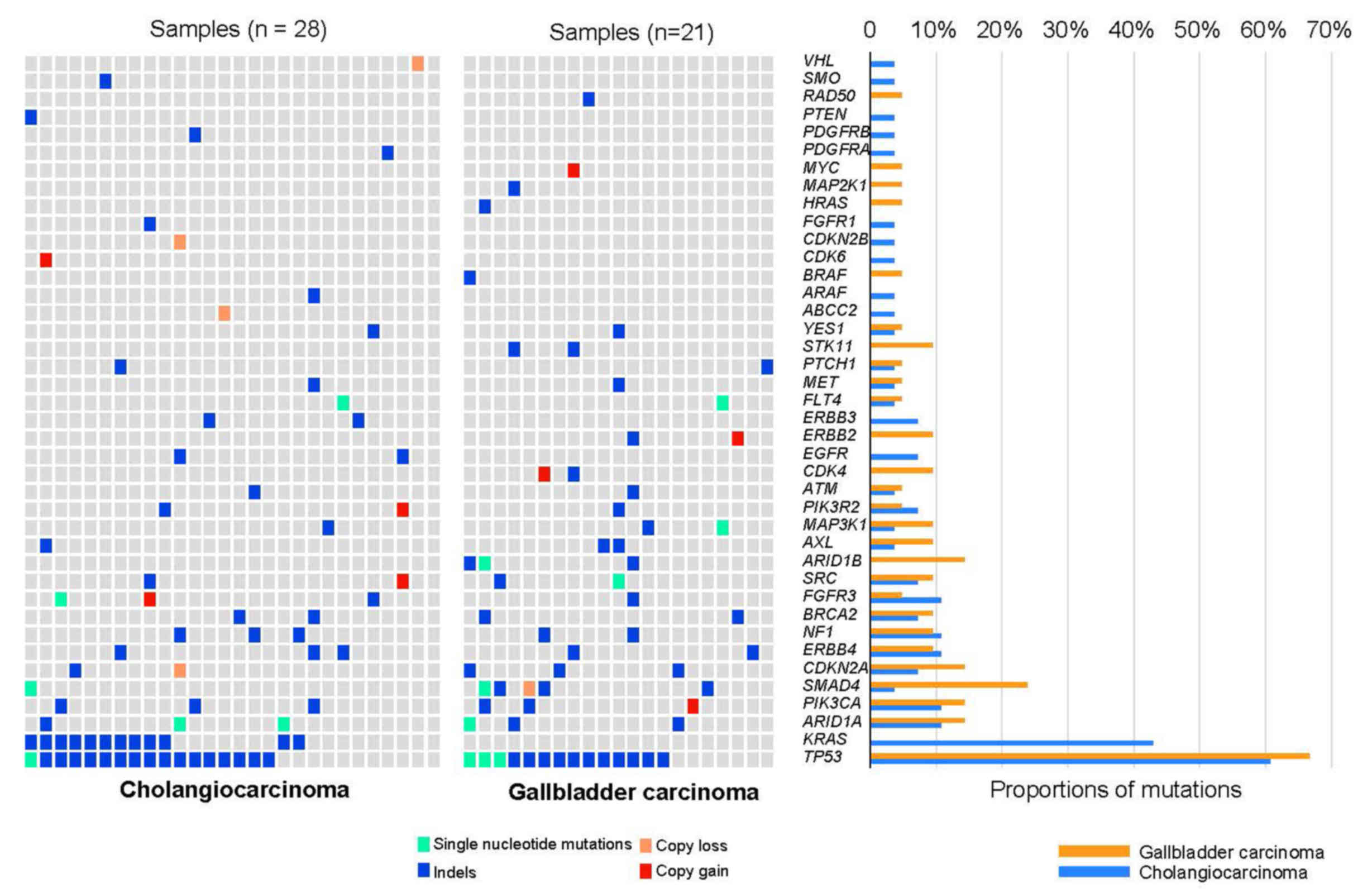
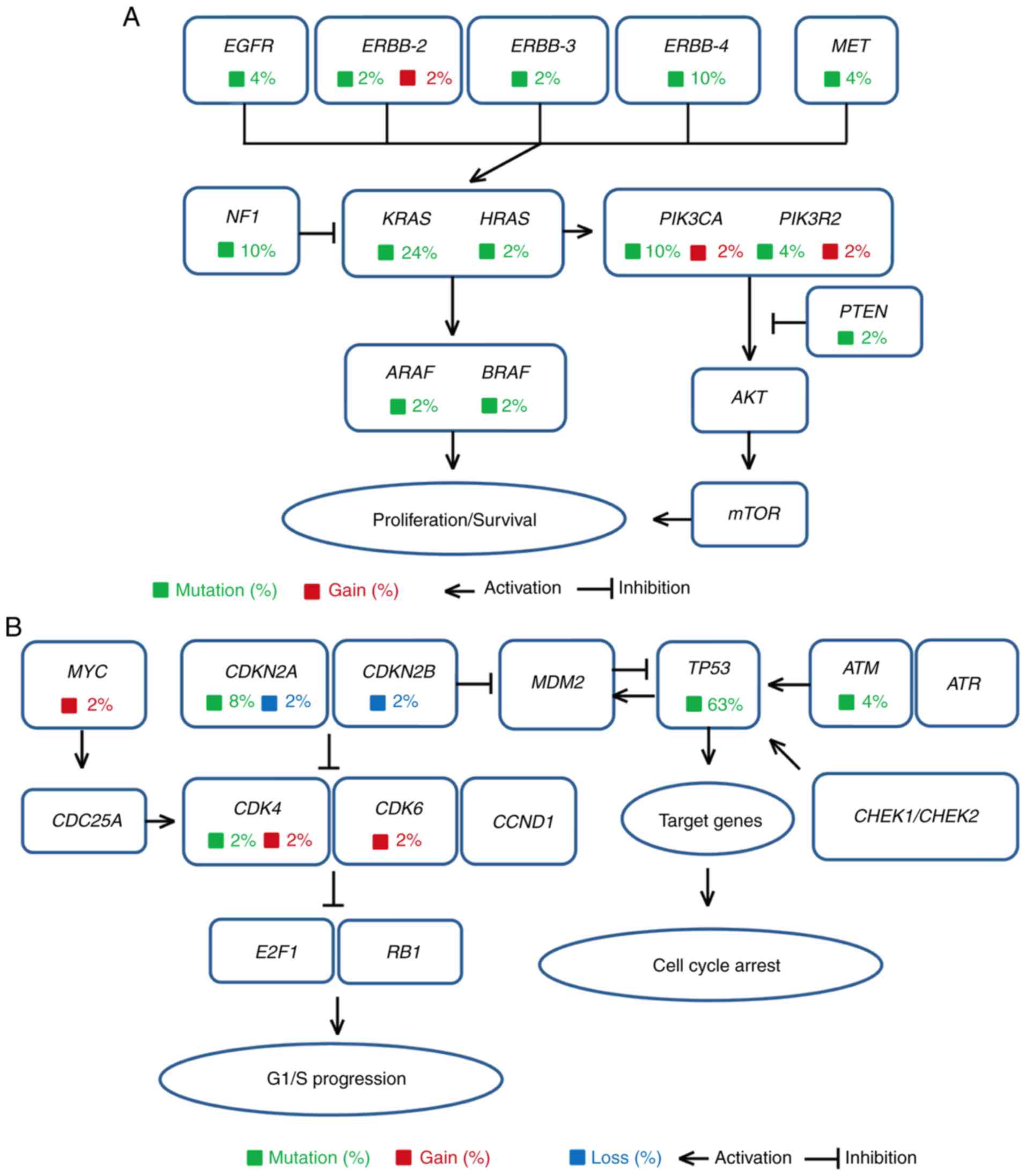
|
1
|
Marcano-Bonilla L, Mohamed EA, Mounajjed T
and Roberts LR: Biliary tract cancers: Epidemiology, molecular
pathogenesis and genetic risk associations. Chin Clin Oncol.
5:612016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Streeter OE Jr, Beron PJ and Iyer PN:
Precision Medicine: Genomic profiles to individualize therapy.
Otolaryngol Clin North Am. 50:765–773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie D, Ren Z, Fan J and Gao Q: Genetic
profiling of intrahepatic cholangiocarcinoma and its clinical
implication in targeted therapy. Am J Cancer Res. 6:577–586.
