CC,
Chou MC and Lee H: DDX3 promotes tumor invasion in colorectal
cancer via the CK1ε/Dvl2 axis. Sci Rep. 6:214832016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu DW, Lin PL, Cheng YW, Huang CC, Wang L
and Lee H: 'KRAS-induced tumor invasion in colorectal cancer via
the β-catenin/ZEB1 axis. Oncotarget. 7:22687–22699. 2016.PubMed/NCBI
|
|
39
|
Wu DW, Lin PL, Wang L, Huang CC and Lee H:
The YAP1/SIX2 axis is required for DDX3-mediated tumor
aggressiveness and cetuximab resistance in KRAS-wild-type
colorectal cancer. Theranostics. 7:1114–1132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heerma van Voss MR, Vesuna F, Trumpi K,
Brilliant J, Berlinicke C, de Leng W, Kranenburg O, Offerhaus GJ,
Bürger H, van der Wall E, et al: Identification of the DEAD box RNA
helicase DDX3 as a therapeutic target in colorectal cancer.
Oncotarget. 6:28312–28326. 2015.PubMed/NCBI
|
|
41
|
Lee CH, Lin SH, Yang SF, Yang SM, Chen MK,
Lee H, Ko JL, Chen CJ and Yeh KT: Low/negative expression of DDX3
may predict poor prognosis in non-smoker patients with oral cancer.
Oral Dis. 20:76–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Samal SK, Routray S, Veeramachaneni GK,
Dash R and Botlagunta M: Ketorolac salt is a newly discovered DDX3
inhibitor to treat oral cancer. Sci Rep. 5:99822015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heerma van Voss MR, van Kempen PM, Noorlag
R, van Diest PJ, Willems SM and Raman V: DDX3 has divergent roles
in head and neck squamous cell carcinomas in smoking versus
non-smoking patients. Oral Dis. 21:270–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, et al: The mutational landscape of head and neck
squamous cell carcinoma. Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilky BA, Kim C, McCarty G, Montgomery EA,
Kammers K, DeVine LR, Cole RN, Raman V and Loeb DM: RNA helicase
DDX3: A novel therapeutic target in Ewing sarcoma. Oncogene.
35:2574–2583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xie M, Vesuna F, Tantravedi S, Bol GM,
Heerma van Voss MR, Nugent K, Malek R, Gabrielson K, van Diest PJ,
Tran PT and Raman V: RK-33 Radio sensitizes prostate cancer cells
by blocking the RNA helicase DDX3. Cancer Res. 76:6340–6350. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun M, Song L, Zhou T, Gillespie GY and
Jope RS: The role of DDX3 in regulating Snail. Biochim Biophys
Acta. 1813:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang S, Yang Z, Li D, Miao X, Yang L, Zou
Q and Yuan Y: The clinical and pathological significance of
nectin-2 and DDX3 expression in pancreatic ductal adenocarcinomas.
Dis Markers. 2015:3795682015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao X, Yang ZL, Xiong L, Zou Q, Yuan Y,
Li J, Liang L, Chen M and Chen S: Nectin-2 and DDX3 are biomarkers
for metastasis and poor prognosis of squamous cell/adenosquamous
carcinomas and adenocarcinoma of gallbladder. Int J Clin Exp
Pathol. 6:179–190. 2013.PubMed/NCBI
|
|
50
|
Pugh TJ, Weeraratne SD, Archer TC,
Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter
SL, Cibulskis K, Erlich RL, et al: Medulloblastoma exome sequencing
uncovers subtype-specific somatic mutations. Nature. 488:106–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Robinson G, Parker M, Kranenburg TA, Lu C,
Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, et al: Novel
mutations target distinct subgroups of medulloblastoma. Nature.
488:43–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY,
Zhang ZG, Pan CM, Hu Y, Cai CP, Dong Y, et al: Exome sequencing
identifies somatic mutations of DDX3X in natural killer/T-cell
lymphoma. Nat Genet. 47:1061–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang L, Lawrence MS, Wan Y, Stojanov P,
Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L,
et al: SF3B1 and other novel cancer genes in chronic lymphocytic
leukemia. N Engl J Med. 365:2497–2506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ojha J, Secreto CR, Rabe KG, Van Dyke DL,
Kortum KM, Slager SL, Shanafelt TD, Fonseca R, Kay NE and Braggio
E: Identification of recurrent truncated DDX3X mutations in chronic
lymphocytic leukaemia. Br J Haematol. 169:445–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cruciat CM, Dolde C, de Groot RE, Ohkawara
B, Reinhard C, Korswagen HC and Niehrs C: RNA helicase DDX3 is a
regulatory subunit of casein kinase 1 in Wnt-β-catenin signaling.
