Introduction
Biliary tract cancer (BTC) is a devastating disease
of the digestive system with poor prognosis, and mainly includes
bile duct carcinoma and gallbladder carcinoma (GBC). The 5-year
survival rate is less than 10% in patients with advanced or
metastatic BTCs according to the National Cancer Database of the
American College of Surgeons and the Surveillance, Epidemiology,
and End Results (SEER) Program (1).
The potentially best treatment for patients with BTCs is radical
resection, but many patients are not suitable for curative surgery.
For patients with advanced BTC undergoing non-radical resection,
the prognosis is very poor. Therefore, the exploration of a new
strategy of more precise and effective treatment is critical to
improve the prognosis of patients with advanced BTCs undergoing
non-radical resection.
Precision medicine means matching the right patients
with the right drugs. The use of genetic mutation testing is often
required prior to issuing a matching prescription. The availability
of appropriate molecular profiling is the key to precision medicine
in routine clinical settings (2). A
few studies have reported genetic mutation profiling in biliary
tract tumors (3–7). In intrahepatic bile duct carcinoma,
genes from the mitogen-activated protein kinase (MAPK) signaling
pathway (8) are frequently mutated,
and gene fusions from the fibroblast growth factor receptor (FGFR)
family are also common (9). The
erythroblastic leukemia viral (v-erb-b) oncogene homolog (ERBB)
family including epidermal growth factor receptor (EGFR, also known
as ERBB1), ERBB2 (also known as HER2), ERBB3 and ERBB4 has a
central role in the tumorigenesis and development (10). The ERBB family receptors are able to
activate several downstream pathways, including the RAS-ERK and
PI3K-AKT pathways (11). In the
Chinese population, alterations in the ERBB family and its
downstream signaling pathway account for up to 36.8% of the
alterations detected in gallbladder cancer, and further
multivariate analyses have revealed that cases with ERBB pathway
mutations have worse outcomes (12). Studies in BTC cell lines have
confirmed that the ERBB pathway may be a suitable candidate for
disruption as part of treatment for BTC patients (13). Many mutated genes in biliary tract
tumors, including ERBB2, PIK3CA, FGFR and IDH1, are
targets for biological drugs. There are several studies indicating
the potential of comprehensive genomic profiling for improving
outcomes in advanced BTC patients (14,15).
However, the clinical efficacy of personalized targeted therapy
based on the genetic alterations found in BTC patients undergoing
non-radical resection has not yet been reported. We aim to
investigate the efficacy and safety of personalized targeted
therapy with excellent potential for actual use based on specific
genetic alterations for patients with advanced BTC after
non-radical resection in this real-world study.
Materials and methods
Patients and data collection
The present study was approved by the Ethics
Committee of the Shanghai Eastern Hepatobiliary Surgery Hospital,
Navy Military Medical University, Shanghai, China (no.
EHBHKY2015-02-010). Forty-nine patients with BTC, which was
confirmed by surgical pathology, were enrolled for targeted deep
sequencing in the Department of Biliary I, Shanghai Eastern
Hepatobiliary Navy Hospital from August 2014 to June 2016 (Table I). Exclusion criteria were stage I
disease (AJCC 7th edition) (http://aboutcancer.com/AJCC_stage.htm) at diagnosis,
no tumor tissue sample available for targeted deep sequencing,
disagreement on conducting target deep sequencing, known or current
evidence of HIV, pregnant or lactating females. Thirty-two patients
had stage IV disease and R2 resection (Table II). To investigate the impact of
targeted medicine on patients with advanced BTC, eligibility
criteria included R2 resection of biliary cancer at TNM stage IV,
at least one measurable lesion at baseline and the detection of
targeted deep sequencing. These BTC patients began drug treatment
one month after the operation. For these patients, a regimen of
mGEMOX (16) (gemcitabine 900
mg/m2 and oxaliplatin 80 mg/m2 i.v. infusion
on days 1 and 8 every 3 weeks until disease progression or
intolerable toxicity) was initially proposed by the local
multidisciplinary team as the standard first-line chemotherapy
(chemotherapy group). In patients found to have at least one
targetable variant and who declined chemotherapy, a biological
agent was recommended as an alternative treatment. The specific
agent was selected according to the potentially targetable altered
gene (targeted therapy group). The information of targetable
altered genes corresponding to the biological agent is shown
(Table SI). When a patient had
some targetable altered genes, we recommended the drugs for
patients according to different levels of evidence and gave them
all the information they required. The highest level of evidence is
that targeted drugs addressing specific gene mutations have been
approved by the US Food and Drug Administration (FDA) or China Food
and Drug Administration (CFDA) in this tumor. The second kind of
evidence is that targeted drugs addressing specific gene mutations
have been approved by the FDA or CFDA in other tumors. The third
kind of evidence is that also drugs specific to this gene mutation
are currently being assessed in clinical trials. The weakest
evidence is some preclinical data about the relationship of the
drug-gene mutation associations. In this real-world clinical study,
the patients made decisions themselves according to the
availability of specific drugs and what they were able to afford.
The recorded data also included clinicopathological features,
operative morbidity, drug administration, number of treatment
cycles, and therapy-related toxicity (Tables III and IV).
 | Table I.Characteristics of the 49 patients
with biliary tract cancer that received surgery. |
Table I.
Characteristics of the 49 patients
with biliary tract cancer that received surgery.
|
Characteristics | Data values |
|---|
| Age, median (range)
in years | 59 (26–72) |
| Sex, n |
|
|
Male | 32 |
|
Female | 17 |
| Cancer type, n |
|
|
Gallbladder carcinoma | 21 |
|
Cholangiocarcinoma | 28 |
| AJCC stage, n |
|
| I | 0 |
| II | 2 |
|
III | 5 |
| IV | 42 |
| Type of surgical
operation, n |
|
| R0 | 8 |
| R1 | 8 |
| R2 | 32 |
|
Biopsy | 1 |
| Treatment, n |
|
|
Targeted therapy | 14 |
|
Chemotherapy | 35 |
| Follow-up, n |
|
|
PFS | 49 |
| OS | 49 |
| NGS detection,
n | 49 |
 | Table II.Characteristics of the 32 patients
with stage IV biliary tract cancer after R2 resection. |
Table II.
