Introduction
Cervical cancer is one of the most common malignant
tumors in females and is a serious threat to the health of females
worldwide (1). In terms of incidence
and death rates, cervical cancer ranks second among all
gynecological malignant tumors and first among all malignant tumors
in females, respectively (2). If
diagnosed during the early stages, patients with cervical cancer
usually have a good prognosis with a 5-year survival rate of 90%
after effective treatments, like surgery, chemotherapy,
radiotherapy, or biotherapy. However, distant metastasis of this
cancer often results in poor prognosis (3). Therefore, reducing and completely
preventing metastasis is key in the treatment of cervical
cancer.
The metastasis of cervical cancer is a process that
involves multiple factors, multiple steps, and a continuous cascade
of reactions (4). In the study of
cervical cancer metastasis, epithelial-mesenchymal transition (EMT)
has garnered attention for being the major molecular mechanism in
the metastatic cascade (5–7). EMT plays a key role in epithelial tumor
progression, invasion, and metastasis. When tumor cells undergo
EMT, they lose their cell polarity and cell-cell adhesion due to
E-cadherin suppression and they break through the basement membrane
thus obtaining mesenchymal properties such as migration and
invasion (8). These cellular
morphological changes enhance tumor cell migration, metastasis, and
aid in the establishment of metastatic sites (9,10). EMT is
a dynamic and complex process that is associated with changes in
multiple growth factors, protein molecules, transcription factors,
as well as the respective pathways they regulate (11). Studies have reported that cadherins
are one of the key components that contribute to cell motility and
invasiveness via EMT (12). The
reduction in E-cadherin expression and its deletion can lead to the
disappearance of cell polarity and decrease cell adhesion (13). E-cadherin is a calcium-dependent
transmembrane glycoprotein, which is negatively correlated with the
progression, local invasion and metastasis of epithelial
carcinomas. The loss of cell polarity is accompanied with an
increase in markers of mesenchymal cell increase such as
N-cadherin, vimentin and fibronectin. Snail is one of the
transcription factors that can suppress the expression of
E-cadherin. It is present in a wide range of human cancers and is
associated with poor prognosis (14).
Matrix metalloproteinases (MMPs) are important proteolytic enzymes,
which are capable of degrading all types of extracellular matrix
proteins and basement membranes (15). The EMT process leads to the initiation
of metastasis (16,17) and enhancement in the invasive ability
of tumor cells (18). Therefore,
suppression of the mesenchymal molecules associated with EMT could
be an effective strategy in abolishing the EMT-triggering effect.
The cervical tumor is prone to distant metastasis and has a deeper
depth of tumor invasion. The PI3K/Akt pathway is a central
regulator of cervical cancer and functions by regulating the cell
cycle and proliferation. PI3K activation phosphorylates and
activates Akt, which in turn activates the transduction of several
downstream signals thus promoting the development of cervical
cancer (19).
At present, surgery, chemotherapy, and radiotherapy
are the most common treatments for cervical cancer. Development of
novel natural therapeutic reagents for cervical cancer is a
fast-growing field of research. According to our previous study,
HMQ-T-F2, a taspine derivative, effectively inhibited cervical HeLa
cell proliferation in vitro and in vivo by
upregulating Axin and suppressing nuclear translocation of
β-catenin (20). However, little is
known about the effect of HMQ-T-F2 on HeLa cell migration. Studies
have revealed that the E-cadherin-catenin complex functions in
cellular adhesion and its loss has been associated with greater
tumor metastasis (21). Based on
these results, the effects of HMQ-T-F2 on migration of the human
cervical cell line, HeLa, were further evaluated. The possible
mechanism of such migration was also explored and a theoretical
basis for the novel treatment of cervical cancer was provided.
Materials and methods
Materials
F2 (purity >98%) was designed and synthesized at
the Health Science Center, Xi'an Jiaotong University. RPMI-1640,
Ribonuclease (RNase), propidium iodide (PI) and Hoechst 33258 were
obtained from Sigma-Aldrich; Merck KGaA. Fetal bovine serum (FBS)
was purchased from HyClone; GE Healthcare Life Sciences. Trypsin
was obtained from AMRESCO, Inc. Penicillin was purchased from
General Pharmaceutical Factory, and streptomycin was purchased from
North China Pharmaceutical. An Annexin V-FITC reagent kit was
purchased from Nanjing KeyGen Biotech Co., Ltd. LRP6 rabbit mAb
(cat. no. 2560S), phospho-LRP6 rabbit mAb (cat. no. 2568S), and
phospho-GSK3β rabbit polyAb (Ser9) (cat. no. 5558T) were purchased
from Cell Signaling Technology, Inc. MMP7 rabbit polyAb (cat. no.
10374-2-AP), MMP3 rabbit polyAb (cat. no. 17873-1-AP), c-Myc rabbit
polyAb (cat. no. 10828-1-AP), Frizzled-8 rabbit polyAb (cat. no.
55093-1-AP), GSK3β rabbit polyAb (cat. no. 22104-1-AP), Mcl-1
rabbit polyAb (cat. no. 16225-1-AP), cyclin B1 rabbit mAb (cat. no.
55004-1-AP), cyclin D1 rabbit polyAb (cat. no. 60186-1-Ig), cyclin
E rabbit polyAb (cat. no. 11554-1-AP), Bax rabbit polyAb (cat. no.
