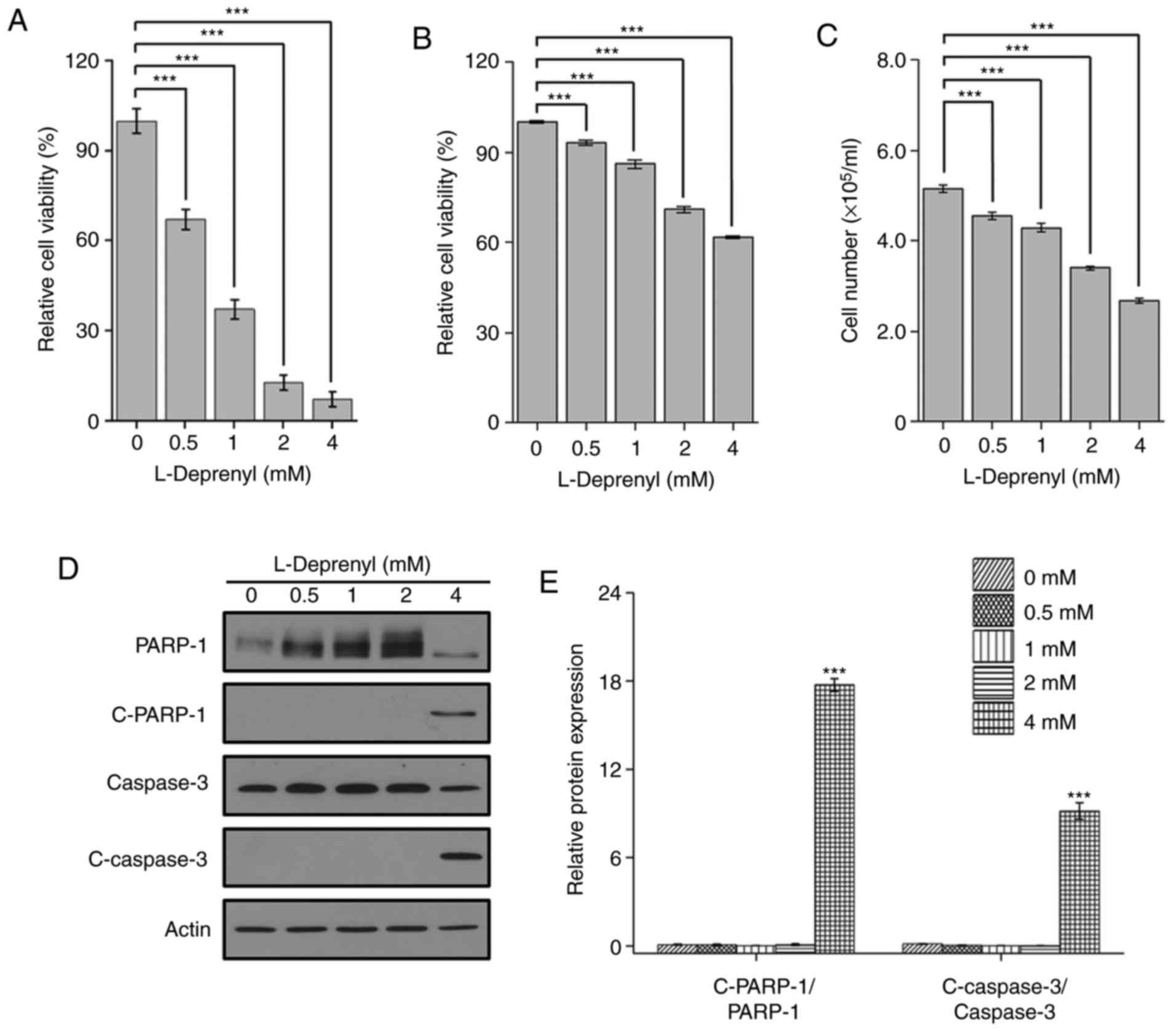
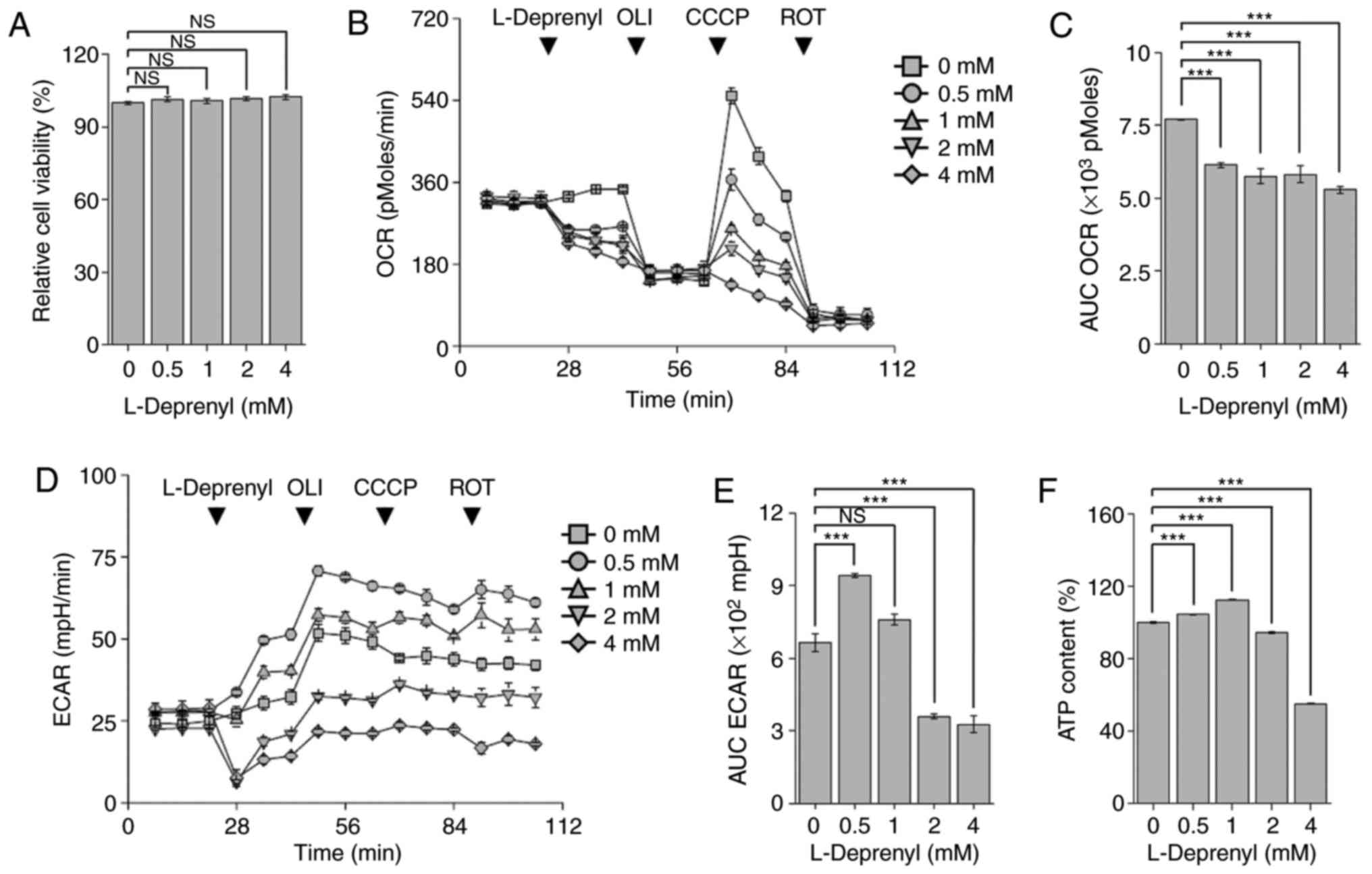
|
1
|
De Kouchkovsky I and Abdul-Hay M: ‘Acute
myeloid leukemia: A comprehensive review and 2016 update’. Blood
Cancer J. 6:e4412016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Löwenberg B and Rowe JM: Introduction to
the review series on advances in acute myeloid leukemia (AML).
Blood. 127:12016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liehr T: Thorough discussion of cancer
research-thoughts against the main stream. Eur J Hum Genet.
25:9022017. View Article : Google Scholar :
|
|
4
|
Wilson CS, Davidson GS, Martin SB, Andries
E, Potter J, Harvey R, Ar K, Xu Y, Kopecky KJ, Ankerst DP, et al:
Gene expression profiling of adult acute myeloid leukemia
identifies novel biologic clusters for risk classification and
outcome prediction. Blood. 108:685–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sallmyr A, Fan J, Datta K, Kim KT, Grosu
D, Shapiro P, Small D and Rassool F: Internal tandem duplication of
FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and
misrepair: Implications for poor prognosis in AML. Blood.
111:3173–3182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adam-Vizi V and Chinopoulos C:
Bioenergetics and the formation of mitochondrial reactive oxygen
species. Trends Pharm Sci. 27:639–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marlein CR, Zaitseva L, Piddock RE,
Robinson SD, Edwards DR, Shafat MS, Zhou Z, Lawes M, Bowles KM and
Rushworth SA: NADPH oxidase-2 derived superoxide drives
mitochondrial transfer from bone marrow stromal cells to leukemic
blasts. Blood. 130:1649–1660. 2017.PubMed/NCBI
|
|
8
|
Watson AS, Riffelmacher T, Stranks A,
Williams O, De Boer J, Cain K, MacFarlane M, McGouran J, Kessler B,
Khandwala S, et al: Autophagy limits proliferation and glycolytic
metabolism in acute myeloid leukemia. Cell Death Discov.
