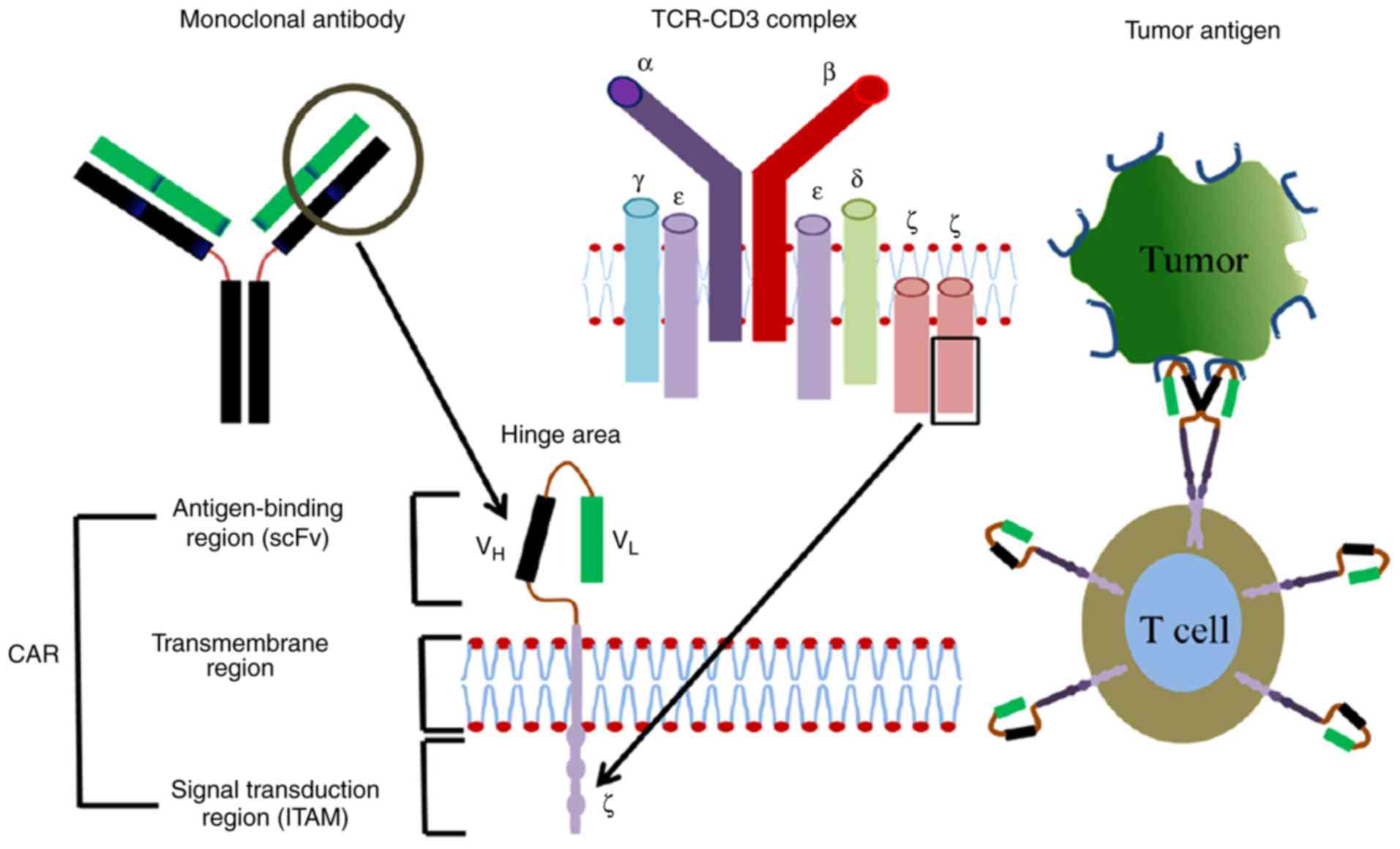
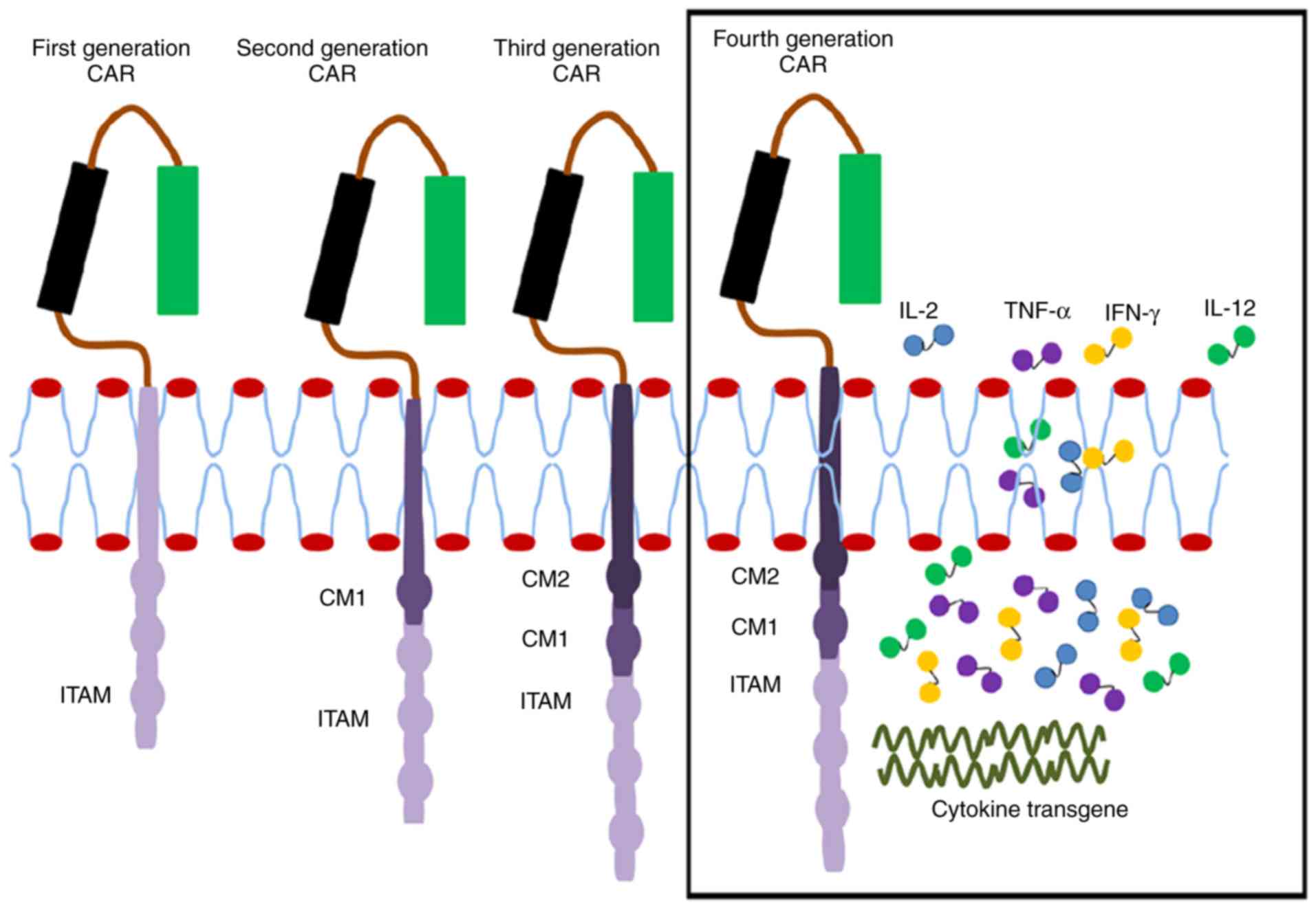
|
1
|
Arruebo M, Vilaboa N, Sáez-Gutierrez B,
Lambea J, Tres A, Valladares M and González-Fernández Á: Assessment
of the evolution of cancer treatment therapies. Cancers.
