Introduction
Anti-angiogenesis is becoming a very promising goal
for cancer therapy (1,2). Angiogenesis is a new passageway from
pre-existing blood vessels and an essential step involved in
physiological and tumor pathological processes (1,3). Tumor
cell growth and metastases processes depend on the induction of a
satisfactory blood support (1,4). Many
chemotherapeutic agents such as paclitaxel (Taxol) inhibit tumor
cell growth, proliferation and induce apoptotic cell death in
cancer treatment. Furthermore, the blocking of angiogenesis
provides a novel therapeutic target against tumor cells (5,6). In
clinical anti-angiogenic therapy, bevacizumab (Avastin) is a
monoclonal antibody for anti-vascular endothelial growth factor
(VEGF) that counteracts the action of VEGF and inhibits tumor
angiogenesis (7,8). Numerous phytochemicals, such as
curcumin or epigallocatechin-3-gallate (EGCG) have been
demonstrated to exert anti-angiogenic bioactivities in several
in vitro and in vivo models (9–11).
Thus, identification of phytochemicals with non-cytotoxic effects
on normal cells and effective anti-angiogenic action could be of
great clinical significance (11–13).
Kaempferol is a flavonoid phytochemical found in
fruits and vegetables and in some traditional Chinese medicines
(TCM) (14–16). Kaempferol has been reported to exert
biological activities such as anti-inflammatory (17,18),
antioxidant (19,20), cardioprotective (19) and antitumor (21–23).
Kaempferol has been demonstrated to provide chemopreventive effects
on different tumor systems including tumor initiation, promotion,
and progression (24,25). Recently, our previous study revealed
that kaempferol caused endoplasmic reticulum stress and
mitochondria-dependent apoptosis in human osteosarcoma U-2 OS cells
(26) and triggered AMPK and
AKT-dependent autophagic cell death in human hepatocarcinoma
SK-HEP-1 cells (27). In addition,
we also demonstrated that kaempferol suppressed U-2 OS cell
metastasis through suppression of the ERK/p38/JNK and AP-1
signaling pathways (28). In an
anti-angiogenic study, our earlier research indicated that
kaempferol induced ROS-mediated p53/ATM-dependent apoptosis in
human umbilical vein endothelial cells (HUVECs) (29). However, there is no available
information regarding the possible major target and anti-angiogenic
mechanism of kaempferol in endothelial cells. In the present study,
we analyzed the anti-angiogenic effects of kaempferol on HUVECs.
Our results demonstrated that kaempferol inhibited HUVEC
proliferation, migration and tube formation. The molecular levels
indicated that kaempferol suppressed VEGF receptor-2 (VEGFR-2)
expression and its downstream signaling cascades (AKT/mTOR and
MEK/ERK) in HUVECs.
Materials and methods
Chemicals and reagents
Kaempferol,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
vascular endothelial growth factor (VEGF), the other chemicals and
reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA)
unless otherwise stated. Medium 200, Low Serum Growth Supplement
(LSGS) and Trypsin-EDTA were obtained from Thermo Fisher
Scientific, Inc. (Carlsbad, CA, USA). The primary antibodies
[VEGFR-2 (cat. no. sc-504), PI3K (cat. no. sc-1637), p-AKT (Ser473)
(cat. no. sc-7985-R), AKT (cat. no. sc-1618), p-mTOR (Ser2448)
(cat. no. sc-101738), mTOR (cat. no. sc-8319), p-MEK1/2
(Ser218/Ser222) (cat. no. sc-7995), MEK1/2 (cat. no. sc-436), p-ERK
(Thr202/Tyr204) (cat. no. sc-16982), ERK (cat. no. sc-135900) and
β-actin (cat. no. sc-47778)] and secondary antibodies against goat
anti-mouse (cat. no. sc-2005)/-rabbit (cat. no. sc-2004) and mouse
anti-goat (cat. no. sc-2354) immunoglobulin (IgG)-horseradish
peroxidase (HRP) were obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Cell culture
HUVECs were obtained from the Bioresources
Collection and Research Center (BCRC), Food Industry Research and
Development Institute (Hsinchu, Taiwan). The cells were cultured in
Medium 200 and LSGS in a humidified atmosphere containing 5%
CO2 and 95% air at 37°C. The cells were applied within
the second to fifth passages, and all assays performed used the
same culture media with 50 ng/ml VEGF.
Cytotoxic assay
VEGF-stimulated HUVECs (1×104 cells/100
µl/well) were seeded into 96-well microplates and then incubated
with or without 50, 100, 150 and 200 µM of kaempferol for 24 h.
Cell viability was detected by MTT assay as previously described
(27,28). Briefly, as soon as kaempferol
exposure was completed, 10 µl MTT solution (5 mg/ml) was added to
each well, and the plate was incubated for an additional 3 h. The
purple crystals were dissolved with the addition of 100 µl DMSO.
The optical density ratio was assessed spectrophotometrically at
570 nm. The percentage of cell viability at each concentration
relative to the untreated control group (% of control) was
plotted.
Wound healing migration assay
HUVECs were plated into 6-well plates and incubated
to 90% confluence for 24 h. A linear wound was scratched using a
200-µl pipette tip through the monolayer before cellular debris was
removed. Then, VEGF-stimulated HUVECs were exposed to 50, 100, 150
and 200 µM of kaempferol for 24 h. The healing process was captured
using a phase-contract microscope after the wound was introduced
prior to kaempferol incubation. Cell migration was determined from
the images of five random fields. The gap size was analyzed by NIH
ImageJ version 1.46 for Windows between the migrating cells from
the opposing wound edge, and the data were expressed as the % of
the initial gap size as previously described (30,31).
Boyden chamber Transwell assay
Cell invasion ability was detected as previously
described (31,32). The Transwell (Millicell Cell Culture
Insert; EMD Millipore, Billerica, MA, USA) with 8-µm polycarbonate
filters was used after being pre-coated with Matrigel (2 mg/ml, 20
µl; BD Biosciences, Bedford, MA, USA) for 2 h at room temperature.
VEGF-stimulated HUVECs (4×103 cells/0.4 ml culture
medium) were seeded onto the upper compartment prior to 50, 100,
150 and 200 µM of kaempferol treatment for 24 h. The cells were
then fixed with 4% paraformaldehyde in PBS and then stained with 2%
crystal violet. The invading cells were counted under a light
microscope before quantification with NIH ImageJ version 1.46 for
Windows.
Tube formation assay
HUVECs were placed at a density of 5×104
cells/well into 24-well flat-bottomed plates after Matrigel (BD
Biosciences) pre-coating at 37°C for 30 min. The VEGF-stimulated
HUVECs (5×104 cells) thereafter were treated with or
without 50, 100, 150 and 200 µM of kaempferol for 24 h. After
exposure, HUVEC tube or network formation was evaluated using a
phase-contrast microscope as previously described (33,34).
VEGFR-2, AKT and ERK1/2 kinase
assay
VEGF-stimulated HUVECs (5×106 cells/75T
flask) were incubated with or without 50, 100, 150 and 200 µM of
kaempferol. After incubation for 6 h, the cells were lysed, and the
activity of VEGFR-2, AKT and ERK1/2 kinase was determined in
accordance with the manufacturer's instructions provided in the AKT
Kinase Assay kit (Nonradioactive), the p44/42 MAP Kinase Assay kit
(Nonradioactive) and the HTScan VEGF Receptor 2 Kinase Assay kit,
respectively (Cell Signaling Technology, Inc., Danvers, MA, USA).
