Introduction
Traditional cancer treatments such as surgery,
radiation therapy and drug treatments, present certain limitations.
As a new treatment strategy, biological immunotherapy has become
the fourth leading tumor-therapy approach. At present, the commonly
used immunotherapy methods include adoptive immunotherapy (T and NK
cells), cytokine, gene and antitumor antibody therapy,
immunological checkpoint (PD-1/PDL1, CTLA-4) blockade therapy and
cancer vaccines (1). Cancer
vaccines, by expressing specific and immunogenic tumor antigens
(polypeptides, DNA and RNA), activate or enhance the antitumor
immunity of the body with the aid of adjuvants such as cytokines
and chemokines to kill and remove tumor cells (2). Cancer vaccines can be divided into the
following categories, according to the different types of antigens:
tumor cell, dendritic cell (DC), peptide and nucleic acid vaccines
(3). Unlike prophylactic vaccines,
commonly used in healthy individuals, therapeutic cancer vaccines
administered to cancer patients are designed to eliminate cancer
cells by enhancing the immune response of the patient (4). This therapeutic vaccination mobilizes
a variety of immune mechanisms to specifically attack and destroy
cancer cells. Thus, compared with traditional treatments, cancer
vaccines, in principle, may be used to inhibit further growth of
advanced cancers and/or recurrent tumors.
The application of nanotechnology has become popular
in cancer vaccine research in recent years (5). Due to their biosafety capacity for
site-specific delivery of antigens and enhancement of the
bioavailability of antigens, nanomaterials are widely used as
cancer vaccine-carriers or adjuvants (6). Nanomaterials can deliver targeted
antigens and adjuvants (7), prevent
their rapid degradation (8) and
increase the retention time of tumor antigens in lymphatic and
tumor tissues (9), thereby
increasing the efficacy and safety of vaccines. Cancer vaccines
produce immune effects that firstly require antigen-presenting
cells (APCs), especially DCs, to effectively ingest and present
tumor-associated antigens (TAAs) (10). Soluble proteins are generally not
easily adsorbed by APCs, but antigen-loaded nanoparticles are
comparable in size to pathogens and are more easily recognized and
ingested by APCs, thereby improving the immunogenicity of vaccines
(11). Currently, some
nanomaterials have demonstrated good carrier or adjuvant activity
in animal studies (12). In the
present review, we present the functions and applications of
nanomaterials in cancer vaccines. The various functions of
nanomaterials in cancer vaccines are depicted in Fig. 1. The advantages and disadvantages of
different cancer vaccines and the corresponding types and functions
of the nanoparticles in the vaccines are summarized in Table I.
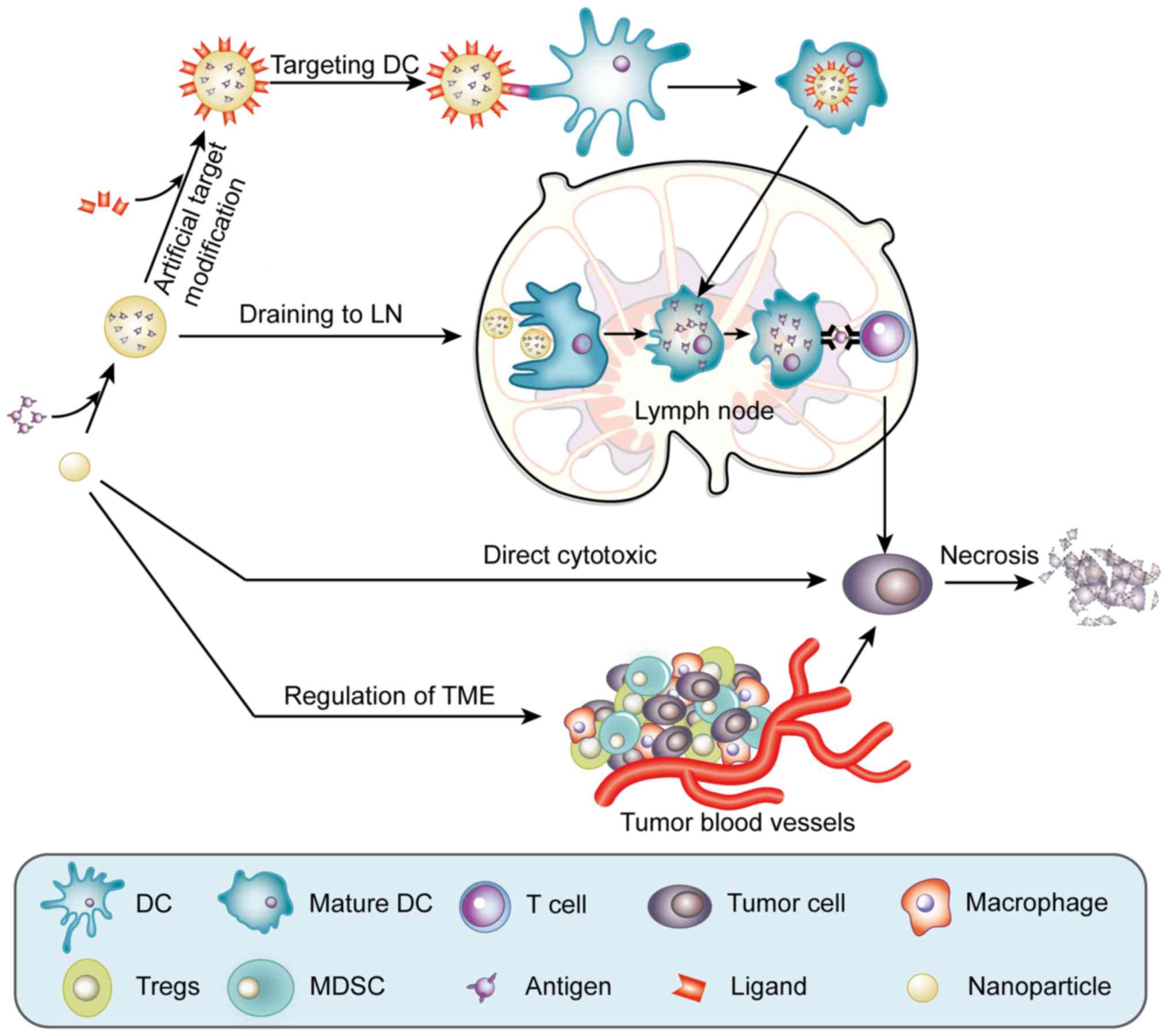 | Figure 1.Functions of multifunctional
nanomaterials in cancer vaccines. Nanoparticles can encapsulate
tumor antigens to protect them from degradation. After the surface
of a nanoparticle is modified with the relevant targeting ligand, a
nanovaccine can target DCs. After peripheral immature DC intake of
nanovaccines, the DC matures and migrates to the lymph nodes. Some
nanoparticles can enhance the drainage of the vaccine to the lymph
nodes, which increases the concentration of nanovaccines in the
lymph nodes and is conducive to DC uptake of the vaccine. In the
lymph nodes, the nanovaccine is presented to T cells, producing
antigen-specific cellular immunity. Furthemore, some nanomaterials
can directly kill tumor cells. In addition, some nanoparticles can
inhibit the development of tumor cells by regulating the TME. DC,
dendritic cell; LN, lymph node; Tregs, regulatory T cells; MDSC,
myeloid-derived suppressor cell. |
 | Table I.Summary of multifunctional
nanomaterials for cancer vaccines. |
Table I.