Characteristics of the 32 patients
with stage IV biliary tract cancer after R2 resection.
|
|
| Conventional
chemotherapy (n=21) |
|---|
|
|
|
|
|---|
|
Characteristics | Targeted therapy
(n=11) | All patients
(n=21) | P-value | With recommendation
(n=13) | P-value |
|---|
| Age, median (range)
in years | 60 (26–65) | 58 (35–66) | 0.645 | 61 (35–66) | 0.897 |
| Sex |
|
|
|
|
|
|
Male | 7 | 13 | 1.000 | 9 | 1.000 |
|
Female | 4 | 8 |
| 4 |
|
| Cancer type |
|
|
|
|
|
|
Gallbladder carcinoma | 6 | 10 | 1.000 | 5 | 0.682 |
|
Cholangiocarcinoma | 5 | 11 |
| 8 |
|
| pTNM stage (AJCC
7th edition) |
|
IVA | 3 | 4 | 0.668 | 2 | 0.630 |
|
IVB | 8 | 17 |
| 11 |
|
| Operative
complication (Clavien-Dindo) |
| Grade
II | 10 | 20 | 1.000 | 13 | 0.458 |
| Grade
IIIa | 1 | 1 |
| 0 |
|
 | Table III.Targeted drugs, the corresponding
altered genes, prognosis and toxicity for the 11 patients with BTC
receiving targeted therapy. |
Table III.
Targeted drugs, the corresponding
altered genes, prognosis and toxicity for the 11 patients with BTC
receiving targeted therapy.
| Patient_ID | Sex | Age (years) | Cancer type | Mutated genes | Recommended
drugs | Drug usage | Dosage | Cycle | Operative
morbidity |
Prognosisa (PD\SD\PR\CR) | Treatment-related
toxicitiesb |
|---|
| BTC-007 | M | 59 | Gallbladder
carcinoma | TP53 | MEK1 | PIK3CA | Trametinib,
everolimus | Everolimus | 10 mg/day (2
months); 5 mg/day (4 months) | 6 | II | SD | Grade 2:
Pruritis/itching |
| BTC-010 | M | 58 |
Cholangiocarcinomas | ERBB3 |
|
| Trastuzumab,
lapatinib | Lapatinib | 1250 mg/day | 3 | II | PR | Grade 2:
Gastrointestinal reaction (vomiting/nausea/diarrhea) |
| BTC-011 | F | 59 |
Cholangiocarcinomas | ERBB4 | PTCH1 | KRAS | Lapatinib,
vismodegib | Lapatinib | 1250 mg/day | 3 | II | PR | Grade 2: Pain |
| BTC-012 | M | 60 |
Cholangiocarcinomas | ERBB3 |
|
| Trastuzumab,
lapatinib | Lapatinib | 1250 mg/day | 2 | II | PD | Grade 2:
Gastrointestinal reaction (constipation/vomiting/nausea) |
| BTC-018 | M | 60 | Gallbladder
carcinoma | MET | PIK3R2 | SRC | Crizotinib,
dasatinib, cabozantinib |
Dasatinib+rapamycin | Dasatinib 140
mg/day; Rapamycin 40 mg/day | 1 | II | SD | Grade 2: Pain |
| BTC-020 | F | 71 | Gallbladder
carcinoma | SRC | TP53 | SMAD4 | Dasatinib | Dasatinib | 140 mg/day | 1 | II | PD | Grade 2: Central
nervous system |
| BTC-026 | M | 65 |
Cholangiocarcinomas | KRAS | PIK3CA | TP53 | Everolimus,
Trametinib | Everolimus | 10 mg/day | 1 | II | SD | Grade 3: Hepatic
damage |
| BTC-034 | M | 62 |
Cholangiocarcinomas | PDGFRA |
|
| Imatinib,
Bevacizumab | Imatinib | 800 mg/day | 6 | II | PR | Grade 3: Hepatic
damage |
| BTC-0840 | M | 63 |
Cholangiocarcinomas | FGFR3 |
|
| Pazopanib | Pazopanib | 800 mg/day | 1 | II | PD | Grade 3:
Platelets |
| BTC-043 | F | 56 | Gallbladder
carcinoma | ERBB2 | FGFR3 | NF1 | Trastuzumab,
Lapatinib | Lapatinib | 1250 mg/day | 4 | II | PD | Grade 2: Hepatic
damage |
| BTC-048 | F | 26 | Gallbladder
carcinoma | RET | CTNNB1 |
| Regorafenib | Regorafenib | 160 mg/day | 5 | IIIa | SD | Grade 3: Fatigue;
Grade 2: Nausea, vomiting, diarrhea, stomatitis |
 | Table IV.Targeted drugs, the corresponding
altered genes, prognosis and toxicity for the 21 patients with BTC
receiving standard chemotherapy. |
Table IV.
Targeted drugs, the corresponding
altered genes, prognosis and toxicity for the 21 patients with BTC
receiving standard chemotherapy.
| Patient_ID | Sex | Age (years) | Cancer type | Mutated genes | Recommended
drugs | Drug usage | Dosage | Cycle | Operative
morbidity |
Prognosisa (PD\SD\PR\CR) | Treatment-related
toxicitiesb |
|---|
| BTC-001 | M | 64 | Gallbladder
carcinoma | STK11 | TP53 | MYC | FANCA | MDM2 | Everolimus,
temsirolimus | mGEMOX | Gemcitabine 900
mg/m2 and oxaliplatin 80 mg/m2 i.v. infusion
on days 1 and 8 every 3 weeks until disease progression or
intolerable toxicity | 2 | II | PD | – |
| BTC-002 | F | 46 |
Cholangiocarcinomas | KRAS | TP53 |
|
|
| None |
|
| 1 | II | PD | – |
| BTC-003 | F | 66 |
Cholangiocarcinomas | KRAS | TP53 | SF3B1 | CDK6 |
| Palbociclib |
|
| 1 | II | PD | – |
| BTC-006 | F | 51 | Gallbladder
carcinoma | PIK3CA | TP53 | AXIN1 | SMAD4 | CCNE1 | Everolimus,
temsirolimus |
|
| 6 | II | PR | – |
| BTC-009 | M | 58 |
Cholangiocarcinomas | ERBB4 | FLT4 | MLL3 |
|
| Lapatinib,
axitinib, pazopanib, sunitinib |
|
| 1 | II | PD | Grade 3: Gastroin
testinal reaction (vomiting/constipation/diarrhea) |
| BTC-016 | F | 63 | Gallbladder
carcinoma | PIK3CA | FLT3 |
|
|
| Rapamycin,
everolimus, sorafinib, sunitinib |
|
| 1 | II | PD | – |
| BTC-017 | M | 35 |
Cholangiocarcinomas | PIK3CA | PDGFRB |
|
|
| Rapamycin,
everolimus |
|
| 2 | II | PD | – |
| BTC-019 | M | 46 |
Cholangiocarcinomas | ERBB4 |
|
|
|
| Lapatinib |
|
| 1 | II | PD | – |
| BTC-021 | M | 66 |
Cholangiocarcinomas | KRAS | APC |
|
|
| Cetuximab
(invalid), panitumumab (invalid), Gefitinib (invalid), Erlotinib
(invalid), everolimus (invalid) |
|
| 1 | II | PD | – |
| BTC-022 | F | 61 |
Cholangiocarcinomas | KRAS | SMAD4 | PTEN | TP53 |
| Trametinib combined
with everolimus, cetuximab (invalid), panitumumab (invalid),
gefitinib (invalid), erlotinib (invalid), everolimus (invalid) |
|
| 12 | II | PR | – |
| BTC-023 | M | 58 | Gallbladder
carcinoma | CDKN2A | BRAF | ARID1B | ARID1A |
| Palbociclib,
sorafinib, trametinib |
|
| 1 | II | PD | – |
| BTC-025 | M | 51 |
Cholangiocarcinomas | EGFR | PIK3R2 | SRC |
|
| Cetuximab,