Science. 339:1436–1441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen HH, Yu HI, Cho WC and Tarn WY: DDX3
modulates cell adhesion and motility and cancer cell metastasis via
Rac1-mediated signaling pathway. Oncogene. 34:2790–2800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu
J, Zhang P, Zhu H, Xu N and Liang S: STC2 promotes the
epithelial-mesenchymal transition of colorectal cancer cells
through AKT-ERK signaling pathways. Oncotarget. 7:71400–71416.
2016.PubMed/NCBI
|
|
58
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun M, Song L, Li Y, Zhou T and Jope RS:
Identification of an antiapoptotic protein complex at death
receptors. Cell Death Differ. 15:1887–1900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen B, Li H, Zeng X, Yang P, Liu X, Zhao
X and Liang S: Roles of microRNA on cancer cell metabolism. J
Transl Med. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen Y, Fu LL, Wen X, Liu B, Huang J, Wang
JH and Wei YQ: Oncogenic and tumor suppressive roles of microRNAs
in apoptosis and autophagy. Apoptosis. 19:1177–1189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao L, Mao Y, Zhao Y and He Y: DDX3X
promotes the biogenesis of a subset of miRNAs and the potential
roles they played in cancer development. Sci Rep. 6:327392016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Valiente-Echeverría F, Hermoso MA and
Soto-Rifo R: RNA helicase DDX3: At the crossroad of viral
replication and antiviral immunity. Rev Med Virol. 25:286–299.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Soulat D, Bürckstümmer T, Westermayer S,
Goncalves A, Bauch A, Stefanovic A, Hantschel O, Bennett KL, Decker
T and Superti-Furga G: The DEAD-box helicase DDX3X is a critical
component of the TANK-binding kinase 1-dependent innate immune
response. EMBO J. 27:2135–2146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gu L, Fullam A, Brennan R and Schröder M:
Human DEAD box helicase 3 couples IκB kinase ε to interferon
regulatory factor 3 activation. Mol Cell Biol. 33:2004–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang X, Wang R, Luo M, Li C, Wang HX, Huan
CC, Qu YR, Liao Y and Mao X: (DEAD)-box RNA helicase 3 modulates
NF-κB signal pathway by controlling the phosphorylation of PP2A-C
subunit. Oncotarget. 8:33197–33213. 2017.PubMed/NCBI
|
|
70
|
Xiang N, He M, Ishaq M, Gao Y, Song F, Guo
L, Ma L, Sun G, Liu D, Guo D and Chen Y: The DEAD-box RNA helicase
DDX3 interacts with NF-κB subunit p65 and suppresses p65-mediated
transcription. PLoS One. 11:e01644712016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dunford A, Weinstock DM, Savova V,
Schumacher SE, Cleary JP, Yoda A, Sullivan TJ, Hess JM, Gimelbrant
AA, Beroukhim R, et al: Tumor-suppressor genes that escape from
X-inactivation contribute to cancer sex bias. Nat Genet. 49:10–16.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Backus KM, Correia BE, Lum KM, Forli S,
Horning BD, González-Páez GE, Chatterjee S, Lanning BR, Teijaro JR,
Olson AJ, et al: Proteome-wide covalent ligand discovery in native
biological systems. Nature. 534:570–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Radi M, Falchi F, Garbelli A, Samuele A,
Bernardo V, Paolucci S, Baldanti F, Schenone S, Manetti F, Maga G
and Botta M: Discovery of the first small molecule inhibitor of
human DDX3 specifically designed to target the RNA binding site:
Towards the next generation HIV-1 inhibitors. Bioorg Med Chem Lett.
22:2094–2098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yedavalli VS, Zhang N, Cai H, Zhang P,
Starost MF, Hosmane RS and Jeang KT: Ring expanded nucleoside
analogues inhibit RNA helicase and intracellular human
immunodeficiency virus type 1 replication. J Med Chem.
51:5043–5051. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Botlagunta M, Kollapalli B, Kakarla L,
Gajarla SP, Gade SP, Dadi CL, Penumadu A and Javeed S: In vitro
anti-cancer activity of doxorubicin against human RNA helicase,
DDX3. Bioinformation. 12:347–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tutt A, Robson M, Garber JE, Domchek SM,
Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler
RK, et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in
patients with BRCA1 or BRCA2 mutations and advanced breast cancer:
A proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schwertman P, Bekker-Jensen S and Mailand
N: Regulation of DNA double-strand break repair by ubiquitin and
ubiquitin-like modifiers. Nat Rev Mol Cell Biol. 17:379–394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bol GM, Khan R, Heerma van Voss MR,
Tantravedi S, Korz D, Kato Y and Raman V: PLGA nanoparticle
formulation of RK-33: An RNA helicase inhibitor against DDX3.
Cancer Chemother Pharmacol. 76:821–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Danhier F, Lecouturier N, Vroman B, Jérôme
C, Marchand-Brynaert J, Feron O and Préat V: Paclitaxel-loaded
PEGylated PLGA-based nanoparticles: In vitro and in vivo
evaluation. J Control Release. 133:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|