Summary of multifunctional
nanomaterials for cancer vaccines.
| Cancer
vaccines | Advantages | Deficiencies | Tumor antigens | Function of
nanomaterials | Nano-materials | Refs. |
|---|
| Tumor cell
vaccine | 1) Provide a
variety of tumor antigens and broad epitopes | 1) Presence of
self-antigens | Whole-cell
antigen | PLGA | Combined with
cancer cell membrane/Delivery of immunoadjuvant | (43,44) |
|
| 2) Comprehensive
specific response | 2) Difficult to
produce industrially |
|
|
|
|
|
|
| 3) Poor
immunogenicity |
|
|
|
|
| Dendritic cell
vaccine | 1) Load many types
of antigens | 1) Complicated
preparation process | DNA | Calcium
phosphate | Delivery of antigen
to DC | (48,49) |
|
| 2) High
presentation efficiency | 2) High cost |
| NPs |
|
|
|
| 3) Could break the
immune tolerance |
| Protein | γ-PGA |
|
|
| Peptide
vaccine | 1) Ease of
production | 1) Subject to
MHC-specificity | Peptide | LZnP NPs | Co-delivery of
peptides and adjuvant | (52) |
|
| 2) High immune
specificity | 2) Poor |
| PELC | Protecting the
antigen/Co-delivery of peptides and adjuvant | (13) |
|
| 3) Good
stability | 3) Easy to produce
immune tolerance |
| Liposome | Co-delivery of
multiple immunostimulants | (16) |
|
|
|
|
| Albumin | Efficient draining
to lymph nodes/Co-delivery of peptides and adjuvant | (9) |
|
|
|
|
| PLGA | Delivery of
antigen/Promoting cross-presentation and lysosomal escape | (23,24) |
| Genetic
vaccine | 1) Ease of
production | 1) Poor delivery
efficiency | DNA | ACNPs | Delivery of
gene | (54) |
|
| 2) Polyvalent
vaccine | 2) Potential safety
issues |
|
|
|
|
|
| 3) Multi-path to
produce CTLs | 3) Could induce
immune tolerance |
|
|
|
|
Multifunctional nanomaterials
Protect and delay antigen release
Relative to conventional cancer vaccines, some
nanomaterials can protect antigens from enzyme-mediated degradation
and are able to delay the release of antigens, which is conducive
to the continued role of the antigen (8).
Song et al (13) combined a PEG-b-PLACL aqueous
solution with Span®85 and squalene to form a stable
isotropic PELC emulsion. Subsequently, the pre-emulsified material
was dispersed in RAH polypeptide antigen solution to form uniform
nanoparticles. Experiments revealed that the PELC formulation could
delay the release of antigens in vitro and in vivo.
PELC formulation protected the antigen from rapid degradation and
enhanced the effectiveness of the vaccine. Furthermore, the vaccine
effectively increased the number of CD4+ T cells and
RAH-specific CD8+ T cells, promoted the secretion of
IFN-γ, enhanced the cytotoxic T-cell response and antitumor effect
and inhibited tumor growth in mice.
Co-delivery of the antigen and the
adjuvant
In cancer vaccine immunotherapy, simultaneous
delivery of the antigen and the adjuvant can improve the efficiency
of cancer vaccines (14).
Nanomaterials deliver antigens into the body, activate the immune
system and induce cellular and humoral immune responses. The main
functions of the adjuvant are to accelerate a lasting immune
response, reduce the dosage of the antigen and activate a specific
cellular immune response.
Standley et al (15) synthesized nanoparticles with
acrylamide as a monomer, that can degrade in the acidic lysosomal
environment and covalently bind CpG and encapsulate ovalbumin (OVA)
to co-deliver antigen and CpG. Compared with free OVA, OVA-NPs or
OVA + CpG, DCs can significantly increase the expression of IL-12
and costimulatory molecules, resulting in stronger antigen-specific
T-cell immune responses. Fox et al (16) used liposomes to carry the
TLR4-agonist glucopyranoside A and the TLR7-agonist imiquimod. The
Th1 phenotype of CD4+ T cells increased significantly
compared with the use of a single agonist. This finding indicates
that nanoparticles that co-deliver multiple immunostimulants
possess a synergistic effect.
Efficient draining to lymph nodes
Lymph nodes located in lymphatic drainage pathways
play an important role in the humoral immune response and immune
cell activity (17). Nanocarriers
can effectively drain the vaccine to the lymphoid tissue, increase
the concentration of the vaccine in the lymph nodes, prolong the
residence time of the vaccine in lymphoid tissue and control the
release of the vaccine and the adjuvant. This function facilitates
the antigen presentation and induces T-cell activation to enhance
the effects of cancer vaccines.
Liu et al (9)
used albumin as a carrier to target a cancer vaccine to the lymph
nodes. In vivo, serum albumin is abundant and can transport
fatty acids from the blood into lymphatic vessels and lymph nodes.
Using this function of albumin, it was conjugated with a lipid, a
peptide antigen and a CpG to form an amphiphilic molecular cancer
vaccine (AMPH vaccine) that was inoculated into tumor-bearing mice.
After inoculation of the structurally optimized CpG-DNA/AMPH
vaccine in mice, it significantly accumulated in the lymph nodes,
thereby enhancing the antitumor effect of T cells, while
significantly reducing systemic toxicity.