panitumumab, erlotinib, nimotuzumab, everolimus |
|
| 4 | II | SD | – |
| BTC-031 | F | 65 | Gallbladder
carcinoma | RB1 | TP53 |
|
|
| None |
|
| 2 | II | PD | – |
| BTC-036 | M | 65 | Gallbladder
carcinoma | RAD50 |
|
|
|
| Cisplatin |
|
| 1 | IIIa | PD | – |
| BTC-039 | F | 52 | Gallbladder
carcinoma | SMAD4 |
|
|
|
| Cisplatin |
|
| 4 | II | SD | Grade 3:
Thrombocy-topenia |
| BTC-041 | F | 55 | Gallbladder
carcinoma | ATRX |
|
|
|
| None |
|
| 3 | II | SD | Grade 3:
Gastrointes tinal reaction (vomiting/nausea) |
| BTC-042 | M | 53 |
Cholangiocarcinomas | TAF1 |
|
|
|
| None |
|
| 2 | II | PD | – |
| BTC-044 | M | 65 |
Cholangiocarcinomas | ATM | NF1 | TP53 | PTPN11 |
| Daunorubicin,
olaparib, rapamycin |
|
| 4 | II | SD | Grade 4: Impairment
of renal function |
| BTC-045 | M | 46 |
Cholangiocarcinomas | IDH2 | MEN1 |
|
|
| None |
|
| 6 | II | PD | Grade 2:
Gastrointes tinal reaction (vomiting/nausea) |
| BTC-047 | M | 55 | Gallbladder
carcinoma | FANCA | KEAP1 | BRCA1 | MAP3K1 |
| None |
|
| 4 | II | SD | Grade 2:
Thrombocy-topenia |
| BTC-049 | M | 64 |
Cholangiocarcinomas | VHL |
|
|
|
| Sorafinib,
sunitinib, bevacizumab |
|
| 3 | II | PD | – |
Study assessment
Progression-free survival (PFS), overall survival
(OS) and disease control rate (DCR) were used to assess the
efficacy of treatments. The PFS of patients was measured as the
duration from the beginning of chemotherapy or precision therapy to
the time of disease progression [according to RECIST 1.1 (17)] or death. OS was defined as the
period from the start of chemotherapy or targeted therapy to death.
The PFS and OS were calculated for all enrolled patients (Table SII). Disease control was defined at
the first response assessment as indicated by imaging. Response and
disease progression were evaluated using the international criteria
proposed by the revised Response Evaluation Criteria in Solid
Tumors (RECIST 1.1) Committee (17). The grade of treatment-related
toxicities was evaluated according to the National Cancer Institute
Common Terminology Criteria for Adverse Events (version 3.0)
(18), in order to assess the
safety of treatments.
Targeted deep sequencing and variant
calling
According to our previous study (19), this panel of targeted deep
sequencing consisted of 4,557 exons of 365 tumor-associated genes,
and 45 introns from 25 genes where frequent gene fusions could be
captured in cancer (Table SIII).
Targeted deep sequencing was carried out on hybridization-captured,
adaptor ligation–based libraries using DNA extracted from 4
formalin-fixed paraffin-embedded (FFPE) sections cut at 10 µm from
49 BTC patients. All of the sequencing assays were performed at the
3D Med Clinical Laboratory Co., Ltd. (Shanghai) and successfully
passed the Proficiency Testing (PT) on NGS solid tumor (NGSST)
developed by the College of American Pathologists (CAP) (https://www.cap.org/). DNA was isolated from FFPE
slides containing at least 20% tumor cells and the library was
prepared using IDT Xgen hybridization buffer (Integrated DNA
Technologies, Inc.) for capture and sequenced on an Illumina
Nextseq 500 (Biostar Technology Ltd.).
Short reads were mapped by BWA 0.7.12-r1039 with
default settings (20). Somatic
single-nucleotide variants (SNVs) were identified using Mutect
1.1.4 with default settings, and Indels (smaller than 50 bp) were
identified by Pindel v0.2.5b8 and VarScan v2.4.0 with default
settings (21–23). For somatic copy number alterations
(CNAs), coverage in the tumor was normalized to that in the matched
control (blood DNA). Our in-house program combined with modified
algorithm was applied to call somatic CNAs (24). Actionable genomic alterations were
defined as being linked to commercially available targeted
therapies or to targeted therapies currently in ongoing clinical
trials. To further annotate the genetic variants found in each
patient, an in-house manually reviewed clinical database (3D
Medicines Inc.) was used to develop plans for targeted therapy.
Statistical methods
The difference in age between the two groups was
tested by using the Student's t test. Fisher's exact test was used
to assess the significance of differences between groups, such as
sex, cancer type, TNM stage, and operative complication. The
heatmap was plotted by R package GenVisR 1.0.2 (25), which was used to demonstrate somatic
mutations spectrum across BTC cohorts. Statistical analyses were
performed using R packages. The log-rank test and Kaplan-Meier
analyses were performed for PFS and OS. P<0.05 was considered
statistically significant. Kaplan-Meier analyses were performed
using SPSS statistical software (version 19.0; SPSS Inc.).
Results
Clinical characteristics of the
patients with biliary tract cancer (BTC)
In the present study, we enrolled 49 BTC patients
with a median age of 59 years (range, 26–72) at diagnosis (Table I) from August 2014 to June 2016 for
targeted deep sequencing. These patients consisted of 32 males and
17 females, and were composed of 21 patients with gallbladder
carcinoma and 28 with bile duct cancer (21 intrahepatic, 5
perihilar, and 2 distal cholangiocarcinomas). Most patients (n=42,
85.7%) had cTNM stage IV disease (AJCC 7th edition) at diagnosis.
Out of the 49 patients, 8 patients with BTCs received radical
resection (R0), while the others (n=41, 83.7%) received R1/R2
resection or biopsy. Follow-up was completed for all 49
patients.
