Introduction
Gliomas, which originate from glial cells, are the
most frequent primary malignant tumors of the central nervous
system (CNS) in humans (1).
Glioblastoma (GBM) is the most aggressive form of human astrocytoma
with a median survival of only 14–15 months (2). It has been reported that isocitrate
dehydrogenase (IDH) 1 or 2 gene mutations are commonly found in
human gliomas (3). In light of the
2016 update of the World Health Organization (WHO) CNS tumor
classification, IDH mutations have been considered novel indicators
in predicting the outcome of glioma patients (4). The IDH1 R132 mutation accounts for
>80% of all IDH mutations (5).
Recent studies have confirmed that wild-type IDH1
(IDH1Wt) and IDH1 mutant (IDH1Mut) gliomas
are biologically different tumor types (6). An increasing number of studies
demonstrate that patients with IDH1Mut GBM exhibit a
better prognosis compared with patients with IDH1Wt GBM
(7,8).
However, a higher proportion of distant relapses were noted in
IDH1Mut compared with IDH1Wt glioma patients.
Therefore, there is an urgent need to understand the molecular
mechanisms underlying glioma tumorigenesis in patients with
different IDH1 mutational status. This can aid the investigation
and development of novel therapeutic strategies.
It has been shown that a hypoxic microenvironment
contributes to glioma growth by inducing the glioma stem cell
phenotype (9). Recent evidence
suggests that IDH1 has a critical role in regulating the
concentration of several antioxidants, such as α-ketoglutarate
(α-KG), nicotinamide adenine dinucleotide phosphate (NADPH) and
glutathione (GSH) (10). Redox
imbalance can result in the inactivation of significant cell cycle
and cell signaling regulators, such as the p53, activator protein 1
and nuclear factor erythroid 2-related factor 2 transcription
factors (11,12). However, adverse effects were noted
following treatment of glioma patients who were stratified
according to their IDH1 mutational status and tumor grade.
Therefore, it is reasonable to suspect that the different IDH1
mutational status may participate in the development of GBM under
hypoxic conditions, although this hypothesis has not been clarified
to date.
RLIP76, which is also known as ralA binding protein
1 (RalBP1), is associated with oxidative stress-induced cell
apoptosis by affecting the intracellular levels of GSH (13,14). It
has been demonstrated that downregulation of RLIP76 in tumor cells
can reverse various tumor biological processes, such as
proliferation, apoptosis and differentiation (15–17). It is
important to note that the expression levels of RLIP76 are closely
associated with the degree of malignancy in gliomas (18–20). It is
also noteworthy that RLIP76 can serve as an oncogene in glioma via
its interaction with important signaling pathways of tumorigenesis,
such as the Rac1, AKT and JNK pathways (18,20).
However, the contribution of the dysregulated RLIP76 expression in
association with the different IDH1 mutational status to GBM
tumorigenesis remains unclear.
The present study investigated the expression levels
of RLIP76 in 124 GBM tissues (98 IDH1Wt and 26
IDH1Mut) using reverse transcription-quantitative PCR
(RT-qPCR), western blot and immunohistochemical assays. The
prognostic value of RLIP76 expression and IDH1 mutational status
for GBM was investigated. The potential roles of RLIP76 on tumor
proliferation and apoptosis in IDH1Wt or
IDH1Mut GBM cells were also explored in a separate set
of in vitro experiments.
Materials and methods
Tissue samples
The present study was granted approval by the
Specialty Committee on Ethics of Biomedicine Research at the Tongji
University. Written informed consent was obtained from all
patients. The selection criteria were previously described
(18). Briefly, the selection
criteria were as follows: i) The subject had a diagnosis of primary
GBM and no history of other tumors; ii) the subject had complete
clinical data, including age, sex, clinical manifestations, mean
tumor diameter (defined as the geometric mean of the three
diameters by MRI scan), extent of resection and adjuvant therapy;
and iii) the subject underwent evaluation by enhanced head MRI
scans for tumor relapse or progression after surgery at least once
every six months. A total of 124 patients who received
glioma-resection surgery were recruited at the Department of
Neurosurgery, Shanghai Tongji Hospital of Tongji University and of
Changzheng Hospital, the Second Military Medical University. The
subjects were recruited from July 2016 to December 2018. The
follow-up was carried out by telephone or mail every 6 months and
the survival time was evaluated until March 2019. GBM was diagnosed
according to the 2016 WHO classification of tumors of the CNS by
two independent experienced pathologists. The clinical
characteristics of the patients with GBM are listed in Table I.
 | Table I.Demographic and clinicopathologic
characteristics of patients with glioblastoma multiforme. |
Table I.
Demographic and clinicopathologic
characteristics of patients with glioblastoma multiforme.
|
|
| RLIP76
expression |
|---|
|
|
|
|
|---|
| Characteristic | Number of patients
(%) | Low | High |
|---|
| Number of
patients | 124 | 62 | 62 |
| Age, years |
|
|
|
|
<60 | 88 (70.97) | 40 | 48 |
|
≥60 | 36 (29.03) | 22 | 14 |
| Sex |
|
|
|
|
Male | 71 (57.26) | 35 | 36 |
|
Female | 53 (42.74) | 27 | 26 |
| Mean tumor
diameter, cm |
|
|
|
|
<5 | 43 (34.69) | 24 | 19 |
| ≥5 | 81 (65.32) | 38 | 43 |
| IDH1 status |
|
|
|
|
Wild-type | 98 (79.03) | 42 | 56 |
|
Mutant | 26 (20.97) | 20 | 6 |
| Resection
degree |
|
|
|
| Gross
total resection | 96 (77.42) | 49 | 47 |
|
Sub-total resection | 24 (19.35) | 11 | 13 |
| Partial
resection | 4 (3.26) | 2 | 2 |
|
Biopsy | 0 (0) | 0 | 0 |
| Survival
status |
|
|
|
|
Alive | 25 (20.16) | 21 | 4 |
|
Dead | 99 (79.84) | 41 | 58 |
| Recurrence |
|
|
|
| No | 18 (14.52) | 15 | 3 |
|
Yes | 106 (85.48) | 47 | 59 |
Cell culture
The U87 cell line (glioblastoma of unknown origin)
was purchased from the American Type Culture Collection (cat. no.
HTB-14™). The cells were grown in DMEM (Sigma-Aldrich; Merck KGaA)
supplemented with 8% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), penicillin G (100 U/ml) and streptomycin (100
g/ml) and maintained at 37°C in humidified air with 5%
CO2. The cells were incubated in anoxic and/or hypoxic
(3–5% O2) environments. Hypoxic conditions were obtained
by replacing oxygen with N2, using a Heracell 150i
CO2 incubator (Thermo Fisher Scientific, Inc.).
Bioinformatic analysis
To examine the expression of RLIP76 in GBM, a
LinkedOmics analysis was performed of The Cancer Genome Atlas
(TCGA) GBM databases according to their IDH1 status. LinkedOmics
(http://www.linkedomics.org) is a
publicly-available portal comprising multi-omics data from all 32
types of cancer in the TCGA database.
To examine the hypoxia-induced gene expression
profiles of IDH1wt and IDH1Mut glioma stem
cell lines, a previously published microarray study was downloaded
from the Gene Expression Omnibus (GEO) database (accession no.
GSE118683) (21). Differentially
expressed genes (DEGs) were identified using the edgeR package
(http://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
in R software; P<0.05 and fold change >2 were considered as
statistically significant. The expressions of all DEGs were
presented in the heatmap.
