Introduction
Liver cancer is one of the most common malignancies
and is the fourth most common cause of cancer-related death
worldwide (1). In 2018, there were
estimated to be 840,000 new cases and 780,000 deaths from liver
cancer worldwide. Hepatocellular carcinoma (HCC),
cholangiocarcinoma and hepatoblastoma (HB) are the main types of
liver cancer. Among them, HB is the most common liver tumor in
children <4 years old; it has a 5-year survival rate of 60–87.7%
(2–4). Generally, liver transplantation,
chemotherapy and surgical resection are the preferred treatment
strategies for patients with HB (5,6).
Although the prognosis of HB has markedly improved in recent
decades, the prognosis remains poor for patients with advanced or
chemotherapy refractory disease (7).
According to several international guidelines,
radiofrequency ablation (RFA) has recently been suggested as a
first-line therapeutic strategy for adult patients with early-stage
liver cancer (8–11). Studies have reported that patients
with liver cancer treated with RFA have a 5-year survival rate of
66–86% (12–14). However, the incidence of recurrence
has been reported to be markedly higher in response to RFA compared
with hepatic resection, and the recurrence rate may be as high as
60–80% within 5 years of RFA (15–17).
Among patients who have undergone hepatic resection, tumor
recurrence after RFA is an important factor affecting patient
prognosis (18,19). Although several studies have
reported the successful RFA treatment of children with liver cancer
(4,20), there remains little focus on RFA for
the treatment of HB.
Long non-coding RNAs (lncRNAs) are arbitrarily
considered to be >200 nucleotides in length and are not
translated into proteins (21–23).
In the past, lncRNAs were considered transcriptional regulation
‘noise’. However, with the development of techniques for
high-throughput sequencing, a large number of newly discovered
dysregulated lncRNAs have been reported to serve an important role
in tumor growth, invasion and metastasis (24–27).
For example, Dong et al reported that upregulated
lncRNA-taurin upregulated 1 (TUG1) serves an important role in
regulating HB cell function, tumor progression and tumor
angiogenesis (28). However, the
biological role of lncRNAs in HB tissues remains widely unknown,
particularly in residual HB tissues after incomplete RFA
treatment.
The connectivity map (CMap) is a wide-ranging drug
perturbation database that contains specific gene expression
profiles from cultured human cells grown with small bioactive
molecules. This database enables the identification of drugs that
affect the same molecular pathway as identified differentially
expressed genes (29–31). Compounds with a highly positive
connectivity score (i.e., ‘score’, 1) may have a highly positive
connection with the same molecular pathways associated with the
differentially expressed genes. Furthermore, a highly negative
connectivity score (i.e., ‘score’, −1) indicates a more negative
connection between the compounds and differentially expressed
genes.
The aim of this study was to analyze the gene
expression profiles of residual HB tissues after incomplete RFA
treatment using microarray data combined with the CMap database, in
order to improve understanding of the processes underlying rapid
proliferation of residual HB tissues and to identify potential
therapeutic targets. A flow chart summarizing the present work is
presented in Fig. 1.
Materials and methods
Instruments and reagents
The Cool-tip™ RFA system was purchased from
Medtronic. NanoDrop ND-1000 was obtained from NanoDrop Technologies
(Thermo Fisher Scientific, Inc.). The Gene Expression Hybridization
kit (cat. no. 5188-5242) was purchased from Agilent Technologies,
Inc. TRIzol® reagent was obtained from Invitrogen
(Thermo Fisher Scientific, Inc.).
Cells
Human HepG2 cells were purchased from Cell Bank of
the Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences. Cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in
atmosphere containing 5% CO2.
Animals
Male athymic BALB/c nude mice (age, 6 weeks; weight,
8–22 g) were obtained from Shanghai SLAC Laboratory Animal Co.,
Ltd. [production permit no. SCXK(Hu)2007-00058]; mice were housed
in specific pathogen-free conditions and no infectious diseases
were detected in the animals. The animals were housed in class
10,000 clean rooms, at room temperature (20–25°C), with 30–70%
humidity and a 12-h artificial light/dark cycle. All experimental
protocols were approved by the Animal Care and Experiment Committee
of Guangxi Medical University (Nanning, China).
Nude mouse subcutaneous xenograft
model
A total of eight immunodeficient nude mice were
subcutaneously transplanted with 100 µl HepG2 tumor cells
(2×106 cells/ml). After 4 weeks, the tumors had an
average diameter of 0.8–1.0 cm and the mice were randomly divided
into two groups for further experimentation. One group of nude mice
(n=4) underwent incomplete ablation via the ultrasound-guided
Cool-tip™ RFA system, after being anesthetized with 1.5%
pentobarbital sodium (60 mg/kg body weight; intraperitoneal
injection). Referring to previous reports, the parameters of
construction of the incomplete ablation model were as follows:
Radiofrequency power was set at 30 W and the temperature was
70±5°C; after continuous ablation for 10 sec, the mice were
euthanized for further analysis (32,33).
Mice in the control group (n=4) did not receive any treatment.
After euthanasia, tumors were immediately removed within 10 min,
images were captured and the tumor samples were divided equally
into two parts (>100 mg). One part was used for pathological
experiments, and the other was stored at −80°C or in liquid
nitrogen after washing with PBS before being used for array
hybridization.
Hematoxylin and eosin (H&E)
staining
Tissues were fixed in 4% paraformaldehyde (Sinopharm
Chemical Reagent Co., Ltd.) at 20°C for >72 h. Subsequently, the
tissues were embedded in paraffin, sliced into 5-µm sections and
underwent H&E staining according to a previously published
protocol (34). A light microscope
(Olympus Corporation) was used to capture the images.
RNA extraction and microarray
hybridization
Total RNA was extracted from each sample using
TRIzol® reagent, according to the manufacturer's
instructions. The quality of RNA was evaluated using a NanoDrop
ND-1000. Subsequently, RNA integrity was assessed using standard
denaturing agarose gel electrophoresis (0.8–2%). Sample labeling
and array hybridization were performed according to the Agilent
One-Color Microarray-Based Gene Expression Analysis protocol
(Agilent Technologies, Inc.). Firstly, the purified mRNA was
obtained by removing ribosomal RNA from total RNA using the
mRNA-ONLY™ Eukaryotic mRNA Isolation kit (Epicentre; Illumina,
Inc.), according to the manufacturer's protocol. Secondly, the
Arraystar Flash RNA Labeling kit (Arraystar, Inc.) was used to
amplify the mRNA and transcribe it into fluorescent cRNA, according
to the manufacturer's protocol. Subsequently, labeled cRNAs were
purified using the RNeasy Mini kit (Qiagen, Inc.), and their
consistency and specific activity were calculated using a NanoDrop
ND-1000, according to the manufacturer's protocol. The instructions
of the Agilent Gene Expression Hybridization kit (cat. no.
5188-5242; Agilent Technologies, Inc.) were followed to perform the
chip hybridization. Finally, the Agilent G2505C DNA Microarray
Scanner (Agilent Technologies, Inc.) was used to wash, fix and scan
the hybridized arrays.
Microarray data analysis
Agilent Feature Extraction software (version
11.0.1.1; Agilent Technologies, Inc.) was applied to extract data
for further analysis. Subsequently, the raw data were normalized
with log2 transformations (GeneSpring GX v12.1; Agilent
Technologies, Inc.). Fold changes >2 and a P<0.05 were
considered statistically significant differences in the expression
of lncRNA and mRNA.
Co-expression network
construction
To further understand the possible role of lncRNAs
and mRNAs in the incompletely ablated HB tissue, a lncRNA-mRNA
co-expression network was constructed according to the correlation
coefficient of the differential expression data. The Pearson
conjunction coefficient was used to evaluate the correlation of the
differentially expressed genes. Genes with an absolute value of
correlation coefficient ≥0.99 and P<0.0001 were selected, and
the lncRNA-mRNA co-expression network was drawn using Cytoscape
3.7.2 (https://cytoscape.org/) (35).
Standard gene set enrichment analysis
(GSEA)
GSEA 3.0 software (http://www.broad.mit.edu/gsea) was used to further
analyze the biological signatures of the gene set in the
incompletely ablated HB tissue (36,37).
The sets, including hallmark gene sets, chemical and genetic
perturbations, canonical pathways, cancer modules, Gene Ontology
(GO) gene sets, oncogenic signatures and immunologic signatures
were collected from the Molecular Signatures Database. The
normalized enrichment score (NES) was used to quantify the
magnitude of enrichment. Using 1,000 permutation test runs, the
cut-off criteria were false discovery rate q-value ≤0.01 and
nominal P≤0.001.