Genetic alterations are enriched in
the ERBB family and cell cycle pathway in patients with BTC
The genomic landscape of the 49 patients established
that TP53 (n=31, 63.3%) variants were most prevalent,
followed by variants in KRAS (n=12, 24.5%), ARID1A
(n=6, 12.2%), PIK3CA (n=6, 12.2%), SMAD4 (n=6,
12.2%), CDKN2A (n=5, 10.2%), and ERBB4 (n=5, 10.2%)
(Fig. 1). Further analysis of copy
number alterations (CNAs) showed low levels of recurrent amplified
genes, such as PIK3CA, SMAD4, FGFR3, SRC, PIK3R2, CDK4,
ERBB2, and CDK6. Among these genes, PIK3CA, ERBB2,
CDK4, and CDK6 may be suitable drug targets for these
BTC patients. In 21 patients with gallbladder cancer (GBC), 8 had
mutations in the ERBB pathway. Further analysis of all of the
alterations demonstrated that these altered genes were highly
enriched in the ERBB family or the cell cycle pathway (Fig. 2A and B).
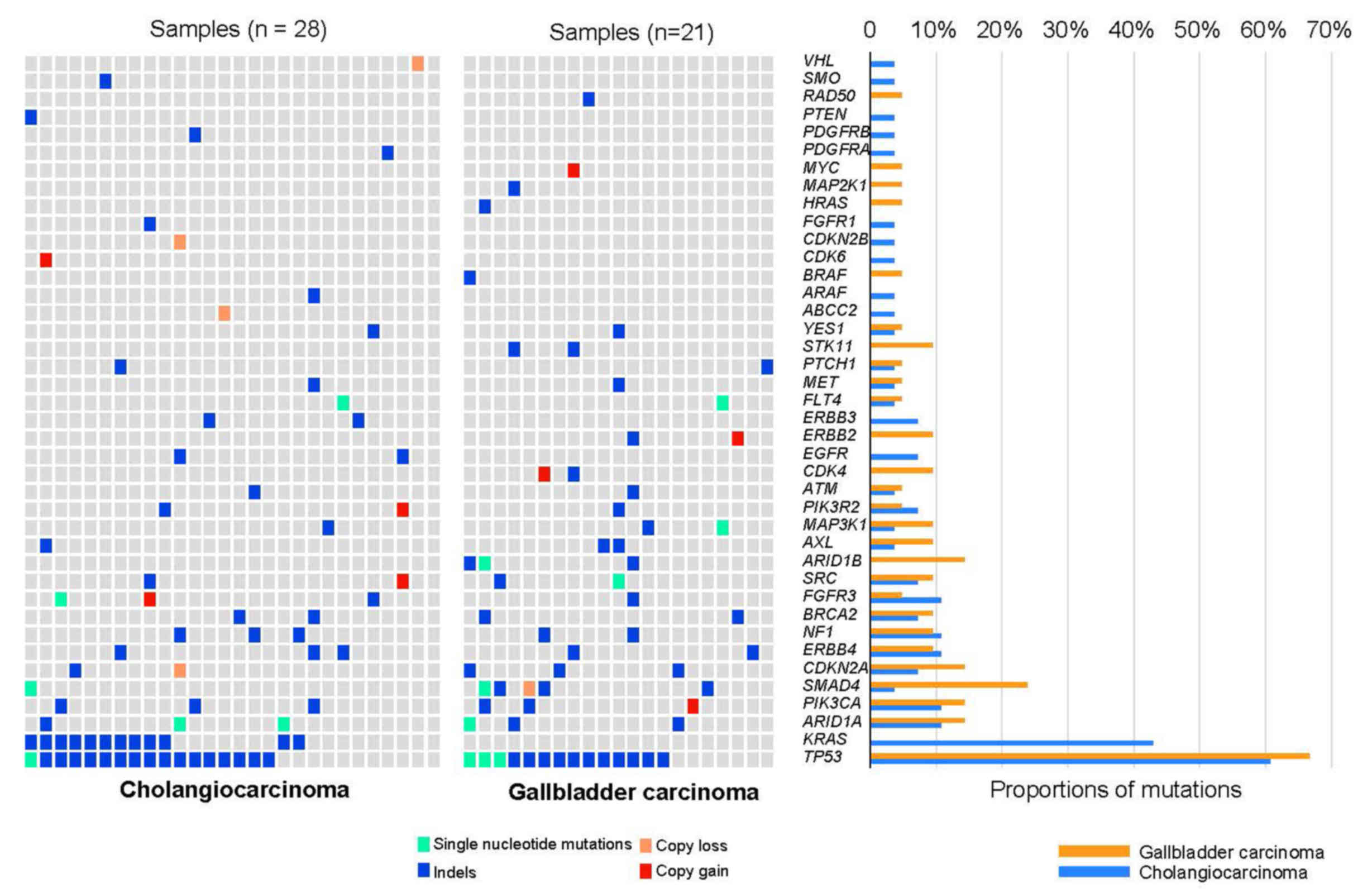 | Figure 1.Mutational landscape of biliary tract
cancers (BTCs). Mutational spectrum of the BTC patients as
determined by targeted deep sequencing (left and middle panels).
Overall, 28 cholangiocarcinomas and 21 gallbladder cancers were
included. The genetic variants landscape showed that TP53, KRAS,
ARID1A, and PIK3CA were frequently mutated. Mutation
subtypes (single nucleotide variant, indel, copy gain and loss) are
denoted by color. The right panel shows the frequency of recurrent
mutated genes. The histogram with different colors shows the
frequency of corresponding genes in cholangiocarcinoma or
gallbladder carcinoma, respectively. The colors indicating the
frequency of corresponding genes in cholangiocarcinoma and
gallbladder carcinoma are reversed in the right panel. TP53,
tumor protein P53; KRAS, KRAS proto-oncogene, GTPase;
ARID1A, AT-rich interaction domain 1A; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
α. |
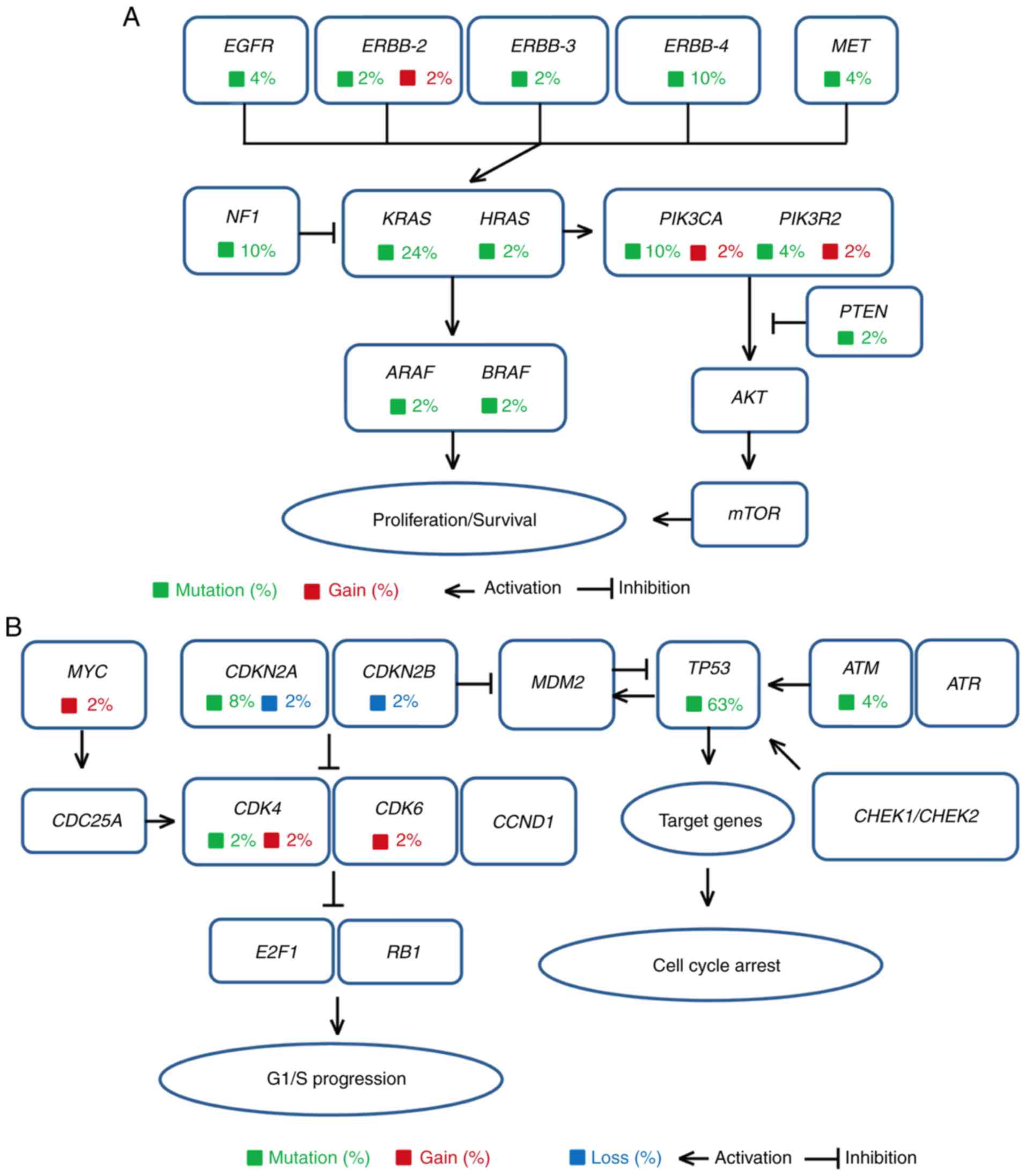 | Figure 2.Cellular signaling pathways
associated with the mutated genes of the biliary tract cancer (BTC)
cases. The mutated genes in 49 patients with BTCs were found to be
mainly distributed in the (A) ERBB family signaling pathway and (B)
cell cycle signaling pathway. Genes responsible for somatic cell
variants and the proportion of mutated genes in 49 patients are
indicated in the signal transduction pathway. Different types of
variants are marked with different colors, such as mutation
(green), gain (red), and loss (blue). EGFR, epidermal growth
factor receptor; ERBB2, Erb-B2 receptor tyrosine kinase;
MET, MET proto-oncogene, receptor tyrosine kinase;
NF1, neurofibromin 1; KRAS, KRAS proto-oncogene,
GTPase; HRAS, HRas proto-oncogene, GTPase; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α;
PIK3R2, phosphoinositide-3-kinase regulatory subunit 2;
ARAF, A-Raf proto-oncogene, serine/threonine kinase;
BRAF, B-Raf proto-oncogene, serine/threonine kinase;
PTEN, phosphatase and tensin homolog; AKT, protein
kinase B; mTOR, mechanistic target of rapamycin kinase;
MYC, MYC proto-oncogene, bHLH transcription factor;
CDKN2A, cyclin dependent kinase inhibitor 2A; CDKN2B,
cyclin dependent kinase inhibitor 2B; MDM2, MDM2