The DAVID bioinformatics resources 6.8 (https://david.ncifcrf.gov) were used to perform gene
ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
analyses on the differentially expressed genes (22,23).
Following the analyses for significance and false discovery rate
(FDR), the GO terms were selected from the significantly enriched
gene sets (P<0.05 and FDR <0.05).
Transfection and stable clone
selection
The IDH1-Flag and IDH1 R132H-Flag plasmids were
constructed into the pCMV-Tag2B vector as previously described
(24). Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for
transfection according to the manufacturer's instructions. The
efficacy of the transfection was tested by examining the expression
of the Flag protein using western blotting. IDH1 siRNA (cat. no.
sc-60829) and siRNA reagent system (cat. no. sc-45064), and RLIP76
siRNA (cat. no. sc-36376) and control siRNA (cat. no. sc-37007),
were purchased from Santa Cruz Biotechnology, Inc., and all
transfections were conducted according to the manufacturer's
instructions. On the 3rd day following transfection, protein
expression was examined by western blotting in order to evaluate
the knockdown efficacy.
RNA isolation and RT-qPCR
Total RNA, including miRNA, was extracted from
cells/tissues with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA was reverse transcribed into cDNA
using the ReverTra Ace qPCR RT kit (FSQ-101; Toyobo Life Science),
according to the manufacturer's protocol. cDNA was amplified using
the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen; Thermo
Fisher Scientific, Inc.) on a 7500 Fast Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR
conditions were: 10 min at 95°C, 1 min at 55°C, followed by 40
cycles of 15 sec at 95°C and 30 sec at 55°C. Relative fold
expression of the target gene was normalized to β-actin and
calculated according to the 2−∆∆Cq method (25). The sequences for the forward and
reverse primers of RLIP76 and β-actin were previously described
(18).
Apoptosis assay
Cell apoptosis was assessed using the ApoScreen
Annexin V Apoptosis Kit (Bender MedSystems, GmbH). After washing
twice with PBS, cells were collected and stained with FITC-Annexin
V and propidium iodide, as per the manufacturer's instructions. All
cells were analyzed by flow cytometry (FACScan; BD Biosciences).
Data analyses were performed using CellQuest software Version 5.0
(BD Biosciences). Experiments were conducted in triplicates.
Western blot analysis
Western blot analyses were performed as described
previously (26). The primary
antibodies used were the following: Anti-Flag antibody (cat. no.
LT0420; LifeTein, LLC), IDH1 (Cell Signaling Technology, Inc.; cat.
no. 8137; 1:500), RLIP76 (Abcam; cat. no. ab56815; 1:1,000),
caspase-9 (Santa Cruz Biotechnology, Inc.; cat. no. sc-17784;
1:500), Bcl-2 (Santa Cruz Biotechnology, Inc.; cat. no. sc-509;
1:500), p53 (Abcam; cat. no. ab-32389; 1:1,000) and β-actin (Abcam;
cat. no. ab-8227; 1:1,000). Western blot analysis was quantified by
normalizing the signal intensity of each sample to that of
β-actin.
Cell proliferation assay
Cell viability was assessed by the Cell Counting
Kit-8 (CCK-8) assay. Briefly, all cells were seeded in
collagen-coated 96-well plates at a density of 5×103
cells/ml and incubated with 10 µl CCK-8 solution for 4 h at 37°C.
The optical density of each sample was recorded with a microplate
reader (Bio-Rad Laboratories, Inc.) at 450 nm. The assays were
performed in triplicate.
Immunohistochemical assays
The immunohistochemical assay (IHC) was conducted as
previously described (18). The
RLIP76 monoclonal antibody (Abcam; cat. no. ab-133549) was used at
a dilution of 1:1,000. The assessment of RLIP76 expression was
conducted as described previously (18). Briefly, RLIP76 expression was divided
into ‘high’ (++, final score 4–6 and +++, final score >6) vs.
‘low’ (+, final score 1–3 and -, final score 0).
Quantification of GSH
GSH was quantified using a GSH-Glo™ Glutathione
Assay kit (cat. no. V6912; Promega Corporation), according to the
manufacturers' instructions, following incubation of
1×104 cells/well in 96-well plates for 1 h at 37°C with
5% CO2 in serum-free HBSS with added Ca2+ and
Mg2+.
Statistical analysis
The experimental data were presented as mean ±
standard deviation. Statistical analysis was performed by the
Student's t-test. Kaplan-Meier survival analysis was used to
compare the overall survival (OS) in glioma patients. Univariate
survival analysis was carried out by the Kaplan-Meier method and
analyzed via the log-rank test to assess differences in survival
between the groups. The Cox proportional hazard model for
multivariate survival analysis was used to assess predictors of
survival. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of RLIP76 in
patients with IDH1Wt and IDH1Mut GBM
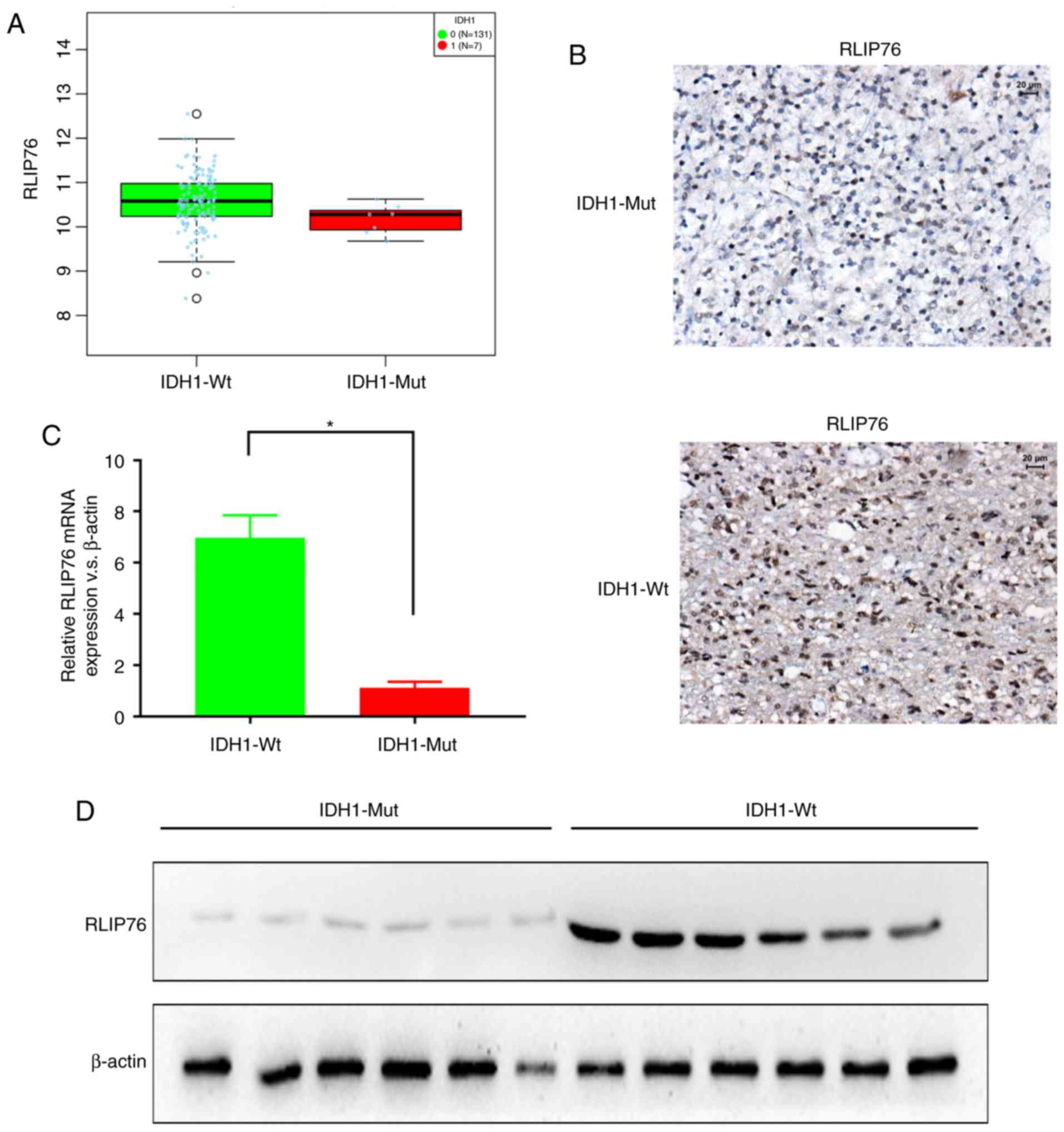
The expression levels of RLIP76 in GBM were
investigated from the TCGA database using LinkedOmics (http://www.linkedomics.org). RLIP76 mRNA expression
levels were significantly higher in the IDH1Wt (n=131)
group compared with the IDH1Mut (n=7) group (Fig. 1A; P=0.0406). Subsequently, the protein
expression levels of RLIP76 were investigated in 124 human GBM
specimens by IHC staining. The GBM tissues were divided into
IDH1Mut (n=26) and IDH1Wt (n=98) groups,
according to their WHO grading. The IHC results revealed that the
IDH1Wt group exhibited higher immunoreactivity for
RLIP76 in their GBM tissues compared with the IDH1Mut
group (Fig. 1B). By RT-qPCR analysis,
it was also revealed that the IDH1Wt group exhibited
significantly higher RLIP76 mRNA expression levels compared with
the IDH1Mut group (Fig.