50599-2-Ig), Bcl-2 rabbit polyAb (cat. no. 12789-1-AP), MMP2 rabbit
mAb (cat. no. 10373-2-AP), MMP9 rabbit mAb (cat. no. 10375-2-AP),
anti-GAPDH (cat. no. 60004-1-Ig), E-cadherin rabbit polyAb (cat.
no. 20874-I-AP), N-cadherin rabbit polyAb (cat. no. 22018-I-AP),
HIF-1α rabbit polyAb (cat. no. 20960-I-AP), Snail rabbit polyAb
(cat. no. 26183-I-AP), phospho-Akt mouse mAb (cat. no. 66444-I-1g),
Akt rabbit polyAb (cat. no. 10176-2-AP), mTOR rabbit polyAb (cat.
no. 20657-I-AP) and vimentin rabbit polyAb (cat. no. A11952-1-AP)
were obtained from ProteinTech Group, Inc. PI3K P110α rabbit mAb
(cat. no. 4249T), PI3K P110β rabbit mAb (cat. no. 3011T), PI3K
P110γ rabbit mAb (cat. no. 5405T), PI3K Class III rabbit mAb (cat.
no. 3358T), p-PI3KP85/P55 rabbit mAb (cat. no. 4228T), PI3KP85
rabbit mAb (cat. no. 4257T), phospho mTOR rabbit mAb (cat. no.
5536P), were all purchased from Cell Signaling Technology, Inc.
Goat anti-rabbit IgG (cat. no. 31579), BCA protein assay reagent
kit, and enhanced chemiluminescent (ECL) plus reagent kit were
obtained from Pierce; Thermo Fisher Scientific, Inc. RIPA Lysis
Buffer was obtained from Applygen Technologies, Inc. Protease
inhibitor cocktail, phosphatase inhibitor cocktail, and DAPI were
purchased from Roche Diagnostics.
Human cell lines
Human cervical cancer cell line HeLa was purchased
from Shanghai Institute of Cell Biology of the Chinese Academy of
Sciences. HeLa cells were cultured in RPMI-1640 medium with 10%
(v/v) FBS and incubated at 37°C in a 5% CO2 atmosphere
with saturated humidity.
Flow cytometric analysis of cell cycle
and apoptosis
The effect of F2 on the cell cycle and apoptosis
were analyzed using flow cytometry. After serum starvation, HeLa
cells treated with F2 for 48 h were collected, washed with cold PBS
and suspended in 70% ice-cold ethanol overnight at −20°C.
Subsequently, the cells were suspended in PBS with 1 ml RNase (50
µg/ml) and 1 ml PI (60 µg/ml) and incubated for 30 min in the
dark.
For cell apoptosis assay, the treated HeLa cells
were harvested, washed with PBS, and sequentially stained with
Annexin V-FITC. After three min, 10 µl PI (20 µg/ml) was added to
these cells and they were incubated in the dark for 10 min.
All stained cells were analyzed using FACS (BD
Biosciences). The data thus obtained were plotted using Modfit LT
software 2.0 (Verity Software house).
Hoechst staining assay
HeLa cells that were treated with F2 for 48 h, were
fixed using 4% paraformaldehyde for 10 min. After washing with PBS,
cells were stained with Hoechst 33258 in the dark for 20 min. The
cells were photographed using an inverted fluorescence microscope
(DM505; Nikon Corp.).
Wound scratch assay
The wound scratch assay was performed in order to
evaluate directional cell migration. Briefly, when HeLa cells grew
to 70–80% confluence, cell monolayers were wounded to form a
scratch using sterile pipette tips (100–200 µl). After washing with
PBS to remove cell debris, cells were incubated in either the
absence or presence of F2 in RPMI-1640 medium with 5% FBS. The
cells were photographed at the beginning (0 h) and then at 24 and
48 h. The migration distance was measured using an image analysis
software (NIS-Elements Viewer 4.2.0; Nikon Corporation) and the
migration rate was calculated.
Transwell migration assay
The HeLa cells were seeded onto a Transwell chamber
and incubated with F2 for 48 h. Then the medium in the chamber was
replaced with serum-free medium and the lower chamber was filled
with RPMI-1640 medium containing 30% FBS as a chemoattractant.
After culturing for 24 h, the cells that did not migrate and
remained at the top of the chamber were removed carefully using a
cotton swab. The migrating cells, that settled at the bottom of the
chamber were fixed using methanol and stained using 0.2% crystal
violet for 15 min. The cells that had migrated were photographed
and counted based on 5-field digital images obtained randomly at a
magnification of ×100.
Western blot analysis
Total protein was lysed using ice-cold RIPA with a
protease inhibitor cocktail and a phosphorylated protease inhibitor
cocktail and was used for HeLa cells that were treated with F2 for
48 h. After quantification using a BCA assay, that was performed in
accordance with the manufacturer's instructions, the protein sample
30 µg was separated by the 10% SDS-PAGE and transferred to a PVDF
membrane. Subsequently, the membranes were blocked using 5% BSA for
2 h at room temperature and incubated with the indicated primary
antibodies that were diluted by 1X TBST buffer overnight at 4°C.
After washing, the membranes were incubated with species-specific
horseradish peroxidase (HRP)-conjugated secondary antibodies for 1
h at 37°C and the resultant antigen-antibody complexes were
visualized using an enhanced chemiluminescence (ECL) kit. The
images were scanned using chemiluminescent and fluorescent imaging
systems (Champchemi™ Professional; cat. no. SG2010084; Beijing Sage
Creation Science Co., Ltd.) and the bands were quantified using
Image-Pro plus software (Image-Pro Plus 5.1; Media Cybernetics,
Inc.). GAPDH was used as an internal control.
Statistical analysis
One-way analysis of variance (ANOVA) with Tukey's
multiple comparison test or Student's unpaired t-test was used to
analyze significance using SPSS 19.0 (IBM Corp.) and GraphPad Prism
5 software (GraphPad Software). A P-value <0.05 was considered
to indicate a statistically significant difference. *P<0.05,
**P<0.01 and ***P<0.001 vs. the control group. Data are
expressed as the means ± SEM.
