Introduction
Gastric cancer (GC) is the second leading cause of
cancer mortality and the fifth most common type of cancer, imposing
a serious health burden for the whole world. It is estimated that
there were 951,600 new cases and 723,100 deaths in 2012 (1,2).
Despite multimodal therapies for GC including surgery, chemotherapy
and ionizing radiation (IR), the prognosis of GC remains dismal
(3,4), which is mainly due to the advanced
stage at diagnosis and the high rate of recurrence. Due to the
complexity of GC, complementary therapies are required to improve
the efficacy of conventional approaches and the survival of GC
patients (5). Radiotherapy plays an
important role in management of unresectable GC. It is a critical
component of adjuvant therapy for GC after surgical resection, and
considered as palliative treatment for relieving local symptoms of
locally advanced GC patients (6,7).
However, during the IR procedure in the treatment of GC,
surrounding organs such as the kidneys, liver, and spleen also
receive irradiation that could lead to toxicity, which limits the
efficacy of radiotherapy in GC (8,9).
Therefore, it is essential to explore effective radiosensitizers to
enhance the IR response and reduce the IR toxicity in GC.
Phytochemicals have become a promising approach in
the management of malignancies (10,11).
[6]-Gingerol, an active phenolic compound derived from ginger,
possesses pharmacological activities including anti-inflammatory,
antioxidant, and antitumor properties (12,13).
In vivo and in vitro studies revealed that
[6]-gingerol was effective in the suppression of carcinogenesis,
angiogenesis and metastasis against various types of cancer
(14–18). However, the chemopreventive effects
of [6]-gingerol on GC have not been fully elucidated. To the best
of our knowledge, there is no evidence on the radiosensitivity
effect and the underlying mechanisms of [6]-gingerol. Therefore, in
the present study we aimed to investigate whether [6]-gingerol can
sensitize GC cells to IR.
Materials and methods
Cell lines and cell culture
The HGC-27 cell line was obtained from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China). Cells were
maintained in a T-25 flask with RPMI-1640 (Gibco, Grand Island, NY,
USA), supplemented with 1% penicillin streptomycin (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) and 10% fetal bovine serum (FBS;
Gibco). The cells were incubated at 37°C, in a 5% CO2
humidified incubator.
Chemicals and reagents
[6]-Gingerol (≥98% purity) was purchased from
Sichuan Weikeqi Biological Technology Co., Ltd. (Sichuan, China).
It was dissolved in DMSO as stock solutions (500 mM) and stored at
−20°C. For every experiment, [6]-gingerol was diluted in complete
cell culture medium to indicated concentrations, with a final DMSO
concentration under 0.1% (v/v). Primary antibodies against cyclin
B1 (cat. no. 55004-1-AP), CDK6 (cat. no. 14052-1-AP), β-actin (cat.
no. HRP-60008) and tubulin (cat. no. HRP-66031) were purchased from
ProteinTech Group, Inc. (Chicago, IL, USA). Antibodies against
caspase-9 (cat. no. 9502), caspase-3 (cat. no. 9665), cleaved
caspase-3 (Asp175) (cat. no. 9664), cytochrome c (cat. no.
4280), cyclin A2 (cat. no. 4656), CDC2 (cat. no. 77055) and cyclin
D1 (cat. no.2978) were purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA). HRP-conjugated goat anti-rabbit IgG (cat.
no. HSA0003) and HRP-conjugated goat anti-mouse IgG (cat. no.
HSA0001) were obtained from Mai Bio Co., Ltd. (Shanghai,
China).
Cell viability assay
The effect of [6]-gingerol on cell viability of
human HGC-27 cells was assessed with Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to
the manufacturer's instructions. Briefly, cells were seeded in
96-well plates at 5×103 cells/well in three replicates
and incubated for 24 h. The medium was removed and the cells were
exposed to [6]-gingerol (50, 100, 200, 400 and 500 µM) or vehicle
(0.1% DMSO) for 48 h. Then 10 µl of CCK-8 solution was added to
each well and incubated at 37°C for 1–4 h. The absorbance was
assessed at 450 nm using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The viable ratio was presented compared
to the vehicle controls (100% active).
Colony formation assay
Cells (2×105) were distributed in 6-well
plates and allowed to adhere for 24 h. Then, the cells were treated
with vehicle control or [6]-gingerol (300 µM) for 24 h, followed by
exposure to different doses of IR. Cells were harvested, counted
and 500 cells of each treatment were seeded into a 60-mm culture
dish with fresh complete culture medium. Following 10–14 days of
incubation, the colonies were stained with crystal violet staining
solution (Beyotime Institute of Biotechnology, Shanghai, China).
The plates were pictured using a digital camera, and the surviving
colonies (colonies containing more than 50 cells under a microscope
in ×100 magnification) were counted by Adobe Photoshop CS6 (Adobe,
San Jose, CA, USA). Cell survival curves were fitted with the
linear-quadratic model using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA).
Flow cytometric analysis of the cell
cycle
Cell cycle distributions were analyzed by assessing
the cellular DNA content. Briefly, cells (2×105/well)
were seeded onto 6-well plates, allowed to incubate for 24 h, and
then the cells were treated with [6]-gingerol (300 µM) or IR (4 Gy)
alone or [6]-gingerol (300 µM) for 24 h followed by IR (4 Gy).
Twenty-four hours after IR, the culture medium was aspirated and
the cells were washed with cold PBS twice and fixed with 70% ethyl
alcohol for >2 h. Then, the cells were washed and resuspendented
in 0.5 ml PI/RNase Staining Buffer (BD Biosciences, San Jose, CA,
USA). After incubation at room temperature for 15 min protected
from light, the cells were analyzed by a flow cytometer (BD
Biosciences).
DAPI staining
The apoptotic nuclear morphological changes were
observed using DAPI staining. Cells (2×105/well) were
seeded onto 6-well plates, and were treated with [6]-gingerol (300
µM) or IR (4 Gy) alone or [6]-gingerol (300 µM) for 24 h followed
by IR (4 Gy). Twenty-four hours after IR, the cells were washed
with PBS and fixed with 4% paraformaldehyde (Nanjing KeyGen
Biotech., Co., Ltd.) for 20 min. Fixed cells were washed with PBS,
and stained with DAPI (Beyotime Institute of Biotechnology)
solution for 10 min in the dark at room temperature, and then the
cells were washed with PBS three times. Images were captured using
a fluorescence microscope.
Flow cytometric analysis of
apoptosis
Apoptosis was analyzed using an Annexin V-FITC/PI
Apoptosis Detection kit (BD Biosciences) according to the
manufacturer's instructions. In brief, cells (2×105)
were plated in 6-well plates and incubated for 24 h. The cells were
then treated with [6]-gingerol (300 µM) or IR (4 Gy) alone or
[6]-gingerol (300 µM) for 24 h followed by IR (4 Gy), and
subsequently, both the floating and the attached cells were
collected, stained and analyzed by a flow cytometer (BD
Biosciences) for apoptosis 24 h post IR.
RNA extraction and quantitative real
time-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol (Invitrogen,
Carlsbad, CA, USA) and was reverse-transcribed into complementary
DNA (cDNA). The sample was subjected to real-time PCR using
Real-Time Quantitative PCR SYBR-Green detection reagent (Takara
Bio, Inc., Tokyo, Japan) and performed by a Fast 7300 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc., Foster
City, CA, USA) according to the manufacturer's instructions. The
primer sequences for p27 and β-actin were as follows: p27 forward,
5′-CAAATGCCGGTTCTGTGGAG-3′ and reverse,
5′-TCCATTCCATGAAGTCAGCGATA-3′; β-actin forward,
5′-CATTGCCGACAGGATGCAG-3′ and reverse,
5′-CTCGTCATACTCCTGCTTGCTG-3′.