Targeting DCs
APCs are of critical importance in transporting
antigens from peripheral circulation to local organized lymphoid
tissue. DCs are the most powerful APCs and are at the heart of
initiation, regulation and maintenance of immune responses. They
can present antigens through MHC class I and MHC class II molecular
pathways, provide sufficient co-stimulatory signals and play a key
role in initiation and regulation of the T-cell antitumor immune
response. DCs express many surface receptors, such as mannose,
DEC-205, CD40, CD11c and DC-SIGN receptors (18). The surface of nanoparticles
encapsulating an antigen can bind to targeting ligands (mannose,
anti-CD11c and anti-DEC205) to specifically deliver the antigen to
DCs, thereby promoting binding and endocytosis of the targeting
ligands to enhance the effect of the vaccine (19,20).
Guo et al (20) developed erythrocyte
membrane-enveloped PLGA nanoparticles containing the antigenic
peptide (hgp10025-33) and the TLR4 agonist monophosphoryl lipid A
(MPLA). The nanoparticle surface was mannose-loaded, allowing the
nanoparticles to be actively targeted by lymphoid organ DCs and
promote accumulation in the lymph nodes. The vaccine was inoculated
into melanoma-bearing mice and prolonged the antitumor time effect,
inhibited tumor growth and metastasis and effectively increased
IFN-γ secretion and CD8+ T cell response.
Promoting cross-presentation and
lysosomal escape
Antigens in cancer vaccines are exogenous antigens.
In theory, the antigens activate CD4+ T cells through an
MHC II presentation pathway in vivo. However, the antitumor
effect is mainly produced by activation of the CD8+ T
cell-mediated immunity and CD4+ T cells play a
supporting role (21). Some
nanovaccines can release antigens into the cytoplasm after being
taken up by DCs. Subsequently the antigen escapes degradation by
lysosomes and thus, can be presented by MHC class I molecules
through the endogenous pathway and the CD8+ T cells can
be activated. Exogenous antigens are presented via MHC I molecules
instead of MHC II molecules, which is called cross-presentation
(22) (Fig. 2). After entering the cell, the
antigen is not damaged by the acidic lysosomal environment and the
large number of enzymes and may produce biological effects to
activate CD8+ cytotoxic T cells through
cross-presentation to kill cancer cells. Therefore, it is important
to assist the antigen escape from lysosomes in order to improve the
vaccine effect. Nanocarriers are often designed based on this
premise.
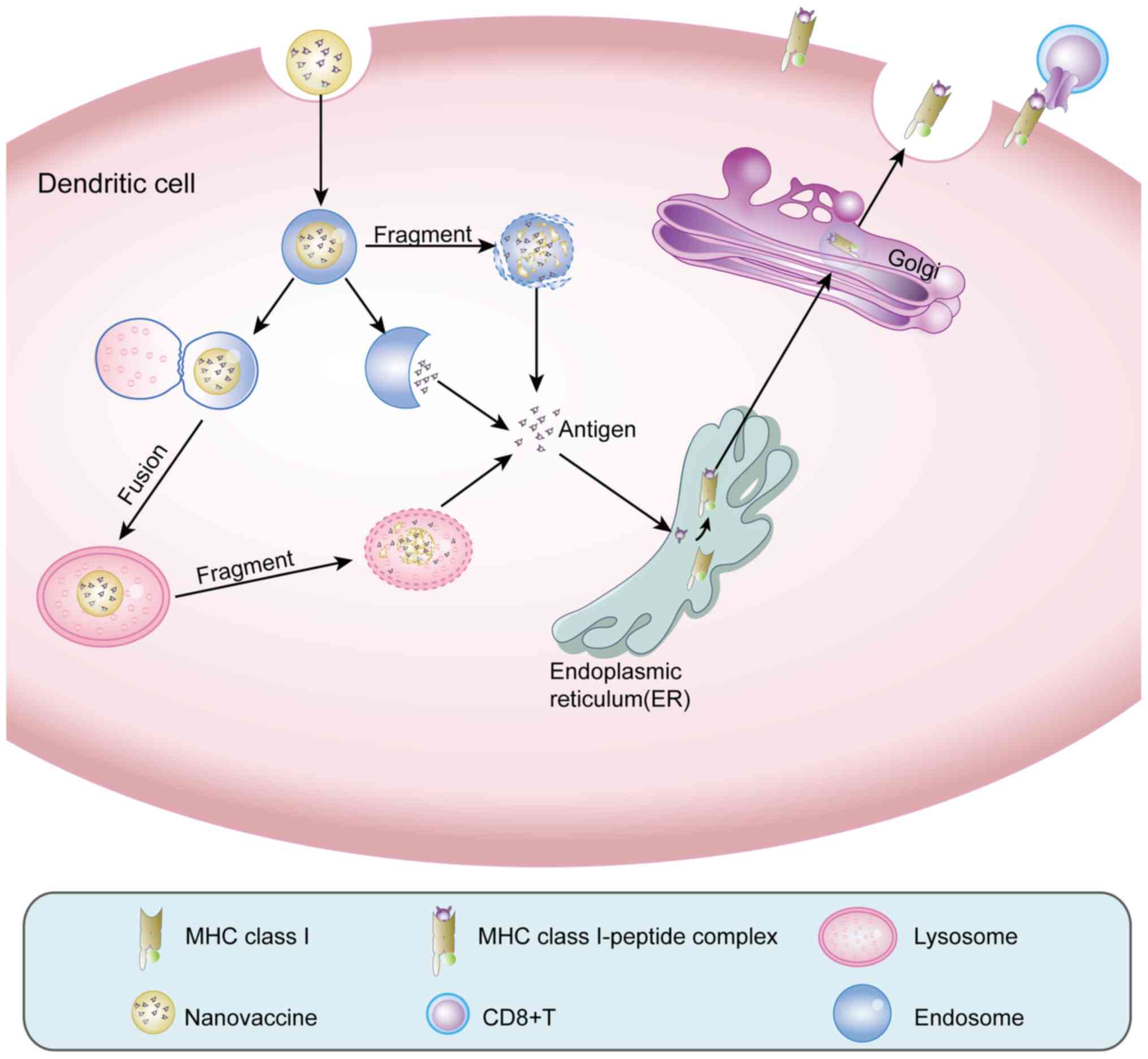 | Figure 2.Lysosomal escape and
cross-presentation. Nanovaccines are phagocytosed by DCs into
endosomes. On one hand, certain nanomaterials can destroy the
endosomal membrane or fuse with the endosome to release the antigen
into the cytoplasm. On the other hand, the endosome fuses with the
lysosomal membrane, delivering the nanovaccine into the lysosome.