Consequently, the purified samples were loaded on 12% SDS-PAGE to
detect targeting proteins by immunoblotting analysis as previously
described (35,36).
Western blot analysis
VEGF-stimulated HUVECs (5×106 cells/75T
flask) were exposed to 50, 100 and 200 µM of kaempferol for 6 h.
After being harvested and lysed, the protein concentration was
assessed with the Bio-Rad Protein Assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Quantified protein lysates (40 µg) were
subjected to 10–12% SDS-polyacrylamide electrophoresis (SDS-PAGE)
gels to separate protein extracts as detailed by our previous
studies (37,38). The primary antibodies (VEGFR-2,
PI3K, p-AKT, AKT, p-mTOR, mTOR, p-MEK1/2, MEK1/2, p-ERK and ERK, at
1:1,000 dilution) were hybridized overnight at 4°C, followed by the
appropriate HRP-conjugated secondary antibodies (1:5,000 dilution)
that were used before the electrochemiluminescence (ECL) reagent
(Immobilon Western HRP substrate kit; Merck Millipore, Temecula,
CA, USA). The densitometric quantification of each blot was carried
out using NIH ImageJ 1.46 software.
Statistical analysis
The data are presented as the means ± standard
deviation (SD) from at least three separate experiments.
Statistical data was analyzed using Student's t-test, and
statistical significance was considered to be P<0.05 and
P<0.001.
Results
Kaempferol reduces HUVEC
viability
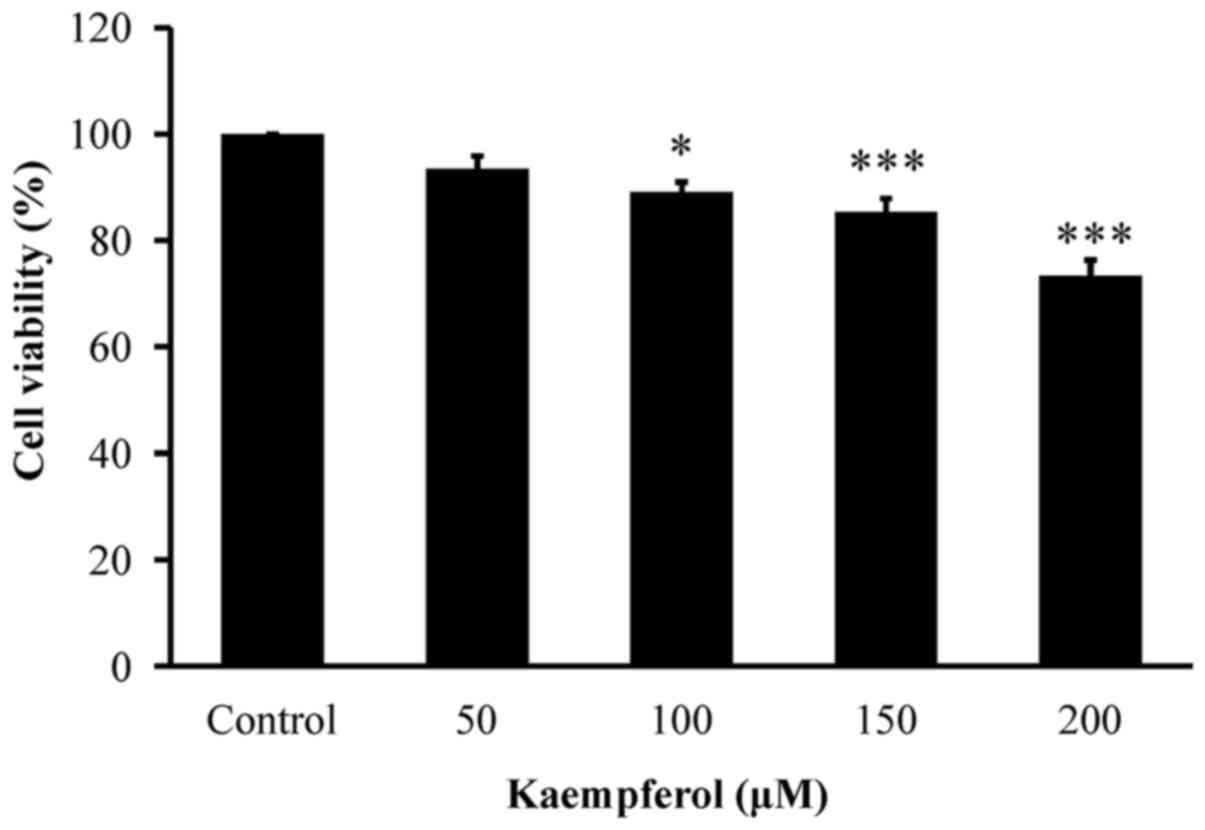
First, VEGF-stimulated HUVECs after 0, 50, 100, 150
and 200 µM of kaempferol exposure for 24 h were assessed for growth
inhibition and cytotoxicity. Our results indicated that kaempferol
significantly decreased viable VEGF-stimulated HUVECs, and this
effect was in a concentration-dependent manner (Fig. 1).
Kaempferol inhibits cell migration and
invasion, as well as disrupts tube formation in VEGF-stimulated
HUVECs
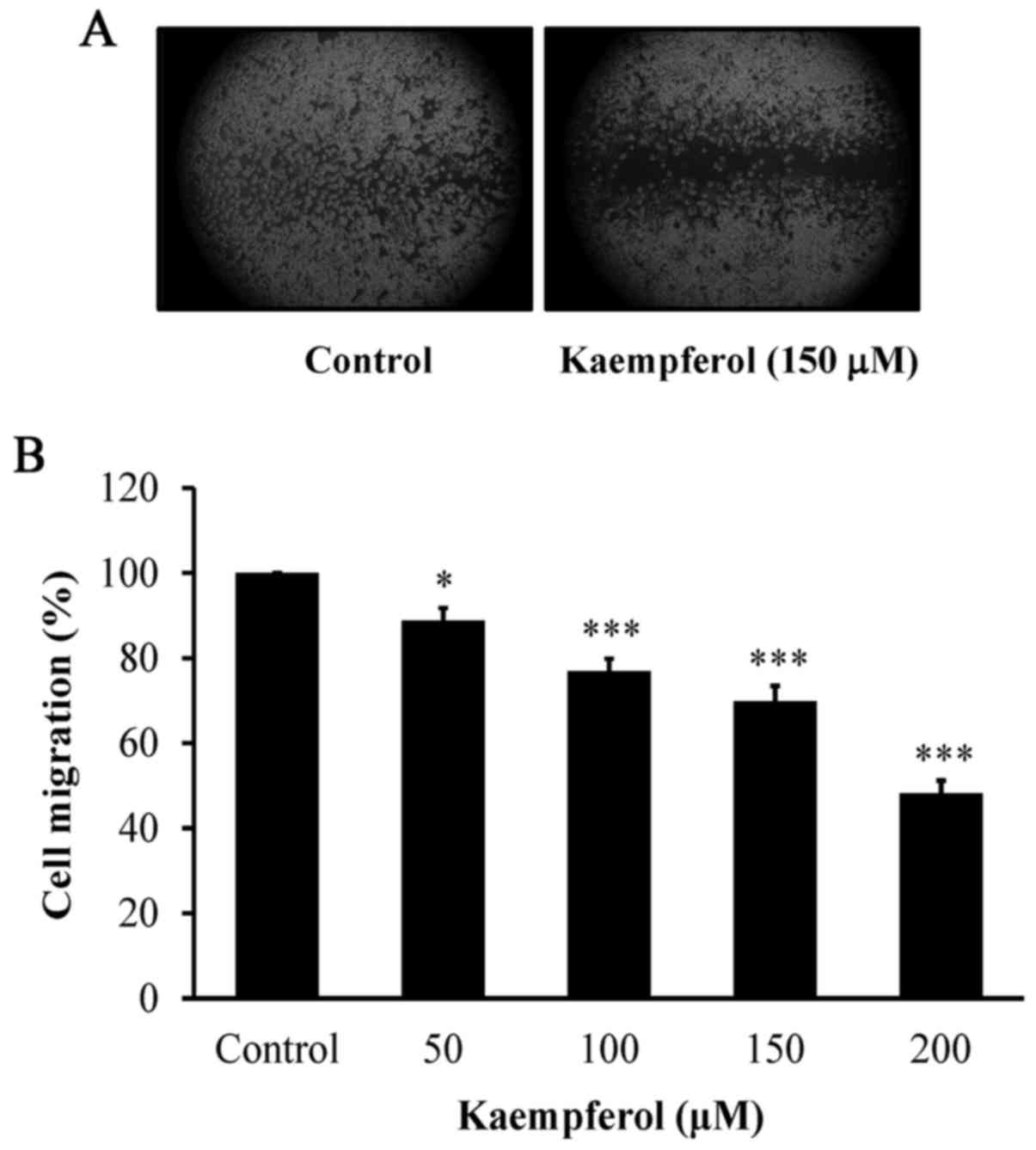
To explore the anti-angiogenic effects of kaempferol
in vitro, its inhibitory influences on VEGF-induced tube
formation and migration were investigated. Our data demonstrated
that kaempferol concentration-dependently suppressed cell migration
as determined by wound healing assay (Fig. 2A and B). Kaempferol also