Functional annotation analysis and
pathway enrichment analysis of the differentially expressed
genes
In addition, bioinformatics analysis, including
Kyoto Encyclopedia of Genes and Genomes (KEGG; www.kegg.jp) pathway analysis and GO analysis, was
performed to explore the roles of the mRNAs differentially
expressed in residual tissue after RFA treatment compared with
untreated HB tissue. The GO project (http://www.geneontology.org) provides the properties
of genes and/or gene products through three domains: Biological
process (BP), cellular component (CC) and molecular function (MF).
R package version 2.8.0 (‘GOstats.’ and ‘GeneAnswers’ packages;
http://www.r-project.org/) is a free
script that synthesizes GO terms and comprehensively visualizes the
results. KEGG is an online database of genomes, enzymatic pathways,
biochemical reactions, diseases and drugs. The pathway database was
used to perform pathway analysis of differentially expressed mRNAs
to infer their molecular functions.
Protein-protein interaction (PPI)
networks and hub genes
The Search Tool for the Retrieval of Interacting
Genes database (http://string-db.org/) was used to
search for affiliations among proteins encoded by the genes that
were differentially expressed in residual HB tissues after
incomplete RFA treatment. The database aims to integrate large
amounts of data on protein-protein associations (38). The minimum required interaction
score for a connection node to be considered was 0.9. Any gene
encoding proteins with >10 connection nodes was regarded as a
hub gene.
CMap analysis
To identify drugs that may affect the same molecular
pathways that were linked to the differentially expressed genes
between the residual HB tissues after incomplete RFA treatment and
untreated HB tissues, the observed differentially expressed genes
and hub genes were compared with the gene expression levels in the
CMap database (http://portals.broadinstitute.org/cmap/). Prior to
conducting the CMap search, the probe IDs of the genes were
converted into the Human Genome HT U133A Array format using the
Batch Query function on the Affymetrix GeneChip website (https://www.affymetrix.com/site/mainPage.affx), and
these data were then inputted into the CMap database. A compound
with a connectivity score ≤-0.75, which indicated a highly negative
association with the expressed genes in the database, was
considered to be a potential drug for RFA-treated liver cancer.
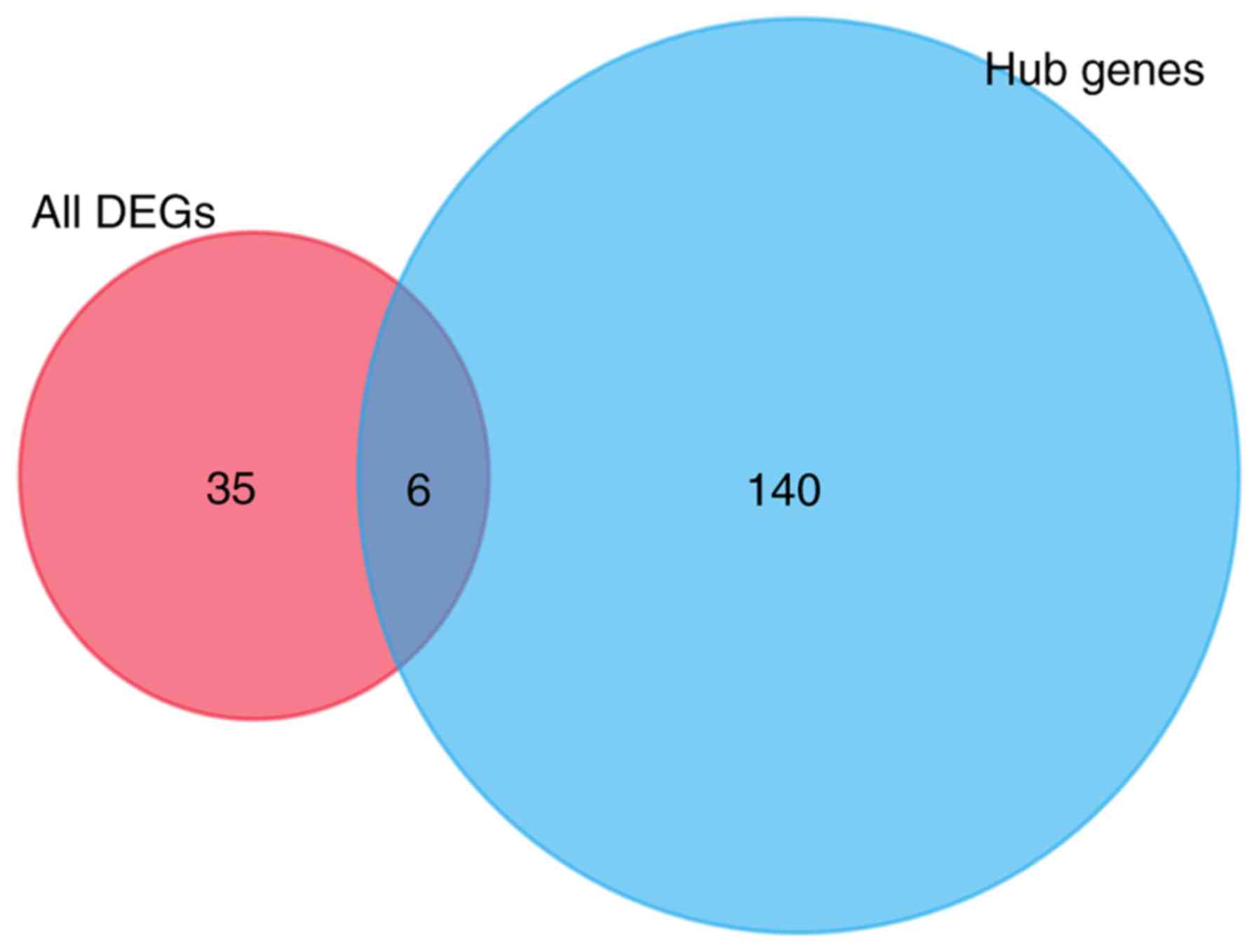
Only the compounds present in both comparison results of DEGs and
hub genes were selected for further research.
Prediction of interactions between
chemicals and proteins
The Search Tool for the Interacting Chemicals
database (STITCH; http://stitch.embl.de) was used to identify
interactions between proteins and the aforementioned compounds,
which may help to identify a novel therapy for HB following
incomplete RFA (39). In the fifth
version of STITCH, the database includes >9,600,000 proteins and
430,000 compounds. The information and SMILES networks of compounds
required for the STITCH interaction analysis were collected from
the PubChem Compounds website (https://pubchem.ncbi.nlm.nih.gov/) for further
analysis. Compounds with clinical application were selected as
candidate drugs for further research.
Molecular docking
Docking was carried out using SYBYL-X 2.0 software
(Certara, Princeton, NJ, USA) to discover novel ligands between the
aforementioned candidate drugs and proteins. The crystal structures
of proteins were obtained from the Research Collaboratory for
Structural Bioinformatics Protein Data Bank (RCSB PDB) (http://www.rcsb.org/) (40). A consensus score (CScore) ≥4 was
used to represent high binding affinities between the proteins and
novel drugs (41,42).