1C). Similar differences were also observed by western
blotting, with regard to the RLIP76 protein expression levels
between the two glioma groups (Fig.
1D). These results indicated that the expression levels of
RLIP76 were significantly upregulated in IDH1Wt gliomas
compared with the IDH1Mut gliomas.
Prognostic value of RLIP76 in
IDH1Wt GBM
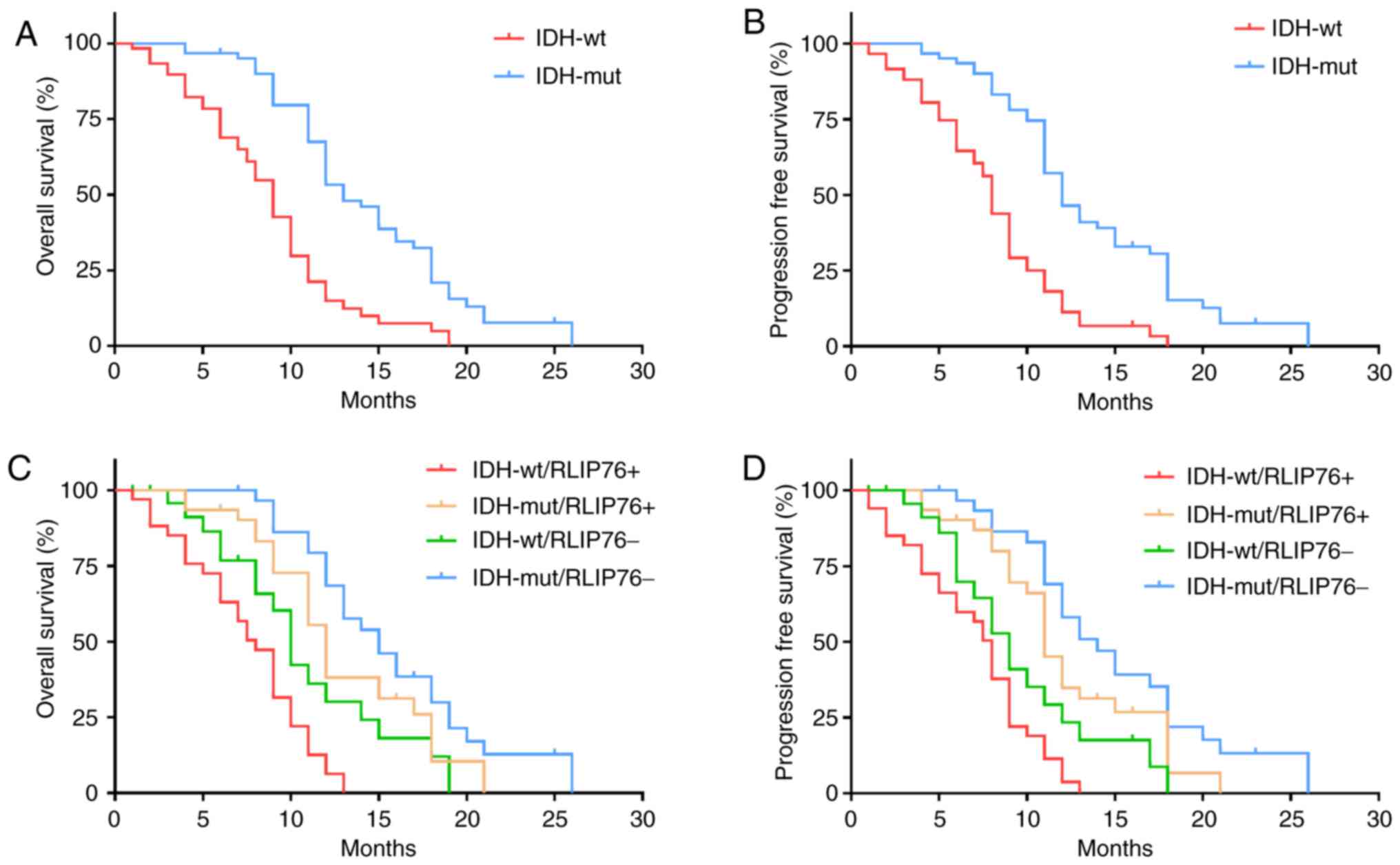
To further explore the prognostic value of RLIP76 in
GBM tissues with different IDH1 mutational status, the patients
(n=124) were divided into the IDH1Mut (n=26) and
IDH1Wt (n=98) groups. The results confirmed that
IDH1Wt was significantly associated with poor OS
(P=0.0241; Fig. 2A) and
progression-free survival (PFS) in patients with GBM (P=0.0178;
Fig. 2B). Kaplan-Meier analysis
further revealed that increased RLIP76 expression levels were
associated with poor OS and PFS in the IDH1Wt group
(P=0.0297 and P=0.0374, respectively), while no significant
difference was noted with regard to the prognostic value of RLIP76
in the IDH1Mut group (P=0.162 and P=0.219, respectively;
Fig. 2C and D). These results
suggested that RLIP76 may exert a specific role in
IDH1Wt GBM that is different from that noted in
IDH1Mut GBM.
Univariate survival analysis indicated that the
expression levels of RLIP76 were a prognostic factor for OS and for
PFS in patients with IDH1Wt glioma (Table II). Multivariate analysis further
confirmed that high expression levels of RLIP76 at diagnosis were a
critical and independent prognostic factor for OS and PFS in
patients with IDH1Wt glioma (Table III).
 | Table II.Univariate analysis of factors
associated with survival of patients with IDH1 wild-type
glioblastoma multiforme. |
Table II.