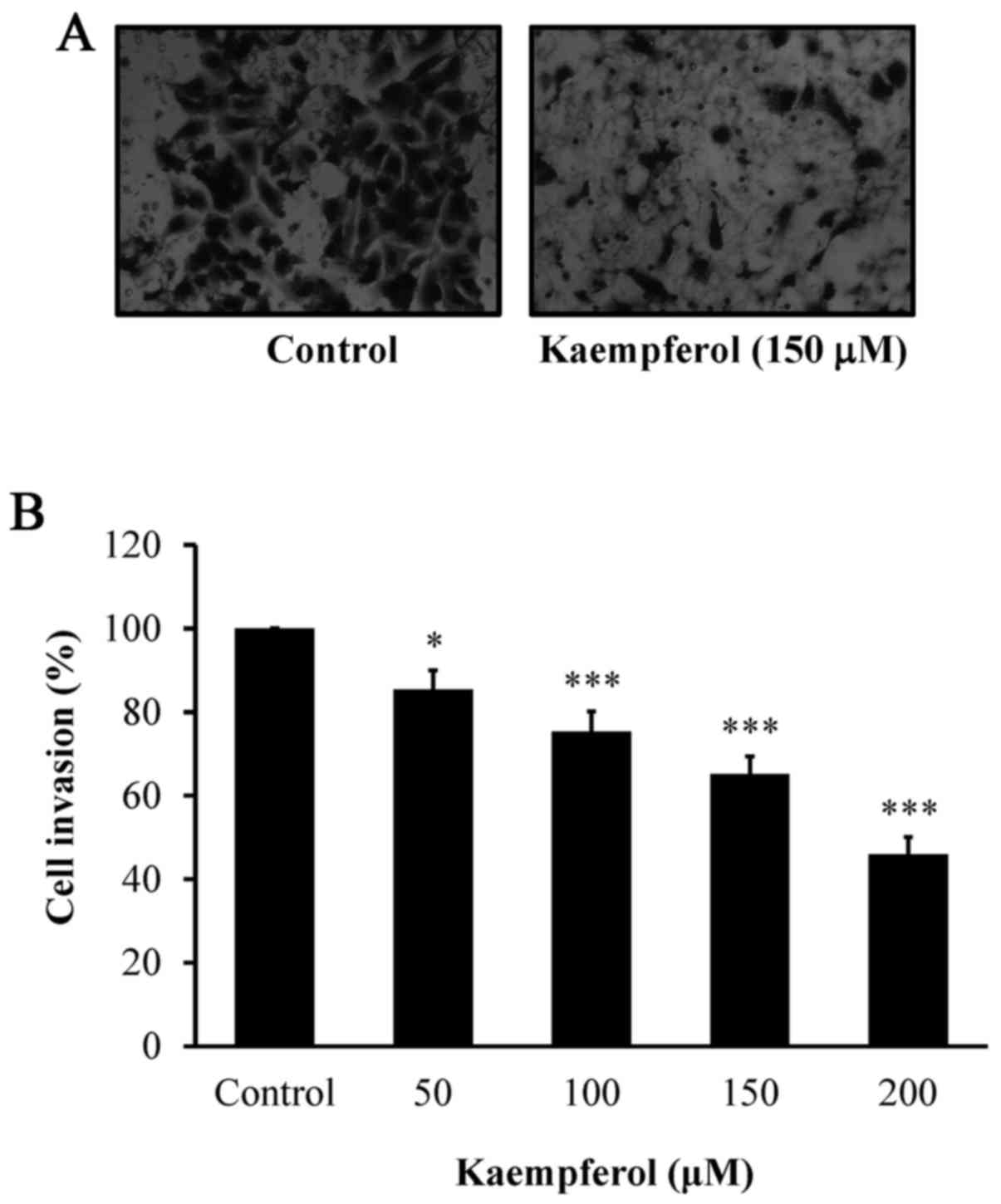
significantly suppressed cell invasion (Fig. 3A and B) in a concentration-dependent
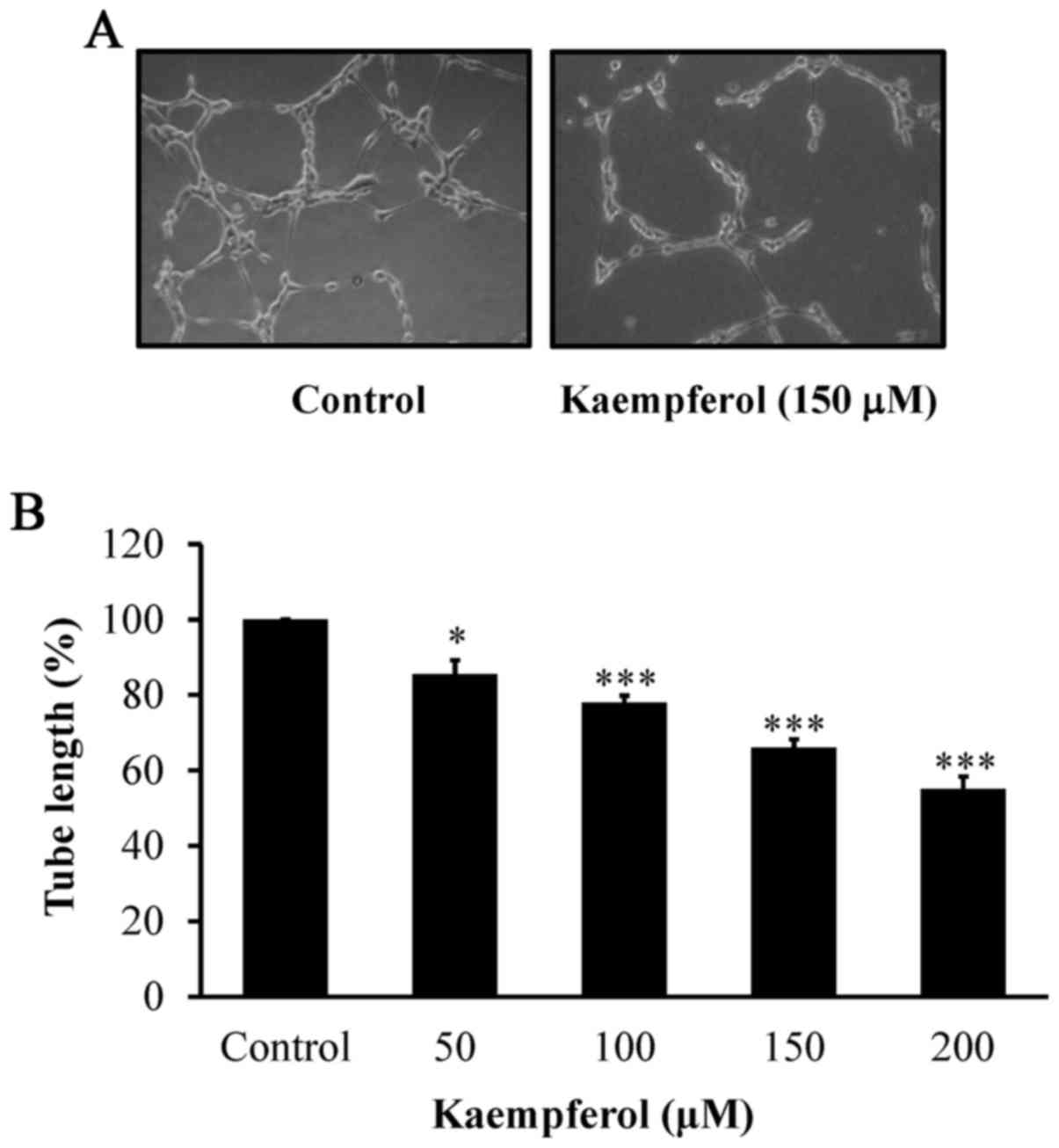
manner. To detect tube formation by endothelial cells, kaempferol
at 50, 100, 150 and 200 µM was added for 24 h. The results revealed
that kaempferol markedly disrupted the tube-like structures and
network formation (Fig. 4A and B),
and this effect was concentration-dependent. Therefore, we
determined that kaempferol exhibits anti-angiogenic effects on
VEGF-stimulated HUVECs in vitro.
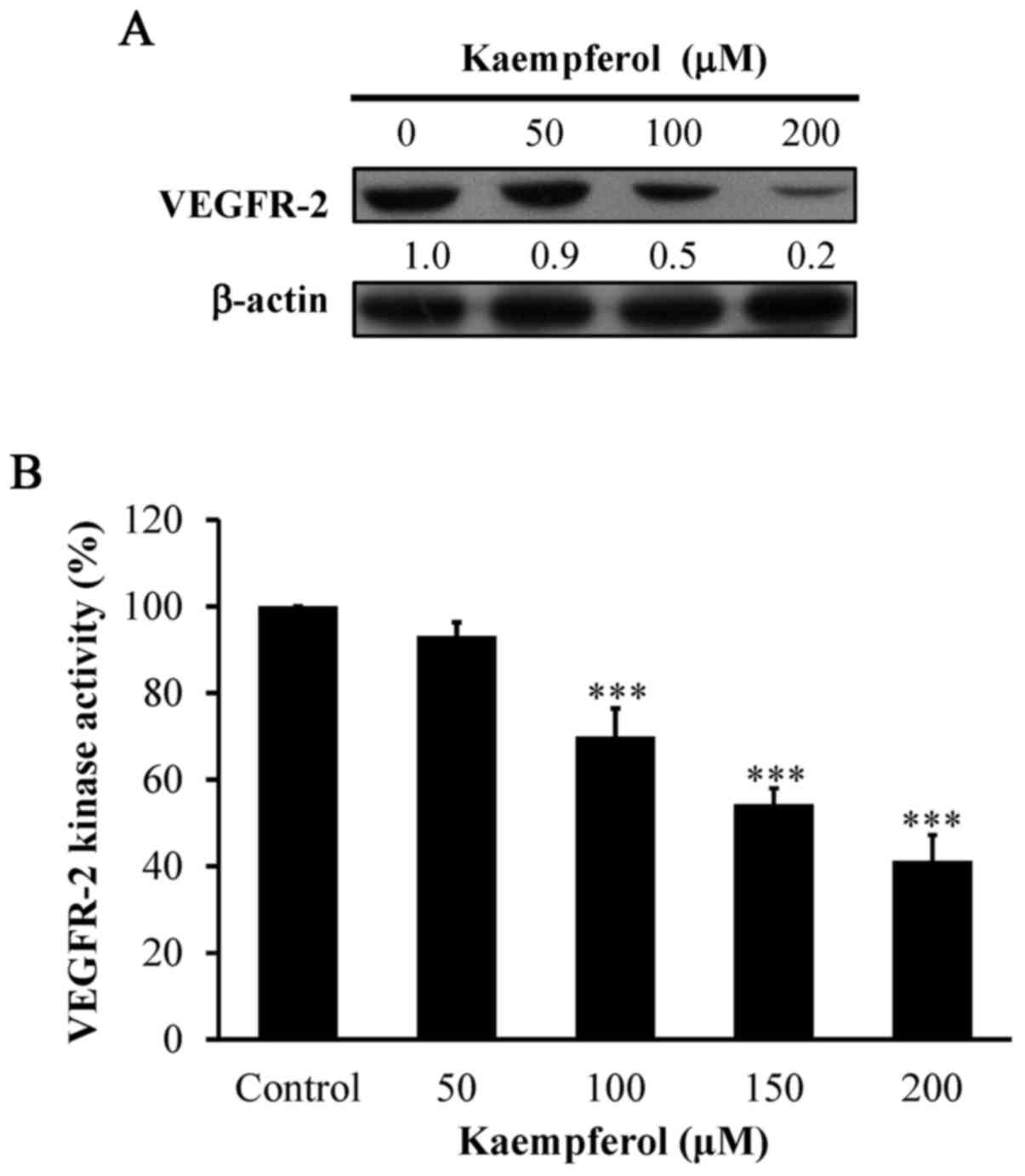
Kaempferol suppresses VEGFR-2
signaling in VEGF-stimulated HUVECs
To clarify whether the angiogenic suppression
requires VEGFR-2 signaling in kaempferol-treated HUVECs, the level
of VEGFR-2 was detected. The protein level of VEGFR-2 (Fig. 5A) and kinase activity (Fig. 5B) were markedly suppressed by
kaempferol exposure in a concentration-dependent manner. Our data
demonstrated that kaempferol-inhibited angiogenesis may be involved
in VEGFR-2 signaling in VEGF-stimulated HUVECs.