2016.PubMed/NCBI
|
|
4
|
Wardell CP, Fujita M, Yamada T, Simbolo M,
Fassan M, Karlic R, Polak P, Kim J, Hatanaka Y, Maejima K, et al:
Genomic characterization of biliary tract cancers identifies driver
genes and predisposing mutations. J Hepatol. 68:959–969. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersen JB, Spee B, Blechacz BR, Avital
I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts
LR, et al: Genomic and genetic characterization of
cholangiocarcinoma identifies therapeutic targets for tyrosine
kinase inhibitors. Gastroenterology. 142:1021.e15–1031.e15. 2012.
View Article : Google Scholar
|
|
6
|
Javle M, Rashid A, Churi C, Kar S, Zuo M,
Eterovic AK, Nogueras-Gonzalez GM, Janku F, Shroff RT, Aloia TA, et
al: Molecular characterization of gallbladder cancer using somatic
mutation profiling. Hum Pathol. 45:701–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papadopoulou K, Murray S, Manousou K,
Tikas I, Dervenis C, Sgouros J, Rontogianni D, Lakis S, Bobos M,
Poulios C, et al: Genotyping and mRNA profiling reveal actionable
molecular targets in biliary tract cancers. Am J Cancer Res.
8:2–15. 2018.PubMed/NCBI
|
|
8
|
Nakamura H, Arai Y, Totoki Y, Shirota T,
Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, et
al: Genomic spectra of biliary tract cancer. Nat Genet.
47:1003–1010. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jain A, Kwong LN and Javle M: Genomic
profiling of biliary tract cancers and implications for clinical
practice. Curr Treat Options Oncol. 17:582016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Berezov A, Wang Q, Zhang G,
Drebin J, Murali R and Greene MI: ErbB receptors: From oncogenes to
targeted cancer therapies. J Clin Invest. 117:2051–2058. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tebbutt N, Pedersen MW and Johns TG:
Targeting the ERBB family in cancer: Couples therapy. Nat Rev
Cancer. 13:663–673. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawamoto T, Ishige K, Thomas M,
Yamashita-Kashima Y, Shu S, Ishikura N, Ariizumi S, Yamamoto M,
Kurosaki K and Shoda J: Overexpression and gene amplification of
EGFR, HER2, and HER3 in biliary tract carcinomas, and the
possibility for therapy with the HER2-targeting antibody
pertuzumab. J Gastroenterol. 50:467–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Javle M, Bekaii-Saab T, Jain A, Wang Y,
Kelley RK, Wang K, Kang HC, Catenacci D, Ali S, Krishnan S, et al:
Biliary cancer: Utility of next-generation sequencing for clinical
management. Cancer. 122:3838–3847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sundar R, Custodio A, Petruckevich A,
Chénard-Poirier M, Ameratunga M, Collins D, Lim J, Kaye SB, Tunariu
N, Banerji U, et al: Clinical outcome of patients with advanced
biliary tract cancer in a dedicated phase I unit. Clin Oncol (R
Coll Radiol). 30:185–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma A, Dwary AD, Mohanti BK, Deo SV,
Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P and
Chaudhary SP: Best supportive care compared with chemotherapy for
unresectable gall bladder cancer: A randomized controlled study. J
Clin Oncol. 28:4581–4586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Briasoulis E, Aravantinos G, Kouvatseas G,
Pappas P, Biziota E, Sainis I, Makatsoris T, Varthalitis I,
Xanthakis I, Vassias A, et al: Dose selection trial of metronomic
oral vinorelbine monotherapy in patients with metastatic cancer: A
hellenic cooperative oncology group clinical translational study.
BMC Cancer. 13:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su D, Zhang D, Chen K, Lu J, Wu J, Cao X,
Ying L, Jin Q, Ye Y, Xie Z, et al: High performance of targeted
next generation sequencing on variance detection in clinical tumor
specimens in comparison with current conventional methods. J Exp
Clin Cancer Res. 36:1212017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Jia P, Li F, Chen H, Ji H, Hucks
D, Dahlman KB, Pao W and Zhao Z: Detecting somatic point mutations
in cancer genome sequencing data: A comparison of mutation callers.