Results
F2 inhibits cervical cancer HeLa cell
migration
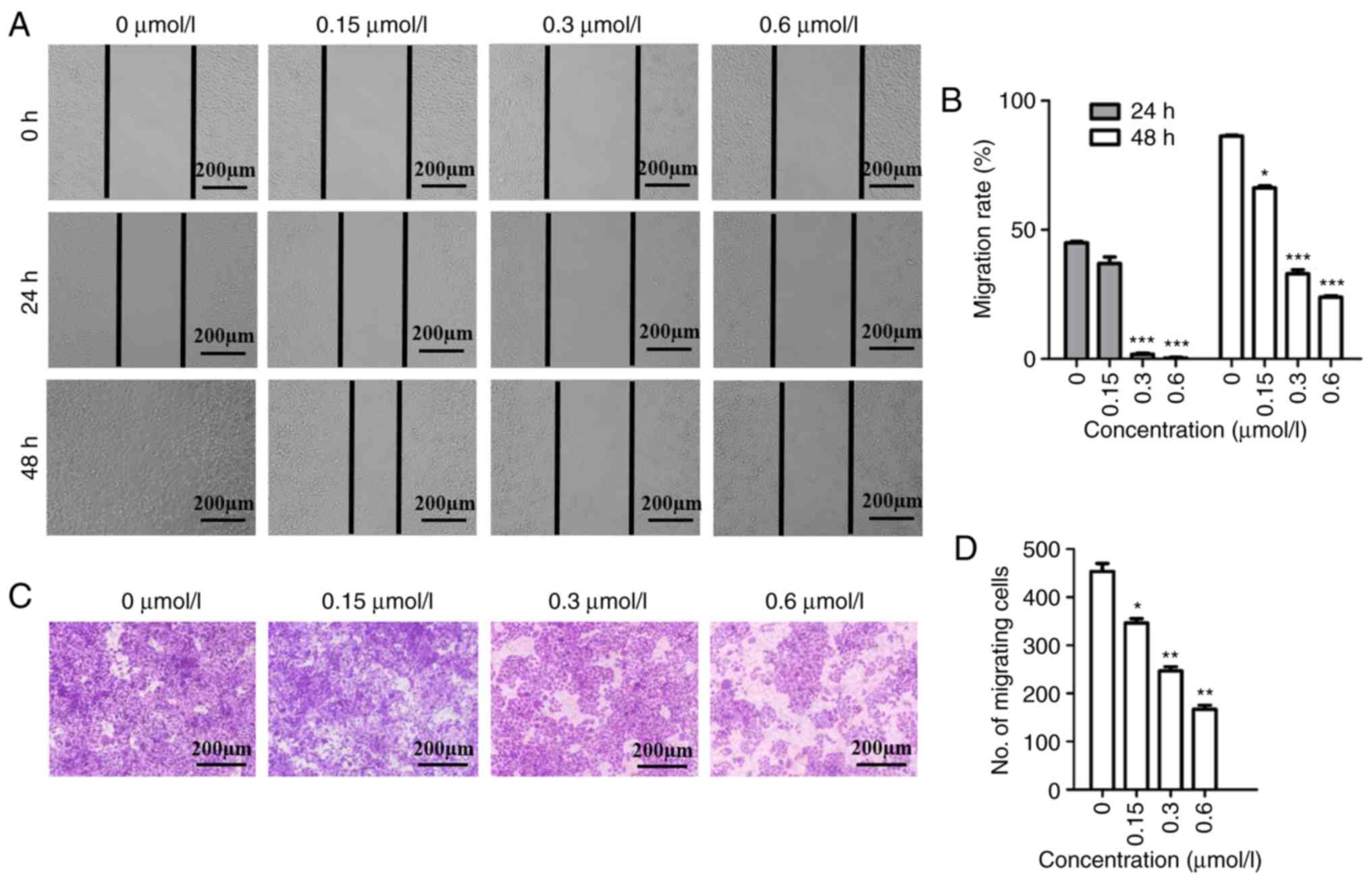
It is known that cell migration is important for the
progression of malignant tumors. The ability of F2 to inhibit
migration was evaluated using wound scratch and Transwell migration
assays. Our previous study concluded that treatment of F2 at a
dosage of 0.6 µmol/l for 48 h had no cytotoxic effect on HeLa cells
(20). In addition, an MTT assay
revealed that F2 had no inhibition on human normal cervical
epithelial cells even at 25 µmol/l, in the previous study. Hence,
F2 was used at a concentration of 0.6 µmol/l for the experiments
and 5% FBS was used to exclude the anti-proliferation effect of F2
in the migration assay for 48 h. As revealed in Fig. 1A and B, HeLa cells migrated to fill
the scratched area after 48 h in the absence of F2, while the
migration rate decreased in a dose-dependent manner upon F2
treatment at 24 and 48 h. According to the Transwell assay results,
the number of HeLa cells migrating through the chamber
significantly decreased in the group that received F2 treatment,
when compared to the control group (Fig.
1C and D). Both these assays confirmed that F2 inhibited HeLa
cell migration.