In the acidic environment of the lysosome, nanomaterials turn the
lysosome into a proton sponge, destroying the lysosome and
releasing the antigen into the cytoplasm. Through these effects,
the exogenous antigens in the nanovaccine are converted into
endogenous antigens. Thus, the antigen can enter the endoplasmic
reticulum and bind to MHC I molecules, which are delivered to the
Golgi apparatus for further modification and are finally expressed
on the cell surface and recognized by CD8+ T cells and
thus, activate CD8+ T cells. DCs, dendritic cells. |
Shen et al (23) demonstrated that PLGA nanoparticles
can enhance exogenous antigen escape from the endosome to the
cytoplasm, leading to presentation of the antigen in conjunction
with MHC I molecules through cross-presentation. Saluja et
al (24) encapsulated the
MART-127-35 peptide (melanoma-associated antigen) with PLGA
nanoparticles and modified the nanoparticle surface with DEC-205
ligands to obtain a nanovaccine capable of targeting DCs. The
results indicated that PLGA nanoparticles promoted the antigen
uptake by DCs and facilitated the lysosomal escape, thus promoting
cross-presentation of the exogenous antigens as well as activation
and proliferation of CD8+ T cells.
Yuba et al (25) synthesized highly pH-sensitive
liposomes that were stable at neutral pH and sensitive to slight
changes in pH in a weakly acidic environment, which rendered them
unstable. The nanomaterials were combined with OVA to treat
tumor-bearing mice. The nanovaccine could effectively be taken up
by DCs, where it then fused with the endosomal membrane in the
acidic environment and was released into the cytoplasm, leading to
cross-presentation, which effectively induced an antigen-specific
cellular immune response.
Regulation of the tumor
microenvironment (TME)
TME refers to the internal environment of tumors,
consisting of tumor, stromal and immune cells, microvessels and
interstitium and is infiltrated by biological molecules (26). Interactions between the TME and
tumor cells have an important effect on tumor growth and metastasis
(27). Due to the special physical
and chemical properties of nanoparticles, which can be enriched in
the TME, tumor blood vessels have enhanced permeability and
retention (EPR) (28).
Nanoparticles in the TME can regulate the cellular and non-cellular
components and then produce antitumor effects.
Targeting tumor-associated macrophages
(TAMs)
TAMs are macrophages that have infiltrated tumor
tissue and are the most abundant immune cells in the TME. Via
cytokines in the TME, macrophages differentiate into different
types of TAMs, mainly divided into the M1 and M2 type (29). M1 TAMs can release a variety of
pro-inflammatory cytokines, immune activators and chemokines and
play an antitumor role through induction of acute inflammation,
immune activation and phagocytosis via the following mechanisms: i)
release of NO and ROS and direct elimination of tumor cells; ii)
antibody-dependent cell-mediated cytotoxicity (ADCC); and iii)
induction of a specific immune response (29). M2 type TAMs exert immunosuppressive
effects through the following mechanisms: i) secretion of
cytokines, such as CXCL-8 and IL-10, that promote tumor cell growth
(30); ii) production of matrix
metalloproteinases (MMPs) that promote tumor invasion and
metastasis; iii) secretion of cytokines, such as IL-10 and TGF-β,
that inhibit T-cell activation and promote the differentiation of
regulatory T cells (Tregs) (31);
and iv) secretion of VEGF, PDGF, and MMPs that promote
neovascularization of tumor cells (32) (Fig.
3).
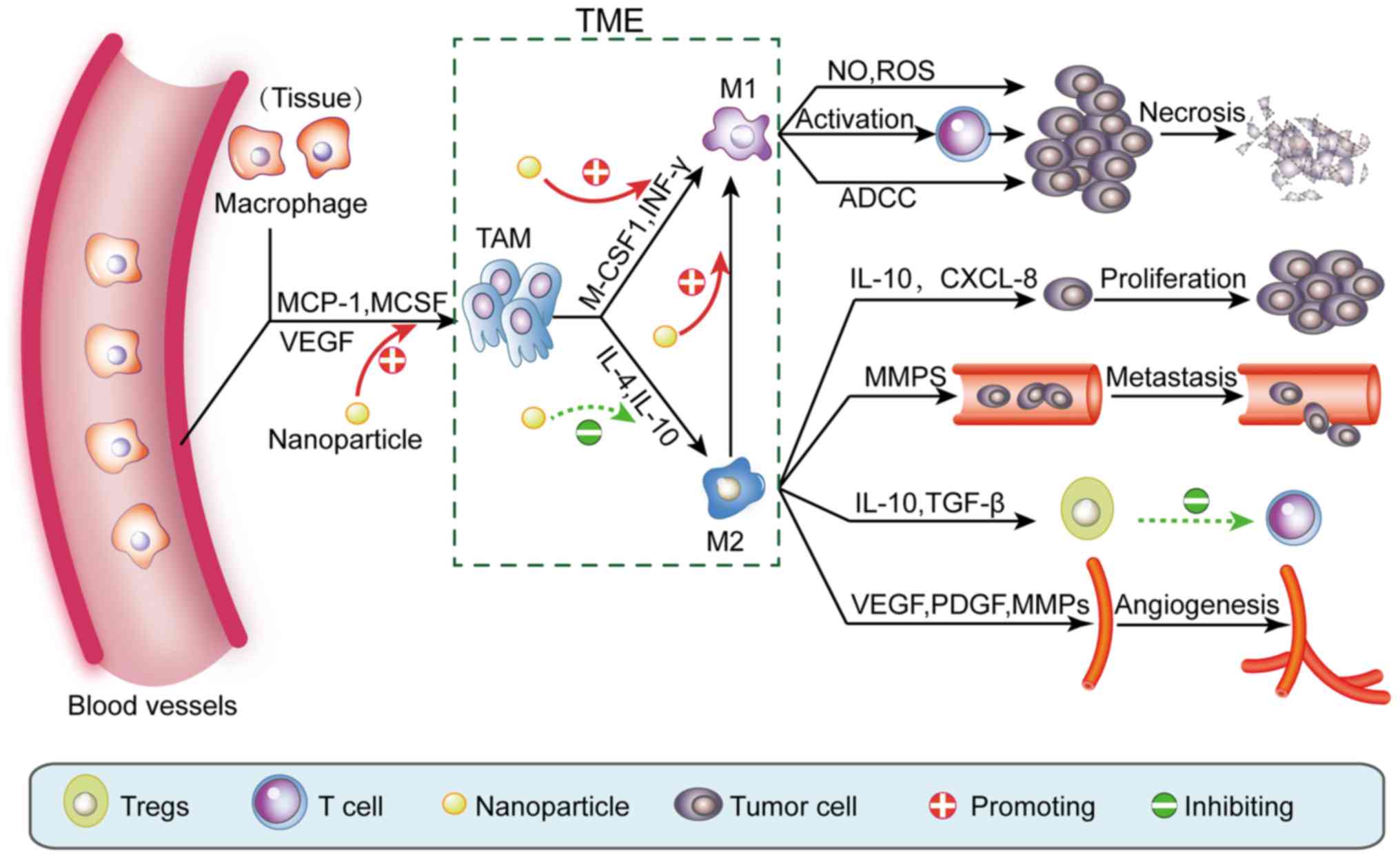 | Figure 3.Nanomaterials inhibit tumors by
regulating TAMs in the TME. Under the action of cytokines, blood
vessels and tissue macrophages are recruited into tumor tissue and
become TAMs. Similarly, under stimulation with different cytokines,
TAMs are polarized to pro-inflammatory M1 macrophages or
anti-inflammatory M2 macrophages. M1 macrophages have antitumor
effects such as: direct elimination of tumor cells through the
release of NO and ROS, ADCC and activation of T cells. In contrast,
M2 macrophages can promote tumor development through i) secretion
of cytokines, such as IL-10 and CXCL-8, which directly promote the
growth of tumor cells; ii) secretion of MMPs that promote tumor
cell infiltration and metastasis; iii) secretion of IL-10 and
TGF-β, which promote Treg differentiation, thereby inhibiting
T-cell activation; and iv) secretion of VEGF, PDGF and MMPs that
promote tumor neovascularization. Nanomaterials can promote the
recruitment of macrophages in the TME and induce TAM polarization
to the M1 rather than the M2 phenotype. Furthermore, nanomaterials
can also promote transformation of M2 macrophages to M1
macrophages. TAMs, tumor-associated macrophages; ADCC,
antibody-dependent cell-mediated cytotoxicity; MMPs, matrix
metalloproteinases; TGF-β, transforming growth factor β; VEGF,
vascular endothelial growth factor; PDGF, platelet-derived growth