Univariate analysis of factors
associated with survival of patients with IDH1 wild-type
glioblastoma multiforme.
| A, Overall
survival |
|---|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.754 | 0.435–1.269 | 0.526 |
| Age (≥60 vs.
<60) | 1.857 | 1.113–2.648 | 0.038 |
| RLIP76 (high vs.
low) | 1.986 | 1.045–2.976 | 0.008 |
| MTD (≥5 cm vs.
<5 cm) | 0.776 | 0.418–1.397 | 0.716 |
| Resection
degree |
|
| 0.039 |
| Total vs.
partly | 0.324 | 0.098–0.912 | 0.023 |
| Subtotal vs.
partly | 0.275 | 0.078–0.936 | 0.032 |
|
| B,
Progression-free survival |
|
|
Variable | HR | 95% CI | P-value |
|
| Sex (male vs.
female) | 0.684 | 0.468–1.078 | 0.493 |
| Age (≥60 vs.
<60) | 1.568 | 1.213–2.336 | 0.042 |
| RLIP76 (high vs.
low) | 1.756 | 1.129–2.743 | 0.008 |
| MTD (≥5 cm vs.
<5 cm) | 0.721 | 0.356–1.463 | 0.841 |
| Resection
degree |
|
| 0.047 |
| Total vs.
partly | 0.411 | 0.0618–0.985 | 0.031 |
| Subtotal vs.
partly | 0.178 | 0.043–0.574 | 0.013 |
 | Table III.Multivariate analysis of factors
associated with survival of patients with IDH1 wild-type
glioblastoma multiforme. |
Table III.
Multivariate analysis of factors
associated with survival of patients with IDH1 wild-type
glioblastoma multiforme.
| A, Overall
survival |
|---|
|
|---|
| Variable | Median survival
(months, 95% CI) | HR | 95% CI | P-value |
|---|
| RLIP76 (high vs.
low) | 11
(6.124–13.142) | 16
(8.187–23.879) | 1.872 | 1.104–3.267 | 0.012 |
| Resection
degree | NA | NA | NA | NA | 0.047 |
| Total
vs. partial | 13
(11.256–14.784) | 4 (0–6.998) | 0.242 | 0.053–0.816 | 0.019 |
|
Subtotal vs. partial | 16
(9.872–20.121) | 4 (0–6.998) | 0.195 | 0.049–0.788 | 0.018 |
| Age (≥60 vs. <60
years) | 8
(3.477–12.236) | 15
(10.121–19.689) | NA | NA | 0.154 |
|
| B,
Progression-free survival |
|
|
Variable | Median survival
(months, 95% CI) | HR | 95% CI | P-value |
|
| RLIP76 (high vs.
low) | 7
(6.124–13.142) | 12
(8.187–23.879) | 1.579 | 1.212–3.978 | 0.021 |
| Resection
degree | NA | NA | NA | NA | 0.034 |
| Total
vs. partial | 11
(8.117–13.168) | 3 (0–5.168) | 0.172 | 0.036–0.775 | 0.026 |
|
Subtotal vs. partial | 12
(7.981–16.336) | 3 (0–5.099) | 0.217 | 0.057–0.819 | 0.031 |
| Age (≥60 vs. <60
years) | 7
(3.117–10.148) | 10
(8.564–16.375) | NA | NA | 0.182 |
Microarray-based GO analysis and
pathway analysis
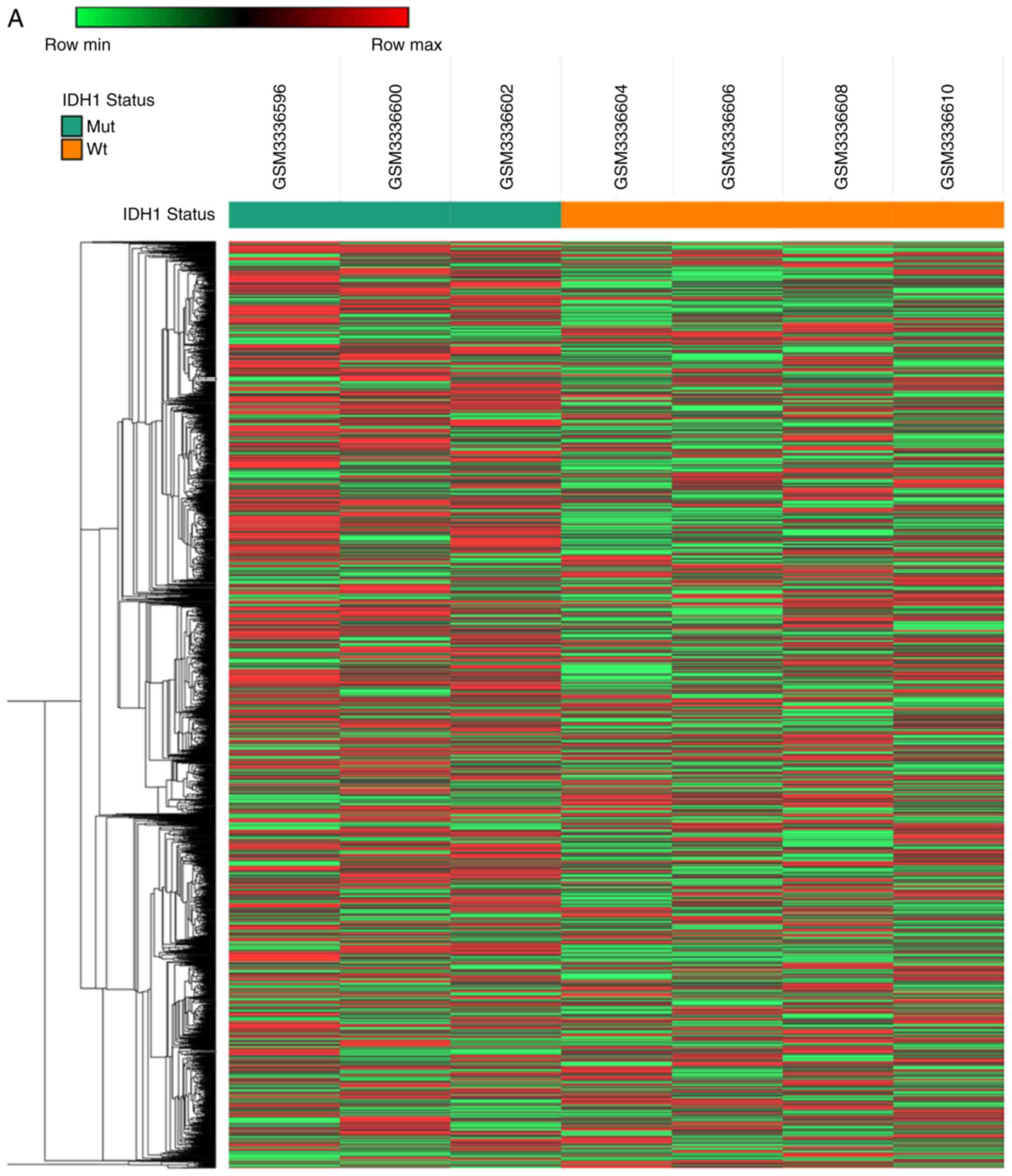
To identify novel oncogenic mRNAs in GBM tissues
with different IDH1 mutational status, the present study analyzed a
previously published microarray study of hypoxia-treated
IDH1wt and IDH1Mut glioma stem cell lines
(full data available at GEO, accession no. GSE118683) (21). The present analysis identified 2,928
mRNAs that were upregulated in IDH1Wt glioma stem cells
compared with IDH1Mut glioma stem cells (fold change
>2 and P<0.05; Fig. 3A) under
hypoxia conditions. In addition, 1,263 mRNAs were downregulated in
IDH1Wt glioma stem cells compared with
IDH1Mut glioma stem cells (fold change >2 and
P<0.05; Fig. 3A).