Western blot analysis
Cells were washed with cold PBS, and lysed in SDS
Lysis Buffer containing protease inhibitors PMSF (all from Beyotime
Institute of Biotechnology). The whole cell lysate was centrifuged
at 18,000 × g for 20 min at 4°C, and the protein concentration was
quantified by the BCA assay (Mai Bio Co., Ltd.). Twenty micrograms
of whole cell lysate was separated on a 12% SDS-PAGE, and then
transferred onto PVDF membranes (EMD Millipore, Billerica, MA,
USA). The membranes were probed with primary antibodies against
cyclin A2 (1:2,000), cyclin B1 (1:3,000), CDC2 (1:3,000), CDK6
(1:3,000), cyclin D1 (1:2,000), capase-9 (1:3,000), caspase-3
(1:3,000), cleaved caspase-3 (1:2,000) and cytochrome c
(1:2,000) at 4°C overnight, followed by incubation with
HRP-conjugated secondary antibodies (1:5,000) for 1 h at room
temperature. The bands were visualized using ECL (EMD
Millipore).
Statistical analysis
SPSS 23.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. All the experiments were performed three
times or more. The data were presented as the mean ± SD and error
bars represent the standard deviation. The statistical analysis of
compared groups was assessed using Student's t-test. The symbols *,
** and *** represent P-values, and P<0.05 was considered to
indicate a statistically significant difference.
Results
[6]-Gingerol inhibits the
proliferation of HGC-27 cells
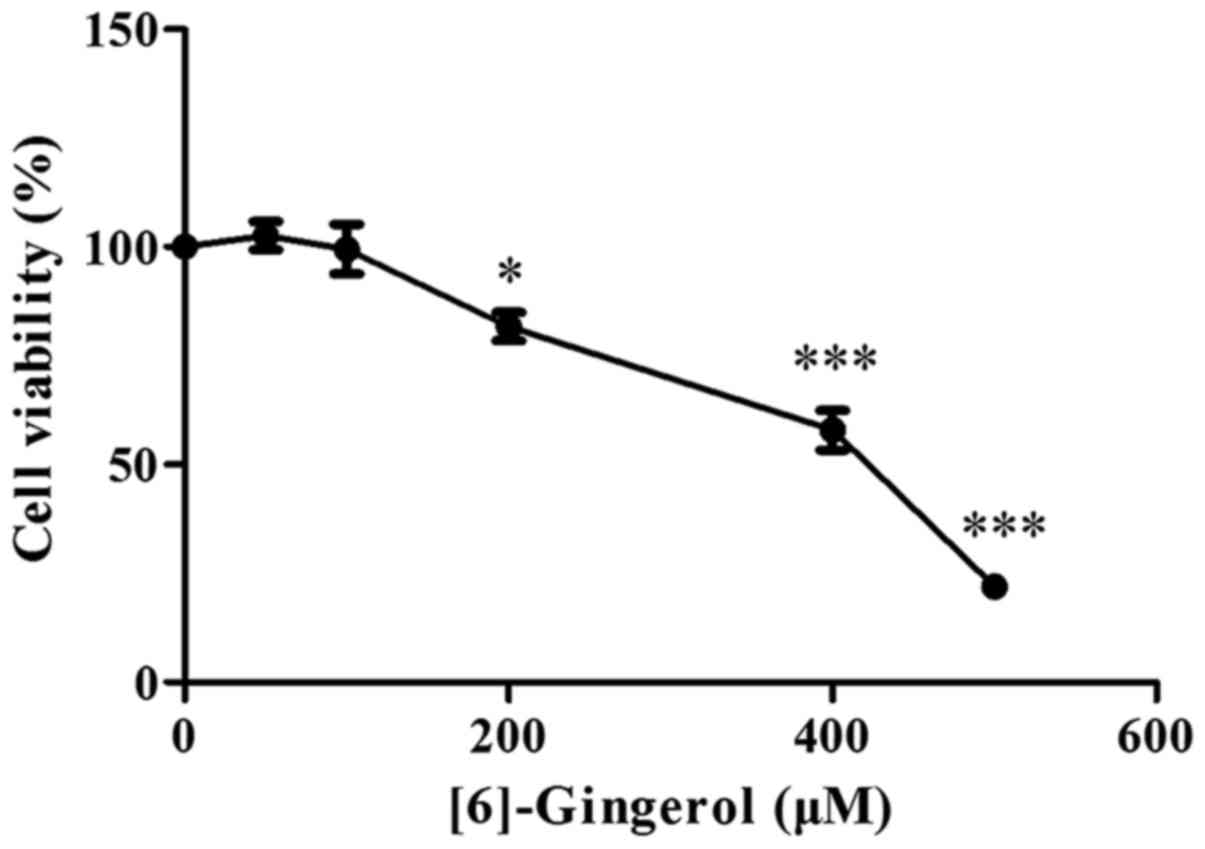
To evaluate the effect of [6]-gingerol on the
proliferation of human GC cell line HGC-27 cells, HGC-27 cells were
exposed to increasing concentrations of [6]-gingerol or vehicle
(0.1% dimethyl sulfoxide) for 48 h and then analyzed using a CCK-8
kit. As shown in Fig. 1,
[6]-gingerol reduced the viability of HGC-27 cells in a
dose-dependent manner, and the half-maximal inhibitory
concentration (IC50) value at 48 h was 386.3 µM.