factor; TME, tumor microenvironment. |
Zanganeh et al (33) found ‘hidden functions’ of
ferumoxytol, which has been approved by the FDA for the treatment
of anemia, such as the production of antitumor effects by
modulating TAMs in the TME, while being non-toxic to normal cells.
Experiments revealed that ferumoxytol can recruit more macrophages
and induce M1 polarization. Notably, apoptotic cancer cells
continue to induce M1 polarization to form an autocrine feedback
loop which maintains the production of tumor cytotoxic substances,
thereby killing tumor cells. In vivo, local and systemic
inoculation of the nanoparticles significantly inhibited tumor cell
growth as well as treated and prevented distant metastasis of the
tumor. Notably, the experiments clarified that the anticancer
mechanism of ferumoxytol includes the promotion of macrophage
recruitment for M1 macrophage polarization, inhibiting M2
macrophages.
Targeting tumor vasculature
The rapid growth of tumors requires
neovascularization to provide adequate nutrient supply. Tumor
neovascularization is dependent on angiogenic factors, mainly
vascular endothelial growth factor (VEGF) and platelet-derived
growth factor (PDGF), which are widely overexpressed in tumors
(34). Therefore, regulation of
angiogenic factors can inhibit tumor growth. Some nanomaterials can
modulate some of the components of the TME without the need for
binding biomolecules. Numerous studies have revealed that
gadolinium-containing fullerene nanoparticles are not toxic to
normal cells, however inhibit tumor growth and metastasis (35,36).
Metal fullerene nanoparticles can act on multiple angiogenic
factors concurrently, effectively inhibiting angiogenesis and
decreasing the microvessel density in tumors (35). In addition, they significantly
decreased the activity of MMPs and promoted the formation of a
fiber cage, which can block signal transduction between TAMs and
tumor cells (36).
Nanoparticles can kill tumor
cells
Some nanomaterials, due to their particular physical
and chemical properties, can kill cancer cells under certain
conditions (37,38). The photothermal effect of certain
nanomaterials, for instance, has become a topic of great interest
in cancer treatment studies (37,39).
Photothermal therapy refers to the use of a material with a high
photothermal conversion efficiency that is injected into the body
and under irradiation of an external light source (usually near
infrared light), the light energy is converted into heat energy
that kills cancer cells.
Guo et al (37) designed HCuSNPs for the combination
of photothermal therapy and immunotherapy. After the recombination,
the compound is more stable, easily remains at the tumor site and
is easily taken up by DCs. Furthermore, photothermal ablation
induced apoptosis of local cancer cells and some tumor antigens
were released into the surrounding environment and synergized with
chitosan-CPG nanocomplexes to activate antigen-specific antitumor
immune responses. Similarly, Duan et al (38) revealed that Zn-pyrophosphate (ZnP)
nanoparticles loaded with the photosensitizer pyrolipid (ZnP@pyro)
can induce apoptosis via light irradiation, destroy the tumor
vasculature and increase tumor immunogenicity.
Application of nanotechnology in cancer
vaccines
Tumor cell vaccines
Tumor cell vaccines include autologous tumor whole
cell vaccines and allogeneic tumor cell vaccines. Autologous tumor
cell vaccines are prepared by isolating tumor cells from patients,
processing them into vaccines in vitro, and then
administering the vaccine to the same patient (40). Allogeneic tumor cell vaccines, which
often contain two or three established human tumor cell lines, can
be used to overcome many of the limitations of autologous tumor
cell vaccines (41). Tumor cell
vaccines can present all the tumor antigens, which induce an immune
response to the tumor antigens. There are many tumor antigens on
the cancer cell membrane surface and it is difficult to achieve
these functions through traditional synthesis technology, although
this would be an ideal vaccine (42). However, due to the low
immunogenicity of tumor vaccines alone, they can only stimulate a
small immune response in the body and therefore, it is essential to
find a suitable adjuvant to enhance the immunogenicity of a tumor
cell vaccine (43).
Fang et al (43) combined purified tumor cell membrane
with PLGA nanoparticles to form cancer cell membrane-coated
nanoparticles (CCNPs). Combination of monophosphoryl lipid A (MPLA)
with CCNPs in a vaccine promoted the maturation of DCs, the uptake
of antigen and induced immune activation. Similarly, Liu et
al (44) designed a
multi-adjuvant whole cell tumor vaccine (WCTV) based on PLGA
nanoparticles modified with a cell penetrating peptide, which could
promote transportation of GM-CSF and IL-2 into tumor cells. The
experimental data revealed that the compound adjuvants promoted DC
recruitment, antigen presentation and T-cell activation.