GO analysis on the targeted genes was conducted
using DAVID 6.8 (https://david.ncifcrf.gov). Based on GO analysis,
~1,234 differentially expressed genes (|fold change|>4 and
P<0.05) were classified (Fig. 3B).
GO analysis revealed that specific biological processes were
enriched, including ‘DNA replication’, ‘cell division’, ‘cell
proliferation’ and the ‘apoptotic process’. In addition to the
biological processes, the differentially expressed genes were also
enriched in the GO terms associated with ‘cellular component’ and
‘molecular function’, such as ‘protein binding’, ‘DNA binding’,
‘ATP binding’ and ‘nucleoplasm’ (Fig.
3B). KEGG pathway enrichment analyses were also performed. The
differentially expressed genes were significantly and predominantly
associated with the ‘metabolic pathway’, a significant process in
the progression of tumor proliferation and apoptosis. Other
enriched pathways involved the ‘cell cycle’, ‘purine metabolism’,
the mTOR and p53 signaling pathways, the ‘long-term potentiation’
and the ‘pyrimidine metabolism’ (Fig.
3C).
Overexpression of IDH1 R132H mutant,
but not IDH1Wt, inhibits cell growth and increases cell
apoptosis via p53-mediated apoptosis in a hypoxic
microenvironment
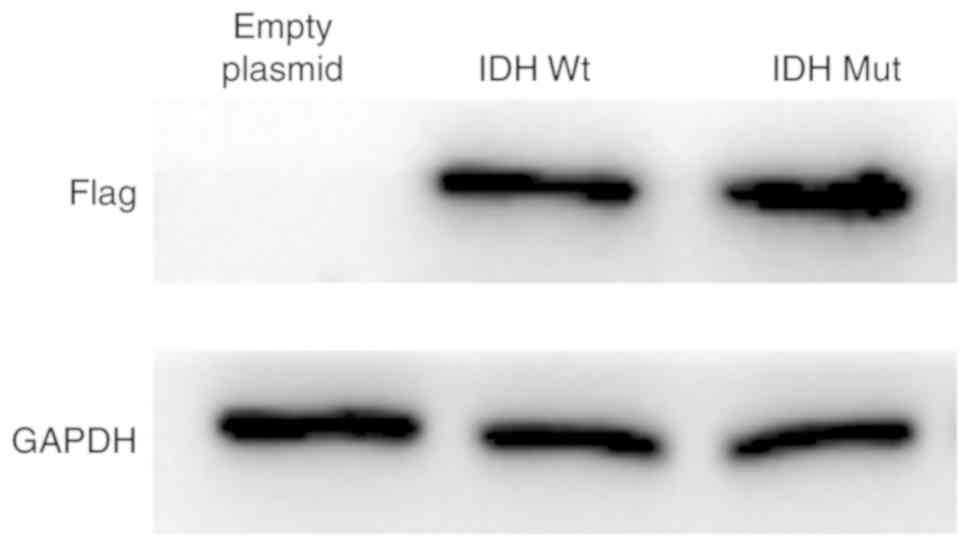
U87 cells that overexpressed either empty vector or
pCMVtag-2B containing IDH1Wt or IDH1Mut were
used to investigate the effects of IDH1Wt and
IDH1Mut proteins on glioma cell growth and apoptosis
under hypoxia. U87 cells with stable overexpression of IDH1 and
IDH1 R132H mutant proteins were successfully established (Fig. 4).
Subsequently, the contribution of the IDH1
mutational status to the growth of U87 cells under hypoxia was
explored by the CCK-8 assay. Overexpression of the IDH1 R132H
mutant protein significantly suppressed cell proliferation in U87
cells compared with cells transfected with empty plasmid or control
parental cells (Fig. 5A). By
contrast, overexpression of the IDH1Wt protein did not
influence cell proliferation compared with cells transfected with
empty plasmid or control parental cells (Fig. 5A). In addition, overexpression of the
IDH1Mut protein resulted in enhanced apoptosis of U87
cells (Fig. 5B). No differences were
noted with regard to cell apoptosis in IDH1Wt cells
compared with the parental control cells (Fig. 5B). These findings suggested that
overexpression of IDH1Mut, but not IDH1Wt,
suppressed cell proliferation and promoted cell apoptosis in GBM
cells under hypoxic conditions.
 | Figure 5.Effects of IDH1 overexpression on the
proliferation and apoptosis of U87 cells. (A) Cell proliferation
was measured by CCK-8 assay at 72 h. (B) Apoptosis rates were
measured by flow cytometry. (C) Representative images and
quantification from western blot analysis for RLIP76, p53,
Caspase-9 and Bcl-2 protein expression levels. (D) Western blotting
results confirming successful knockdown of IDH1 in U87 cells by
siRNA transfection. (E) Proliferation of control cells and cells
transfected with IDH1 siRNA was measured by CCK-8 assay at 72 h.
(F) Apoptosis rates of control cells and IDH1 siRNA-transfected
cells were measured by flow cytometry. (G) Representative images
and quantification from western blot analysis for RLIP76, p53,
Caspase-9 and Bcl-2 protein expression levels in control cells and
IDH1 siRNA-transfected cells. *P<0.05 and **P<0.01 vs.
control. IDH1, IDH1, isocitrate dehydrogenase 1; CCK-8, Cell
Counting Kit-8; RLIP76, ralA binding protein 1; siRNA, small
interfering RNA; Wt, wild-type; Mut, mutant; OD, optical density;
GFP, green fluorescent protein; PI, propidium iodide. |
To further explore the mechanism by which the IDH1
R132H mutant protein mediated cell growth inhibition and apoptosis,
the expression levels of RLIP76, p53, caspase-9 and Bcl-2 proteins
were detected by western blotting. The bioinformatic analysis of
the GSE118683 database performed in the present study indicated
that the expression levels of the aforementioned genes were
significantly altered between IDH1Mut and
IDH1Wt GBM cells under hypoxic conditions (data not
shown). Western blot analysis confirmed that the IDH1 R132H
mutant-overexpressing cells exhibited higher protein expression
levels of p53 and caspase-9, and lower protein expression levels of
Bcl-2 and RLIP76, compared with the
IDH1Wt-overexpressing and the control cells (Fig. 5C). Notably, no difference was observed
in the protein expression levels of RLIP76 between the control and
IDH1Mut groups. These results suggested that the
antitumor effects of the IDH1 R132H mutant proteins on GBM were
associated with an induction of the p53-dependent apoptotic pathway
under hypoxia.