[6]-Gingerol sensitizes HGC-27 cells
to IR
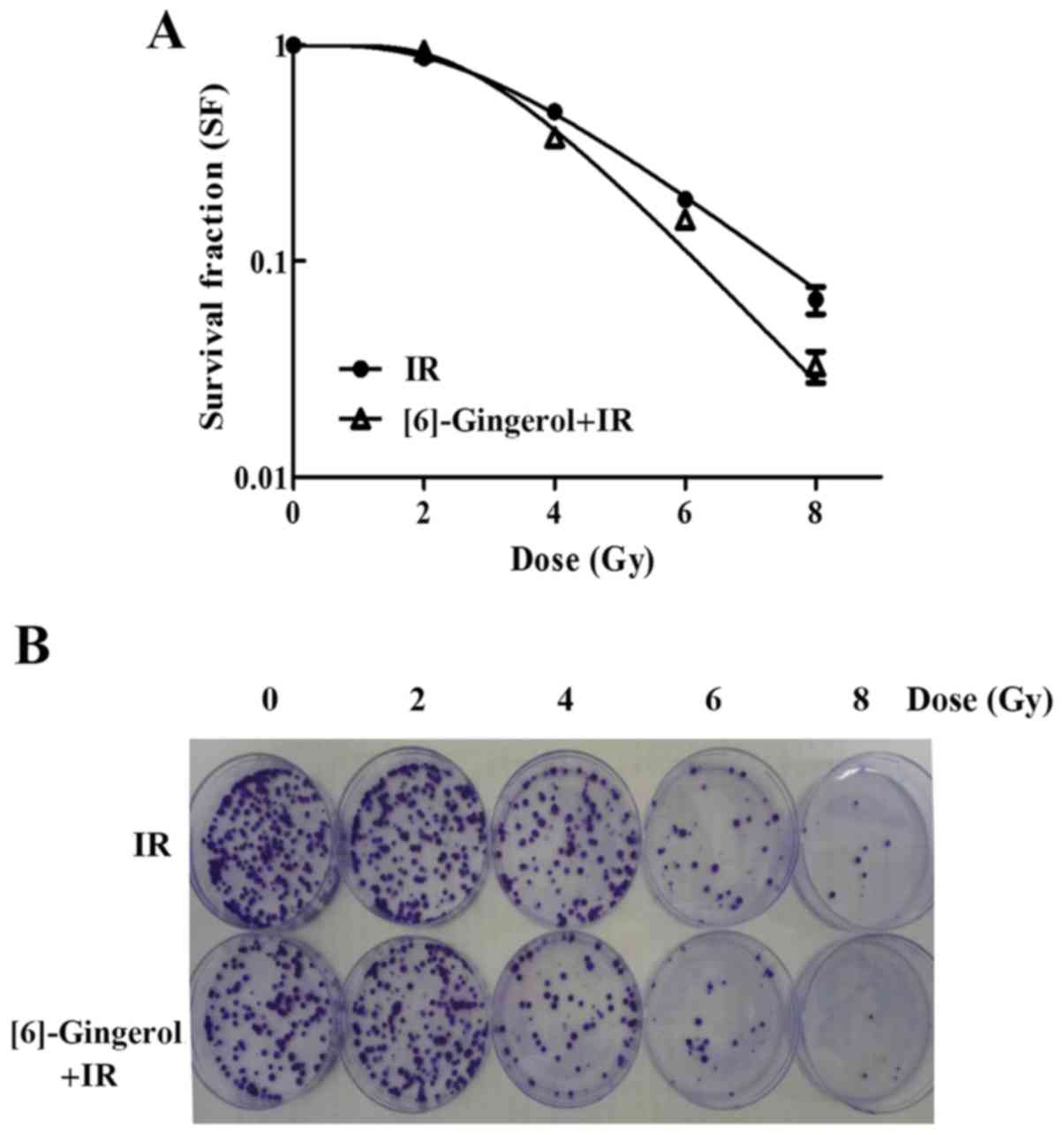
To determine the radiosensitivity of [6]-gingerol in
HGC-27 cells, a clonogenic survival assay was performed. A
concentration below the IC50 value was chosen for this
assay. Cells were pretreated with [6]-gingerol (300 µM) or vehicle
for 24 h before being exposed to IR treatment. As shown in Fig. 2A and B, we found that [6]-gingerol
sensitized HGC-27 cells to IR. Survival fractions (SFs) of the
combination group at 4, 6 and 8 Gy were decreased from 49.2 to
37.3%, 19.35 to 15.8% and 6.6 to 3.3% respectively, compared with
the IR group alone (P<0.05). The mean lethal dose (D0) value was
decreased from 1.92 to 1.38 Gy, and the sensitization enhancement
ratio (SER) was 1.39. These results revealed that [6]-gingerol
enhanced the radiosensitivity of HGC-27 cells.
[6]-Gingerol enhances IR-induced G2/M
phase arrest
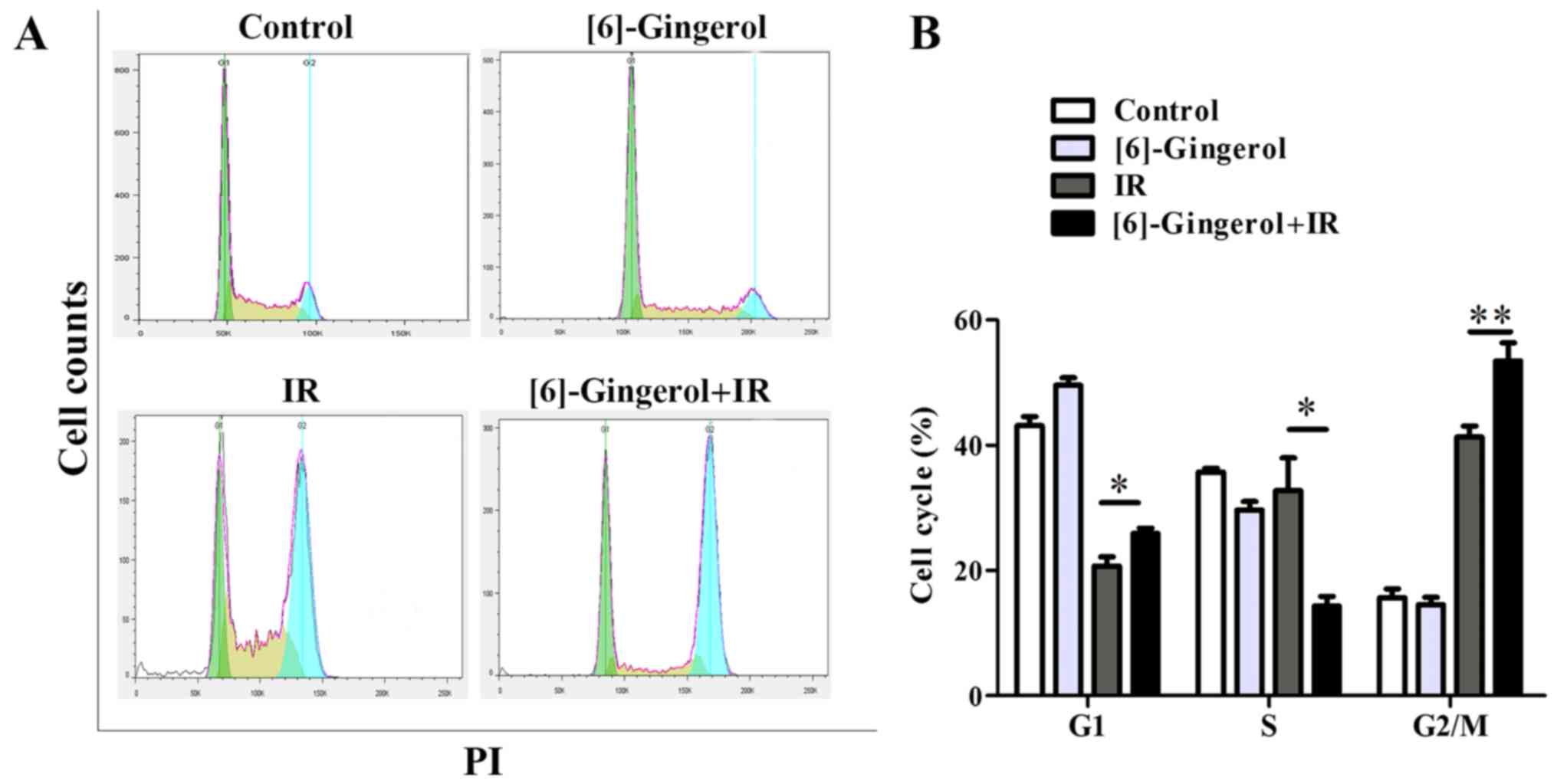
To investigate the mechanisms by which [6]-gingerol
induced the radiosensitization effect in HGC-27 cells, we first
examined the effect of [6]-gingerol or IR alone and in combination
on the cell cycle progression. Cells were treated with [6]-gingerol
(300 µM) or IR (4 Gy), or preincubated with [6]-gingerol (300 µM)
for 24 h followed by 4 Gy of IR exposure. Cells were then collected
and analyzed by flow cytometry. As shown in Fig. 3A and B, [6]-gingerol alone arrested
the cell population at the G1 phase (43.1% in the control vs. 49.5%
in [6]-gingerol alone; P=0.005), and decreased the S phase cell
proportion (35.7% in the control vs. 29.7% in [6]-gingerol alone;
P=0.006), while the G2 phase was not affected (15.7% in the control
vs. 14.5% in [6]-gingerol alone; P=0.34). However, when
[6]-gingerol was combined with IR, the G2/M phase blocking was
significantly enhanced compared with the IR group (41.3% in IR
alone vs. 53.5% in [6]-gingerol+IR; P=0.006). The S phase
population of the cell cycle was decreased in the combination group
compared with the IR group (32.8% in IR vs. 14.3% in
[6]-gingerol+IR; P=0.02). Populations in the G2/M phase are known
to be more sensitive to IR, while cells in the S phase are
relatively resistant to IR, therefore the results imply that the
radiosensitization effect of [6]-gingerol on HGC-27 cells may
partly be due to the G2/M arrest of the cell cycle.