proto-oncogene; TP53, tumor protein P53; ATM, ATM
serine/threonine kinase; ATR, ATR serine/threonine kinase;
CDC25A, cell division cycle 25A; CDK4, cyclin
dependent kinase 4; CDK6, cyclin dependent kinase 6;
CCND1, cyclin D1; CHEK1, checkpoint kinase 1;
CHEK2, checkpoint kinase 2; E2F1, E2F transcription
factor 1; RB1, RB transcriptional corepressor 1. |
Personalized targeted therapy in
advanced BTC patients with R2 resection
Because tumor staging and resection margins can
significantly impact the prognosis of patients with BTCs (26,27),
only 32 patients who met the eligibility criteria of having stage
IV disease and R2 resection were enrolled in the treatment study
(Table II). The patient
characteristics, age, sex, cancer type, pTNM stage, and operative
morbidity, were similar between the targeted therapy and
conventional chemotherapy groups (Table II). In the targeted therapy group,
11 patients received corresponding targeted therapy as an
alternative to chemotherapy (Tables
III and IV). The mutated
genes, recommended drugs, drug administration (drug usage, dosage,
and cycle), operative morbidity, prognosis, and treatment-related
toxicities of the two groups are described in Tables III and IV. The targeted drugs lapatinib,
everolimus, dasatinib, imatinib, pazopanib, and regorafenib were
administered to 11 BTC patients in the targeted therapy group,
while mGEMOX was given to 21 patients in the chemotherapy group.
The administration of these targeted agents and chemotherapeutic
regimens was performed by the local multidisciplinary team in this
study.
According to the revised RECIST guideline (version
1.1), the targeted therapy group had partial response (PR) in 3
patients, stable disease (SD) in 4 patients, and progressive
disease (PD) in the remaining 4 patients, while the chemotherapy
group had 2 patients with PR, 5 with SD, and 14 with PD (Tables III and IV). The targeted therapy group had a
63.6% disease control rate (DCR), while the chemotherapy group had
a 33.3% DCR. Moreover, the targeted therapy group had a median PFS
of 4.5 months (2.5–20.5 months), and a median OS of 12.9 months
(4.7–24.8 months) (Fig. 3A and B),
while the chemotherapy group had a median PFS of 1.5 months
(0.5–11.6 months) and a median OS of 4.1 months (1.3–18.4 months)
(Fig. 3C and D). Subgroup analysis
of 13 patients from the chemotherapy group who received
recommendations of targeted therapy, showed that they had a median
PFS of 1.5 months (2.5–20.5 months), a median OS of 2.8 months
(4.7–24.8 months) and a DCR of 30.8% (Fig. S1 and Table IV).
Safety of personalized targeted
therapy
In the targeted therapy group, there were 4 patients
(BTC-026, BTC-034, BTC-040, and BTC-048) who experienced Grade 3
treatment-related toxicity, including hepatic damage,
thrombocytopenia, and fatigue (Table
III). The chemotherapy group had 3 patients (BTC-009, BTC-039,
and BTC-041) with Grade 3 treatment-related toxicities and 1
patient (BTC-044) with Grade 4 renal impairment (Table IV). In addition, 36.4% patients in
targeted therapy group experienced Grade >2 treatment-related
toxicity, while 19.0% patients in the conventional chemotherapy
group did.