F2 inhibits Wnt signaling proteins in
HeLa cells
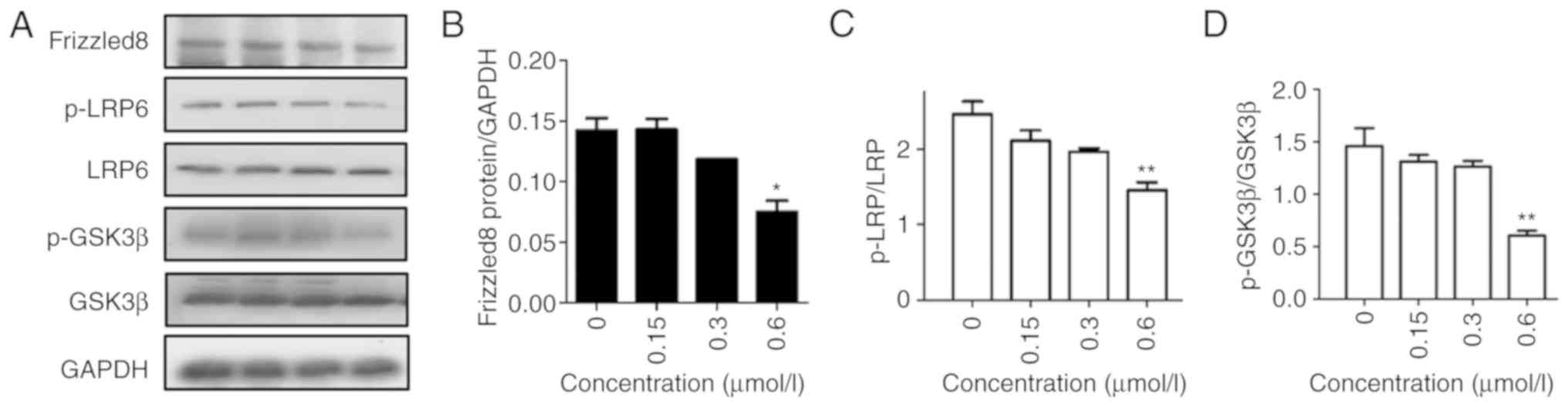
β-catenin is an important molecule in the Wnt
signaling pathway. In our previous study, it was revealed that F2
arrests the translocation of β-catenin to the nucleus in a
dose-dependent manner. Thus, the expression of Wnt signaling
proteins was investigated after exposure to F2 for 48 h in order to
determine whether inhibition of migration by F2 was associated with
Wnt signaling. The data revealed that the expression of Frizzled-8,
phosphorylation of LRP-5/6, and phosphorylation of GSK3β decreased
in a concentration-dependent manner (Fig.
2), indicating that F2 inhibited the migration via the Wnt
signaling pathway.
F2 inhibits EMT-related molecules in
HeLa cells
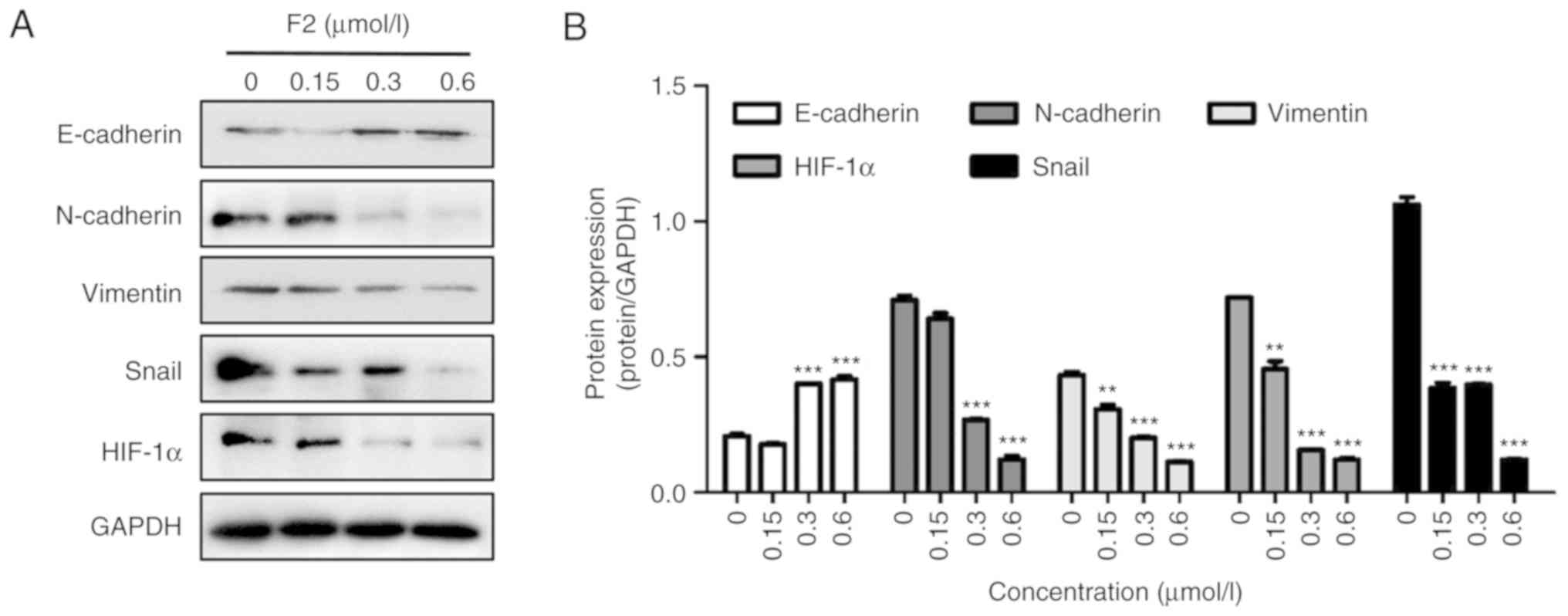
Induction of EMT is the initial process by which
epithelial cancer cells acquire motility, thus promoting migration
and invasion (22). A western blot
assay was performed in order to determine the effect of F2 on the
expression of EMT-related proteins. As revealed in Fig. 3, F2 significantly increased the
expression of E-cadherin, and decreased the expression of
N-cadherin, vimentin and Snail. These results indicated that F2 can
inhibit the migration of HeLa cells by reversing EMT.
HIF-1α plays an important role in tumor cell
metastasis and cancer function (23).
Using a western blot assay, it was revealed that F2 decreased
HIF-1α expression (Fig. 3). HIF-1α
promotes tumor metastasis by increasing the expression of MMPs that
play an important role in metastatic foci formation (23). Western blot analysis demonstrated that
the expression of MMP2, MMP3, MMP7, and MMP9 was downregulated in
HeLa cells treated with F2 (Fig. 4).