[6]-Gingerol regulates the levels of
IR-induced cell cycle-associated proteins and p27 mRNA
expression
To demonstrate the mechanisms underlying the
enhanced IR-induced G2/M arrest by [6]-gingerol, we then examined
the expression levels of G2/M transition regulators including
cyclin B1, cyclin A2, CDC2 and p27. As shown in Fig. 4, we observed that the protein levels
of cyclin B1, cyclin A2, and CDC2 were downregulated and p27 mRNA
expression was upregulated in the [6]-gingerol+IR group compared
with the IR alone group. Since the previous cellular results
revealed G1 phase arrest in the combination treatment group, we
also investigated G1 phase-associated checkpoints, and determined
that cyclin D1 was downregulated however, CDK6 remained unchanged.
The data were consistent with the results of the cellular cell
distribution analysis.
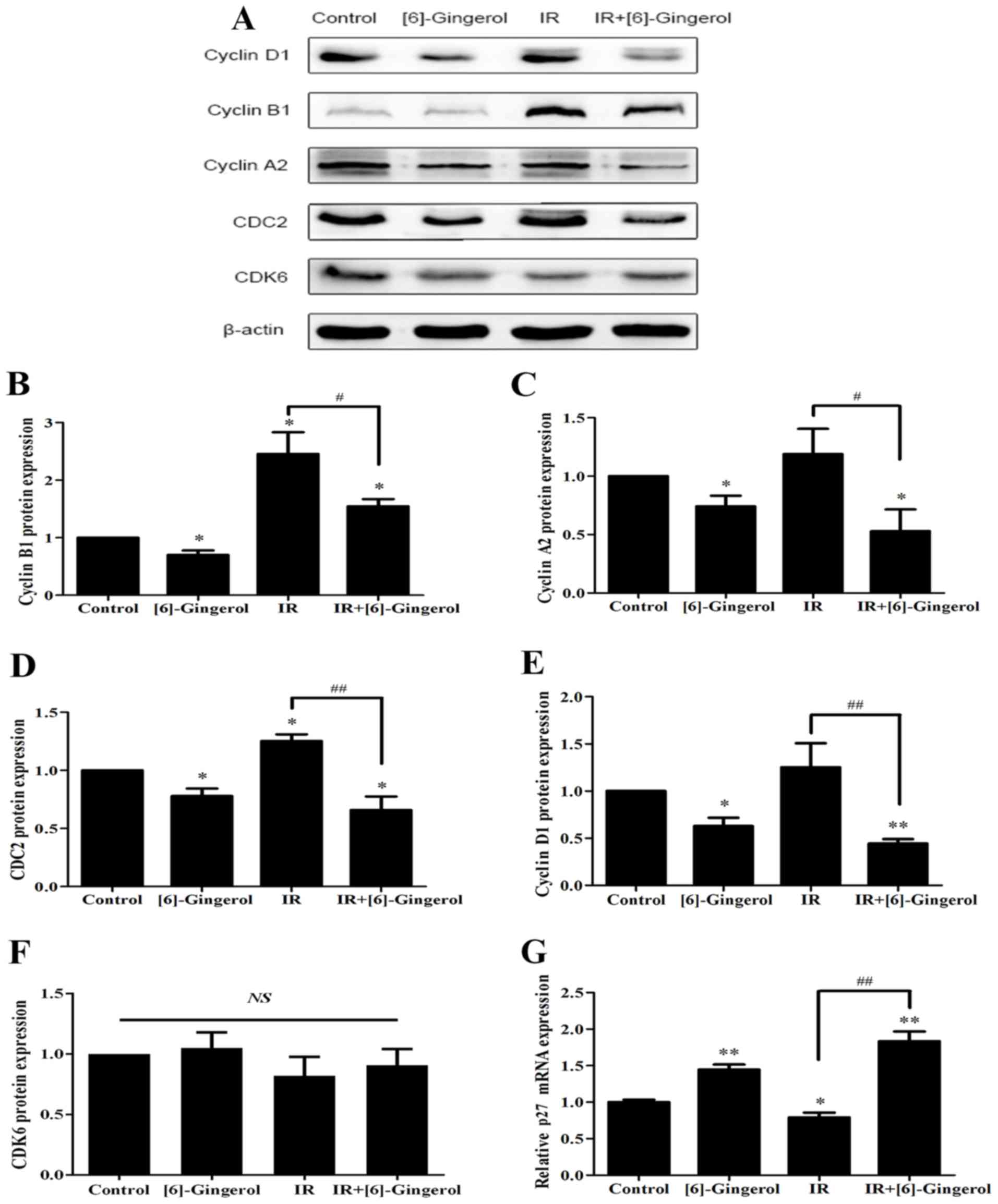 | Figure 4.Effects of [6]-gingerol on IR-induced
cell cycle regulatory proteins and p27 mRNA expression. HGC-27
cells were treated with the vehicle, [6]-gingerol (300 µM), 4 Gy of
IR alone, or exposed to IR (4 Gy) post [6]-gingerol (300 µM)
incubation. (A) After treatment, the cells were harvested and total
cell lysates were subjected to western blotting. The levels of
cyclin D1, cyclin B1, cyclin A2, CDC2, CDK6 and β-actin were
analyzed. Typical images of three independent experiments were
presented. (B-F) Statistical analysis of the protein expression
levels. (G) The relative mRNA level of p27 in the indicated
treatments was assessed by qRT-PCR. *Significant difference between
the indicated groups; **P<0.01, *P<0.05.