Results
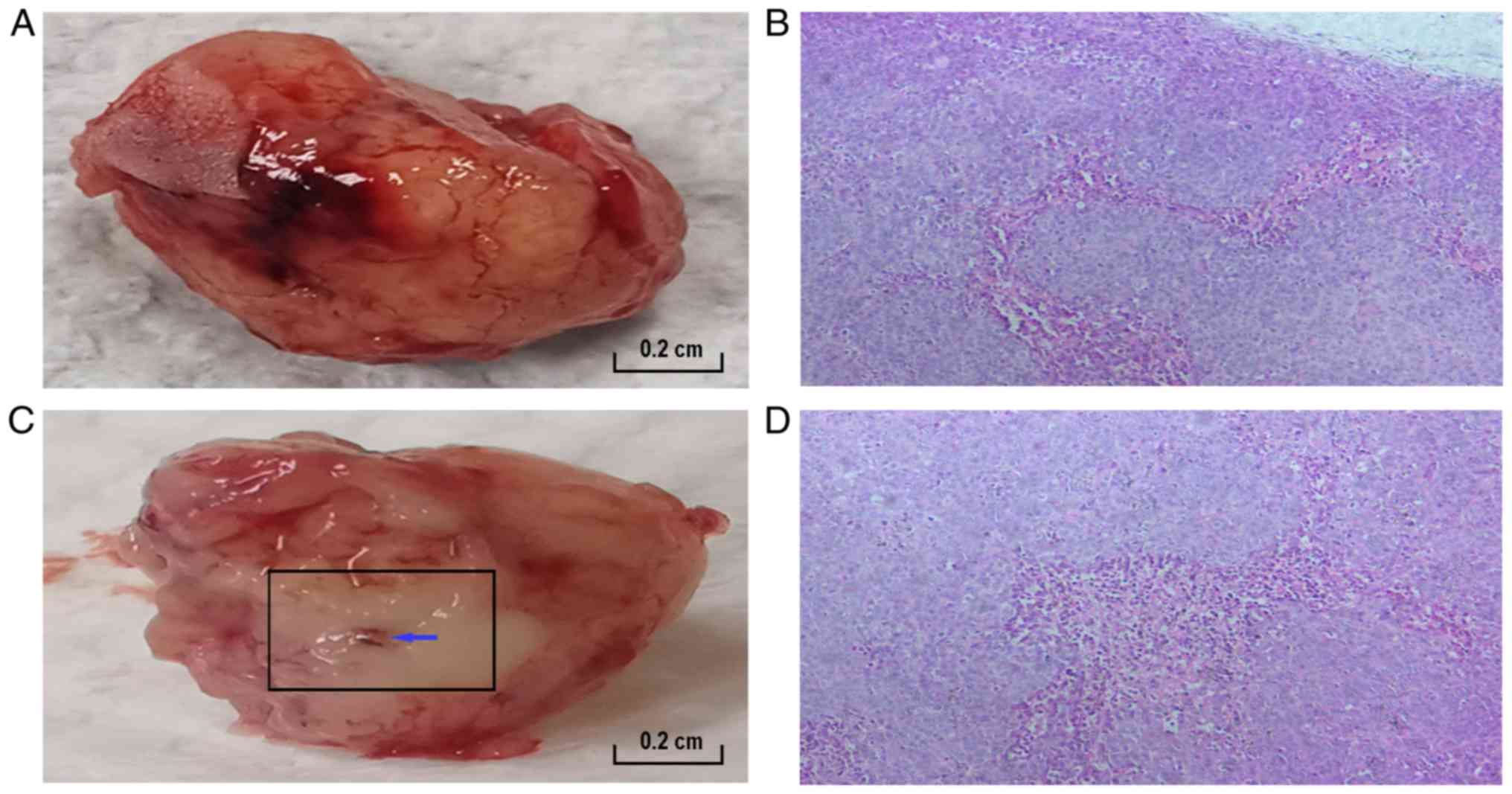
Pathological examination of the tumor
tissue after RFA
In the untreated group, the tumor was red to the
naked eye (Fig. 2A), whereas in the
RFA-treated group, the tumor exhibited a white burning appearance
(Fig. 2C). Subsequently, the two
groups underwent pathological examination using H&E staining
(Fig. 2B and D). A small number of
fragmented dark blue nuclear structures were detected at the
junction of the necrotic zone and the marginal zone in the
RFA-treated group (magnification, ×200). Light pink/dark red
staining indicates vascular tissue (magnification, ×200).
Expression profile of lncRNAs and
mRNAs in incomplete RFA residual tissues of a subcutaneous HepG2
cell-based tumor transplantation nude mouse model
To identify lncRNAs and mRNAs, the expression of
which was modified by RFA treatment of HB, a subcutaneous tumor
transplantation model of nude mice was generated using HepG2 cells;
one group was treated with incomplete RFA and the other received no
treatment.
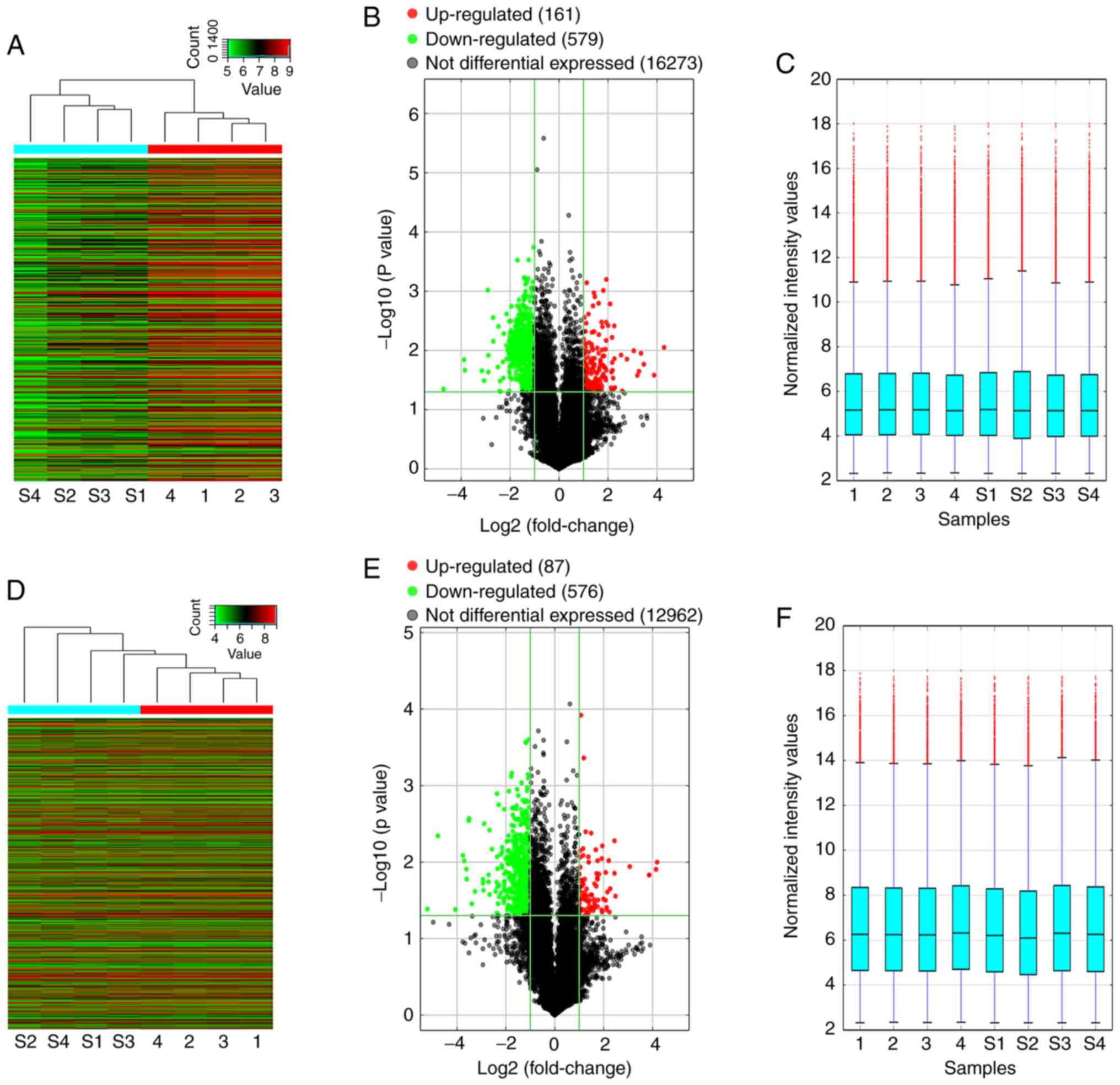
Fold-changes (incomplete RFA-treated nude mice vs.
untreated nude mice subcutaneously transplanted with HepG2 cells)
and P-values were calculated after normalizing the in vivo
expression data (Fig. 3A and D).
Using the GeneSpring GX v12.1 software package, the median
expression levels of the lncRNAs and mRNAs in the experimental and
control groups were ~5.2 and 6.2, respectively (Fig. 3C and F). Overall, 740 lncRNAs and
663 mRNAs were significantly differentially expressed in the four
incomplete RFA-treated samples compared with the untreated samples
(fold change ≥2.0, P<0.05; Fig. 3B
and E). Among these, 161 lncRNAs and 87 mRNAs were
significantly enriched in the experimental group, whereas 579
lncRNAs and 576 mRNAs had significantly lower expression in the
experimental group. The expression of lncRNA RP11-150O12.5 (fold
change, 19.60; P=0.044) was significantly upregulated, but the
expression of AK094929 (fold change, 26.38; P=0.008) was
significantly downregulated. These differentially expressed lncRNAs
may act as the chief regulators in the recurrence and progression
of HB after incomplete RFA treatment.