3:3279–3330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ethun CG, Bilen MA, Jani AB, Maithel SK,
Ogan K and Master VA: Frailty and cancer: Implications for oncology
surgery, medical oncology, and radiation oncology. CA Cancer J
Clin. 67:362–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alfarouk KO, Stock C-M, Taylor S, Walsh M,
Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO,
Harguindey S, et al: Resistance to cancer chemotherapy: Failure in
drug response from ADME to P-gp. Cancer Cell Int. 15:712015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Lv L and Yang K: Chemotherapy
targeting cancer stem cells. Am J Cancer Res. 5:880–893.
2015.PubMed/NCBI
|
|
5
|
Levitzki A: Targeting the immune system to
fight cancer using chemical receptor homing vectors carrying
polyinosine/cytosine (PolyIC). Front Oncol. 2:42012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ZY, Ma F, Huang H and He CY:
Synthetic immunity to break down the bottleneck of cancer
immunotherapy. Sci Bull. 60:977–985. 2015. View Article : Google Scholar
|
|
7
|
Smith AJ, Oertle J, Warren D and Prato D:
Chimeric antigen receptor (CAR) T cell therapy for malignant
cancers: Summary and perspective. J Cell Immunother. 2:59–68. 2016.
View Article : Google Scholar
|
|
8
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Lymphocytes and the cellular basis of
adaptive immunity. New York: Garland Science; 2002
|
|
9
|
Mak TW and Saunders ME: The immune
response: Basic and clinical principles. Academic Press; 2005
|
|
10
|
Dembic Z: The cytokines of the immune
system: The role of cytokines in disease related to immune
response. Academic Press; 2015, View Article : Google Scholar
|
|
11
|
Pennock ND, White JT, Cross EW, Cheney EE,
Tamburini BA and Kedl RM: T cell responses: Naive to memory and
everything in between. Adv Physiol Educ. 37:273–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hassanipour-Azgomi S,
Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S and
Salehiniya H: Incidence and mortality of prostate cancer and their
relationship with the human development index worldwide. Prostate
Int. 4:118–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haas GP, Delongchamps N, Brawley OW, Wang
CY and de la Roza G: The worldwide epidemiology of prostate cancer:
Perspectives from autopsy studies. Can J Urol. 15:3866–3871.
2008.PubMed/NCBI
|
|
15
|
Brawley OW: Trends in prostate cancer in
the United States. J Natl Cancer Inst Monogr. 2012:152–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cotter K, Konety B and Ordonez MA:
Contemporary management of prostate cancer. F1000Res. 5:2016.
|
|
17
|
Lynch JH, Batuello JT, Crawford ED,
Gomella LG, Kaufman J, Petrylak DP and Joel AB: Therapeutic
strategies for localized prostate cancer. Rev Urol. 3 Suppl
2:S39–S48. 2001.PubMed/NCBI
|
|
18
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative intent. Eur
Urol. 71:618–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bander NH, Nanus DM, Milowsky MI,
Kostakoglu L, Vallabahajosula S and Goldsmith SJ: Targeted systemic
therapy of prostate cancer with a monoclonal antibody to
prostate-specific membrane antigen. Semin Oncol. 30:667–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morrissey KM, Yuraszeck T, Li CC, Zhang Y
and Kasichayanula S: Immunotherapy and novel combinations in
oncology: Current landscape, challenges, and opportunities. Clin
Transl Sci. 9:89–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khalil DN, Budhu S, Gasmi B, Zappasodi R,
Hirschhorn-Cymerman D, Plitt T, De Henau O, Zamarin D, Holmgaard
RB, Murphy JT, et al: The new era of cancer immunotherapy:
Manipulating T-cell activity to overcome malignancy. Adv Cancer
Res. 128:1–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu S, Li A, Liu Q, Li T, Yuan X, Han X and
Wu K: Chimeric antigen receptor T cells: A novel therapy for solid
tumors. J Hematol Oncol. 10:782017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mirzaei HR, Rodriguez A, Shepphird J,
Brown CE and Badie B: Chimeric antigen receptors T cell therapy in
solid tumor: Challenges and clinical applications. Front Immunol.