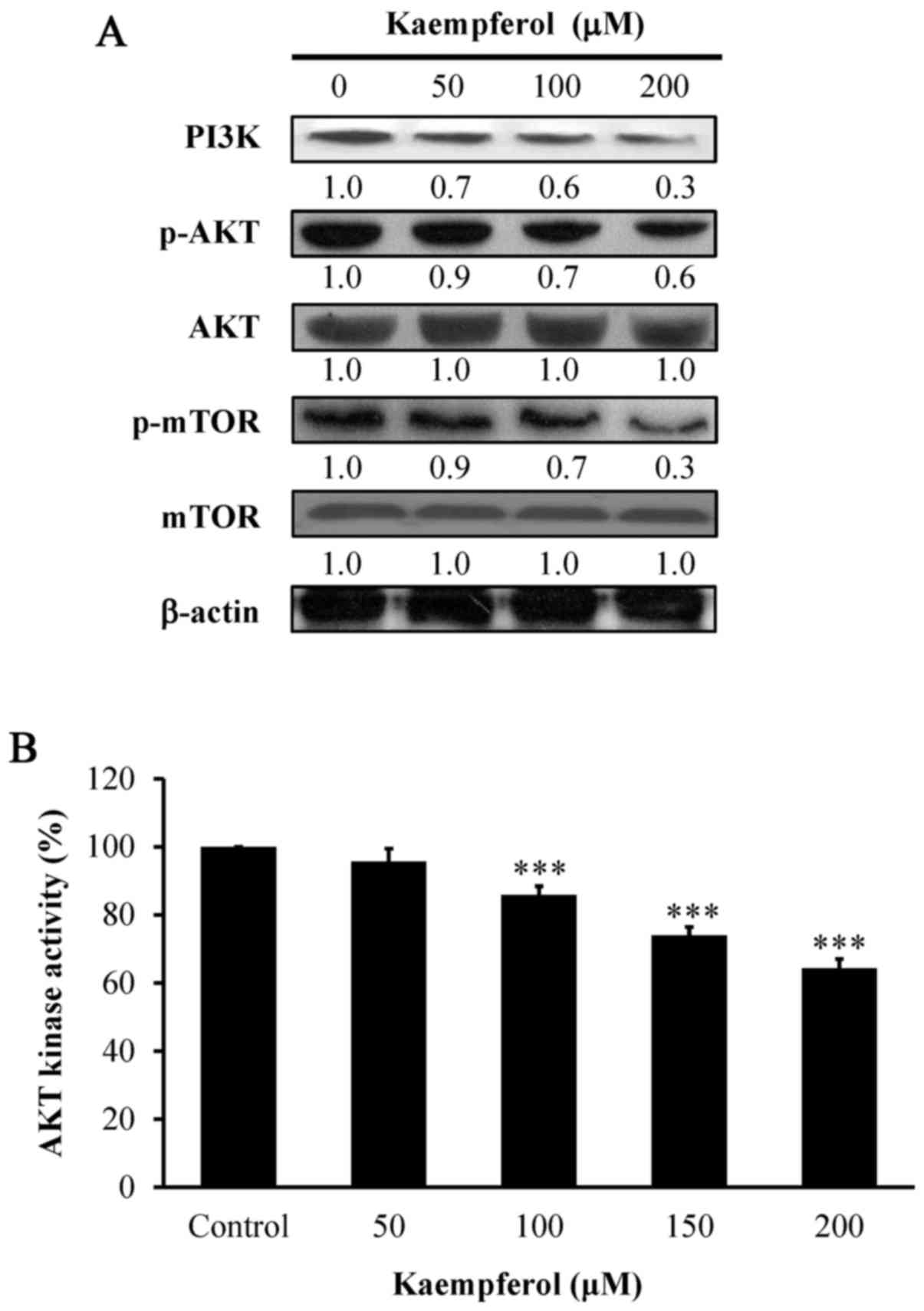
Kaempferol alters abundance of
PI3K/AKT/mTOR signaling in VEGF-stimulated HUVECs
To determine the major pathway involved in the
anti-angiogenic effect of kaempferol, we detected PI3K/AKT/mTOR
signaling after kaempferol treatment at 6 h. Our results indicated
that the protein levels of PI3K, and phosphorylation of both AKT
and mTOR were significantly decreased in a concentration-dependent
manner (Fig. 6A). The results
revealed that PI3K/AKT/mTOR signaling contributed to the
kaempferol-induced angiogenic effects on VEGF-stimulated
HUVECs.
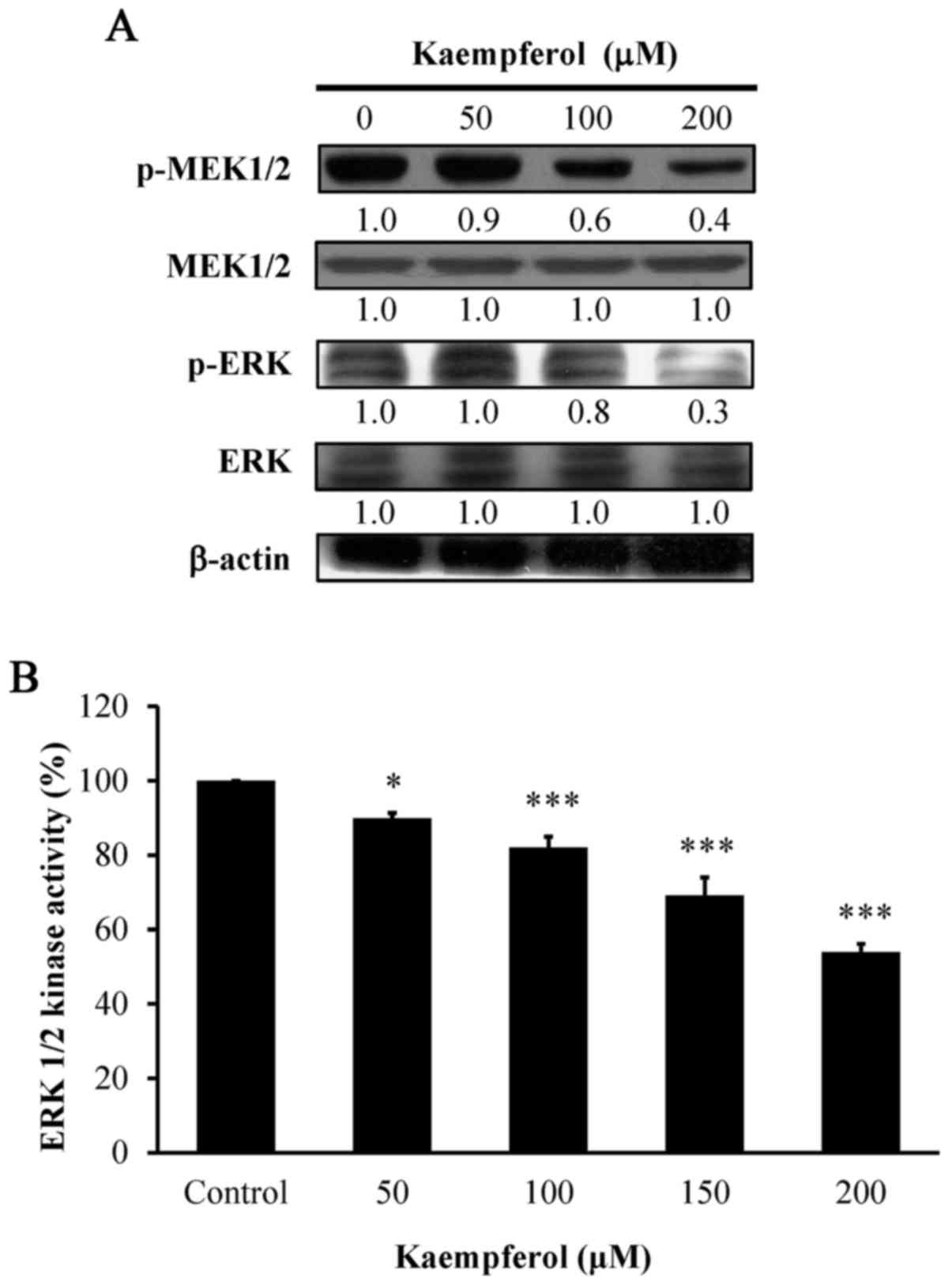
Kaempferol affects the protein levels
of phosphorylation of MEK and ERK signaling in VEGF-stimulated
HUVECs
We next aimed to clarify whether kaempferol-induced
anti-angiogenesis in HUVECs was mediated mainly through
phosphorylation of the MEK and ERK pathways. To demonstrate this,
we further investigated the protein levels of phosphorylated MEK
and phosphorylated ERK and determined that both were significantly
decreased in a concentration-dependent manner after kaempferol
exposure (Fig. 7A). The finding
demonstrated that kaempferol attenuated angiogenesis on
VEGF-stimulated HUVECs through the MEK1/2 and ERK pathways.