Three BTC cases of personalized
targeted therapy
In this cohort, 11 patients received the
corresponding targeted therapies based on their genetic variants.
Positive clinical responses were observed in 3 patients and are
described herein. A 26-year-old female patient (BTC-048) was
diagnosed with Stage IVb Grade 2 gallbladder adenocarcinoma with
metastases to the peritoneum in December 2014 (Table III and Fig. S2A). Based on her genetic
alterations (Fig. 4A), a targeted
drug, regorafenib, targeting RET (160 mg/day) was recommended and
was administered on January 25, 2015. On April 21, 2015, the sum of
the diameters of all of the measurable lesions was decreased by
29.2% (SD) according to RECIST 1.1 (Fig. S2B). The patient's performance
status was found to have improved a month later, with a reported
increase in body weight and decrease in abdominal pain. The patient
continued to experience stable disease based on CT imaging
(Fig. S2C). However, the patient
ceased regorafenib treatment on July 18, 2017 due to Grade 3
fatigue and Grade 2 nausea, vomiting, diarrhea, and stomatitis. On
July 30, the CT images showed PD and the sum of the diameters had
increased to the pre-treatment RECIST measurements (Fig. S2D). For this reason, a PFS period
of 6 months was recorded for this patient from initiation of
treatment in January to disease progression in July (Fig. 4B).
There were also 2 patients with BTC, who received
personalized precise therapy with satisfying results. One patient
(BTC-010) with stage IVa intrahepatic cholangiocarcinoma who
underwent R2 resection, harbored an ERBB3 p.R1127H mutation and the
ERBB inhibitor lapatinib was administered (Table III). The PFS and OS of this
patient were 20.5 and 24.8 months, respectively. The other
intrahepatic cholangiocarcinoma patient (BTC-034), also with stage
IVa disease, carried a PDGFRA p.T1066I mutation and was treated
with imatinib as recommended (Table
III). The PFS and OS were 7.5 and 15.8 months respectively.
Discussion
In the present study, the landscape of genomic
variants in 49 Chinese biliary tract cancer (BTC) patients was
obtained. Several studies on whole-exome sequencing and whole
genome sequencing of BTCs have recently been published (3,12,28–30).
TP53 and KRAS were reported as the mostly frequently
mutated genes in previous studies (9,31), and
the majority of the variants are single nucleotide variants. These
findings are consistent with our results. However, we found a
higher frequency of CDKN2A loss in comparison to Western
cohorts (14). High BRCA and
IDH mutations were reported in cholangiocarcinoma of Western
populations (3–5,14),
while no such mutations were found in our study. These
aforementioned studies only described the genomic variant landscape
and the relationship between prognosis and genomic variants. The
use of this genomic profiling information to guide clinical
treatment has not been available to use (14,15).
Our study focused on advanced BTC patients with non-radical
resection, and we assessed the clinical efficacy and safety of
personalized targeted therapy guided by targeted deep sequencing in
these patients.
In recent years, biomarker-driven clinical trials
have been carried out in a wide variety of cancers. Targeted deep
sequencing that can achieve high sequencing depth is crucial to
accurately identify genomic variants in formalin-fixed
paraffin-embedded samples with low tumor cell content and high
heterogeneity (32–34), and has also been recognized as a
practical method for clinical genetic alteration detection in many
types of cancers (35–37). Nevertheless, no studies have been
reported on the application of genomic profiling information to
guide the precision treatment for a group of advanced BTC patients
with non-radical resection. Our study was designed to use targeted
deep sequencing for the detection of genetic mutations to guide
clinical decision-making in advanced BTC patients with non-radical
resection. The personalized targeted therapy group had a median PFS
of 4.5 months, a median OS of 12.9 months and a 63.6% DCR, while
the chemotherapy group had a median PFS of 1.5 months, a median OS
of 4.1 months, and a 33.3% DCR. These results may provide
preliminary evidence to support the development of a novel
treatment strategy of personalized targeted therapy for advanced
BTC patients with non-radical resection.
Gemcitabine plus cisplatin (GC) is the standard
treatment for advanced BTC for this decade, demonstrating a median
OS of gemcitabine regimen of 8.1 months and GC of 11.7 months,
respectively (38). The OS of GC
reported is longer than that explored in our study. However, there
are some differences between their research and ours. Regarding
group selection, we focused on the patients with R2 resection,
while they choose patients who did not receive surgery. The two
sets of patients are not comparable. The staging system is also
different. The 32 patients we used to analyze prognosis were all
stage IV patients (with metastatic tumors) in our study, while part
of their group was made up of patients with locally advanced cancer
but no metastatic tumors. The prognosis of these patients by stage
is quite different. Our study more closely reflects real-world
clinical practice for advanced BTC with non-radical resection, in
which the standardization of drug use and other factors are not as
strict as in clinical drug trials.
Treatment-related toxicity is a crucial factor that
influences clinical drug use and effects (39,40).
In this study, all of the patients in the targeted therapy group
experienced Grade 2 or 3 treatment-related toxicities, while five
patients in conventional chemotherapy group did. When Grade 2 and 3
toxicity occurred, the drugs could be continuously used by
adjusting the drug dosage and drug properties. In both groups, some
patients with Grade 2 or 3 toxicities continued taking medicine by
reducing the dosage or making other changes. Only one patient in
the chemotherapy group with Grade 4 renal impairment stopped taking
the drugs. Most patients were on medication regimens for only a
short time and the patients in the chemotherapy group did not
experience any treatment-related toxicity because of the rapid
progression of the disease, which does not mean that these
chemotherapeutic drugs had low toxicity. Overall, both targeted
therapy and chemotherapy were found to pose some risk of toxicity
for BTC patients in real-world clinical practice. The key is
finding a way to reduce treatment-related toxicity through drug
adjustment or other means so that BTC patients can continue and
complete their medication regimens.
In our cohort, 8 of 21 (38.1%) GBC patients had
mutations in the ERBB pathway. It has also been reported that
approximately 36.8% of GBC patients have aberrant ERBB signaling,
and multivariate analyses revealed that patients with ERBB pathway
mutations had worse outcomes (12).
However, there are no clinical studies that have explored if
interrogating ERBB signaling can improve the prognoses of such GBC
patients. In this study, 3 advanced GBC patients who received
therapy specifically targeting alterations in the ERBB pathway
achieved marginally longer PFS and OS (Fig. S3), in comparison to the 5 GBC
patients who underwent conventional chemotherapy. Despite the small
sample size of GBC patients treated with targeted therapy, the
preliminary results have shed light on precision therapy for GBC
patients with mutations in the ERBB signaling pathway.