#Significant difference between the IR and
[6]-gingerol+IR treatment groups; ##P<0.01,
#P<0.05. IR, ionizing radiation; NS, no statistical
significance. |
[6]-Gingerol increases IR-induced
apoptosis
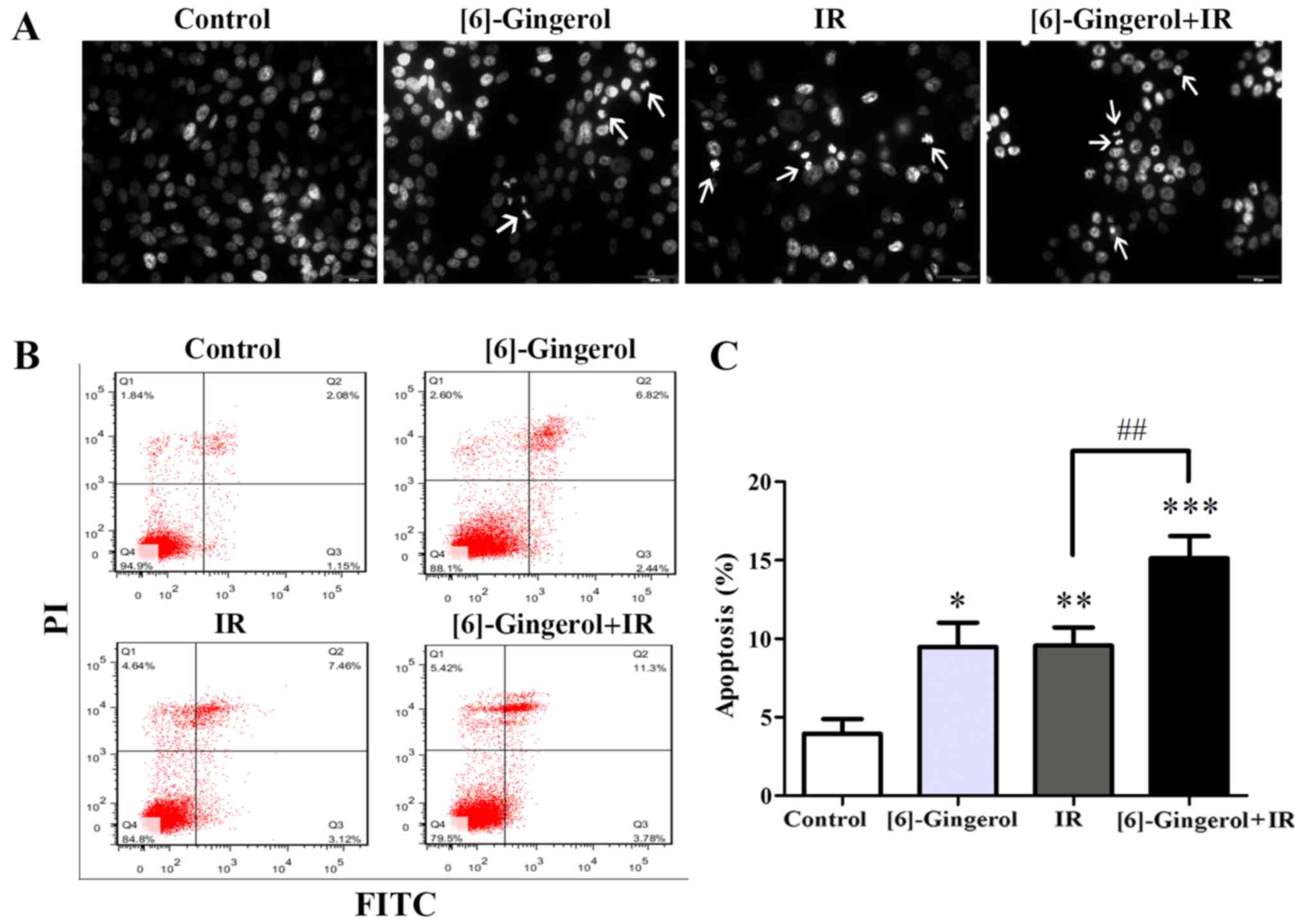
We next investigated whether [6]-gingerol could
increase IR-induced apoptosis. Cells were treated with vehicle
control, [6]-gingerol (300 µM), IR (4 Gy), and [6]-gingerol (300
µM) + IR (4 Gy), and then were stained with DAPI. As shown in
Fig. 5A, apoptotic cells with
condensed chromatin and fragmented nuclei were clearly visible in
both single-treatment of [6]-gingerol or IR and in the combination
treatment, but not in the control. Apoptosis was further analyzed
by Annexin V/PI-staining. As shown in Fig. 5B and C, [6]-gingerol or IR alone
induced apoptosis of HGC-27 cells, and the apoptosis rates were
9.5±1.6 and 9.6%±1.2% respectively, compared with the vehicle
control (3.9±1.0%). [6]-Gingerol pretreatment significantly
increased IR-induced cell apoptosis compared with IR alone in
HGC-27 cells (9.6±1.2% in IR alone vs. 15.1±1.4% in
[6]-gingerol+IR; P=0.007). These results revealed that [6]-gingerol
increased the apoptosis induction of IR in HGC-27 cells.
[6]-Gingerol enhances the levels and
activities of IR-induced apoptosis regulatory proteins
To elucidate the mechanisms of the
[6]-gingerol-enhanced IR-induced cell apoptosis, we performed
western blot analysis to examine the protein levels of several key
regulatory molecules including caspase-9, cleaved-caspase-9,
caspase-3, cleaved-caspase-3 and cytochrome c, which are
initiators and executors of the apoptotic process. Fig. 6 revealed that the levels of
procaspase-9 (47 kDa), and cytochrome c were upregulated in
the cells treated with IR or [6]-gingerol alone compared with the
control, meanwhile, the cleaved fragments of caspase-9 (35 kDa)
were also observed. Procaspase-3 (35 kDa) was decreased and
cleaved-caspase-3 (17/19 kDa) was markedly increased in the
combination treatment group. Furthermore, the combination treatment
of [6]-gingerol with IR was more effective in upregulating those
proteins levels compared with either treatment alone. These
findings were consistent with the previous flow cytometric data of
apoptosis, indicating that [6]-gingerol enhanced IR-induced
apoptosis via the activation of caspase-9, caspase-3 and the
release of cytochrome c.
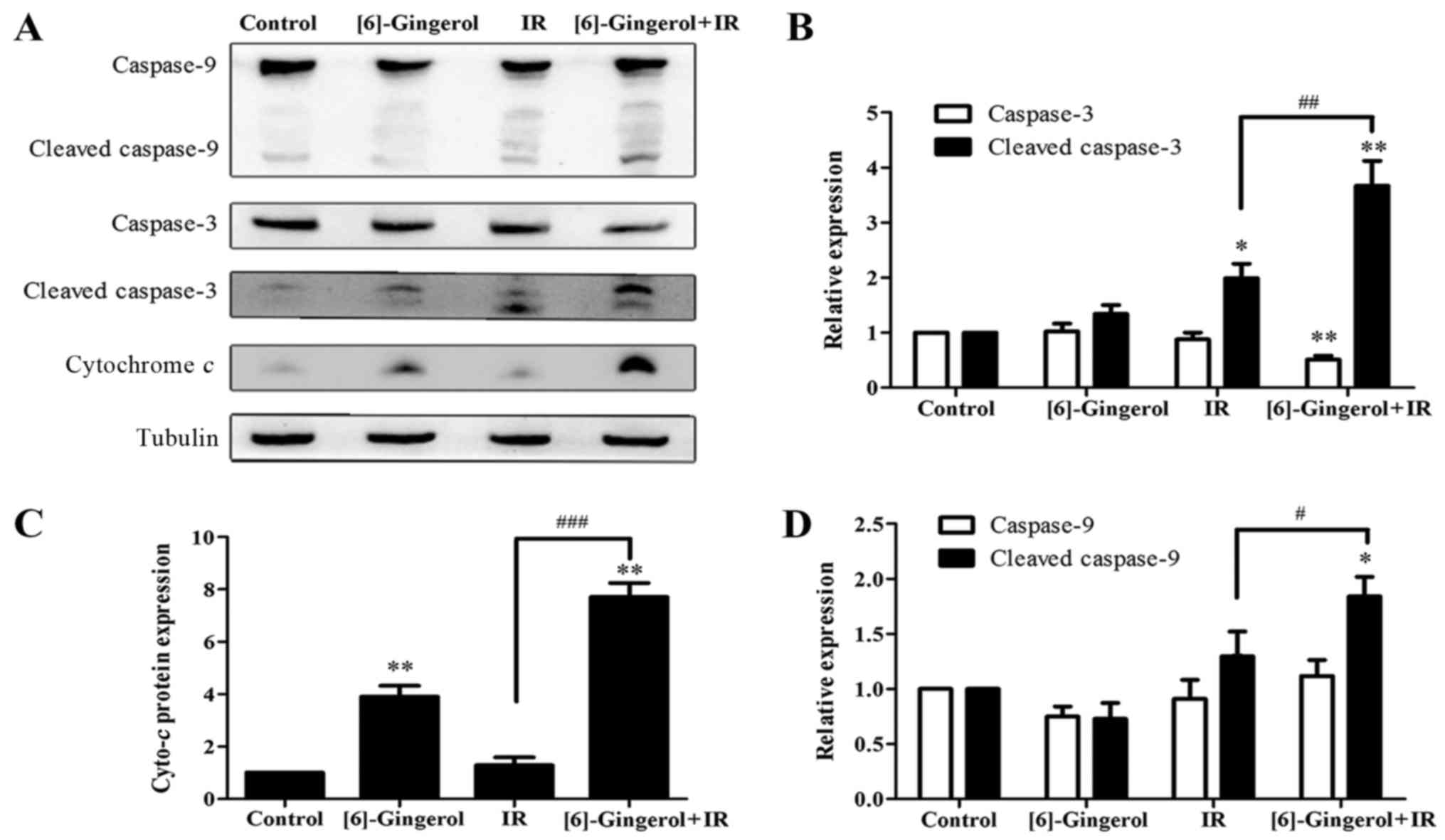 | Figure 6.Effects of [6]-gingerol on the
IR-induced levels and activities of proteins involved in apoptosis.