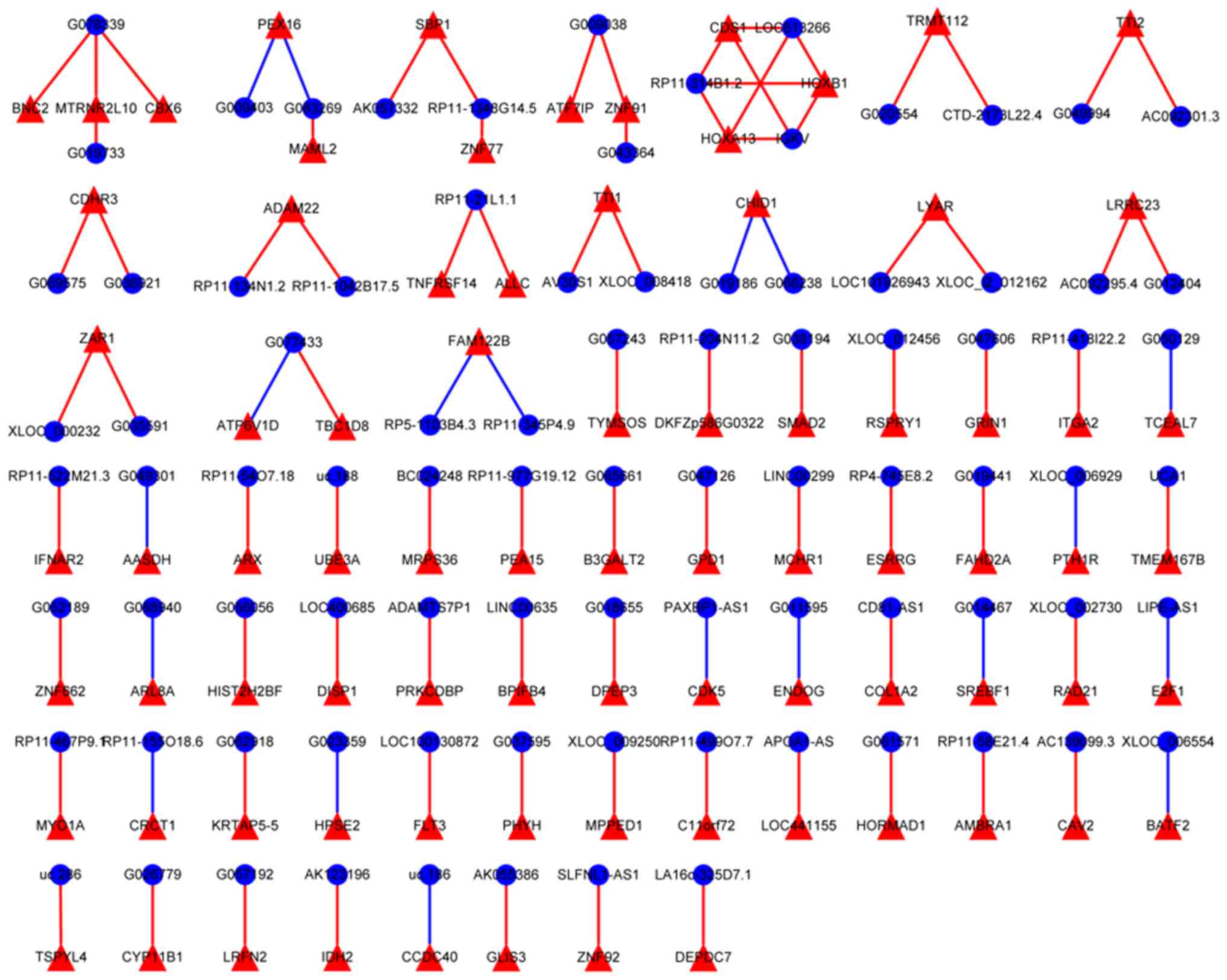
Construction of the lncRNA-mRNA
co-expression network
A co-expression network was constructed to more
comprehensively understand the relationship between the lncRNA and
mRNA expression levels in the incompletely ablated HB tissue
(Fig. 4). Overall, 1,201 pairs of
co-expression correlations between lncRNAs and mRNAs were
collected; these pairs included 387 mRNAs (43 upregulated and 344
downregulated) and 468 lncRNAs (78 upregulated and 390
downregulated). Within this co-expression network, there were 975
positive relationships and 226 negative relationships. AV30S1-TTI1
and IGKV-HOXA13 were the most positive relationships.
XLOC_006929-PTH1R was the most negative relationship.
GSEA
GSEA is an approach used to identify chemical and
genetic perturbations, pathways, cancer modules, GO terms,
oncogenic signatures and immunologic signatures related to all
expressed genes. In all of the assessed gene sets, there were eight
gene sets that exhibited significant enrichment in the incompletely
ablated HB tissue compared with the control tissue, including
TUMORS PCA2 [Normalized Enrichment Score (NES)=2.13], IL6
DEPRIVATION (NES=2.14), EARLY T LYMPHOCYTE (NES=2.15), ENDOCHONDRAL
BONE MORPHOGENESIS (NES=2.09), TRAF6KO EFF CD8 TCELL (NES=2.09),
NEOCORTEX BASAL RADIAL GLIA (NES=2.10), IMMATURE B LYMPHOCYTE
(NES=2.07) and MYC TARGETS (NES=2.06) (Fig. 5).
Functional annotation of the
differentially expressed genes
GO and KEGG analyses were conducted to predict the
functions and interacting pathways of the differentially expressed
genes in the incomplete ablation HB tissue. With regards to GO BP
terms, the upregulated genes were most enriched in ‘antigen
processing and presentation of endogenous antigen’ (GO:0019883),
‘embryonic morphogenesis’ (GO:0048598), ‘antigen processing and
presentation of exogenous peptide antigen via MHC class I,
TAP-dependent’ (GO:0002479), ‘protein localization to chromatin’
(GO:0071168) and ‘antigen processing and presentation of exogenous
peptide antigen via MHC class I’ (GO:0042590) (Fig. 6A). In addition, the downregulated
genes were particularly enriched in ‘regulation of lipid metabolic
process’ (GO:0019216), ‘phosphorus metabolic process’ (GO:0006793),
‘phosphate-containing compound metabolic process’ (GO:0006796),
‘regulation of cellular metabolic process’ (GO:0031323) and
‘nucleobase-containing compound metabolic process’ (GO:0006139)
(Fig. 6B). For MF, the upregulated
genes were most enriched in ‘voltage-gated cation channel activity’
(GO:0022843), ‘voltage-gated ion channel activity’ (GO:0005244),
‘voltage-gated channel activity’ (GO:0022832), ‘cation channel
activity’ (GO:0005261) and ‘metal ion transmembrane transporter
activity’ (GO:0046873) (Fig. 6C).
The downregulated genes were most enriched in ‘SNARE binding’
(GO:0000149), ‘syntaxin binding’ (GO:0019905), ‘tRNA binding’
(GO:0000049), ‘protein methyltransferase activity’ (GO:0008276) and
‘p53 binding’ (GO:0002039) (Fig.
6D). For CC, the upregulated genes were most enriched in ‘9+0
non-motile cilium’ (GO:0097731), ‘photoreceptor cell cilium’
(GO:0097733), ‘endosome lumen’ (GO:0031904), ‘transmembrane
transporter complex’ (GO:1902495) and ‘nuclear heterochromatin’
(GO:0005720) (Fig. 6E). The
downregulated genes were most enriched in ‘intracellular’
(GO:0005622), ‘intracellular membrane-bounded organelle’
(GO:0043231), ‘organelle inner membrane’ (GO:0019866), ‘nucleus’
(GO:0005634) and ‘obsolete intracellular part’ (GO:0044424)
(Fig. 6F). Furthermore, the
upregulated genes were enriched in the following pathways: ‘MAPK
signaling pathway’, ‘acute myeloid leukemia’, ‘arrhythmogenic right
ventricular cardiomyopathy (ARVC)’, ‘thyroid hormone synthesis’ and
‘pancreatic cancer’ (Fig. 7A), and
the downregulated genes were enriched in ‘human papillomavirus
infection’, ‘regulation of lipolysis in adipocytes’, ‘glycerolipid
metabolism’, ‘ECM-receptor interaction’ and ‘cell cycle’ pathways
(Fig. 7B).