Furthermore, we observed that most BTC patients who
progressed rapidly such that those in the chemotherapy group
experienced only 1–2 cycles of chemotherapy (Table IV). Some BTC patients experienced
treatment-related toxicity and had to stop taking chemotherapy
drugs. Overall, BTC patients with PR and SD underwent more cycles
than those with PD did. Here, BTC-045 was an outlier. BTC-045 had
PD and underwent six cycles of chemotherapy. To some extent,
chemotherapy cycles are related with disease progression in this
real-world clinical study.
The present research has several limitations. Some
recommended targeted drugs are difficult to obtain because some of
them have been approved by the United States Food and Drug
Administration (FDA) but not by the China Food and Drug
Administration (CFDA). In consideration of high medical costs, many
patients chose the cheaper option, even if another option was
favored that may have been more effective. Furthermore, genetic
testing did not show which mutations were related to the resistance
of chemotherapy in the present study. We do not have access to any
other information on BTC patients with targetable altered genes
resistant to chemotherapy. In addition, toxicity caused a dose
reduction in the targeted drugs, optimization of the medication
plan, or finally discontinuation in the medication in this study,
which affected the evaluation of the drug effect. Although this
real-world clinical study included the greatest number of patients
with R2 resection undergoing personalized precision therapy of any
such study, total sample size and the proportion of BTC patients
taking the medicine were relatively small. Large umbrella trials of
personalized precision therapy are needed to confirm our findings.
Despite various limitations, this study reflects real-world
clinical practice as it relates to personalized targeted therapy
guided by targeted deep sequencing in patients with advanced BTC
undergoing non-radical resection in China.
In conclusion, the results of this clinical study
suggest that targeted deep sequencing offers a promising method of
detecting actionable genetic alterations in BTC cases for precision
therapy. This study provides preliminary evidence that personalized
targeted therapy based on actionable genetic alterations may
benefit patients with advanced BTC undergoing non-radical
resection. Large umbrella trials covering personalized precision
therapy are needed to confirm the clinical efficacy and safety of
this therapeutic strategy for patients with advanced BTC undergoing
non-radical resection.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Yizhou Ye, Chao Sun,
Zishou Zhou, and Xinkai Cao for their assistance with data
collection and analysis for this study. In addition, the authors
would like to thank all of the patients and their families for
their support of this study.
Funding
This study is partially supported by the following
grants: The Science and Technology Commission of Shanghai
Municipality (no. 15411951900), the National Natural Science
Foundation of China (no. 81472280), and the Special Fund for the
Application and Transformation of Precision Medicine at the Second
Military Medical University (no. 2017JZ11).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
FF designed the targeted therapy study, contributed
to data interpretation and wrote the manuscript. QC designed the
targeted therapy study of gallbladder carcinoma patients and wrote
the manuscript; DZ designed the targeted deep sequencing study,
contributed to data interpretation and composed the manuscript. BL
performed the targeted therapy study of gallbladder carcinoma
patients and interpreted the data; HQ analyzed the data from
targeted deep sequencing and contributed to data interpretation. CX
collected tumor tissue from cholangiocarcinoma patients and
contributed to data interpretation; MH analyzed the clinical
information and interpreted the data. YY collected the tumor tissue
from gallbladder carcinoma patients and collected the clinical
information; ZL performed the extraction of genomic DNA from tumor
tissue samples and contributed to data interpretation. JYL
performed the quality control analyses on targeted deep sequencing
and interpreted the data; ZQ performed the targeted therapy study
of cholangiocarcinoma patients and interpreted the data. LX
discussed the hypothesis, interpreted data, and composed the
manuscript; CL performed the construction of sequencing library,
analyzed the clinical information of patients, and contributed to
data interpretation. FL performed the analyses on the SNV/Indel and
copy number variations of tumor tissue samples, contributed to data
interpretation, and composed the manuscript; BY led the project,
conceived the study, and composed the manuscript; XJ conceived the
study and participated in its design and coordination. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
This study protocol was approved by the
Institutional Review Board of Shanghai Eastern Hepatobiliary
Surgery Hospital, Navy Military Medical University (no.
EHBHKY2015-02-010). Written informed consent was obtained from all
of the BTC patients.
Patient consent for publication
Not applicable.
Competing interests
All of the authors affiliated with 3D Medicines Inc.
are current or former employees. The other authors have no
potential or actual conflicts of interest to disclose.
Glossary
Abbreviations
Abbreviations:
|
BTC
|
biliary tract cancer
|
|
NGS
|
next generation sequencing
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
DCR
|
disease control rate
|
|
GBC
|
gallbladder carcinoma
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
NSCLC
|
non-small cell lung cancer
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
References
|
1
|
Marcano-Bonilla L, Mohamed EA, Mounajjed T
and Roberts LR: Biliary tract cancers: Epidemiology, molecular
pathogenesis and genetic risk associations. Chin Clin Oncol.
5:612016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Streeter OE Jr, Beron PJ and Iyer PN:
Precision Medicine: Genomic profiles to individualize therapy.
Otolaryngol Clin North Am. 50:765–773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie D, Ren Z, Fan J and Gao Q: Genetic
profiling of intrahepatic cholangiocarcinoma and its clinical
implication in targeted therapy. Am J Cancer Res. 6:577–586.
2016.PubMed/NCBI
|
|
4
|
Wardell CP, Fujita M, Yamada T, Simbolo M,
Fassan M, Karlic R, Polak P, Kim J, Hatanaka Y, Maejima K, et al:
Genomic characterization of biliary tract cancers identifies driver
genes and predisposing mutations. J Hepatol. 68:959–969. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersen JB, Spee B, Blechacz BR, Avital
I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts
LR, et al: Genomic and genetic characterization of
cholangiocarcinoma identifies therapeutic targets for tyrosine
kinase inhibitors. Gastroenterology. 142:1021.e15–1031.e15. 2012.
View Article : Google Scholar
|
|
6
|
Javle M, Rashid A, Churi C, Kar S, Zuo M,
Eterovic AK, Nogueras-Gonzalez GM, Janku F, Shroff RT, Aloia TA, et
al: Molecular characterization of gallbladder cancer using somatic
mutation profiling. Hum Pathol. 45:701–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papadopoulou K, Murray S, Manousou K,
Tikas I, Dervenis C, Sgouros J, Rontogianni D, Lakis S, Bobos M,
Poulios C, et al: Genotyping and mRNA profiling reveal actionable
molecular targets in biliary tract cancers. Am J Cancer Res.
8:2–15. 2018.PubMed/NCBI
|
|
8
|
Nakamura H, Arai Y, Totoki Y, Shirota T,
Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, et
al: Genomic spectra of biliary tract cancer. Nat Genet.