In addition, c-Myc protein expression decreased after F2 treatment
(Fig. 4). MMPs and c-Myc are the
nuclear targets of the Wnt/β-catenin signaling pathway. These
observations were consistent with our previous study, which
reported that F2 markedly inhibited HeLa cells by targeting
β-catenin.
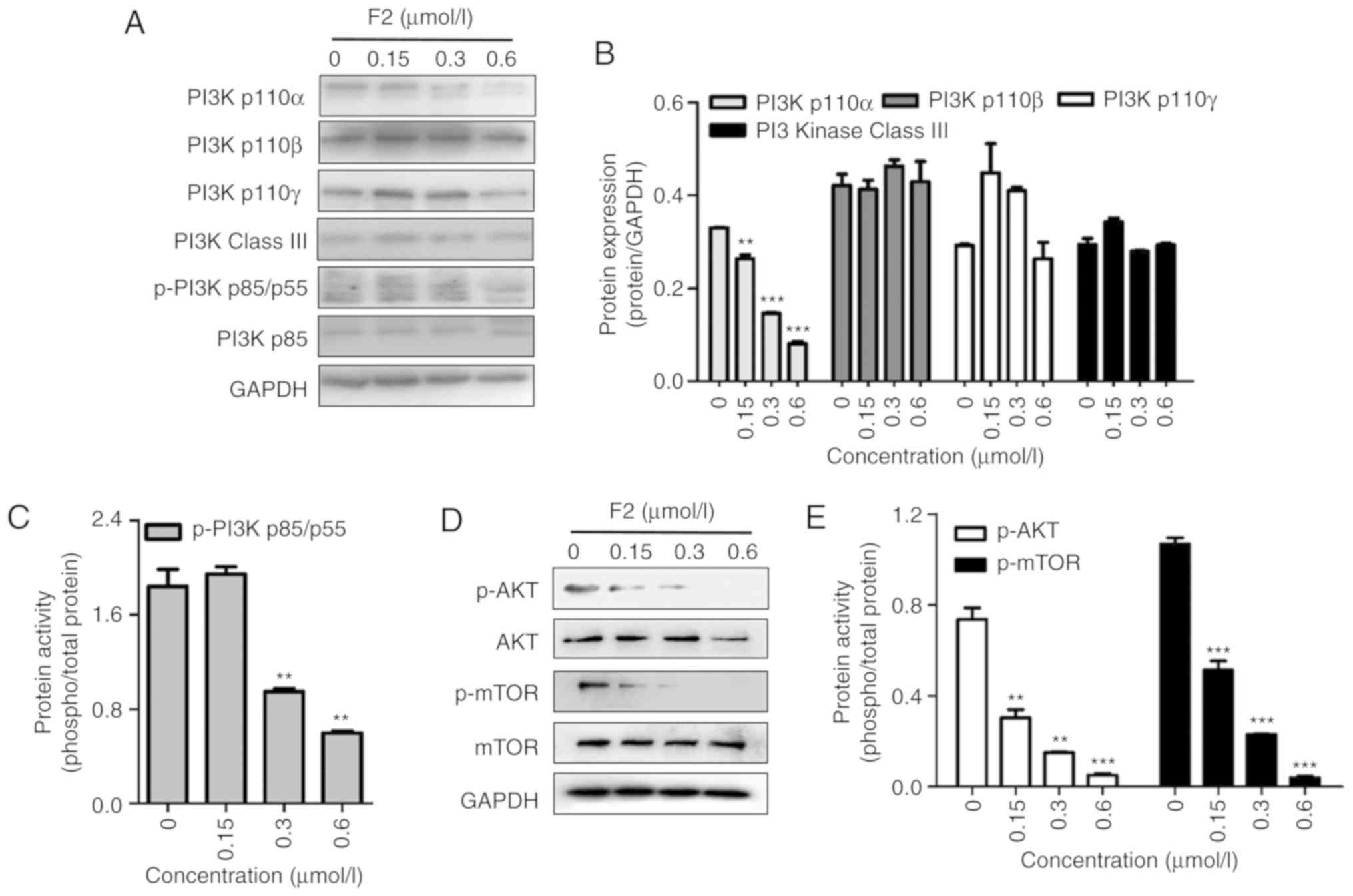
F2 impairs the PI3K/Akt signaling
pathway
The PI3K/Akt pathway is involved in the regulation
of HIF-1α and is closely related to cell proliferation. Western
blot analysis revealed that F2 inhibited the PI3K/Akt signaling
pathway in a dose-dependent manner along with the inhibition of
HIF-1α expression. As revealed in Fig.
5, F2 inhibited the PI3Kp110α subunit and the phosphorylation
of PI3Kp85/p55, Akt and mTOR, which are important molecules in PI3K
signaling.
F2 induces the accumulation of HeLa
cells in the S phase
PI staining and flow cytometry were used to analyze
the cell cycle distribution in the HeLa cells following treatment
with F2. The results revealed that F2 significantly arrested the
HeLa cells in their S phase. The HeLa cells were treated with 0,
0.15, 0.3 and 0.6 µmol/l of F2 for 48 h, and the percentage of
cells in the S phase increased from 38.07% in vehicle controls to
40.76, 48.96 and 62.38%, respectively. Conversely, the percentage
of cells in the G1 phase decreased from 51.47 to 50.7, 45.72 and
31.85%, while the percentage of cells in the G2/M phase decreased
from 10.47 to 8.55, 5.34 and 5.78%, respectively (Fig. 6A and B).
To explore the mechanisms underlying F2-mediated S
phase arrest, the effect of F2 on key cell cycle-related proteins
was examined. It is well known that cyclin E is closely correlated
with S phase arrest, and the present results revealed that F2
upregulated the expression of cyclin E (Fig. 6C and D). In addition, treatment with
F2 also downregulated the expression of cyclin D1 and cyclin B1,
which are closely related to the G1 and G2/M phase, respectively.