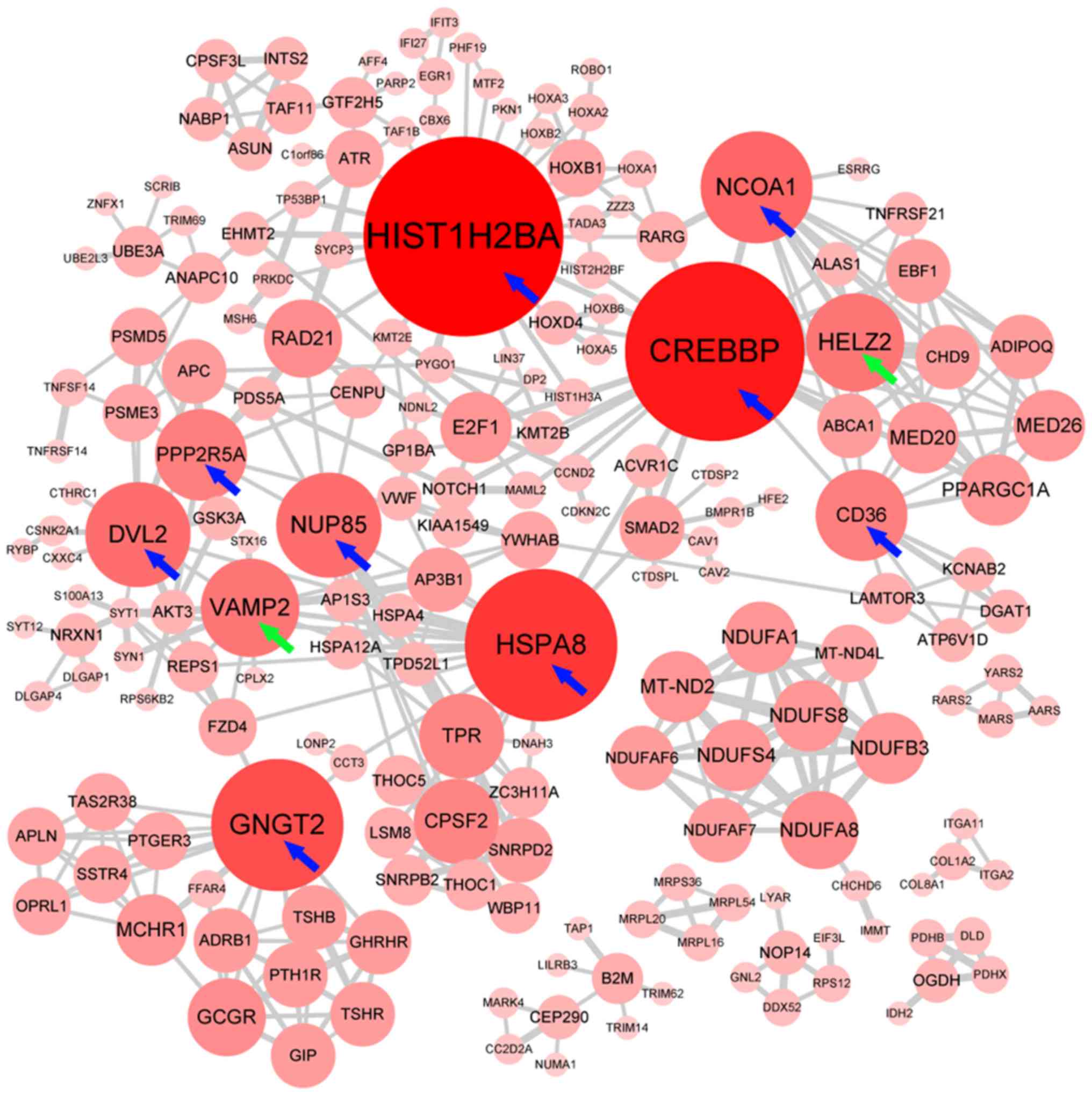
PPI network and hub genes for the
differentially expressed genes
Hiding the disconnected nodes, a PPI network
comprising 247 nodes and 462 edges was generated to view the
interactions among the 663 differentially expressed genes (Fig. 8). Finally, 11 hub genes, including
two upregulated (VAMP2 and HELZ2) and nine downregulated genes
(HIST1H2BA, CREBBP, HSPA8, GNGT2, NCOA1, DVL2, NUP85, CD36 and
PPP2R5A) were identified from the PPI network.
Repurposed drugs for the treatment of
RFA-treated HB
After removing duplicates, 41 compounds with
connectivity scores ≤-0.75, which indicated that the compounds may
negatively affect the differentially expressed genes, were
identified from the CMap database. In addition, 146 compounds with
connectivity scores ≤-0.75 were selected from the database using
the hub genes imputed. Finally, only six drugs that appeared at the
intersection of the aforementioned results were selected as
candidate drugs: Valproic acid, metformin, tanespimycin,
wortmannin, fulvestrant and MK-886 (Fig. 9).
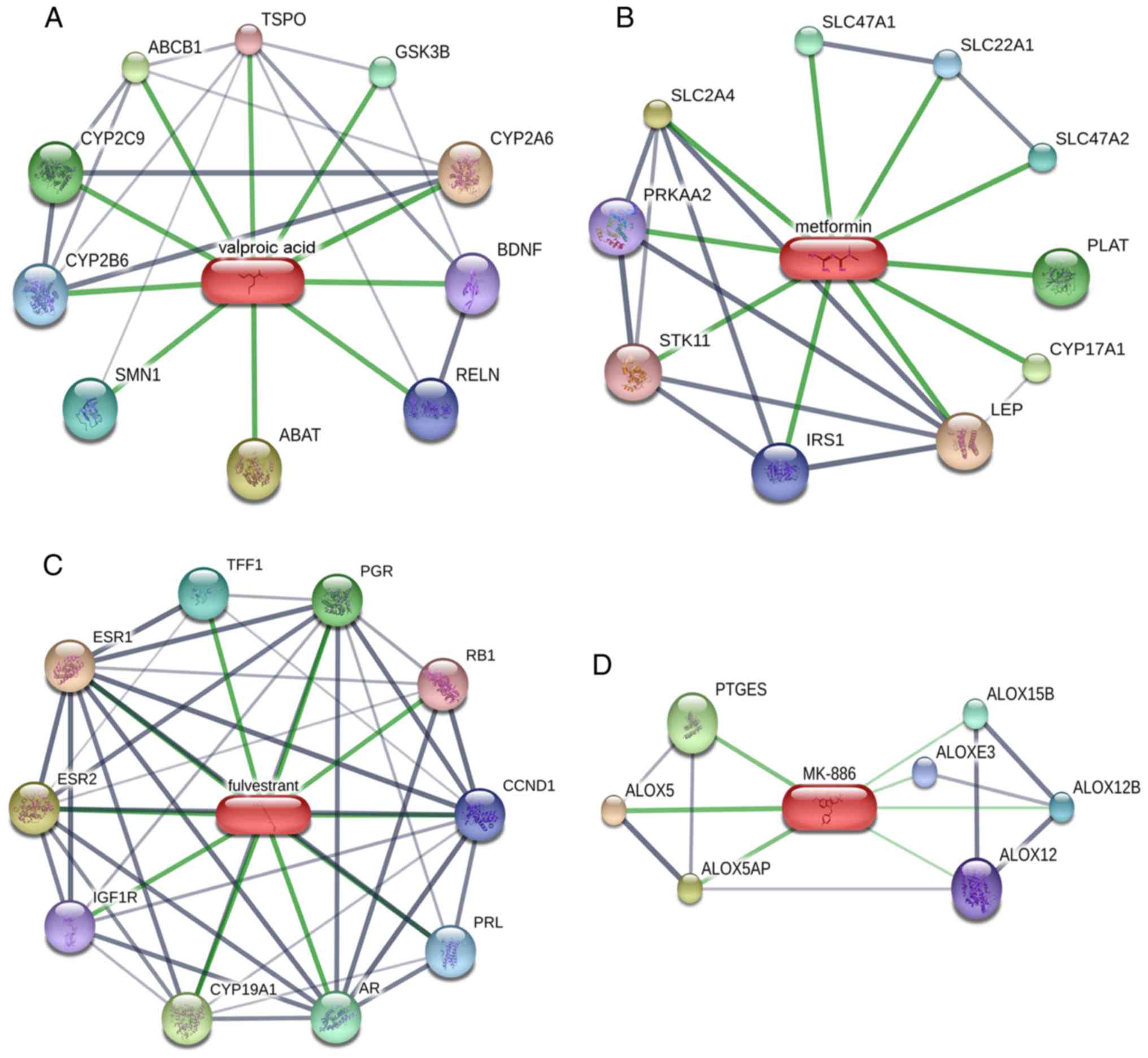
Construction of a drug-target network
for the six candidate drugs
To identify the interactions between proteins and
chemicals, a drug-target network was constructed for the six
candidate drugs using the STITCH database (Fig. 10). The results identified four
drug-target networks containing a total of 37 genes, which may be
relevant for HB therapy. Additionally, two drug-target networks
were not available: Tanespimycin and wortmannin. These compounds
and target proteins may provide potential treatment direction in
the future.