Dendritic cell vaccines
The use of DCs as the carrier in a DC cancer vaccine
is recognized as one approach for tumor immunotherapy. A tumor
antigen-loaded DC vaccine is considered to have enhanced potential
for tumor immunotherapy (45).
However, these vaccines mainly use virus or virus-like particles
(VLPs) as carriers to transfer antigens into DCs. Thus, they often
cause an associated immune response, producing specific
neutralizing antibodies to prevent viral infection and therefore,
their use is risk-bearing (46).
Because of their unique physicochemical properties, nanomaterials
have exhibited great potential as vaccine carriers/adjuvants to
enhance the immunogenicity of antigens by promoting antigen uptake
by DCs, protecting antigens from enzymatic degradation and
regulating immune cells (47).
Zeng et al (48) transfected DCs with AFP1 cDNA (with a
signal peptide) and AFP2 cDNA (without a signal peptide) using
calcium phosphate nanoparticles and induced maturation with
recombinant mouse granulocyte macrophage colony-stimulating factor
(rmGM-CSF), creating a DC vaccine. The in vivo and in
vitro results revealed that the DC vaccine induced activation
and proliferation of T lymphocytes to promote secretion of the
cytokine IFN-γ, which induced a highly effective and specific
immune response to liver cancer. In addition, Matsuo et al
(49) encapsulated OVA in
biodegradable nanoparticles (γ-PGA NPs) and delivered it to DCs
in vitro. The in vivo experimental results revealed
that the use of OVA/γ-PGA NP-pulsed DCs induced TAA-specific CTLs,
resulting in a strong antitumor effect.
Peptide vaccines
Cancer peptide vaccines have the advantage of direct
stimulation, high specificity of the immune response, no autoimmune
response or immunosuppression, as well as lack of carcinogenic risk
and have wide prospective applications (50). However, cancer peptide vaccines also
have shortcomings, such as weak immunogenicity, a short half-life
and being prone to immune tolerance. Nanomaterials can be used as
carriers in cancer peptide vaccines and as vehicles for co-delivery
of antigens and immune adjuvants to target the immune system, which
increases peptide vaccine immune function (51).
Zhuang et al (52) used lipid-coated zinc phosphate
hybrid nanoparticles (LZnP NPs) to deliver polypeptides
(TRP2180-188 and HGP10025-33) and Toll-like receptor 4 agonists.
The combination of H-2K (b) and H-2D (b)-restricted peptides
provided multiple epitopes as targets of specific MHC alleles and
then, the immune system was better able to monitor the tumor. The
data revealed that the LZnP nanovaccine increased the secretion of
cytokines and the population of CD8+ T cells with IFN-γ
secretion. The antitumor effect of the nanovaccine was significant
in the treatment and prevention of a melanoma-mouse model compared
with free antigens and a single peptide-loaded nanovaccine
(52).
Genetic vaccines
Genetic vaccines, also known as nucleic acid
vaccines, contain recombinant genes encoding a tumor antigen that
are inserted into vectors and then introduced into host cells,
which then express the exogenous antigenic proteins that can induce
an immune response against the antigen, thereby preventing and
treating the disease. Genetic vaccines are divided into DNA
vaccines and RNA vaccines, however DNA vaccines are the most
researched nucleic acid vaccines. DNA vaccines are promising
therapeutic vaccines for tumors, but conventional vaccination with
plasmids is less than ideal and has potential safety concerns
(53). To this end, the use of
synthetic novel non-viral vector materials for the delivery of DNA
vaccines has become an intensely studied area of research (54,55).
Liu et al (54) designed alginic acid-coated chitosan
nanoparticles (ACNPs) as an oral delivery carrier for the
legumain-DNA vaccine. The experimental data revealed that ACNPs
were better than chitosan nanoparticles (CNPs) in protecting DNA
from degradation in an acidic solution (pH 1.5). Furthermore, the
results indicated that the vaccine could avoid degradation of DNA
by gastric acid and was effectively taken up and expressed by APCs
and the tumor volume was significantly reduced.
Li et al (55) used four DNA strands that
self-assembled into DNA nanostructures with well-defined structures
and uniform sizes. Unmethylated CpG bound to the end of DNA strands
by base pairing to form three-dimensional DNA tetrahedra carrying
an adjuvant. Experiments demonstrated that the DNA nanostructures
effectively entered macrophage-like RAW264.7 cells without
transfection reagents. DNA nanostructures have high plasticity, a
precise structure, stable properties, low toxicity, and resistance
to nuclease degradation. Therefore, DNA nanostructures provide an
unprecedented opportunity for the design of safe and effective
nanovaccine carriers.
Conclusions and future perspectives
In the field of tumor immunotherapy, animal
experiments have revealed that multifunctional nanomaterials
combined with cancer vaccines are an effective antitumor treatment
regimen. Multifunctional nanodelivery systems can simultaneously
deliver tumor antigens and nanoadjuvants to draining lymph nodes
and prolong antigen release time, triggering effective and lasting
antitumor immune effects. The discovery of effective TAAs and their
targeted delivery to APCs are keys to the development of immune
responses to cancer vaccines. However, a strong antitumor immune
effect may cause a potential autoimmune response, leading to severe
autoimmune diseases. Therefore, it is necessary to further seek
more effective tumor-specific antigens, better targeting of
nanomaterials and to achieve personalized therapy for patients with
new specific antigens. Simultaneously, it is difficult to eliminate
the immune suppression effect of the TME and the selection of
appropriate TAAs and epitopes also limits the development of cancer
vaccines. At present, some nanomaterial-based cancer vaccines have
entered clinical trials, such as phase 1 trials for PAN-301-1 in
prostate cancer and Lipovaxin-MM in melanoma. However, most
nanomaterial-based cancer vaccines remain in the experimental
animal-model stage. To translate these studies from experimental
animal models to clinical application, it is necessary to develop a
safe and effective nano-adjuvant. Simultaneously, it is necessary
to produce nanoparticles in a controllable, reproducible and
scalable manner, which will be the main challenge for application
of nanomaterials in cancer vaccines.