8:18502017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohammed S, Sukumaran S, Bajgain P,
Watanabe N, Heslop HE, Rooney CM, Brenner MK, Fisher WE, Leen AM
and Vera JF: Improving chimeric antigen receptor-modified T cell
function by reversing the immunosuppressive tumor microenvironment
of pancreatic cancer. Mol Ther. 25:249–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abate-Daga D and Davila ML: CAR models:
Next-generation CAR modifications for enhanced T-cell function. Mol
Ther Oncolytics. 3:160142016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin L, Lai Y, Zhao R, Wei X, Weng J, Lai
P, Li B, Lin S, Wang S, Wu Q, et al: Incorporation of a hinge
domain improves the expansion of chimeric antigen receptor T cells.
J Hematol Oncol. 10:682017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sommermeyer D, Hill T, Shamah SM, Salter
AI, Chen Y, Mohler KM and Riddell SR: Fully human CD19-specific
chimeric antigen receptors for T-cell therapy. Leukemia.
31:2191–2199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monnier PP, Vigouroux RJ and Tassew NG: In
vivo applications of single chain Fv (variable domain) (scFv)
fragments. Antibodies. 2:193–208. 2013. View Article : Google Scholar
|
|
29
|
Whitlow M, Bell BA, Feng S-L, Filpula D,
Hardman KD, Hubert SL, Rollence ML, Wood JF, Schott ME, Milenic DE,
et al: An improved linker for single-chain Fv with reduced
aggregation and enhanced proteolytic stability. Protein Eng.
6:989–995. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alfthan K, Takkinen K, Sizmann D,
Söderlund H and Teeri TT: Properties of a single-chain antibody
containing different linker peptides. Protein Eng. 8:725–731. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zidovetzki R, Rost B and Pecht I: Role of
transmembrane domains in the functions of B-and T-cell receptors.
Immunol Lett. 64:97–107. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dotti G, Gottschalk S, Savoldo B and
Brenner MK: Design and development of therapies using chimeric
antigen receptor-expressing T cells. Immunol Rev. 257:107–126.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janeway CA Jr, Travers P, Walport M and
Shlomchik M: Principles of innate and adaptive immunity
immunobiologyGarland Science. New York: 2001
|
|
34
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Molecular Biology of the Cell. 4th edition.
Garland Science; New York, NY: 2002
|
|
35
|
Artyomov MN, Lis M, Devadas S, Davis MM
and Chakraborty AK: CD4 and CD8 binding to MHC molecules primarily
acts to enhance Lck delivery. Proc Natl Acad Sci USA.
107:16916–16921. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi YE, Yu HN, Yoon CH and Bae YS:
Tumor-mediated down-regulation of MHC class II in DC development is
attributable to the epigenetic control of the CIITA type I
promoter. Eur J Immunol. 39:858–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. New Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Porter DL, Levine BL, Bunin N, Stadtmauer
EA, Luger SM, Goldstein S, Loren A, Phillips J, Nasta S, Perl A, et
al: A phase 1 trial of donor lymphocyte infusions expanded and
activated ex vivo via CD3/CD28 costimulation. Blood. 107:1325–1331.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jacobson CA and Ritz J: Time to put the
CAR-T before the horse. Blood. 118:4761–4762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cartellieri M, Bachmann M, Feldmann A,
Bippes C, Stamova S, Wehner R, Temme A and Schmitz M: Chimeric
antigen receptor-engineered T cells for immunotherapy of cancer. J
Biomed Biotechnol. 2010:9563042010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haji-Fatahaliha M, Hosseini M, Akbarian A,
Sadreddini S, Jadidi-Niaragh F and Yousefi M: CAR-modified T-cell
therapy for cancer: An updated review. Artif Cells Nanomed
Biotechnol. 44:1339–1349. 2016.PubMed/NCBI
|
|
42
|
Kulemzin S, Kuznetsova V, Mamonkin M and
Taranin A: Engineering chimeric antigen receptors. Acta Naturae.