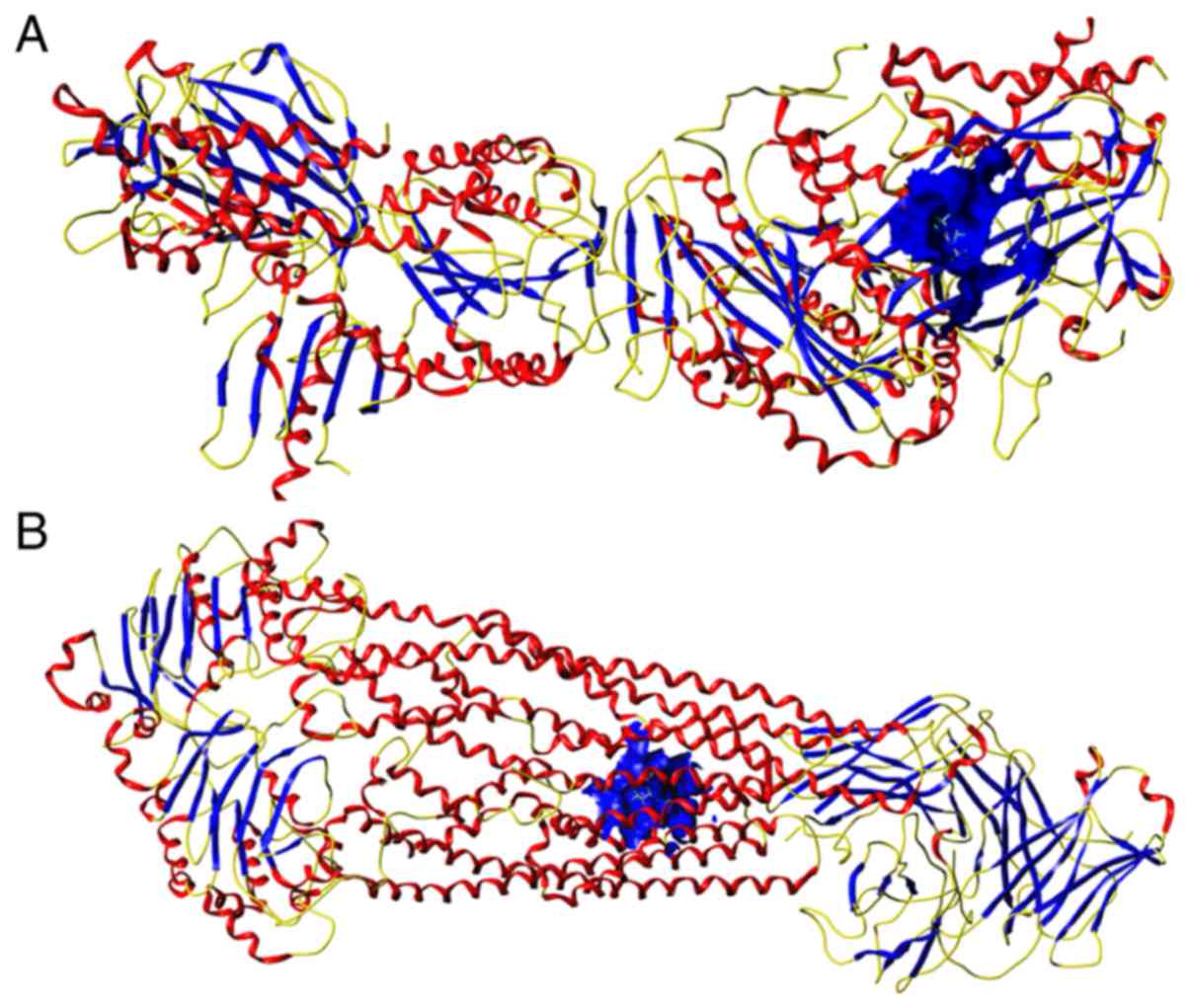
Molecular docking study
Two pairs of compounds and target proteins with the
highest combined score [solute carrier family 2 member 4 (SLC2A4)
and metformin, combined score 0.99; ATP-dependent translocase ABCB1
(ABCB1) and valproic acid, combined score 0.95] were selected to
verify the binding affinities using molecular docking analysis. The
CScore of the affinities between SLC2A4 (PDB code: 5VNM) and
metformin was 4 (Fig. 11A), and
the CScore of the affinities between ABCB1 (PDB code: 6QEX) and
valproic acid was 5 (Fig. 11B).
These findings may provide new insights about the selection of
future drugs.
Discussion
HB is the predominant type of pediatric liver tumor,
and has two primary pathological types: Epithelial and mixed
(2). Epithelial cells are the major
histological cell type in HB and this type of cancer usually has a
good prognosis (43). However, the
small-cell undifferentiated subtype of HB has a poor prognosis
(2,44,45).
RFA has been widely used for treating liver cancer, particularly
patients with early detection of cancer. However, because of
residual tumor cells, microscopic metastasis and vascular invasion,
this type of therapy is often unable to offer complete tumor
clearance (46). Notably, after
sub-lethal treatment of cancer, surviving tumor cells have a
significantly enhanced ability to invade and metastasize (19,47,48).
Increasing evidence has shown that lncRNAs may be involved in
tumorigenesis and disease progression in HB. Dong et al
(28,49) reported that knockdown of
lncRNA-CRNDE and lncRNA-TUG1 reduces growth and angiogenesis in HB
cells, which may be a prospective target in the diagnosis and
therapy of HB. In addition, Zhang et al reported that lncRNA
OIP5-AS1, as a competing endogenous RNA, may inhibit cell
proliferation, metastasis and epithelial to mesenchymal transition
in HB (50). However, few studies
have described the changes in lncRNA expression in incompletely
ablated HB tissue.
In the present study, HepG2 tumor cells were
subcutaneously transplanted into eight male nude mice.
Subsequently, four nude mice were randomly selected as the
experimental group and subjected to incomplete ablation treatment,
which served as an in vivo model for residual HB cells
following insufficient RFA. After microarray analysis, it was
determined that a total of 740 lncRNAs and 663 mRNAs were
significantly differentially expressed compared with in the
untreated nude mouse subcutaneous xenograft model. A co-expression
network was then constructed to predict the target genes and
factors regulated by the lncRNAs. Simultaneously, GSEA was
performed to further analyze the chemical and genetic
perturbations, pathways, cancer modules, GO terms, oncogenic
signatures and immunologic signatures related to all expressed
genes. GO term analysis, KEGG pathway analysis and a PPI network
analysis were then performed to understand the function and
pathways of the differentially expressed genes. The results
revealed that the genes were enriched in T lymphocytes, CD8
effector T cells, B cell lymphoma tumors, etc. The results from GO
term and KEGG pathway analyses indicated that the differentially
expressed genes were involved in functions including antigen
processing, as well as the presentation of endogenous antigens,
regulation of cellular metabolic processes, MAPK signaling and cell
cycle regulation. These results indicated that the differentially
expressed mRNAs may have an important role in the invasion and
metastasis of incompletely ablated HB cells through the regulation
of immunologic activity, energy changes and cellular
metabolism.
While there are numerous approaches for treating
patients with HB, complete surgery is still highly recommended
(2). However, the use of RFA
therapy in HB is a good alternative (4,51–53).
RFA therapy combined with surgery and/or chemotherapy may be a
promising and effective way to treat children with HB (52,53).
However, research has shown that after RFA in patients with
early-stage liver cancer (Barcelona Clinic Liver Cancer stage 0 or
A), the frequency of aggressive intrasegmental recurrence is
markedly increased to 15% and this is an important factor affecting
patient prognosis (47). In this
study, six compounds were identified as candidate drugs for the
treatment of patients with aggressive intrasegmental recurrence:
Valproic acid, metformin, tanespimycin, wortmannin, fulvestrant and
MK-886.