47:1003–1010. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jain A, Kwong LN and Javle M: Genomic
profiling of biliary tract cancers and implications for clinical
practice. Curr Treat Options Oncol. 17:582016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Berezov A, Wang Q, Zhang G,
Drebin J, Murali R and Greene MI: ErbB receptors: From oncogenes to
targeted cancer therapies. J Clin Invest. 117:2051–2058. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tebbutt N, Pedersen MW and Johns TG:
Targeting the ERBB family in cancer: Couples therapy. Nat Rev
Cancer. 13:663–673. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawamoto T, Ishige K, Thomas M,
Yamashita-Kashima Y, Shu S, Ishikura N, Ariizumi S, Yamamoto M,
Kurosaki K and Shoda J: Overexpression and gene amplification of
EGFR, HER2, and HER3 in biliary tract carcinomas, and the
possibility for therapy with the HER2-targeting antibody
pertuzumab. J Gastroenterol. 50:467–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Javle M, Bekaii-Saab T, Jain A, Wang Y,
Kelley RK, Wang K, Kang HC, Catenacci D, Ali S, Krishnan S, et al:
Biliary cancer: Utility of next-generation sequencing for clinical
management. Cancer. 122:3838–3847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sundar R, Custodio A, Petruckevich A,
Chénard-Poirier M, Ameratunga M, Collins D, Lim J, Kaye SB, Tunariu
N, Banerji U, et al: Clinical outcome of patients with advanced
biliary tract cancer in a dedicated phase I unit. Clin Oncol (R
Coll Radiol). 30:185–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma A, Dwary AD, Mohanti BK, Deo SV,
Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P and
Chaudhary SP: Best supportive care compared with chemotherapy for
unresectable gall bladder cancer: A randomized controlled study. J
Clin Oncol. 28:4581–4586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Briasoulis E, Aravantinos G, Kouvatseas G,
Pappas P, Biziota E, Sainis I, Makatsoris T, Varthalitis I,
Xanthakis I, Vassias A, et al: Dose selection trial of metronomic
oral vinorelbine monotherapy in patients with metastatic cancer: A
hellenic cooperative oncology group clinical translational study.
BMC Cancer. 13:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su D, Zhang D, Chen K, Lu J, Wu J, Cao X,
Ying L, Jin Q, Ye Y, Xie Z, et al: High performance of targeted
next generation sequencing on variance detection in clinical tumor
specimens in comparison with current conventional methods. J Exp
Clin Cancer Res. 36:1212017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Jia P, Li F, Chen H, Ji H, Hucks
D, Dahlman KB, Pao W and Zhao Z: Detecting somatic point mutations
in cancer genome sequencing data: A comparison of mutation callers.
Genome Med. 5:912013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye K, Schulz MH, Long Q, Apweiler R and
Ning Z: Pindel: A pattern growth approach to detect break points of
large deletions and medium sized insertions from paired-end short
reads. Bioinformatics. 25:2865–2871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koboldt DC, Chen K, Wylie T, Larson DE,
McLellan MD, Mardis ER, Weinstock GM, Wilson RK and Ding L:
VarScan: Variant detection in massively parallel sequencing of
individual and pooled samples. Bioinformatics. 25:2283–2285. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casey BJ, Somerville LH, Gotlib IH, Ayduk
O, Franklin NT, Askrend MK, Jonides J, Berman MG, Wilson NL,
Teslovich T, et al: Behavioral and neural correlates of delay of
gratification 40 years later. Proc Natl Acad Sci USA.
108:14998–5003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skidmore ZL, Wagner AH, Lesurf R, Campbell
KM, Kunisaki J, Griffith OL and Griffith M: GenVisR: Genomic
visualizations in R. Bioinformatics. 32:3012–3014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung W, Jang JY, Kang MJ, Chang YR, Shin
YC, Chang J and Kim SW: Effects of surgical methods and tumor
location on survival and recurrence patterns after curative
resection in patients with T2 gallbladder cancer. Gut Liver.
10:140–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: Clinical and
prognostic significance of preoperative plasma hyperfibrinogenemia
in gallbladder cancer patients following surgical resection: A
retrospective and in vitro study. BMC Cancer. 14:5662014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersen JB and Thorgeirsson SS: Genetic
profiling of intrahepatic cholangiocarcinoma. Curr Opin
Gastroenterol. 28:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira DV, Zhang S, Chen X, Calvisi DF
and Andersen JB: Molecular profiling of intrahepatic
cholangiocarcinoma: The search for new therapeutic targets. Expert
Rev Gastroenterol Hepatol. 11:349–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chong DQ and Zhu AX: The landscape of
targeted therapies for cholangiocarcinoma: Current status and
emerging targets. Oncotarget. 7:46750–46767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee H and Ross JS: The potential role of
comprehensive genomic profiling to guide targeted therapy for
patients with biliary cancer. Therap Adv Gastroenterol. 10:507–520.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagle N, Berger MF, Davis MJ, Blumenstiel
B, Defelice M, Pochanard P, Ducar M, Van Hummelen P, Macconaill LE,
Hahn WC, et al: High-throughput detection of actionable genomic
alterations in clinical tumor samples by targeted, massively
parallel sequencing. Cancer Discov. 2:82–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klein CJ and Foroud TM: Neurology
individualized medicine: When to use next-generation sequencing
panels. Mayo Clin Proc. 92:292–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
LaDuca H, Farwell KD, Vuong H, Lu HM, Mu
W, Shahmirzadi L, Tang S, Chen J, Bhide S and Chao EC: Exome
sequencing covers >98% of mutations identified on targeted next
generation sequencing panels. PLoS One. 12:e01708432017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kou T, Kanai M, Yamamoto Y, Kamada M,
Nakatsui M, Sakuma T, Mochizuki H, Hiroshima A, Sugiyama A,
Nakamura E, et al: Clinical sequencing using a next-generation
sequencing-based multiplex gene assay in patients with advanced
solid tumors. Cancer Sci. 108:1440–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ge S, Li B, Li Y, Li Z, Liu Z, Chen Z, Wu
J, Gao J and Shen L: Genomic alterations in advanced gastric cancer
endoscopic biopsy samples using targeted next-generation
sequencing. Am J Cancer Res. 7:1540–1553. 2017.PubMed/NCBI
|
|
37
|
Au CH, Wa A, Ho DN, Chan TL and Ma ES:
Clinical evaluation of panel testing by next-generation sequencing
(NGS) for gene mutations in myeloid neoplasms. Diagn Pathol.
11:112016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perović Mihanović M, Haque NS, Rutherford
GW, Zekan Š and Begovac J: Toxicity-related antiretroviral drug
treatment modifications in individuals starting therapy: A cohort
analysis of time patterns, sex, and other risk factors. Med Sci
Monit. 19:483–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bar-Ad V, Ohri N and Werner-Wasik M:
Esophagitis, treatment-related toxicity in non-small cell lung
cancer. Rev Recent Clin Trials. 7:31–35. 2012. View Article : Google Scholar : PubMed/NCBI
|


