Thus, the data revealed that F2 induced HeLa cell cycle arrest at
the S phase.
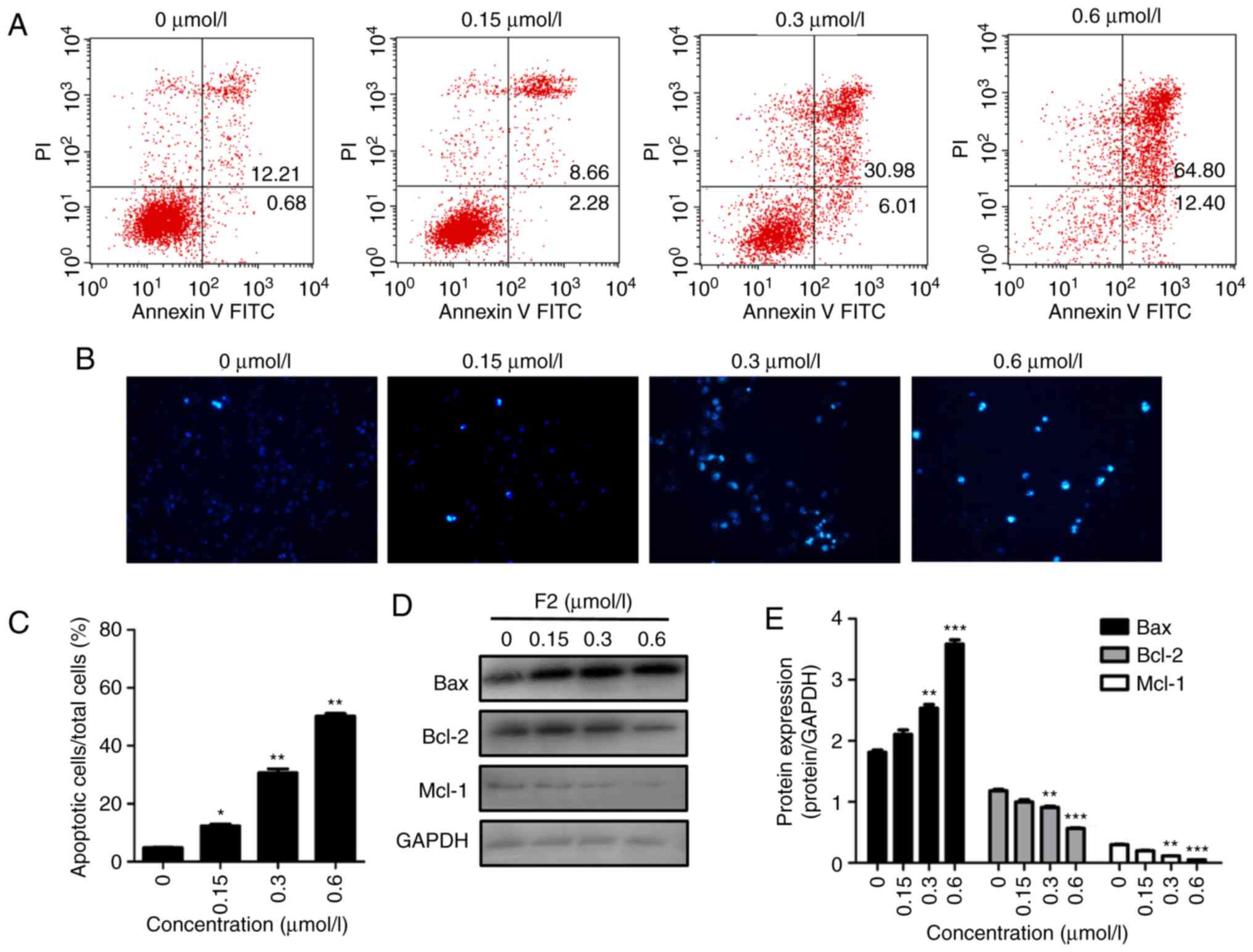
F2 induces HeLa cell apoptosis and
regulates cell apoptosis-regulatory molecules
Annexin V-PI staining and flow cytometric analysis
were used to determine whether F2 inhibits HeLa cell proliferation
by inducing cell apoptosis. As revealed in Fig. 7A, the FACS results revealed that the
percentage of apoptotic cells (the sum of lower right quadrant and
upper right quadrant) in the vehicle group was 12.89%, and that the
percentage of apoptotic cells increased after treatment with F2.
The percentage of apoptotic cells in F2-treated groups were 10.94,
36.99 and 77.20, respectively. In addition, Hoechst staining was
applied to observe the morphology of HeLa cells. The results
indicated that F2 induced the condensed bright blue apoptotic
nuclei in HeLa cells (Fig. 7B and C).
Induction of apoptosis was further confirmed by upregulation of the
pre-apoptotic protein Bax and downregulation of the anti-apoptotic
proteins, Bcl-2 and Mcl-1 (Fig. 7D and
E). The data indicated that F2 activity was related to
programmed cell death.
Discussion
Every year there are >500,000 new cases of
cervical cancer and ~200,000 cervical cancer-related deaths
worldwide, with the majority of these occurring in developing
countries (24). Although existing
cervical cancer screening techniques and HPV have improved the rate
at which this disease is diagnosed to some extent, reoccurrence and
metastasis still result in poor outcomes (25,26).
Therefore, novel and efficient therapeutic targets for cancer
metastasis are required.
Our previous study demonstrated that β-catenin is an
important target of F2, and it was confirmed that F2 regulates the
β-catenin destruction complex that consists of Axin, APC, and CK1
(20). It is well known that
β-catenin is a key player in abnormal Wnt signaling and is the most
important oncogene in cervical cancer biology due to its ability to
regulate cell migration. In order to elucidate the mechanism of
inhibition of HeLa cell migration by F2, wound healing and
Transwell assays were used. The results revealed that F2 could
inhibit the migration of HeLa cells. The activation of
Wnt/β-catenin signaling in cancers mostly occurs as a result of the
binding of the Wnt ligand with its receptor LRP5/6 and Frizzled8.