Knockdown of IDH1 inhibits cell growth
and enhances cell apoptosis via induction of the RLIP76-dependent
apoptotic pathway under hypoxia
U87 cells were transfected with IDH1 siRNA and
western blotting results demonstrated that the protein expression
levels of IDH1 were successfully reduced (Fig. 5D). Following IDH1 siRNA transfection,
U87 cell proliferation was significantly suppressed under hypoxic
conditions compared with that of the control cells, as demonstrated
by the CCK-8 assay (Fig. 5E). In
addition, flow cytometric analysis indicated that the apoptosis
rate was significantly increased in U87 cells following IDH1
silencing compared with that of the control cells (Fig. 5F). These results indicated that IDH1
functioned as a tumor oncogene in U87 cells under hypoxic
conditions.
Since RLIP76 has a critical role in the prognosis of
IDH1Wt GBM (Fig. 2C), the
expression levels of RLIP76, p53, caspase-9 and Bcl-2 were measured
in order to investigate their ability to regulate tumor progression
under hypoxia. Knockdown of IDH1 resulted in a significant decrease
in RLIP76 expression levels without affecting p53 expression in U87
cells (Fig. 5G). This result was not
noted in the tissues containing the IDH1 R132H mutant phenotype
(Fig. 5C). The data demonstrated that
although the IDH1 R132H mutant phenotype exerted similar antitumor
effects on GBM with those noted in the presence of IDH1 knockdown,
it promoted apoptosis under hypoxia via a distinct mechanism of
action. The IDH1 R132H mutant protein promoted p53-induced
apoptosis, whereas IDH1 knockdown inhibited the RLIP76-dependent
apoptotic pathway. This may partially explain why RLIP76 was a
better prognostic indicator in IDH1Wt GBM compared with
IDH1Mut GBM.
Knockdown of RLIP76 inhibits cell
proliferation and decreases GSH levels in IDH1Wt, but
not in IDH1Mut, glioma cells under hypoxia
Transfection of parental control, IDH1Wt
or IDH1Mut U87 cells with RLIP76 siRNA significantly
suppressed RLIP76 protein expression (Fig. 6A). RLIP76 knockdown significantly
inhibited cell growth and promoted the induction of apoptosis in
IDH1Wt U87 cells, although it did not affect the
proliferation and apoptosis of IDH1Mut U87 cells under
hypoxia (Fig. 6B and C). The results
suggested that RLIP76 expression contributed to the malignant
progression of IDH1Wt glioma cells under hypoxic
conditions.
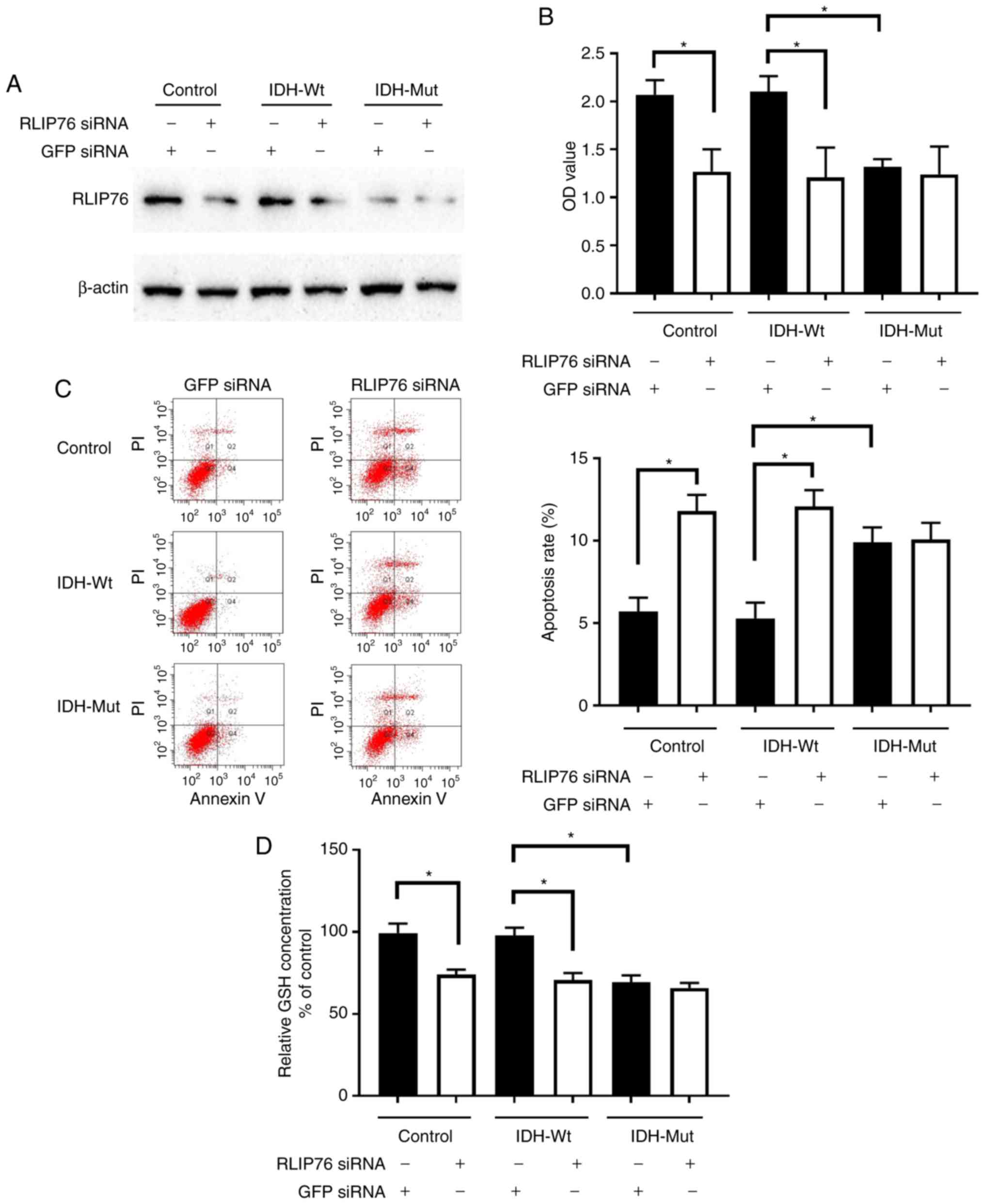 | Figure 6.Effects of RLIP76 knockdown on
proliferation and apoptosis of U87 cells overexpressing Wt or Mut
IDH1. Control cells and cells stably overexpressing Wt or R132H Mut
IDH1 were transfected with either a control siRNA or a
RLIP76-specific siRNA for 72 h. (A) Western blotting results
confirming successful knockdown of RLIP76 after siRNA transfection.
(B) Cell proliferation was measured by Cell Counting Kit-8 assay.
(C) Apoptosis rates were measured by flow cytometry. Representative
plots and quantification is shown. (D) GSH levels were measured
using a commercial glutathione assay kit. *P<0.05, with
comparisons indicated by brackets. RLIP76, ralA binding protein 1;
IDH1, IDH1, isocitrate dehydrogenase 1; Wt, wild-type; Mut, mutant;
siRNA, small interfering RNA; GSH, glutathione; GFP, green
fluorescent protein; OD, optical density; PI, propidium iodide. |
The present bioinformatic analysis revealed that the
differential gene expression noted in IDH1Mut glioma
cells was mainly associated with the metabolic pathway (Fig. 3). In addition, a previous study has
reported that RLIP76 has a critical role in the regulation of the
metabolic pathway required for GSH detoxification (27). In light of the aforementioned
findings, the expression levels of GSH were investigated. GSH is
considered the main antioxidative defense mechanism that prevents
intracellular damage under hypoxia. IDH1Mut cells
exhibited a dramatic decrease in GSH levels compared with those of
the control and IDH1Wt cells under hypoxia. In addition,
knockdown of RLIP76 in IDH1Wt glioma cells significantly
decreased GSH levels. However, GSH levels were not significantly
different in IDH1Mut cells following transfection with
either control or RLIP76 siRNA. These results suggested that RLIP76
may influence the metabolic pathway of IDH1Wt glioma
cells by regulating GSH levels in the hypoxic microenvironment.