With the development of nanobiotechnology and the
optimization of nanomaterials, surface modification and
functionalization of nanoparticles will lead to more ideal
nanomaterials and in turn to breakthroughs in the diagnosis,
treatment and even prevention of human tumors. Furthermore, recent
biomedical breakthroughs in tumor neoplastic antigen screening,
such as tumor DNA/RNA exon sequencing and identification of new
antigens, have contributed to the advancement of individualized
immunotherapy for tumors. With optimized nanoadjuvants that can
bind to TAAs with stronger specificity, combined with
immune-related molecules, such as cytokines or immunopotentiators,
nanocancer vaccines with targeted, safe and timed quantitative
release can be obtained and promise to become the future focus of
studies that further address immunotherapy challenges.
Concurrently, the light and heat sensitivity and the magnetic
properties of nanomaterials combined with immunotherapy for cancer
treatment present novel therapeutic options. In the near future,
nanomaterial-based cancer vaccines will be combined with surgical
resection, radiotherapy, chemotherapy and other traditional
treatment programs and are expected to play greater roles in
clinical tumor treatment.
Acknowledgements
The present review was supported by the following
grants: the NSFC 81301958 and the Science and Technology Commission
Foundation of Sichuan Province LY-58.
References
|
1
|
Qasim W and Thrasher AJ: Progress and
prospects for engineered T cell therapies. Br J Haematol.
166:818–829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ribas A, Butterfield LH, Glaspy JA and
Economou JS: Current developments in cancer vaccines and cellular
immunotherapy. J Clin Oncol. 21:2415–2432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aly HA: Cancer therapy and vaccination. J
Immunol Methods. 382:1–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lollini PL, Cavallo F, Nanni P and Forni
G: Vaccines for tumour prevention. Nat Rev Cancer. 6:204–216. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith DM, Simon JK and Baker JR Jr:
Applications of nanotechnology for immunology. Nat Rev Immunol.
13:592–605. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pashine A, Valiante NM and Ulmer JB:
Targeting the innate immune response with improved vaccine
adjuvants. Nat Med. 11 Suppl:S63–S68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlosser E, Mueller M, Fischer S, Basta
S, Busch DH, Gander B and Groettrup M: TLR ligands and antigen need
to be coencapsulated into the same biodegradable microsphere for
the generation of potent cytotoxic T lymphocyte responses. Vaccine.
26:1626–1637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Temmerman ML, Rejman J, Demeester J,
Irvine DJ, Gander B and De Smedt SC: Particulate vaccines: On the
quest for optimal delivery and immune response. Drug Discov Today.
16:569–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Moynihan KD, Zheng Y, Szeto GL, Li
AV, Huang B, Van Egeren DS, Park C and Irvine DJ: Structure-based
programming of lymph-node targeting in molecular vaccines. Nature.
507:519–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palucka K and Banchereau J:
Dendritic-cell-based therapeutic cancer vaccines. Immunity.
39:38–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scheerlinck JP and Greenwood DL:
Virus-sized vaccine delivery systems. Drug Discov Today.
13:882–887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldberg MS: Immunoengineering: How
nanotechnology can enhance cancer immunotherapy. Cell. 161:201–204.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song YC, Cheng HY, Leng CH, Chiang SK, Lin
CW, Chong P, Huang MH and Liu SJ: A novel emulsion-type adjuvant
containing CpG oligodeoxynucleotides enhances CD8+
T-cell-mediated anti-tumor immunity. J Control Release.
173:158–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer NO, Rasley A, Corzett M, Hwang MH,
Hoeprich PD and Blanchette CD: Colocalized delivery of adjuvant and
antigen using nanolipoprotein particles enhances the immune
response to recombinant antigens. J Am Chem Soc. 135:2044–2047.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Standley SM, Mende I, Goh SL, Kwon YJ,
Beaudette TT, Engleman EG and Fréchet JM: Incorporation of CpG
oligonucleotide ligand into protein-loaded particle vaccines
promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem.
18:77–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fox CB, Sivananthan SJ, Duthie MS, Vergara
J, Guderian JA, Moon E, Coblentz D, Reed SG and Carter D: A
nanoliposome delivery system to synergistically trigger TLR4 AND
TLR7. J Nanobiotechnology. 12:172014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zinkernagel RM, Ehl S, Aichele P, Oehen S,
Kündig T and Hengartner H: Antigen localisation regulates immune
responses in a dose- and time-dependent fashion: A geographical
view of immune reactivity. Immunol Rev. 156:199–209. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reddy ST, Swartz MA and Hubbell JA:
Targeting dendritic cells with biomaterials: Developing the next
generation of vaccines. Trends Immunol. 27:573–579. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui Z, Han SJ and Huang L: Coating of
mannan on LPD particles containing HPV E7 peptide significantly
enhances immunity against HPV-positive tumor. Pharm Res.
21:1018–1025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao
Y, Kong M, Qi Y, Tan S and Zhang Z: Erythrocyte membrane-enveloped
polymeric nanoparticles as nanovaccine for induction of antitumor
immunity against melanoma. ACS Nano. 9:6918–6933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perez-Diez A, Joncker NT, Choi K, Chan WF,
Anderson CC, Lantz O and Matzinger P: CD4 cells can be more
efficient at tumor rejection than CD8 cells. Blood. 109:5346–5354.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delamarre L, Pack M, Chang H, Mellman I
and Trombetta ES: Differential lysosomal proteolysis in
antigen-presenting cells determines antigen fate. Science.
307:1630–1634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen H, Ackerman AL, Cody V, Giodini A,
Hinson ER, Cresswell P, Edelson RL, Saltzman WM and Hanlon DJ:
Enhanced and prolonged cross-presentation following endosomal
escape of exogenous antigens encapsulated in biodegradable
nanoparticles. Immunology. 117:78–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saluja SS, Hanlon DJ, Sharp FA, Hong E,
Khalil D, Robinson E, Tigelaar R, Fahmy TM and Edelson RL:
Targeting human dendritic cells via DEC-205 using PLGA
nanoparticles leads to enhanced cross-presentation of a
melanoma-associated antigen. Int J Nanomedicine. 9:5231–5246.
2014.PubMed/NCBI
|
|
25
|
Yuba E, Kono Y, Harada A, Yokoyama S, Arai
M, Kubo K and Kono K: The application of pH-sensitive
polymer-lipids to antigen delivery for cancer immunotherapy.
Biomaterials. 34:5711–5721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Joyce JA: Therapeutic targeting of the
tumor microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohla S: Tumor microenvironment. J Cell
Biochem. 101:801–804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Danhier F, Feron O and Préat V: To exploit
the tumor microenvironment: Passive and active tumor targeting of
nanocarriers for anti-cancer drug delivery. J Control Release.