It also presents the inactivation of GSK-3β, which leads to the
increase in the activity and quantitative stabilization of
β-catenin. The present results revealed that F2 suppressed
phosphorylation of LRP5/6 and expression of Frizzled8. LRP5/6 and
Frizzled8 are transmembrane receptors of the Wnt/Frizzled8 pathway.
In addition, the inhibition of F2 on phosphorylated LRP5/6 resulted
in the suppression of the phosphorylation of GSK3β.
Furthermore, several studies have confirmed that the
Wnt signaling pathways regulate the process of EMT in cancer. The
key factor of the Wnt signaling pathway, β-catenin, induces EMT by
increasing E-cadherin suppressors (27,28). EMT
is reported to promote the invasive ability and metastasis of
cervical cancer and is positively associated with poor prognosis
(29). When Wnt signaling is
activated, the number of EMT target proteins increase, and vice
versa. These findings reveal that the crosstalk between
Wnt/β-catenin signaling and the EMT process forms a positive
feedback loop (30). Therefore, when
EMT-related proteins were evaluated using western blotting, the
results revealed that F2 inhibited the expression of Snail. In
addition, F2 also significantly upregulated the expression of
epithelial marker E-cadherin, and downregulated the expression of
interstitial markers N-cadherin and vimentin. These results
indicated that F2 can inhibit the migration of HeLa cells by
reversing EMT. The overexpression of HIF-1α protein has been
reported in several human cancers including cervical, breast, and
ovarian cancer (31–33). Clinically, high levels of HIF-1α have
several functions in cancer biology including angiogenesis, cell
survival, tumor metastases and overcoming hypoxia (23). Using western blot analysis, it was
revealed that F2 decreased HIF-1α expression. HIF-1α promotes the
metastasis of malignant tumors by increasing the levels of MMPs
(34). MMPs are also known to play a
major role in different cell behaviors such as cell proliferation,
migration, infiltration, differentiation, angiogenesis and
metastatic foci formation (15).
Western blot analysis revealed that the expression of MMP2, MMP3,
MMP7, MMP9 and c-Myc were downregulated in HeLa cells treated with
F2. In addition, MMPs and c-Myc are the nuclear targets of the
Wnt/β-catenin signaling pathway. These observations were consistent
with our previous study that revealed that F2 significantly
inhibited HeLa cells by targeting β-catenin. The PI3K/Akt/mTOR
signaling pathway is involved in the development and progression of
tumors. It is aberrantly activated in cervical cancer and is
closely related to tumor recurrence and metastasis (35). PI3K/Akt/mTOR signaling is also an
intracellular signaling pathway that regulates the cell cycle and
can induce the occurrence of EMT (36). In the present study, it was revealed
that F2 inhibited the phosphorylation of PI3Kp85/p55 and its
downstream signaling molecules, Akt and mTOR. Thus, the results of
our present and previous studies, collectively, revealed that F2
inhibited the migration and proliferation of HeLa cells by
reversing EMT, and negatively regulating the Wnt/β-catenin and
PI3K/Akt signaling pathways.
In addition, the present research results revealed
that F2 arrested the HeLa cell cycle at the S phase. Certain key
proteins involved in regulating cell cycle transition were also
studied. The results revealed that F2 induced HeLa cell arrest and
was also associated with the downregulation of cyclin D1 and cyclin
B1 and upregulation of cyclin E. The results also revealed that F2
induced HeLa cell apoptosis by downregulating Bcl-2 and Mcl-1 and
upregulating Bax. These data partly reflect the ability of F2 to
inhibit HeLa cell proliferation. Nevertheless, further studies are
needed on the mechanism of whcih F2 suppresses migration of the
human cervical cancer HeLa cells and the druggability of F2.
In conclusion, the present findings demonstrated
that F2 can impair migration and proliferation of the human
cervical cancer HeLa cells. F2 induced HeLa cell arrest at the S
phase and apoptosis. F2 reversed EMT, thus potentially inhibiting
both the Wnt/β-catenin and PI3K/Akt signaling pathways, and
downregulating HIF-1α expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81503101 and
81773772), the Fundamental Research Funds for the Central
Universities (xjj2018167) and the National Science Foundation for
Post-Doctoral Scientists of China (grant no. 2019M653670).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
BD was responsible for collection of data, data
analysis and interpretation, and manuscript writing. RY and MF
carried out the experiments. TY and BW analyzed the data and
reviewed the manuscript. BD and YZ was responsible for the
conception and design of the study, and financial support. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A and Colombo N; ESMO Guidelines Committee, :
Cervical cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28 (Suppl):iv72–iv83. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fomenko Y, Cialkowska-Rysz A, Muravlyova
L, Sirota V and Sapar B: Assessment of direct results of cervical
cancer combined treatment. Georgian Med News. 21–24.
2018.PubMed/NCBI
|
|
3
|
Zhang L, Qian H, Sha M, Luan Z, Lin M,
Yuan D, Li X, Huang J and Ye L: Downregulation of HOTAIR expression
mediated anti-metastatic effect of artesunate on cervical cancer by
inhibiting COX-2 expression. PLoS One. 11:e01648382016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu SC, Yu CC, Hsu LS, Chen KS, Su MY and
Chen PN: Berberine reverses epithelial-tomesenchymal transition and
inhibits metastasis and tumor-induced angiogenesis in human
cervical cancer cells. Mol Pharmacol. 86:609–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, He S, Guo P, Guo X and Zheng J:
Microrna-1297 inhibits metastasis and epithelial-mesenchymal
transition by targeting aeg-1 in cervical cancer. Oncol Rep.