Valproic acid is a fatty acid with anticonvulsant
and anti-manic properties that has marked therapeutic value for the
treatment of bipolar disorder and epilepsy, particularly
generalized epilepsies (54). A
recent discovery reported a novel ability of valproic acid, in that
it can inhibit histone deacetylase and may serve an important role
in cancer treatment (54–56). Zhu et al reported that,
combined with sorafenib, valproic acid may inhibit growth and
induce apoptosis of HCC cells (57). However, valproic acid is also known
to cause liver injury. Metformin is a biguanide hypoglycaemic agent
that is a first-line therapeutic strategy for the treatment of type
2 diabetes (58). In 2001,
Schneider et al first reported the activity of metformin as
an antitumor agent in hamsters (59). To date, the value of metformin in
the treatment of cancer has been further explored in areas such as
liver cancer, ovarian cancer, breast cancer, prostate cancer and
colorectal cancer (60–64). Zhang et al (65) reported that metformin and curcumin
in combination effectively inhibit the growth, metastasis and
angiogenesis of HCC. Tsai et al (66) reported that therapeutic programs
combined with metformin and rapamycin could enhance autophagic cell
death in HCC. Tanespimycin is a benzoquinone antineoplastic
antibiotic that has been widely investigated for the treatment of
leukaemia and solid tumors, such as breast cancer (67), gastric cancer (68) and pancreatic cancer (69). Wortmannin is an androstadiene
metabolite isolated from Penicillium wortmannii. PX-866 is
an analogue of wortmannin and has stable antitumor activity
(70). Fulvestrant is a pure
estrogen receptor antagonist and has been widely used in the
treatment of advanced breast cancer (71,72).
MK-886 is a member of the class of indoles, and evidence has
suggested that it can cause cell death in several tumor cell types,
such as gastric cancer cells, prostate cancer cells and lung tumor
cells (73–75).
In conclusion, this study generated a model to
imitate residual HepG2 cells following incomplete RFA treatment
using a nude mouse subcutaneous xenograft model. Using microarray
data analysis, profiles of lncRNAs and mRNAs were obtained in
incompletely ablated HB cells. By analyzing those data in relation
to data from the CMap database, six compounds were identified as
potential drugs for the treatment of aggressive intrasegmental
recurrence in patients with HB.
Acknowledgements
Not applicable.
Funding
This work was funded by the Fund of National Natural
Science Foundation of China (grant no. NSFC81860319), the Fund of
Innovation Project of Guangxi Graduate Education (grant no.
YCSW2019114) and the Fund of Guangxi Key R&D Project Plan
(grant no. AB17195020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JBP, YH and HY made substantial contributions to the
conception and design of the study. XDW and CYZ made substantial
contributions to the acquisition and analysis of data. CYZ, QQ and
HYL made substantial contributions to the interpretation of data.
XDW and HYL were involved in drafting the manuscript. JBP, XDW and
HYL were involved in critically revising it for important
intellectual content. JBP, YH and HY gave final approval for the
version of the manuscript to be published. Each author sufficiently
participated in the work to take public responsibility for
appropriate portions of the content and agreed to be accountable
for all aspects of the work to ensure that questions regarding the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The Animal Care and Experiment Committee of Guangxi
Medical University approved the protocol of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kremer N, Walther AE and Tiao GM:
Management of hepatoblastoma: An update. Curr Opin Pediatr.
26:362–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiao GL, Li L, Cheng W, Ge J, Zhang Z and
Wei Y: Predictors of survival after resection of children with
hepatoblastoma: A single Asian center experience. Eur J Surg Oncol.
40:1533–1539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu B, Zhou L, Huang G, Zhong Z, Jiang C,
Shan Q, Xu M, Kuang M and Xie X: First experience of
ultrasound-guided percutaneous ablation for recurrent
hepatoblastoma after liver resection in children. Sci Rep.
5:168052015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwala S, Gupta A, Bansal D, Vora T,
Prasad M, Arora B, Kapoor G, Chinnaswamy G, Radhakrishnan V, Laskar
S, et al: Management of hepatoblastoma: ICMR consensus document.
Indian J Pediatr. 84:456–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyers RL, Tiao G, de Ville de Goyet J,
Superina R and Aronson DC: Hepatoblastoma state of the art:
Pre-treatment extent of disease, surgical resection guidelines and
the role of liver transplantation. Curr Opin Pediatr. 26:29–36.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sumazin P, Chen Y, Treviño LR, Sarabia SF,
Hampton OA, Patel K, Mistretta TA, Zorman B, Thompson P, Heczey A,
et al: Genomic analysis of hepatoblastoma identifies distinct
molecular and prognostic subgroups. Hepatology. 65:104–121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
European Association For The Study Of The
Liver: European Organisation For Research And Treatment Of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korean Liver Cancer Study Group (KLCSG);
National Cancer Center, Korea (NCC), . 2014 KLCSG-NCC Korea
practice guideline for the management of hepatocellular carcinoma.
Gut Liver. 9:267–317. 2015.
|
|
11
|
Clavien PA, Lesurtel M, Bossuyt PM, Gores
GJ, Langer B and Perrier A; OLT for HCC Consensus Group, :
Recommendations for liver transplantation for hepatocellular
carcinoma: An international consensus conference report. Lancet
Oncol. 13:e11–e22. 2012. View Article : Google Scholar
|
|
12
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar :
|
|
13
|
Vitale A, Peck-Radosavljevic M, Giannini
EG, Vibert E, Sieghart W, Van Poucke S and Pawlik TM: Personalized
treatment of patients with very early hepatocellular carcinoma. J
Hepatol. 66:412–423. 2017. View Article : Google Scholar
|
|
14
|
European Association for the Study of the
Liver. Electronic address, . easloffice@easloffice.eu; European
Association for the Study of the Liver: EASL clinical practice
guidelines: Management of hepatocellular carcinoma. J Hepatol.
69:182–236. 2018. View Article : Google Scholar
|
|
15
|
Xu XL, Liu XD, Liang M and Luo BM:
Radiofrequency ablation versus hepatic resection for small
hepatocellular carcinoma: Systematic review of randomized
controlled trials with meta-analysis and trial sequential analysis.
Radiology. 287:461–472. 2018. View Article : Google Scholar
|
|
16
|
N'Kontchou G, Mahamoudi A, Aout M,
Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier
N, Beaugrand M and Seror O: Radiofrequency ablation of
hepatocellular carcinoma: Long-term results and prognostic factors
in 235 Western patients with cirrhosis. Hepatology. 50:1475–1483.
2009. View Article : Google Scholar
|
|
17
|
Livraghi T, Meloni F, Di Stasi M, Rolle E,
Solbiati L, Tinelli C and Rossi S: Sustained complete response and
complications rates after radiofrequency ablation of very early
hepatocellular carcinoma in cirrhosis: Is resection still the
treatment of choice? Hepatology. 47:82–89. 2008. View Article : Google Scholar
|
|
18
|
Jiang K, Chen J, Liu Y, Liu J, Liu A, Dong
J and Huang Z: Heat-irrigate effect' of radiofrequency ablation on
relevant regional hepatocyte in living swine liver-initial study on
pathology. Cell Biochem Biophys. 72:37–41. 2015. View Article : Google Scholar
|
|
19
|
Kang TW, Lim HK and Cha DI: Aggressive
tumor recurrence after radiofrequency ablation for hepatocellular
carcinoma. Clin Mol Hepatol. 23:95–101. 2017. View Article : Google Scholar :
|
|
20
|
Yevich S, Calandri M, Gravel G, Fresneau
B, Brugières L, Valteau-Couanet D, Branchereau S, Chardot C, Aerts
I, de Baere T, et al: Reiterative radiofrequency ablation in the
management of pediatric patients with hepatoblastoma metastases to
the lung, liver, or bone. Cardiovasc Intervent Radiol. 42:41–47.
2019. View Article : Google Scholar
|
|
21
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar :
|
|
22
|
St Laurent G, Wahlestedt C and Kapranov P:
The Landscape of long noncoding RNA classification. Trends Genet.
31:239–251. 2015. View Article : Google Scholar :
|
|
23
|
Devaux Y, Zangrando J, Schroen B, Creemers
EE, Pedrazzini T, Chang CP, Dorn GW II, Thum T and Heymans S;
Cardiolinc network, : Long noncoding RNAs in cardiac development
and ageing. Nat Rev Cardiol. 12:415–425. 2015. View Article : Google Scholar
|
|
24
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar
|
|
25
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar :
|
|
26
|
Huo X, Han S, Wu G, Latchoumanin O, Zhou
G, Hebbard L, George J and Qiao L: Dysregulated long noncoding RNAs
(lncRNAs) in hepatocellular carcinoma: Implications for
tumorigenesis, disease progression, and liver cancer stem cells.
Mol Cancer. 16:1652017. View Article : Google Scholar :
|
|
27
|
Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu
H and Lu D: LncRNA HOTAIR promotes human liver cancer stem cell
malignant growth through downregulation of SETD2. Oncotarget.
6:27847–27864. 2015.
|
|
28
|
Dong R, Liu GB, Liu BH, Chen G, Li K,
Zheng S and Dong KR: Targeting long non-coding RNA-TUG1 inhibits
tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis.