Cells were seeded to 6-well plates and treated with the vehicle,
[6]-gingerol (300 µM), IR (4 Gy) alone, or pretreated with
[6]-gingerol for 24 h before being exposed to IR. Twenty-four hours
after 4 Gy of IR, the cells were collected and whole cell lysates
were immunoblotted with antibodies against caspase-9, caspase-3,
cleaved-caspase-3, cytochrome c, using tubulin as a control.
(A) Representative data is shown of three independent experiments.
(B-D) Statistical analysis of the protein expression levels.
**P<0.01, *P<0.05. #Significant difference between
the IR and [6]-gingerol+IR treatment groups;
###P<0.001, ##P<0.01,
#P<0.05. IR, ionizing radiation. |
Discussion
GC places a big burden on societies due to its high
incidence and poor prognosis. Radiotherapy is an important modality
for the treatment of GC, however, the efficacy is limited due to
the intrinsic and extrinsic IR resistance and toxicity to normal
tissues. Existing radiosensitization approaches like combining
chemotherapeutic drugs such as cisplatin, 5-fluorouracil with IR
can produce synergistic effects but also increase side effects in
some cases (19). Therefore,
searching for new radiosensitizers is of great importance.
Phytochemicals have drawn wide attention as promising
radiosensitizers in the course of radiotherapy (20–23).
[6]-Gingerol has demonstrated potential chemopreventive ability in
various cancer types. For the first time, our study investigated
the possibility of [6]-gingerol as a radiosensitizer in GC
cells.
In the present study, we first investigated the
effect of [6]-gingerol alone on the proliferation of HGC-27 GC
cells and determined that [6]-gingerol could inhibit cell viability
in a dose-dependent manner. To determine the potential of
[6]-gingerol as a radiosensitizer, we next chose [6]-gingerol at
300 µM (<IC50) for a colony formation assay.
According to the survival curve, radiobiological parameters were
calculated. In the field of radiobiology, the combined therapeutic
effects based on drug and ionizing irradiation is obtained by the
SFs (24–26). SER is commonly used as a direct
reflection of radiosensitivity. SER was calculated by dividing the
D0 value of the IR alone group by the D0 value of the combined
[6]-gingerol and IR group (the D0 value refers to mean lethal
dose). In the present study, at 4, 6 and 8 Gy, the SFs of the
combination treatment were decreased compared with IR alone; the D0
value of the combination group was relatively lower suggesting that
the reasonable lower doses of X-ray can also kill tumor cells when
coupled with [6]-gingerol; the SER was 1.39. Therefore,
pretreatment with [6]-gingerol could sensitize HGC-27 cells to
IR.
There are several factors that influence IR
sensitivity, including the modulation of cell apoptosis, cycle
distribution, hypoxia, DNA damage repair and signaling pathways
(27–29). G2/M phase is the most
radio-sensitive stage of the cell cycle, therefore drugs that can
induce G2/M arrest are potential radiosensitizers. Previous studies
reported chemotherapeutic agents that enhanced the radiosensitivity
of cancer cells by accumulating the G2/M population, such as
zerumbone and docetaxel (20,30).
[6]-Gingerol was reported to induce cell cycle arrest in various
cancers. A study by Rastogi et al reported that [6]-gingerol
induced G2/M cell cycle arrest in cervical cancer cells (31), and another study by Lee et al
revealed that [6]-gingerol caused cell cycle arrest at the G1 phase
in colorectal cancer cells (16).
However, the effect of [6]-gingerol on cell cycle distribution of
GC cells remains unknown. In the present study, treatment with
[6]-gingerol (300 µM) alone arrested cells at the G1 phase, and the
alteration of the G2/M phase was slight. Notably, when [6]-gingerol
was combined with IR, the G2/M phase [the most radiosensitive stage
of the cell cycle (32)] was
significantly increased compared to IR alone, with the S phase
(relatively resistant to IR) decreased and the G1 phase (less
sensitive to IR) increased. Cell cycle progression is primarily
regulated by activation of cyclins and cyclin-dependent kinases
(Cdks), and inhibition of these checkpoints may have the potential
to mediate radiosensitization (33). The cyclin B/CDC2 complex is
responsible for the phosphorylation and activation of enzymes that
are required for normal mitosis and is considered as a crucial
checkpoint for G2 to M phase transition (34). The impairment of the cyclin B/CDC2
complex activity blocked G2/M transition. Cyclin A is also
essential for G2 progression (35).
The CDK inhibitors (CKIs) are important negative regulators of cell
cycle progression. They interact with cyclin/CDK complexes and
inhibit their activities. p27, a CDK inhibitor, binds to CDC2 and
inhibits its activity (36). In the
present study, we analyzed the involved regulatory proteins by
western blotting and found that [6]-gingerol decreased cyclin B1,
cyclin A2 and CDC2 expression and increased the mRNA expression of
p27. Pretreatment with [6]-gingerol before IR exposure
downregulated the cell protein levels of cyclin B1, cyclin A2, and
CDC2 compared with IR alone. qRT-PCR revealed that the p27 mRNA
level was markedly enhanced by the combination treatment, thus we
hypothesized that the increase of p27 may contribute to the
decrease of CDC2. How p27 interacts with CDC2 and influences its
expression is worth studying in the future. Therefore, we suggest
that [6]-gingerol enhances IR-induced arrest at the G2/M phase
through inhibition of G2/M checkpoints. We also observed G1 phase
blocking and an S phase decrease, and the G1 phase was associated
with cyclin D1 downregulation. The CDK4/6-cyclin D1 complex is a
central checkpoint of G1 progression and G1/S transition (37). Thus, the decrease of cyclin D1 and
the resultant G1 arrest may also mediate the radiosensitization of
[6]-gingerol in HGC-27 cells.
Apoptosis is a main form of cell death after IR.