9:6–14. 2017.PubMed/NCBI
|
|
43
|
Chmielewski M and Abken H: TRUCKs: The
fourth generation of CARs. Expert Opin Biol Ther. 15:1145–1154.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chmielewski M, Kopecky C, Hombach AA and
Abken H: IL-12 release by engineered T cells expressing chimeric
antigen receptors can effectively Muster an antigen-independent
macrophage response on tumor cells that have shut down tumor
antigen expression. Cancer Res. 71:5697–5706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Scarfò I and Maus MV: Current approaches
to increase CAR T cell potency in solid tumors: Targeting the tumor
microenvironment. J Immunother Cancer. 5:282017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Anassi E and Ndefo UA: Sipuleucel-T
(provenge) injection: The first immunotherapy agent (vaccine) for
hormone-refractory prostate cancer. P T. 36:197–202.
2011.PubMed/NCBI
|
|
47
|
Westdorp H, Sköld AE, Snijer BA, Franik S,
Mulder SF, Major PP, Foley R, Gerritsen WR and de Vries IJM:
Immunotherapy for prostate cancer: Lessons from responses to
tumor-associated antigens. Front Immunol. 5:1912014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ge C, Li R, Song X and Qin S: Advances in
evidence-based cancer adoptive cell therapy. Chin Clin Oncol.
6:182017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kiessling A, Wehner R, Füssel S, Bachmann
M, Wirth MP and Schmitz M: Tumor-associated antigens for specific
immunotherapy of prostate cancer. Cancers. 4:193–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Murphy GP, Greene TG, Tino WT, Boynton AL
and Holmes EH: Isolation and characterization of monoclonal
antibodies specific for the extracellular domain of prostate
specific membrane antigen. J Urol. 160:2396–2401. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sokoloff RL, Norton KC, Gasior CL, Marker
KM and Grauer LS: A dual-monoclonal sandwich assay for
prostate-specific membrane antigen: Levels in tissues, seminal
fluid and urine. Prostate. 43:150–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Saeki N, Gu J, Yoshida T and Wu X:
Prostate stem cell antigen: A Jekyll and Hyde molecule? Clin Cancer
Res. 16:3533–3538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Silver DA, Pellicer I, Fair WR, Heston W
and Cordon-Cardo C: Prostate-specific membrane antigen expression
in normal and malignant human tissues. Clin Cancer Res. 3:81–85.
1997.PubMed/NCBI
|
|
54
|
NCBI: PSCA prostate stem cell antigen.
NCBI. 2010.
|
|
55
|
Kloss CC, Condomines M, Cartellieri M,
Bachmann M and Sadelain M: Combinatorial antigen recognition with
balanced signaling promotes selective tumor eradication by
engineered T cells. Nat Biotechnol. 31:71–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duong CP, Westwood JA, Berry LJ, Darcy PK
and Kershaw MH: Enhancing the specificity of T-cell cultures for
adoptive immunotherapy of cancer. Immunotherapy. 3:33–48. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feldmann A, Arndt C, Bergmann R, Loff S,
Cartellieri M, Bachmann D, Aliperta R, Hetzenecker M, Ludwig F,
Albert S, et al: Retargeting of T lymphocytes to PSCA- or PSMA
positive prostate cancer cells using the novel modular chimeric
antigen receptor platform technology ‘UniCAR’. Oncotarget.
8:31368–31385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bühler P, Molnar E, Dopfer EP, Wolf P,
Gierschner D, Wetterauer U, Schamel WW and Elsässer-Beile U:
Target-dependent T-cell activation by coligation with a PSMA × CD3
diabody induces lysis of prostate cancer cells. J Immunother.
32:565–573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bühler P, Wolf P, Gierschner D, Schaber I,
Katzenwadel A, Schultze-Seemann W, Wetterauer U, Tacke M, Swamy M,
Schamel W, et al: A bispecific diabody directed against
prostate-specific membrane antigen and CD3 induces T-cell mediated
lysis of prostate cancer cells. Cancer Immunol, Immunother.