Discussion
GBM represents one of the most malignant forms of
astrocytoma in humans. It has been reported that IDH1Wt
glioma patients exhibit poorer prognosis and lower functional
connectivity compared with IDH1Mut glioma patients
(28). The present study confirmed
that the IDH1Wt phenotype was a prognostic factor for
poor disease outcome in patients with GBM. Previous studies have
demonstrated that IDH1 mutations result in depleted levels of
crucial antioxidant molecules, such as GSH, NADPH and α-KG. GSH is
regarded as the major intracellular free radical scavenger and the
elimination of GSH-conjugates (GS-E) is critical for cell survival,
since the accumulation of GS-E results in cell toxicity. RLIP76 has
demonstrated dinitrophenyl-S-glutathione conjugate-dependent ATPase
(DNP-SG ATPase) activity, which accounts for up to 80% of the GS-E
efflux and is the major member of the cell detoxification system.
It has been shown that the GS-E detoxification process is
reversible. The present study demonstrated that the expression
levels of RLIP76 in the IDH1Mut specimens were
significantly lower compared with those in the IDH1Wt
glioma specimens. The present results indicated that IDH mutation
may inhibit RLIP76 expression levels. However, the precise
mechanism of the regulation of RLIP76 expression by the IDH
mutation remains unclear.
The traditional WHO classification has been revised
in 2016 and includes novel molecular markers in addition to
histological evaluation. IDH1 mutation is one of the most robust
markers used in glioma patients. IDH1 is frequently mutated in low
grade glioma and secondary GBM. However, these IDH1 mutations are
rare in primary GBM (29).
IDH1-driven metabolic reprogramming has been regarded as a critical
progress for retaining the glioma stem cell compartment (24). Diminished IDH1 activity results in
exhaustion of reduced glutathione and stimulates the production of
reactive oxygen species (ROS) (24).
ROS-induced lipid peroxidation that occurs in plasma and
mitochondrial membranes is the major stimulus for the production of
4-hydroxynonenal (4-HNE) (30). It is
believed that the metabolic pathway for 4-HNE detoxification is
activated by RLIP76. This pathway may represent an ideal
gene-targeting strategy for malignant tumor treatment (31). Therefore, it is reasonable to
speculate that RLIP76 regulates numerous cellular signaling
pathways, notably the IDH1-induced metabolic pathway, under hypoxic
conditions, and by doing so it may ultimately promote tumor
development. Bioinformatic analysis in the present study
demonstrated that the metabolic pathway was significantly altered
between IDH1wt GBM and IDH1Mut GBM tissues
under hypoxia. In addition, the current data revealed that high
levels of RLIP76 expression were associated with lower OS and PFS
in patients with IDH1Wt GBM. RLIP76 expression is not an
ideal marker for prognosis prediction in the IDH1Mut
group. This may be due to the considerable differences between the
metabolic adaptation in IDH1Wt and IDH1Mut
GBM, which are driven by IDH1 activity (3). However, these issues need to be further
clarified in future studies.
The fast and incurable tumor recurrence is more
common in IDH1Wt GBM subjects and requires effective
treatment approaches (32). However,
the markers available to predict prognosis of patients with
IDH1Wt GBM are insufficient. Several studies point to
the application of RLIP76 as a prognostic marker of various
malignant cancers, such as melanoma, kidney, colon and prostate
cancer (16,33,34). In
addition, it was previously demonstrated that RLIP76 expression
exhibited elevated levels in high-grade gliomas (18). This finding led to the current
investigation that RLIP76 could be used as a prognostic marker for
GBM with a different IDH1 mutational status on the basis of the
2016 WHO classification. In the present study, statistical analysis
indicated that RLIP76 was a critical factor for OS and PFS in the
IDH1Wt, but not in the IDH1Mut group. The
findings suggested that RLIP76 expression could be employed to
predict the clinical outcome of IDH1Wt GBM.
RLIP76 functions as an oncogene by increasing the
expression of the Rac1/JNK pathway proteins and by activating the
PI3K/AKT pathway in glioma (18,20).
Notably, RLIP76 regulates apoptosis independent of the p53 status
in malignant glioma and neuroblastoma (18,35). The
present study demonstrated that the decrease in IDH1 activity,
either by siRNA transfection or by upregulation of the IDH1 R132H
mutant protein, could suppress cell growth and enhance cell
apoptosis in a hypoxic microenvironment. To identify the molecular
mechanisms of this process in U87 cells, the expression levels of
Bcl-2, caspase-9, p53 and RLIP76 proteins were investigated and the
results indicated that the IDH1 R132H mutant protein promoted
p53-induced apoptosis, while the IDH1Wt protein
suppressed cell apoptosis via the RLIP76-dependent pathway.
Notably, knockdown of IDH1 significantly inhibited RLIP76
expression, without affecting p53 expression. It is possible that
RLIP76 had a critical role in IDH1Wt-mediated apoptosis,
whereas p53 status was highly associated with IDH1 mutation-induced
apoptosis under hypoxic conditions. Mounting evidence indicates
that IDH1Wt and IDH1Mut gliomas are
biologically different tumor types (36,37). In
agreement with this hypothesis, the results of the present study
provided experimental evidence that IDH1Wt and
IDH1Mut regulated the induction of GBM apoptosis via
distinct biological mechanisms. These data suggested that agents
that specifically target RLIP76 in IDH1Wt, but not in
IDH1Mut cell types, may be promising therapeutic agents
for GBM treatment.
Taken collectively, the present study demonstrated
that RLIP76 may be an ideal prognostic biomarker of
IDH1Wt GBM. In addition, the present findings improved
our understanding on the fundamental function of IDH1 mutation in
glioma. RLIP76 may be used to further stratify patients with
IDH1Wt glioma into high and low risk subjects, in order
to optimize their treatment.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Science
Foundation of China (grant no. 81802489), the Fundamental Research
Funds for the Central Universities (grant no. 22120180353), the
Shanghai Natural Science Foundation (grant nos. 18ZR1434500,
19ZR1448900 and 18411962500) and the Scientific Research Initial
Funding of Shanghai Tongji Hospital (grant no. RCQD1704).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and LZ performed the experiments. QW and YC wrote
the manuscript. JG, HC, CZ collected and interpreted the data and
performed the bioinformatics analysis. JQ and CL provided technical
assistance. QW, JQ and CL designed the study and revised the
manuscript. All authors read and approved the final
manuscript..
Ethics approval and consent to
participate
The present study was granted approval by the
Specialty Committee on Ethics of Biomedicine Research at the Tongji
University. Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lim M, Xia Y, Bettegowda C and Weller M:
Current state of immunotherapy for glioblastoma. Nat Rev Clin
Oncol. 15:422–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaminska B, Czapski B, Guzik R, Król SK
and Gielniewski B: Consequences of IDH1/2 mutations in gliomas and
an assessment of inhibitors targeting mutated IDH proteins.