Kaempferol inhibits AKT and ERK
kinases in VEGF-stimulated HUVECs
To determine whether AKT and ERK activities are
involved in HUVECs, we assessed the kinase activities of AKT and
ERK. AKT kinase (Fig. 6B) and
ERK1/2 kinase (Fig. 7B) activities
were concentration-dependently suppressed by kaempferol exposure.
Therefore, we provide direct evidence that kaempferol inhibited
angiogenic effects by blocking AKT and ERK signaling in
VEGF-stimulated HUVECs.
Discussion
Flavonoids are important phytochemicals found in
foods like fruits, vegetables, wine and tea (14–16,39).
Notably, kaempferol is a flavonoid phytochemical, which exists in a
variety of fruits and vegetables, including onions, kale, broccoli,
apples, cherries, berries, tea and red wine (40,41).
Kaempferol has multiple bioactivities, including antitumor effects,
an antioxidant activity, and an anti-inflammatory function
(17–20). In addition, kaempferol has induced
apoptotic and autophagic cell death and/or cell cycle arrest in
various tumor cell lines, including colon, liver, gastric and
bladder cancer cells (25,27,42–47).
Kim et al (48) first
demonstrated that kaempferol modulated angiogenesis and
immune-endothelial cell adhesion. Zhao et al (49) revealed that kaempferol from Pu-erh
tea had anti-colorectal tumor cells and anti-angiogenesis effects
on HUVECs. However, the target and molecular mechanism involved in
the anti-angiogenic effects of kaempferol are still unknown.
Notably, cell migration, invasion, tube formation and proliferation
of endothelial cells are necessary processes during tumor
angiogenesis (1,12,50).
In the present study, we are the first to report that kaempferol at
50, 100, 150 and 200 µM inhibited VEGF-stimulated HUVEC cell
proliferation (Fig. 1), inhibited
cell migration (Fig. 2) and
invasion (Fig. 3), and these
effects were vital factors in angiogenic activity. Markedly,
kaempferol inhibited tube formation (Fig. 4) in VEGF-stimulated HUVECs. Our
results revealed that kaempferol triggered anti-angiogenic activity
in VEGF-stimulated HUVECs, and this finding is in agreement with
our previous study by our research group (29).
It is well known that vascular endothelial growth
factor (VEGF) stimulates VEGF receptor further to activate its
kinase activity which is a serious step in initiated tumor
angiogenesis (51). Suppression of
angiogenesis through the blocking of the VEGF/VEGFR signaling
pathway has developed as a potential approach in antitumor therapy
(51,52). VEGFR family members include KDR
(kinase insert domain-containing receptor; VEGFR-2), FLT1 (Fms-like
tyrosine kinase; VEGFR-1), and FLT4 (VEGFR-3) (51). VEGFR-2 binds VEGF-A, which is
expressed in vascular endothelial cells and hematopoietic stem
cells (53). In the present study,
we focused on VEGFR-2 and its downstream signaling in
kaempferol-treated HUVECs. Our results demonstrated that kaempferol
triggered anti-angiogenic activity in VEGF-stimulated HUVECs by
decreasing the VEGFR-2 protein level (Fig. 5A) and kinase activity (Fig. 5B). It has been documented that Y1175
and Y1214 in human VEGFR-2 are the main auto-phosphorylation sites
following VEGF binding, and the activation of several downstream
pathways, including PI3K/AKT and MEK/ERK levels (54,55).
Our results revealed that kaempferol also reduced VEGFR-2
downstream protein levels, including PI3K, p-AKT, p-mTOR (Fig. 6A) and p-MEK1/2, p-ERK1/2 signaling
(Fig. 7A). In addition, kaempferol
also reduced AKT and ERK1/2 kinase activity (Figs. 6B and 7B). Our findings revealed that the
kaempferol-inhibited angiogenic effects on VEGF-stimulated HUVECs
may require VEGR-2 signaling.
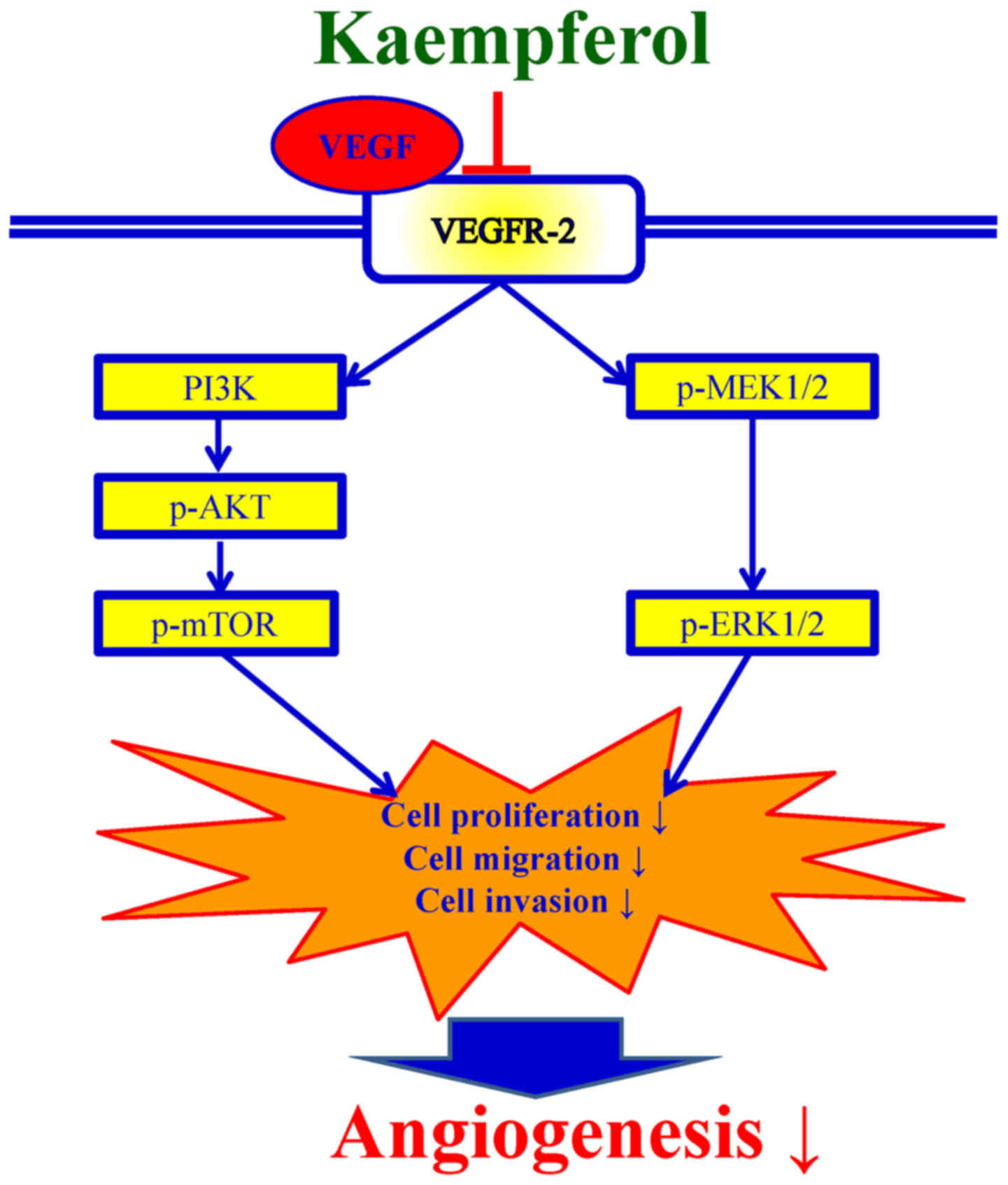
In conclusion, these data clearly revealed the
molecular signaling pathway in VEGF-stimulated HUVECs induced by
kaempferol as summarized in Fig. 8.