57:43–52. 2008. View Article : Google Scholar
|
|
60
|
Ma Q, Safar M, Holmes E, Wang Y, Boynton
AL and Junghans RP: Anti-prostate specific membrane antigen
designer T cells for prostate cancer therapy. Prostate. 61:12–25.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai H, Wang Y, Lu X and Han W: Chimeric
antigen receptors modified T-cells for cancer therapy. J Natl
Cancer Inst. 108:djv4392016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kiniwa Y, Miyahara Y, Wang HY, Peng W,
Peng G, Wheeler TM, Thompson TC, Old LJ and Wang RF:
CD8+ Foxp+ regulatory T cells mediate
immunosuppression in prostate cancer. Clin Cancer Res.
13:6947–6958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vergati M, Cereda V, Madan RA, Gulley JL,
Huen NY, Rogers CJ, Hance KW, Arlen PM, Schlom J and Tsangsa KY:
Analysis of circulating regulatory T cells in patients with
metastatic prostate cancer pre- versus post-vaccination. Cancer
Immunol Immunother. 60:197–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cao Z and Kyprianou N: Mechanisms
navigating the TGF-β pathway in prostate cancer. Asian J Urol.
2:11–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lopez-Bujanda Z and Drake CG:
Myeloid-derived cells in prostate cancer progression: Phenotype and
prospective therapies. J Leukocyte Biol. 102:393–406. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Koehler H, Kofler D, Hombach A and Abken
H: CD28 costimulation overcomes transforming growth
factor-β-mediated repression of proliferation of redirected human
CD4+ and CD8+ T cells in an antitumor cell
attack. Cancer Res. 67:2265–2273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Loskog A, Giandomenico V, Rossig C, Pule
M, Dotti G and Brenner M: Addition of the CD28 signaling domain to
chimeric T-cell receptors enhances chimeric T-cell resistance to T
regulatory cells. Leukemia. 20:1819–1828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ha S, Ruoff R, Kahoud N, Franke TF and
Logan SK: Androgen receptor levels are upregulated by Akt in
prostate cancer. Endocr Relat Cancer. 18:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mikhailova M, Wang Y, Bedolla R, Lu XH,
Kreisberg JI and Ghosh PM: AKT regulates androgen
receptor-dependent growth and PSA expression in prostate cancer.
Adv Exp Med Biol. 617:397–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gosmann C, Frazer IH, Mattarollo SR and
Blumenthal A: IL-18, but not IL-12, induces production of IFN-γ in
the immunosuppressive environment of HPV16 E7 transgenic
hyperplastic skin. J Invest Dermatol. 134:2562–2569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiang T, Zhou C and Ren S: Role of IL-2 in
cancer immunotherapy. Oncoimmunology. 5:e11634622016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhao J, Zhao J and Perlman S: Differential
effects of IL-12 on Tregs and non-Treg T cells: Roles of IFN-γ,
IL-2 and IL-2R. PLoS One. 7:e462412012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rivière I and Sadelain M: Chimeric antigen
receptors: A cell and gene therapy perspective. Mol Ther.
25:1117–1124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sadelain M, Brentjens R and Rivière I: The
basic principles of chimeric antigen receptor (CAR) design. Cancer
Discov. 3:388–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang C, Liu J, Zhong JF and Zhang X:
Engineering CAR-T cells. Biomark Res. 5:222017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bonifant CL, Jackson HJ, Brentjens RJ and
Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther
Oncolytics. 3:160112016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. New Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Curran KJ, Pegram HJ and Brentjens RJ:
Chimeric antigen receptors for T cell immunotherapy: Current
understanding and future directions. J Gene Med. 14:405–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tey SK: Adoptive T-cell therapy: Adverse
events and safety switches. Clin Transl Immunol. 3:e172014.
View Article : Google Scholar
|
|
81
|
Ligtenberg MA, Mougiakakos D, Mukhopadhyay
M, Witt K, Lladser A, Chmielewski M, Riet T, Abken H and Kiessling
R: Coexpressed catalase protects chimeric antigen
receptor-redirected t cells as well as bystander cells from
oxidative stress-induced loss of antitumor activity. J Immunol.
196:759–766. 2016. View Article : Google Scholar : PubMed/NCBI
|