1:150082015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robottom BJ: Efficacy, safety, and patient
preference of monoamine oxidase B inhibitors in the treatment of
Parkinson's disease. Patient Prefer Adherence. 5:57–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Youdim MB, Edmondson D and Tipton KF: The
therapeutic potential of monoamine oxidase inhibitors. Nat Rev
Neurosci. 7:295–309. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magyar K and Szende B: (−)-Deprenyl, A
selective MAO-B inhibitor, with apoptotic and anti-apoptotic
properties. Neurotoxicology. 25:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
ThyagaRajan S, Tran L, Molinaro CA,
Gridley DS, Felten DL and Bellinger DL: Prevention of mammary tumor
development through neuroimmunomodulation in the spleen and lymph
nodes of old female sprague-dawley rats by L-Deprenyl.
Neuroimmunomodulation. 20:141–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lajkó E, Polgár L, Láng O, Lengyel J,
Kőhidai L and Magyar K: Basic cell physiological activities (cell
adhesion, chemotaxis and proliferation) induced by selegiline and
its derivatives in Mono Mac 6 human monocytes. J Neural Transm
(Vienna). 119:545–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chi Y, Lindgren V, Quigley S and Gaitonde
S: Acute myelogenous leukemia with t(6;9)(p23;q34) and marrow
basophilia: An overview. Arch Pathol Lab Med. 132:1835–1837.
2008.PubMed/NCBI
|
|
16
|
Lee BH, Tothova Z, Levine RL, Anderson K,
Buza-Vidas N, Cullen DE, McDowell EP, Adelsperger J, Fröhling S,
Huntly BJ, et al: FLT3 mutations confer enhanced proliferation and
survival properties to multipotent progenitors in a murine model of
chronic myelomonocytic leukemia. Cancer Cell. 12:367–380. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharpe MA and Baskin DS: Monoamine oxidase
B levels are highly expressed in human gliomas and are correlated
with the expression of HiF-1α and with transcription factors Sp1
and Sp3. Oncotarget. 7:3379–3393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jayanthi S, Deng X, Noailles PA, Ladenheim
B and Cadet JL: Methamphetamine induces neuronal apoptosis via
cross-talks between endoplasmic reticulum and
mitochondria-dependent death cascades. FASEB J. 18:238–251. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfeiffer T, Schuster S and Bonhoeffer S:
Cooperation and competition in the evolution of ATP-producing
pathways. Science. 292:504–507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oliver D: The structure and activity of
selegiline, its functional groups, and congeners. Bioorg Chem.
2002.
|
|
22
|
Sriskanthadevan S, Jeyaraju DV, Chung TE,
Prabha S, Xu W, Skrtic M, Jhas B, Hurren R, Gronda M, Wang X, et
al: AML cells have low spare reserve capacity in their respiratory
chain that renders them susceptible to oxidative metabolic stress.
Blood. 125:2120–2130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samudio I, Harmancey R, Fiegl M,
Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W,
Duvvuri S, Taegtmeyer H and Andreeff M: Pharmacologic inhibition of
fatty acid oxidation sensitizes human leukemia cells to apoptosis
induction. J Clin Invest. 120:142–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacque N, Ronchetti AM, Larrue C, Meunier
G, Birsen R, Willems L, Saland E, Decroocq J, Maciel TT, Lambert M,
et al: Targeting glutaminolysis has antileukemic activity in acute
myeloid leukemia and synergizes with BCL-2 inhibition. Blood.
126:1346–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yen K, Travins J, Wang F, David MD, Artin
E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, et al:
AG-221, a first-in-class therapy targeting acute myeloid leukemia
harboring oncogenic IDH2 mutations. Cancer Discov. 7:478–493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang F, Travins J, DeLaBarre B,
Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A,
Liu W, Gliser C, et al: Targeted inhibition of mutant IDH2 in
leukemia cells induces cellular differentiation. Science.
340:622–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
ThyagaRajan S, Felten SY and Felten DL:
Antitumor effect of L-deprenyl in rats with carcinogen-induced
mammary tumors. Cancer Lett. 123:177–183. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thyagarajan S, Meites J and Quadri SK:
Deprenyl reinitiates estrous cycles, reduces serum prolactin, and
decreases the incidence of mammary and pituitary tumors in old
acyclic rats. Endocrinology. 136:1103–1110. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
ThyagaRajan S and Quadri SK: L-Deprenyl
inhibits tumor growth, reduces serum prolactin, and suppresses
brain monoamine metabolism in rats with carcinogen-induced mammary
tumors. Endocrine. 10:225–232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tatton WG and Chalmers-Redman RM:
Modulation of gene expression rather than monoamine oxidase
inhibition: (−)-deprenyl-related compounds in controlling
neurodegeneration. Neurology. 47:S171–S183. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simon L, Szilágyi G, Bori Z, Telek G,
Magyar K and Nagy Z: Low dose (−)deprenyl is cytoprotective: It
maintains mitochondrial membrane potential and eliminates oxygen
radicals. Life Sci. 78:225–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi H, Maruyama W, Akao Y, Takahashi T,
Iwasa K, Youdim MB and Naoi M: N-Propargylamine protects SH-SY5Y
cells from apoptosis induced by an endogenous neurotoxin,
N-methyl(R)salsolinol, through stabilization of mitochondrial
membrane and induction of anti-apoptotic Bcl-2. J Neural Trans.
113:21–32. 2006. View Article : Google Scholar
|
|
33
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|