Molecules. 24(pii): E9682019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stancheva G, Goranova T, Laleva M,
Kamenova M, Mitkova A, Velinov N, Poptodorov G, Mitev V, Kaneva R
and Gabrovsky N: IDH1/IDH2 but not TP53 mutations predict prognosis
in Bulgarian glioblastoma patients. Biomed Res Int.
2014:6547272014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medeiros BC, Fathi AT, DiNardo CD, Pollyea
DA, Chan SM and Swords R: Isocitrate dehydrogenase mutations in
myeloid malignancies. Leukemia. 31:272–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akyerli CB, Yüksel S, Can Ö, Erson-Omay
EZ, Oktay Y, Coşgun E, Ülgen E, Erdemgil Y, Sav A, von Deimling A,
et al: Use of telomerase promoter mutations to mark specific
molecular subsets with reciprocal clinical behavior in IDH mutant
and IDH wild-type diffuse gliomas. J Neurosurg. 128:1102–1114.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hata N, Hatae R, Yoshimoto K, Murata H,
Kuga D, Akagi Y, Sangatsuda Y, Suzuki SO, Iwaki T, Mizoguchi M and
Iihara K: Insular primary glioblastomas with IDH mutations:
Clinical and biological specificities. Neuropathology. 37:200–206.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Labussiere M, Boisselier B, Mokhtari K, Di
Stefano AL, Rahimian A, Rossetto M, Ciccarino P, Saulnier O,
Paterra R, Marie Y, et al: Combined analysis of TERT, EGFR, and IDH
status defines distinct prognostic glioblastoma classes. Neurology.
83:1200–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen JE, Lumibao J, Blazek A, Gaskins HR
and Harley B: Hypoxia activates enhanced invasive potential and
endogenous hyaluronic acid production by glioblastoma cells.
Biomater Sci. 6:854–862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gandhi N and Das GM: Metabolic
reprogramming in breast cancer and its therapeutic implications.
Cells. 8(pii): E892019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cox AG, Tsomides A, Kim AJ, Saunders D,
Hwang KL, Evason KJ, Heidel J, Brown KK, Yuan M, Lien EC, et al:
Selenoprotein H is an essential regulator of redox homeostasis that
cooperates with p53 in development and tumorigenesis. Proc Natl
Acad Sci USA. 113:E5562–E5571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McArdle A, Pollock N, Staunton CA and
Jackson MJ: Aberrant redox signalling and stress response in
age-related muscle decline: Role in inter- and intra-cellular
signalling. Free Radic Biol Med. 132:50–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singhal SS, Singh SP, Singhal P, Horne D,
Singhal J and Awasthi S: Antioxidant role of glutathione
S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated
signaling. Toxicol Appl Pharmacol. 289:361–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singhal SS, Yadav S, Roth C and Singhal J:
RLIP76: A novel glutathione-conjugate and multi-drug transporter.
Biochem Pharmacol. 77:761–769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagaprashantha LD, Singhal J, Li H, Warden
C, Liu X, Horne D, Awasthi S, Salgia R and Singhal SS:
2′-Hydroxyflavanone effectively targets RLIP76-mediated drug
transport and regulates critical signaling networks in breast
cancer. Oncotarget. 9:18053–18068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu N and Du CH: RLIP76 silencing inhibits
cell proliferation and invasion in melanoma cell line A375. Eur Rev
Med Pharmacol Sci. 21:2054–2060. 2017.PubMed/NCBI
|
|
17
|
Wang W, Liu J, Qi J, Zhang J, Zhu Q and
Qin C: RLIP76 increases apoptosis through Akt/mTOR signaling
pathway in gastric cancer. Oncol Rep. 36:2216–2224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Wang JY, Zhang XP, Lv ZW, Fu D, Lu
YC, Hu GH, Luo C and Chen JX: RLIP76 is overexpressed in human
glioblastomas and is required for proliferation, tumorigenesis and
suppression of apoptosis. Carcinogenesis. 34:916–926. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Qian J, Wang J, Luo C, Chen J, Hu
G and Lu Y: Knockdown of RLIP76 expression by RNA interference
inhibits invasion, induces cell cycle arrest, and increases
chemosensitivity to the anticancer drug temozolomide in glioma
cells. J Neurooncol. 112:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Cai Z, Liang Q, Wang Q, Lu Y, Hu
L and Hu G: RLIP76 depletion enhances autophagic flux in U251
cells. Cell Mol Neurobiol. 37:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dao Trong P, Rosch S, Mairbaurl H, Pusch
S, Unterberg A, Herold-Mende C and Warta R: Identification of a
prognostic hypoxia-associated gene set in IDH-mutant glioma. Int J
Mol Sci. 19(pii): E29032018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calvert AE, Chalastanis A, Wu Y, Hurley
LA, Kouri FM, Bi Y, Kachman M, May JL, Bartom E, Hua Y, et al:
Cancer-associated IDH1 promotes growth and resistance to targeted
therapies in the absence of mutation. Cell Rep. 19:1858–1873. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Wang Q, Cui Y, Liu ZY, Zhao W,
Wang CL, Dong Y, Hou L, Hu G, Luo C, et al: Knockdown of cyclin D1
inhibits proliferation, induces apoptosis, and attenuates the
invasive capacity of human glioblastoma cells. J Neurooncol.
106:473–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma R, Singhal SS, Cheng J, Yang Y,
Sharma A, Zimniak P, Awasthi S and Awasthi YC: RLIP76 is the major
ATP-dependent transporter of glutathione-conjugates and doxorubicin
in human erythrocytes. Arch Biochem Biophys. 391:171–179. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Derks J, Kulik S, Wesseling P, Numan T,
Hillebrand A, van Dellen E, de Witt Hamer PC, Geurts JJG,
Reijneveld JC, Stam CJ, et al: Understanding cognitive functioning
in glioma patients: The relevance of IDH-mutation status and
functional connectivity. Brain Behav. 9:e012042019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horbinski C: What do we know about IDH1/2
mutations so far, and how do we use it? Acta Neuropathol.
125:621–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong H and Yin H: Role of lipid
peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing
on mitochondria. Redox Biol. 4:193–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gasparovic AC, Milkovic L, Sunjic SB and
Zarkovic N: Cancer growth regulation by 4-hydroxynonenal. Free
Radic Biol Med. 111:226–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang PF, Song HW, Cai HQ, Kong LW, Yao K,
Jiang T, Li SW and Yan CX: Preoperative inflammation markers and
IDH mutation status predict glioblastoma patient survival.
Oncotarget. 8:50117–50123. 2017.PubMed/NCBI
|
|
33
|
Zhang Y, Song X, Gong W, Zhu Z, Liu X, Hou
Q, Sun Y, Chai J, Zou L and Guan J: RLIP76 blockade by siRNA
inhibits proliferation, enhances apoptosis, and suppresses invasion
in HT29 colon cancer cells. Cell Biochem Biophys. 71:579–585. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singhal SS, Singhal J, Yadav S, Sahu M,
Awasthi YC and Awasthi S: RLIP76: A target for kidney cancer
therapy. Cancer Res. 69:4244–4251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singhal SS, Nagaprashantha L, Singhal P,
Singhal S, Singhal J, Awasthi S and Horne D: RLIP76 inhibition: A
promising developmental therapy for neuroblastoma. Pharm Res.
34:1673–1682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parker SJ and Metallo CM: Metabolic
consequences of oncogenic IDH mutations. Pharmacol Ther. 152:54–62.
2015. View Article : Google Scholar : PubMed/NCBI
|