These findings provide evidence demonstrating the anti-angiogenic
activity of kaempferol, and we suggest that kaempferol which is a
phytochemical may act as an angiogenesis inhibitor for cancer
treatment in the near future.
Acknowledgements
Not applicable.
References
|
1
|
Varinska L, Kubatka P, Mojzis J, Zulli A,
Gazdikova K, Zubor P, Büsselberg D, Caprnda M, Opatrilova R,
Gasparova I, et al: Angiomodulators in cancer therapy: New
perspectives. Biomed Pharmacother. 89:578–590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aalders KC, Tryfonidis K, Senkus E and
Cardoso F: Anti-angiogenic treatment in breast cancer: Facts,
successes, failures and future perspectives. Cancer Treat Rev.
53:98–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markowska A, Sajdak S, Markowska J and
Huczyński A: Angiogenesis and cancer stem cells: New perspectives
on therapy of ovarian cancer. Eur J Med Chem. 142:87–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lupo G, Caporarello N, Olivieri M,
Cristaldi M, Motta C, Bramanti V, Avola R, Salmeri M, Nicoletti F
and Anfuso CD: Anti-angiogenic therapy in cancer: Downsides and new
pivots for precision medicine. Front Pharmacol. 7:5192017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ai B, Bie Z, Zhang S and Li A: Paclitaxel
targets VEGF-mediated angiogenesis in ovarian cancer treatment. Am
J Cancer Res. 6:1624–1635. 2016.PubMed/NCBI
|
|
6
|
Allegrini G, Coltelli L, Orlandi P,
Fontana A, Camerini A, Ferro A, Cazzaniga M, Casadei V, Lucchesi S,
Bona E, et al: Pharmacogenetic interaction analysis of VEGFR-2 and
IL-8 polymorphisms in advanced breast cancer patients treated with
paclitaxel and bevacizumab. Pharmacogenomics. 15:1985–1999. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sini V, Cassano A, Corsi D, De Laurentiis
M, Gamucci T, Mauri M, Naso G, Roselli M, Ruggeri EM, Tonini G, et
al: Bevacizumab as first-line treatment in HER2-negative advanced
breast cancer: Pros and cons. Tumori. 102:472–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pentheroudakis G, Kotoula V, Kouvatseas G,
Charalambous E, Dionysopoulos D, Zagouri F, Koutras A, Papazisis K,
Pectasides D, Samantas E, et al: Association of VEGF-A splice
variant mRNA expression with outcome in bevacizumab-treated
patients with metastatic breast cancer. Clin Breast Cancer.
14:330–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abd El-Rahman SS, Shehab G and Nashaat H:
Epigallocatechin-3-Gallate: The prospective targeting of cancer
stem cells and preventing metastasis of chemically-induced mammary
cancer in rats. Am J Med Sci. 354:54–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walczak K, Marciniak S and Rajtar G:
Cancer chemoprevention-selected molecular mechanisms. Postepy Hig
Med Dosw. 71:149–161. 2017. View Article : Google Scholar
|
|
11
|
Saberi-Karimian M, Katsiki N, Caraglia M,
Boccellino M, Majeed M and Sahebkar A: Vascular endothelial growth
factor: An important molecular target of curcumin. Crit Rev Food
Sci Nutr. 1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhattacharjee S and Mandal DP:
Angiogenesis modulation: The ‘spice effect’. J Environ Pathol
Toxicol Oncol. 31:273–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Shen P, Zhou J and Lu Y: Diet
phytochemicals and cutaneous carcinoma chemoprevention: A review.
Pharmacol Res. 119:327–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kilari EK and Putta S: Biological and
phytopharmacological descriptions of litchi chinensis. Pharmacogn
Rev. 10:60–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami A and Ohnishi K: Target molecules
of food phytochemicals: Food science bound for the next dimension.
Food Funct. 3:462–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang R, Ai X, Duan Y, Xue M, He W, Wang
C, Xu T, Xu M, Liu B, Li C, et al: Kaempferol ameliorates H9N2
swine influenza virus-induced acute lung injury by inactivation of
TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed
Pharmacother. 89:660–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhuang Z, Ye G and Huang B: Kaempferol
alleviates the interleukin-1β-induced inflammation in rat
osteoarthritis chondrocytes via suppression of NF-κB. Med Sci
Monit. 23:3925–3931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duan L, Ding W, Liu X, Cheng X, Cai J, Hua
E and Jiang H: Biosynthesis and engineering of kaempferol in
Saccharomyces cerevisiae. Microb Cell Fact. 16:1652017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han S, Nguyen Hanh TT, Hur J, Kim NM, Kim
SB, Hwang KH, Moon YH, Kang C, Chung B, Kim YM, et al: Synthesis
and characterization of novel astragalin galactosides using
β-galactosidase from Bacillus circulans. Enzyme Microb
Technol. 103:59–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hung TW, Chen PN, Wu HC, Wu SW, Tsai PY,
Hsieh YS and Chang HR: Kaempferol inhibits the invasion and
migration of renal cancer cells through the downregulation of AKT
and FAK pathways. Int J Med Sci. 14:984–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kashafi E, Moradzadeh M, Mohamadkhani A
and Erfanian S: Kaempferol increases apoptosis in human cervical
cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed
Pharmacother. 89:573–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee GA, Choi KC and Hwang KA: Kaempferol,
a phytoestrogen, suppressed triclosan-induced
epithelial-mesenchymal transition and metastatic-related behaviors
of MCF-7 breast cancer cells. Environ Toxicol Pharmacol. 49:48–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okoye FB, Sawadogo WR, Sendker J, Aly AH,
Quandt B, Wray V, Hensel A, Esimone CO, Debbab A, Diederich M and
Proksch P: Flavonoid glycosides from Olax mannii: Structure
elucidation and effect on the nuclear factor kappa B pathway. J
Ethnopharmacol. 176:27–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song W, Dang Q, Xu D, Chen Y, Zhu G, Wu K,
Zeng J, Long Q, Wang X, He D and Li L: Kaempferol induces cell
cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38
signaling. Oncol Rep. 31:1350–1356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang WW, Tsai SC, Peng SF, Lin MW, Chiang
JH, Chiu YJ, Fushiya S, Tseng MT and Yang JS: Kaempferol induces
autophagy through AMPK and AKT signaling molecules and causes G2/M
arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human
hepatic cancer cells. Int J Oncol. 42:2069–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF,
Tsuzuki M, Amagaya S, Huang WW and Yang JS: Kaempferol suppresses
cell metastasis via inhibition of the ERK-p38-JNK and AP-1
signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep.