38:3121–3129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sathyanarayanan A, Chandrasekaran KS and
Karunagaran D: Microrna-145 modulates epithelial-mesenchymal
transition and suppresses proliferation, migration and invasion by
targeting sip1 in human cervical cancer cells. Cell Oncol (Dordr).
40:119–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi X, Zhang L and Lu X: New insights into
the epithelial-to-mesenchymal transition in cancer. Trend Pharmacol
Sci. 37:246–248. 2016. View Article : Google Scholar
|
|
9
|
Franco-Chuaire ML, Magda Carolina SC and
Chuaire-Noack L: Epithelial mesenchymal transition (EMT):
Principles and clinical impact in cancer therapy. Invest Clin.
54:186–205. 2013.PubMed/NCBI
|
|
10
|
Wang Y, Wen M, Kwon Y, Xu Y, Liu Y, Zhang
P, He X, Wang Q, Huang Y, Jen KY, et al: CUL4A induces
epithelial-mesenchymal transition and promotes cancer metastasis by
regulating ZEB1 expression. Cancer Res. 74:520–531. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li HQ and Ke Y: Mechanism of
epithelial-mesenchymal transition. Chin Pharmacol Bull.
33:1342–1344. 2017.
|
|
12
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gos M, Miloszewska J and Przybyszewska M:
Epithelial- mesenchymal transition in cancer progression. Postepy
Biochem. 55:121–128. 2009.(In Polish). PubMed/NCBI
|
|
14
|
Sethi S, Macoska J, Chen W and Sarkar FH:
Molecular signature of epithelialmesenchymal transition (EMT) in
human prostate cancer bone metastasis. Am J Transl Res. 3:90–99.
2010.PubMed/NCBI
|
|
15
|
Li W, Li S, Deng L, Yang S, Li M, Long S,
Chen S, Lin F and Xiao L: Decreased MT1-MMP in gastric cancer
suppressed cell migration and invasion via regulating MMPs and EMT.
Tumor Biol. 36:6883–6889. 2015. View Article : Google Scholar
|
|
16
|
Hugo HJ, Kokkinos MI, Blick T, Ackland ML,
Thompson EW and Newgreen DF: Defining the E-cadherin repressor
interactome in epithelial mesenchymal transition: The PM C42 model
as a case study. Cells Tissues Organs. 193:23–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen PT, Kudo Y, Yoshida M, Iizuka S,
Ogawa I and Takata T: N-cadherin expression is correlated with
metastasis of spindle cell carcinoma of head and neck region. J
Oral Pathol Med. 40:77–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YL, Liu HF, Shi XJ and Wang Y:
Antiproliferative activity of Farnesol in HeLa cervical cancer
cells is mediated via apoptosis induction, loss of mitochondrial
membrane protential and PI3K/Akt signaling pathway. J Buon.
23:752–757. 2018.PubMed/NCBI
|
|
20
|
Dai B, Yang T, Ma Y, Ma N, Shi X, Zhang D,
Zhang J and Zhang Y: HMQ-T-F2 exert antitumour effects by
upregulation of Axin in human cervical HeLa cells. J Cell Mol Med.
22:2955–2959. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beavon IR: The E-cadherin-catenin complex
in tumour metastasis: Structure, function and regulation. Eur J
Cancer. 36:1607–1620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bahnson A, Athanassiou C, Koebler D, Qian
L, Shun T, Shields D, Yu H, Wang H, Goff J, Cheng T, et al:
Automated measurement of cell motility and proliferation. BMC Cell
Biol. 6:192005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 10:721–732. 2003. View Article : Google Scholar
|
|
24
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan J, Zhang Y, Ren C, Shi W and Chen L:
Involvement of nuclear protein C23 in activation of EGFR signaling
in cervical cancer. Tumour Biol. 37:905–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yee GP, de Souza P and Khachigian LM:
Current and potential treatments for cervical cancer. Curr Cancer
Drug Targets. 13:205–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sánchez-Tilló E, de Barrios O, Siles L,
Cuatrecasas M, Castells A and Postigo A: β-catenin/TCF4 complex
induces the epithelial-to-mesenchymal transition (EMT)-activator
ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA.
108:19204–19209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Solanas G, Porta-de-la-Riva M, Agustí C,
Casagolda D, Sánchez-Aguilera F, Larriba MJ, Pons F, Peiró S,
Escrivà M, Muñoz A, et al: E-cadherin controls beta-catenin and
NF-kappaB transcriptional activity in mesenchymal gene expression.
J Cell Sci. 121:2224–2234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Itsrole in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon YJ, Ye DJ, Baek HS and Chun YJ:
7,12-Dimethylbenz[α]anthracene increases cell proliferation and
invasion through induction of Wnt/β-catenin signaling and EMT
process. Environ Toxicol. 33:729–742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer. 93:266–276. 2001. View Article : Google Scholar
|
|
32
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Birner P, Schindl M, Obermair A,
Breitenecker G and Oberhuber G: Expression of hypoxia-inducible
factor 1alpha in epithelial ovarian tumors: Its impact on prognosis
and on response to chemotherapy. Clin Cancer Res. 7:1661–1668.
2001.PubMed/NCBI
|
|
34
|
Song IS, Wang AG, Yoon SY, Kim JM, Kim JH,
Lee DS and Kim NS: Regulation of glucose metabolism-related genes
and VEGF by HIF-1alpha and HIF-1beta, but not HIF-2alpha, in
gastric cancer. Exp Mol Med. 41:51–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bachelder RE, Yoon SO, Franci C, de
Herreros AG and Mercurio AM: Glycogen synthase kinase-3 is an
endogenous inhibitor of Snail transcription: Implications for the
epithelial to mensenchymal transition. J Cell Biol. 168:29–33.
2005. View Article : Google Scholar : PubMed/NCBI
|