Genome Med. 5:912013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye K, Schulz MH, Long Q, Apweiler R and
Ning Z: Pindel: A pattern growth approach to detect break points of
large deletions and medium sized insertions from paired-end short
reads. Bioinformatics. 25:2865–2871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koboldt DC, Chen K, Wylie T, Larson DE,
McLellan MD, Mardis ER, Weinstock GM, Wilson RK and Ding L:
VarScan: Variant detection in massively parallel sequencing of
individual and pooled samples. Bioinformatics. 25:2283–2285. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casey BJ, Somerville LH, Gotlib IH, Ayduk
O, Franklin NT, Askrend MK, Jonides J, Berman MG, Wilson NL,
Teslovich T, et al: Behavioral and neural correlates of delay of
gratification 40 years later. Proc Natl Acad Sci USA.
108:14998–5003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skidmore ZL, Wagner AH, Lesurf R, Campbell
KM, Kunisaki J, Griffith OL and Griffith M: GenVisR: Genomic
visualizations in R. Bioinformatics. 32:3012–3014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung W, Jang JY, Kang MJ, Chang YR, Shin
YC, Chang J and Kim SW: Effects of surgical methods and tumor
location on survival and recurrence patterns after curative
resection in patients with T2 gallbladder cancer. Gut Liver.
10:140–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: Clinical and
prognostic significance of preoperative plasma hyperfibrinogenemia
in gallbladder cancer patients following surgical resection: A
retrospective and in vitro study. BMC Cancer. 14:5662014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersen JB and Thorgeirsson SS: Genetic
profiling of intrahepatic cholangiocarcinoma. Curr Opin
Gastroenterol. 28:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira DV, Zhang S, Chen X, Calvisi DF
and Andersen JB: Molecular profiling of intrahepatic
cholangiocarcinoma: The search for new therapeutic targets. Expert
Rev Gastroenterol Hepatol. 11:349–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chong DQ and Zhu AX: The landscape of
targeted therapies for cholangiocarcinoma: Current status and
emerging targets. Oncotarget. 7:46750–46767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee H and Ross JS: The potential role of
comprehensive genomic profiling to guide targeted therapy for
patients with biliary cancer. Therap Adv Gastroenterol. 10:507–520.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagle N, Berger MF, Davis MJ, Blumenstiel
B, Defelice M, Pochanard P, Ducar M, Van Hummelen P, Macconaill LE,
Hahn WC, et al: High-throughput detection of actionable genomic
alterations in clinical tumor samples by targeted, massively
parallel sequencing. Cancer Discov. 2:82–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klein CJ and Foroud TM: Neurology
individualized medicine: When to use next-generation sequencing
panels. Mayo Clin Proc. 92:292–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
LaDuca H, Farwell KD, Vuong H, Lu HM, Mu
W, Shahmirzadi L, Tang S, Chen J, Bhide S and Chao EC: Exome
sequencing covers >98% of mutations identified on targeted next
generation sequencing panels. PLoS One. 12:e01708432017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kou T, Kanai M, Yamamoto Y, Kamada M,
Nakatsui M, Sakuma T, Mochizuki H, Hiroshima A, Sugiyama A,
Nakamura E, et al: Clinical sequencing using a next-generation
sequencing-based multiplex gene assay in patients with advanced
solid tumors. Cancer Sci. 108:1440–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ge S, Li B, Li Y, Li Z, Liu Z, Chen Z, Wu
J, Gao J and Shen L: Genomic alterations in advanced gastric cancer
endoscopic biopsy samples using targeted next-generation
sequencing. Am J Cancer Res. 7:1540–1553. 2017.PubMed/NCBI
|
|
37
|
Au CH, Wa A, Ho DN, Chan TL and Ma ES:
Clinical evaluation of panel testing by next-generation sequencing
(NGS) for gene mutations in myeloid neoplasms. Diagn Pathol.
11:112016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perović Mihanović M, Haque NS, Rutherford
GW, Zekan Š and Begovac J: Toxicity-related antiretroviral drug
treatment modifications in individuals starting therapy: A cohort
analysis of time patterns, sex, and other risk factors. Med Sci
Monit. 19:483–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bar-Ad V, Ohri N and Werner-Wasik M:
Esophagitis, treatment-related toxicity in non-small cell lung
cancer. Rev Recent Clin Trials. 7:31–35. 2012. View Article : Google Scholar : PubMed/NCBI
|