Radiosensitizers could enhance the therapeutic effect of
radiotherapy by inducing apoptosis (38). Previous studies have shown that
[6]-gingerol induced apoptosis in various cancer cells through
several mechanisms. One study revealed that [6]-gingerol enhanced
TRAIL-induced apoptosis but alone inhibited viability only slightly
in GC cells (39). Conversely, our
study determined that [6]-gingerol alone inhibited cell viability
in a dose-dependent manner, and induced apoptosis in GC cells. When
combined with IR, [6]-gingerol decreased clonogenic survival and
increased IR-induced apoptosis. The two major apoptosis pathways
are the extrinsic pathway or death receptor-mediated pathway and
the intrinsic pathway or mitochondrial-mediated pathway. In the
mitochondrial pathway, cytochrome c is released from the
mitochondrial intermembrane into the cytoplasm, which stimulates
apoptosis. Then, cytochrome c interacts with Apaf-1, binding
and activating caspase-9 proenzymes, an initiator caspase.
Caspase-3, a key effector caspase, is activated by active
caspase-9, followed by activation of the rest of the caspase
cascades and apoptosis induction (40). In the present study, the western
blotting results revealed that [6]-gingerol alone increased cleaved
capsase-3 and cytochrome c, but the influence on cleaved
caspase-9 was not significant. In fact, in addition to caspase-9,
caspase-3 can be activated by caspase-8, which plays a role in the
extrinsic pathway. Previous studies have reported that [6]-gingerol
could sensitize TRAIL-induced apoptotic cancer cell death through
caspase-8 and caspase-3 activation (41). These results indicated that
[6]-gingerol can induce apoptosis and that the manner in which
[6]-gingerol induces and modulates apoptosis is complex, which
inspires us to perform more studies in the future. When
[6]-gingerol was combined with IR, cleaved caspase-9, cleaved
caspase-3 and cytochrome c were markedly increased compared
with either [6]-gingerol or IR alone, indicating that [6]-gingerol
sensitized HGC-27 GC cells to IR through activation of caspases.
Radiation induces DNA damage directly and also generates abnormally
elevated ROS, which can cause large amounts of DNA damage, thereby
increasing the apoptosis effect of radiation on the tumor (24,42).
The present study revealed that [6]-gingerol enhanced IR-induced
apoptosis, and cytochrome c, a critical molecule in the
mitochondrial-mediated apoptosis pathway, was significantly
increased in the combination group, which hinted to possible
mitochondrial damage. Yet the specific changes of the mitochondria
and through what mechanism these damages can be induced warrant
further study in our future research.
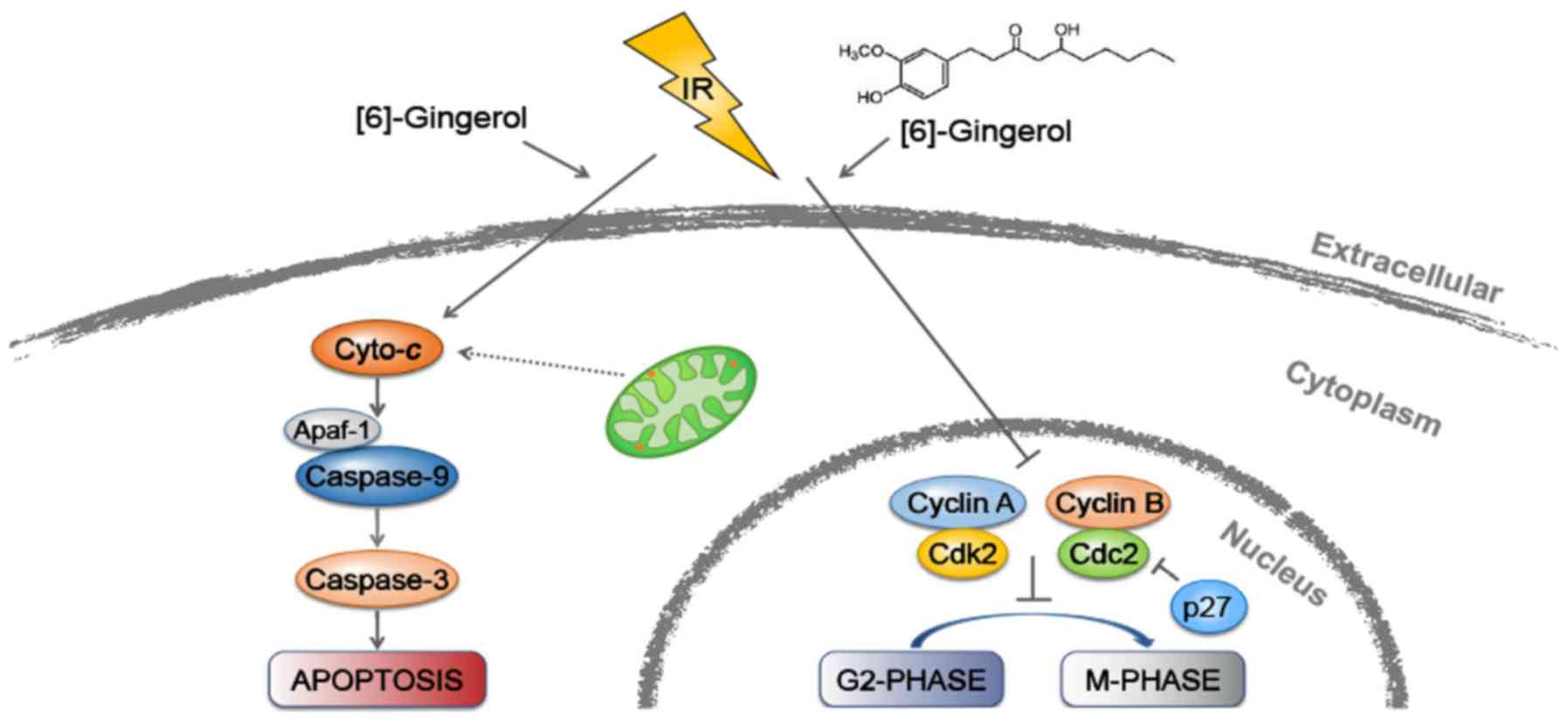
In conclusion, as depicted in Fig. 7, our study revealed for the first
time that [6]-gingerol could sensitize GC cells to IR. Moreover, we
demonstrated that the radiosensitization effect of [6]-gingerol on
HGC-27 cells was mediated through induction of G2/M arrest and
apoptosis. Recently, the application of natural phytochemicals in
cancer control and management has gained general acceptance.
[6]-Gingerol is the most abundant bioactive compound of ginger, and
it is readily available and inexpensive. Therefore, the use of
[6]-gingerol as a radiosensitizer would provide a promising future
for GC radiotherapy and bring great benefits for GC patients. To
confirm the radiosensitization effect of [6]-gingerol, further
studies focusing on the distinct molecular mechanisms, animal
experiments and clinical trials are warranted.
Acknowledgements
We thank all the members of our laboratory for their
helpful technical support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe
C, et al: The global burden of cancer 2013. JAMA Oncol. 1:505–527.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Sun LL, Meng YL, Song GY, Hu JJ,
Lu P and Ji B: Survival trends in gastric cancer patients of
Northeast China. World J Gastroenterol. 17:3257–3262.
2011.PubMed/NCBI
|
|
5
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong RK, Jang R and Darling G:
Postoperative chemoradiotherapy vs. preoperative chemoradiotherapy
for locally advanced (operable) gastric cancer: Clarifying the role
and technique of radiotherapy. J Gastrointest Oncol. 6:89–107.