148:135–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murdoch C, Giannoudis A and Lewis CE:
Mechanisms regulating the recruitment of macrophages into hypoxic
areas of tumors and other ischemic tissues. Blood. 104:2224–2234.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zanganeh S, Hutter G, Spitler R, Lenkov O,
Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M,
et al: Iron oxide nanoparticles inhibit tumour growth by inducing
pro-inflammatory macrophage polarization in tumour tissues. Nat
Nanotechnol. 11:986–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Connolly DT, Heuvelman DM, Nelson R,
Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM and
Feder J: Tumor vascular permeability factor stimulates endothelial
cell growth and angiogenesis. J Clin Invest. 84:1470–1478. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng H, Xing G, Sun B, Zhao F, Lei H, Li
W, Song Y, Chen Z, Yuan H, Wang X, et al: Potent angiogenesis
inhibition by the particulate form of fullerene derivatives. ACS
Nano. 4:2773–2783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng H, Xing G, Blanco E, Song Y, Zhao L,
Sun B, Li X, Wang PC, Korotcov A, Li W, et al: Gadolinium
metallofullerenol nanoparticles inhibit cancer metastasis through
matrix metalloproteinase inhibition: Imprisoning instead of
poisoning cancer cells. Nanomedicine (Lond). 8:136–146. 2012.
View Article : Google Scholar
|
|
37
|
Guo L, Yan DD, Yang D, Li Y, Wang X,
Zalewski O, Yan B and Lu W: Combinatorial photothermal and immuno
cancer therapy using chitosan-coated hollow copper sulfide
nanoparticles. ACS Nano. 8:5670–5681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duan X, Chan C, Guo N, Han W, Weichselbaum
RR and Lin W: Photodynamic therapy mediated by nontoxic core-shell
nanoparticles synergizes with immune checkpoint blockade to elicit
antitumor immunity and antimetastatic effect on breast cancer. J Am
Chem Soc. 138:16686–16695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Q, Wang C, Zhan Z, He W, Cheng Z, Li
Y and Liu Z: Near-infrared dye bound albumin with separated imaging
and therapy wavelength channels for imaging-guided photothermal
therapy. Biomaterials. 35:8206–8214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berger M, Kreutz FT, Horst JL, Baldi AC
and Koff WJ: Phase I study with an autologous tumor cell vaccine
for locally advanced or metastatic prostate cancer. J Pharm Pharm
Sci. 10:144–152. 2007.PubMed/NCBI
|
|
41
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fields RC, Shimizu K and Mulé JJ: Murine
dendritic cells pulsed with whole tumor lysates mediate potent
antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci
USA. 95:pp. 9482–9487. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fang RH, Hu CM, Luk BT, Gao W, Copp JA,
Tai Y, O'Connor DE and Zhang L: Cancer cell membrane-coated
nanoparticles for anticancer vaccination and drug delivery. Nano
Lett. 14:2181–2188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu SY, Wei W, Yue H, Ni DZ, Yue ZG, Wang
S, Fu Q, Wang YQ, Ma GH and Su ZG: Nanoparticles-based
multi-adjuvant whole cell tumor vaccine for cancer immunotherapy.
Biomaterials. 34:8291–8300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ueda Y, Itoh T, Fuji N, Harada S, Fujiki
H, Shimizu K, Shiozaki A, Iwamoto A, Shimizu T, Mazda O, et al:
Successful induction of clinically competent dendritic cells from
granulocyte colony-stimulating factor-mobilized monocytes for
cancer vaccine therapy. Cancer Immunol Immunother. 56:381–389.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han JA, Kang YJ, Shin C, Ra JS, Shin HH,
Hong SY, Do Y and Kang S: Ferritin protein cage nanoparticles as
versatile antigen delivery nanoplatforms for dendritic cell
(DC)-based vaccine development. Nanomedicine (Lond). 10:561–569.
2014. View Article : Google Scholar
|
|
47
|
Dobrovolskaia MA and McNeil SE:
Immunological properties of engineered nanomaterials. Nat
Nanotechnol. 2:469–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zeng B, Liao AJ, Lu FG, Fang WY and Wang
J: Inhibition of the growth of hepatocarcinoma xenograft in Balb/c
mice induced by dendritic cells immunized with AFP cDNA fragement.
Zhonghua Zhong Liu Za Zhi. 32:98–102. 2010.(In Chinese). PubMed/NCBI
|
|
49
|
Matsuo K, Ishii Y, Matsuo K, Yoshinaga T,
Akashi M, Mukai Y, Yoshioka Y, Okada N and Nakagawa S: The utility
of poly(γ-glutamic acid) nanoparticles as antigen delivery carriers
in dendritic cell-based cancer immunotherapy. Biol Pharm Bull.
33:2003–2007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang J, Zhang Q, Li K, Yin H and Zheng JN:
Composite peptide-based vaccines for cancer immunotherapy (Review).
Int J Mol Med. 35:17–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cruz LJ, Rueda F, Cordobilla B, Simón L,
Hosta L, Albericio F and Domingo JC: Targeting nanosystems to human
DCs via Fc receptor as an effective strategy to deliver antigen for
immunotherapy. Mol Pharm. 8:104–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhuang X, Wu T, Zhao Y, Hu X, Bao Y, Guo
Y, Song Q, Li G, Tan S and Zhang Z: Lipid-enveloped zinc phosphate
hybrid nanoparticles for codelivery of H-2K(b) and
H-2D(b)-restricted antigenic peptides and monophosphoryl lipid A to
induce antitumor immunity against melanoma. J Control Release.
228:26–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Senovilla L, Vacchelli E, Garcia P,
Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G and
Galluzzi L: Trial watch: DNA vaccines for cancer therapy.
OncoImmunology. 2:e238032013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Z, Lv D, Liu S, Gong J, Wang D, Xiong
M, Chen X, Xiang R and Tan X: Alginic acid-coated chitosan
nanoparticles loaded with legumain DNA vaccine: Effect against
breast cancer in mice. PLoS One. 8:e601902013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li J, Pei H, Zhu B, Liang L, Wei M, He Y,
Chen N, Li D, Huang Q and Fan C: Self-assembled multivalent DNA
nanostructures for noninvasive intracellular delivery of
immunostimulatory CpG oligonucleotides. ACS Nano. 5:8783–8789.
2011. View Article : Google Scholar : PubMed/NCBI
|

