30:925–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee CF, Yang JS, Tsai FJ, Chiang NN, Lu
CC, Huang YS, Chen C and Chen FA: Kaempferol induces
ATM/p53-mediated death receptor and mitochondrial apoptosis in
human umbilical vein endothelial cells. Int J Oncol. 48:2007–2014.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang JS, Lin CA, Lu CC, Wen YF, Tsai FJ
and Tsai SC: Carboxamide analog ITR-284 evokes apoptosis and
inhibits migration ability in human lung adenocarcinoma A549 cells.
Oncol Rep. 37:1786–1792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai SC, Tsai MH, Chiu CF, Lu CC, Kuo SC,
Chang NW and Yang JS: AMPK-dependent signaling modulates the
suppression of invasion and migration by fenofibrate in CAL 27 oral
cancer cells through NF-κB pathway. Environ Toxicol. 31:866–876.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goodwin CR, Lal B, Zhou X, Ho S, Xia S,
Taeger A, Murray J and Laterra J: Cyr61 mediates hepatocyte growth
factor-dependent tumor cell growth, migration, and Akt activation.
Cancer Res. 70:2932–2941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu CC, Chen HP, Chiang JH, Jin YA, Kuo SC,
Wu TS, Hour MJ, Yang JS and Chiu YJ: Quinazoline analog HMJ-30
inhibits angiogenesis: Involvement of endothelial cell apoptosis
through ROS-JNK-mediated death receptor 5 signaling. Oncol Rep.
32:597–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujita H, Gomori A, Fujioka Y, Kataoka Y,
Tanaka K, Hashimoto A, Suzuki T, Ito K, Haruma T, Yamamoto-Yokoi H,
et al: High potency VEGFRs/MET/FMS triple blockade by TAS-115
concomitantly suppresses tumor progression and bone destruction in
tumor-induced bone disease model with lung carcinoma cells. PLoS
One. 11:e01648302016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pu K, Yuan L, Chen L, Wang A, Zhou X,
Zhang H and Zhu Y: Identification of VEGFR2-binding peptides using
high throughput bacterial display methods and functional
assessment. Curr Cancer Drug Targets. 15:158–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee MR, Lin C, Lu CC, Kuo SC, Tsao JW,
Juan YN, Chiu HY, Lee FY, Yang JS and Tsai FJ: YC-1 induces G0/G1
phase arrest and mitochondria-dependent apoptosis in
cisplatin-resistant human oral cancer CAR cells. Biomedicine.
7:122017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Davatgaran-Taghipour Y, Masoomzadeh S,
Farzaei MH, Bahramsoltani R, Karimi-Soureh Z, Rahimi R and
Abdollahi M: Polyphenol nanoformulations for cancer therapy:
Experimental evidence and clinical perspective. Int J Nanomedicine.
12:2689–2702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu RH: Health-promoting components of
fruits and vegetables in the diet. Adv Nutr. 4 Suppl:S384–S392.
2013. View Article : Google Scholar
|
|
41
|
Arif H, Sohail A, Farhan M, Rehman AA,
Ahmad A and Hadi SM: Flavonoids-induced redox cycling of copper
ions leads to generation of reactive oxygen species: A potential
role in cancer chemoprevention. Int J Biol Macromol. 106:569–578.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi EJ and Ahn WS: Kaempferol induced the
apoptosis via cell cycle arrest in human breast cancer MDA-MB-453
cells. Nutr Res Pract. 2:322–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee HS, Cho HJ, Yu R, Lee KW, Chun HS and
Park JH: Mechanisms underlying apoptosis-inducing effects of
Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci.
15:2722–2737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Che J, Liang B, Zhang Y, Wang Y, Tang J
and Shi G: Kaempferol alleviates ox-LDL-induced apoptosis by
up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in
human endothelial cells. Cardiovasc Pathol. 31:57–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Varshney R, Gupta S and Roy P:
Cytoprotective effect of kaempferol against palmitic acid-induced
pancreatic β-cell death through modulation of autophagy via
AMPK/mTOR signaling pathway. Mol Cell Endocrinol. 448:1–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cho IH, Choi YJ, Gong JH, Shin D, Kang MK
and Kang YH: Astragalin inhibits autophagy-associated airway
epithelial fibrosis. Respir Res. 16:512015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang HC, Syu KY and Lin JK: Chemical
composition of Solanum nigrum linn extract and induction of
autophagy by leaf water extract and its major flavonoids in AU565
breast cancer cells. J Agric Food Chem. 58:8699–8708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim JD, Liu L, Guo W and Meydani M:
Chemical structure of flavonols in relation to modulation of
angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem.
17:165–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao X, Song JL, Kim JD, Lee JS and Park
KY: Fermented Pu-erh tea increases in vitro anticancer activities
in HT-29 cells and has antiangiogenetic effects on HUVECs. J
Environ Pathol Toxicol Oncol. 32:275–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yüksel Ş, Akyerli Boylu C and Yakicier
Cengiz M: Angiogenesis, invasion, and metastasis characteristics of
hepatocellular carcinoma. J Gastrointest Cancer. Aug 7–2017.
View Article : Google Scholar
|
|
51
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chebib R, Verlingue L, Cozic N, Faron M,
Burtin P, Boige V, Hollebecque A and Malka D: Angiogenesis
inhibition in the second-line treatment of metastatic colorectal
cancer: A systematic review and pooled analysis. Semin Oncol.
44:114–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu M, Xiong H, Xu Y, Xiong X, Zou H, Zheng
M, Wang X and Zhou X: Association between VEGF-A and VEGFR-2
polymorphisms and response to treatment of neovascular AMD with
anti-VEGF agents: A meta-analysis. Br J Ophthalmol. 101:976–984.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li W, Man XY, Li CM, Chen JQ, Zhou J, Cai
SQ, Lu ZF and Zheng M: VEGF induces proliferation of human hair
follicle dermal papilla cells through VEGFR-2-mediated activation
of ERK. Exp Cell Res. 318:1633–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Z, Neiva KG, Lingen MW, Ellis LM and
Nör JE: VEGF-dependent tumor angiogenesis requires inverse and
reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ.
17:499–512. 2010. View Article : Google Scholar : PubMed/NCBI
|