2015.PubMed/NCBI
|
|
7
|
Pang X, Wei W, Leng W, Chen Q, Xia H, Chen
L and Li R: Radiotherapy for gastric cancer: A systematic review
and meta-analysis. Tumour Biol. 35:387–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trip AK, Sikorska K, van Sandick JW, Heeg
M, Cats A, Boot H, Jansen EP and Verheij M: Radiation-induced
dose-dependent changes of the spleen following postoperative
chemoradiotherapy for gastric cancer. Radiother Oncol. 116:239–244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dawson LA, Kavanagh BD, Paulino AC, Das
SK, Miften M, Li XA, Pan C, Ten Haken RK and Schultheiss TE:
Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys.
76 Suppl:S108–S115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu XQ, Sun Y, Lau E, Zhao M and Su SB:
Advances in synergistic combinations of Chinese herbal medicine for
the treatment of cancer. Curr Cancer Drug Targets. 16:346–356.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baliga MS, Haniadka R, Pereira MM, D'Souza
JJ, Pallaty PL, Bhat HP and Popuri S: Update on the chemopreventive
effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr.
51:499–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oyagbemi AA, Saba AB and Azeez OI:
Molecular targets of [6]-gingerol: Its potential roles in cancer
chemoprevention. Biofactors. 36:169–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park KK, Chun KS, Lee JM, Lee SS and Surh
YJ: Inhibitory effects of [6]-gingerol, a major pungent principle
of ginger, on phorbol ester-induced inflammation, epidermal
ornithine decarboxylase activity and skin tumor promotion in ICR
mice. Cancer Lett. 129:139–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng CJ, Wu CF, Huang HW, Ho CT and Yen
GC: Anti-invasion effects of 6-shogaol and 6-gingerol, two active
components in ginger, on human hepatocarcinoma cells. Mol Nutr Food
Res. 54:1618–1627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SH, Cekanova M and Baek SJ: Multiple
mechanisms are involved in 6-gingerol-induced cell growth arrest
and apoptosis in human colorectal cancer cells. Mol Carcinog.
47:197–208. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HS, Seo EY, Kang NE and Kim WK:
[6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer
cells. J Nutr Biochem. 19:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EC, Min JK, Kim TY, Lee SJ, Yang HO,
Han S, Kim YM and Kwon YG: [6]-Gingerol, a pungent ingredient of
ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys
Res Commun. 335:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins GS, O'Cathail SM, Muschel RJ and
McKenna WG: Drug radiotherapy combinations: Review of previous
failures and reasons for future optimism. Cancer Treat Rev.
41:105–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deorukhkar A, Ahuja N, Mercado AL,
Diagaradjane P, Raju U, Patel N, Mohindra P, Diep N, Guha S and
Krishnan S: Zerumbone increases oxidative stress in a
thiol-dependent ROS-independent manner to increase DNA damage and
sensitize colorectal cancer cells to radiation. Cancer Med.
4:278–292. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orr WS, Denbo JW, Saab KR, Ng CY, Wu J, Li
K, Garner JM, Morton CL, Du Z, Pfeffer LM, et al: Curcumin
potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-kB
activity. PLoS One. 8:513092013. View Article : Google Scholar
|
|
22
|
Liu JS, Che XM, Chang S, Qiu GL, He SC,
Fan L, Zhao W, Zhang ZL and Wang SF: β-elemene enhances the
radiosensitivity of gastric cancer cells by inhibiting Pak1
activation. World J Gastroenterol. 21:9945–9956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun M, Pan D, Chen Y, Li Y, Gao K and Hu
B: Coroglaucigenin enhances the radiosensitivity of human lung
cancer cells through Nrf2/ROS pathway. Oncotarget. 8:32807–32820.
2017.PubMed/NCBI
|
|
24
|
Yao JX, Yao ZF, Li ZF and Liu YB:
Radio-sensitization by Piper longumine of human breast adenoma
MDA-MB-231 cells in vitro. Asian Pac J Cancer Prev. 15:3211–3217.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang P, Wang L, Rodriguez-Aguayo C, Yuan
Y, Debeb BG, Chen D, Sun Y, You MJ, Liu Y, Dean DC, et al: miR-205
acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat
Commun. 5:56712014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azad A, Lim Yin S, D'Costa Z, Jones K,
Diana A, Sansom OJ, Kruger P, Liu S, McKenna WG, Dushek O, et al:
PD-L1 blockade enhances response of pancreatic ductal
adenocarcinoma to radiotherapy. EMBO Mol Med. 9:167–180. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rey S, Schito L, Koritzinsky M and Wouters
BG: Molecular targeting of hypoxia in radiotherapy. Adv Drug Deliv
Rev. 109:45–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berdis AJ: Current and emerging strategies
to increase the efficacy of ionizing radiation in the treatment of
cancer. Expert Opin Drug Discov. 9:167–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding M, Zhang E, He R and Wang X: Newly
developed strategies for improving sensitivity to radiation by
targeting signal pathways in cancer therapy. Cancer Sci.
104:1401–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyanaga S, Ninomiya I, Tsukada T, Okamoto
K, Harada S, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H,
et al: Concentration-dependent radiosensitizing effect of docetaxel
in esophageal squamous cell carcinoma cells. Int J Oncol.
48:517–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rastogi N, Duggal S, Singh SK, Porwal K,
Srivastava VK, Maurya R, Bhatt ML and Mishra DP: Proteasome
inhibition mediates p53 reactivation and anti-cancer activity of
6-gingerol in cervical cancer cells. Oncotarget. 6:43310–43325.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dillon MT, Good JS and Harrington KJ:
Selective targeting of the G2/M cell cycle checkpoint to improve
the therapeutic index of radiotherapy. Clin Oncol (R Coll Radiol).
26:257–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan Y, Black CP and Cowan KH:
Irradiation-induced G2/M checkpoint response requires ERK1/2
activation. Oncogene. 26:4689–4698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smits VA and Medema RH: Checking out the
G(2)/M transition. Biochim Biophys Acta. 1519:1–12. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furuno N, den Elzen N and Pines J: Human
cyclin A is required for mitosis until mid prophase. J Cell Biol.
147:295–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Payne SR, Zhang S, Tsuchiya K, Moser R,
Gurley KE, Longton G, deBoer J and Kemp CJ: p27kip1 deficiency
impairs G2/M arrest in response to DNA damage, leading to an
increase in genetic instability. Mol Cell Biol. 28:258–268. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng M, Zeng C, Lu X, He X, Zhang R, Qiu
Q, Zheng G, Jia X, Liu H and He Z: miR-218 suppresses gastric
cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis
in a feedback loop. Cancer Lett. 403:175–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim BM and Hong Y, Lee S, Liu P, Lim JH,
Lee YH, Lee TH, Chang KT and Hong Y: Therapeutic implications for
overcoming radiation resistance in cancer therapy. Int J Mol Sci.
16:26880–26913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishiguro K, Ando T, Maeda O, Ohmiya N,
Niwa Y, Kadomatsu K and Goto H: Ginger ingredients reduce viability
of gastric cancer cells via distinct mechanisms. Biochem Biophys
Res Commun. 362:218–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee DH, Kim DW, Jung CH, Lee YJ and Park
D: Gingerol sensitizes TRAIL-induced apoptotic cell death of
glioblastoma cells. Toxicol Appl Pharmacol. 279:253–265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang B, Wang Y and Su Y: Peroxiredoxins,
a novel target in cancer radiotherapy. Cancer Lett. 286:154–160.
2009. View Article : Google Scholar : PubMed/NCBI
|