7:e22782016. View Article : Google Scholar :
|
|
29
|
Musa A, Ghoraie LS, Zhang SD, Glazko G,
Yli-Harja O, Dehmer M, Haibe-Kains B and Emmert-Streib F: A review
of connectivity map and computational approaches in
pharmacogenomics. Brief Bioinform. 19:506–523. 2018.
|
|
30
|
Lamb J: The connectivity map: A new tool
for biomedical research. Nat Rev Cancer. 7:54–60. 2007. View Article : Google Scholar
|
|
31
|
Brum AM, van de Peppel J, Nguyen L, Aliev
A, Schreuders-Koedam M, Gajadien T, van der Leije CS, van Kerkwijk
A, Eijken M, van Leeuwen JPTM and van der Eerden BCJ: Using the
connectivity map to discover compounds influencing human osteoblast
differentiation. J Cell Physiol. 233:4895–4906. 2018. View Article : Google Scholar
|
|
32
|
Hakimé A, Hines-Peralta A, Peddi H, Atkins
MB, Sukhatme VP, Signoretti S, Regan M and Goldberg SN: Combination
of radiofrequency ablation with antiangiogenic therapy for tumor
ablation efficacy: Study in mice. Radiology. 244:464–470. 2007.
View Article : Google Scholar
|
|
33
|
Zhang N, Wang L, Chai ZT, Zhu ZM, Zhu XD,
Ma DN, Zhang QB, Zhao YM, Wang M, Ao JY, et al: Incomplete
radiofrequency ablation enhances invasiveness and metastasis of
residual cancer of hepatocellular carcinoma cell HCCLM3 via
activating β-catenin signaling. PLoS One. 9:e1159492014. View Article : Google Scholar :
|
|
34
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:pdb.prot4986. 2008.
|
|
35
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar :
|
|
36
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar
|
|
37
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar
|
|
38
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar
|
|
39
|
Szklarczyk D, Santos A, von Mering C,
Jensen LJ, Bork P and Kuhn M: STITCH 5: Augmenting protein-chemical
interaction networks with tissue and affinity data. Nucleic Acids
Res. 44:D380–D384. 2016. View Article : Google Scholar
|
|
40
|
Burley SK, Berman HM, Christie C, Duarte
JM, Feng Z, Westbrook J, Young J and Zardecki C: RCSB Protein Data
Bank: Sustaining a living digital data resource that enables
breakthroughs in scientific research and biomedical education.
Protein Sci. 27:316–330. 2018. View Article : Google Scholar
|
|
41
|
Meng XY, Zhang HX, Mezei M and Cui M:
Molecular docking: A powerful approach for structure-based drug
discovery. Curr Comput Aided Drug Des. 7:146–157. 2011. View Article : Google Scholar :
|
|
42
|
Clark RD, Strizhev A, Leonard JM, Blake JF
and Matthew JB: Consensus scoring for ligand/protein interactions.
J Mol Graph Model. 20:281–295. 2002. View Article : Google Scholar
|
|
43
|
Weinberg AG and Finegold MJ: Primary
hepatic tumors of childhood. Hum Pathol. 14:512–537. 1983.
View Article : Google Scholar
|
|
44
|
Haas JE, Feusner JH and Finegold MJ: Small
cell undifferentiated histology in hepatoblastoma may be
unfavorable. Cancer. 92:3130–3134. 2001. View Article : Google Scholar
|
|
45
|
Dong R, Jia D, Xue P, Cui X, Li K, Zheng
S, He X and Dong K: Genome-wide analysis of long noncoding RNA
(lncRNA) expression in hepatoblastoma tissues. PLoS One.
9:e855992014. View Article : Google Scholar :
|
|
46
|
Umeda Y, Matsuda H, Sadamori H, Matsukawa
H, Yagi T and Fujiwara T: A prognostic model and treatment strategy
for intrahepatic recurrence of hepatocellular carcinoma after
curative resection. World J Surg. 35:170–177. 2011. View Article : Google Scholar
|
|
47
|
Kang TW, Lim HK, Lee MW, Kim YS, Rhim H,
Lee WJ, Gwak GY, Paik YH, Lim HY and Kim MJ: Aggressive
intrasegmental recurrence of hepatocellular carcinoma after
radiofrequency ablation: Risk factors and clinical significance.
Radiology. 276:274–285. 2015. View Article : Google Scholar
|
|
48
|
Shiozawa K, Watanabe M, Takahashi M, Wakui
N, Iida K and Sumino Y: Analysis of patients with rapid aggressive
tumor progression of hepatocellular carcinoma after percutaneous
radiofrequency ablation. Hepatogastroenterology. 56:1689–1695.
2009.
|
|
49
|
Dong R, Liu XQ, Zhang BB, Liu BH, Zheng S
and Dong KR: Long non-coding RNA-CRNDE: A novel regulator of tumor
growth and angiogenesis in hepatoblastoma. Oncotarget.
8:42087–42097. 2017.
|
|
50
|
Zhang Z, Liu F, Yang F and Liu Y: Kockdown
of OIP5-AS1 expression inhibits proliferation, metastasis and EMT
progress in hepatoblastoma cells through up-regulating miR-186a-5p
and down-regulating ZEB1. Biomed Pharmacother. 101:14–23. 2018.
View Article : Google Scholar
|
|
51
|
van Laarhoven S, van Baren R, Tamminga RY
and de Jong KP: Radiofrequency ablation in the treatment of liver
tumors in children. J Pediatr Surg. 47:e7–e12. 2012. View Article : Google Scholar
|
|
52
|
Ye J, Shu Q, Li M and Jiang TA:
Percutaneous radiofrequency ablation for treatment of
hepatoblastoma recurrence. Pediatr Radiol. 38:1021–1023. 2008.
View Article : Google Scholar
|
|
53
|
Gómez FM, Patel PA, Stuart S and Roebuck
DJ: Systematic review of ablation techniques for the treatment of
malignant or aggressive benign lesions in children. Pediatr Radiol.
44:1281–1289. 2014. View Article : Google Scholar
|
|
54
|
Tomson T, Battino D and Perucca E:
Valproic acid after five decades of use in epilepsy: Time to
reconsider the indications of a time-honoured drug. Lancet Neurol.
15:210–218. 2016. View Article : Google Scholar
|
|
55
|
Terbach N and Williams RS:
Structure-function studies for the panacea, valproic acid. Biochem
Soc Trans. 37:1126–1132. 2009. View Article : Google Scholar
|
|
56
|
Tomson T, Battino D and Perucca E: The
remarkable story of valproic acid. Lancet Neurol. 15:1412016.
View Article : Google Scholar
|
|
57
|
Zhu W, Liang Q, Yang X, Yu Y, Shen X and
Sun G: Combination of sorafenib and Valproic acid synergistically
induces cell apoptosis and inhibits hepatocellular carcinoma growth
via down-regulating Notch3 and pAkt. Am J Cancer Res. 7:2503–2514.
2017.
|
|
58
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R and
Matthews DR: Management of hyperglycaemia in type 2 diabetes: A
patient-centered approach. Position statement of the American
Diabetes Association (ADA) and the European Association for the
Study of Diabetes (EASD). Diabetologia. 55:1577–1596. 2012.
View Article : Google Scholar
|
|
59
|
Schneider MB, Matsuzaki H, Haorah J,
Ulrich A, Standop J, Ding XZ, Adrian TE and Pour PM: Prevention of
pancreatic cancer induction in hamsters by metformin.
Gastroenterology. 120:1263–1270. 2001. View Article : Google Scholar
|
|
60
|
Donadon V, Balbi M, Mas MD, Casarin P and
Zanette G: Metformin and reduced risk of hepatocellular carcinoma
in diabetic patients with chronic liver disease. Liver Int.
30:750–758. 2010. View Article : Google Scholar
|
|
61
|
Tseng CH: Metformin reduces ovarian cancer
risk in Taiwanese women with type 2 diabetes mellitus. Diabetes
Metab Res Rev. 31:619–626. 2015. View Article : Google Scholar
|
|
62
|
Campagnoli C, Pasanisi P, Abbà C,
Ambroggio S, Biglia N, Brucato T, Colombero R, Danese S, Donadio M,
Venturelli E, et al: Effect of different doses of metformin on
serum testosterone and insulin in non-diabetic women with breast
cancer: A randomized study. Clin Breast Cancer. 12:175–182. 2012.
View Article : Google Scholar
|
|
63
|
Tseng CH: Metformin significantly reduces
incident prostate cancer risk in Taiwanese men with type 2 diabetes
mellitus. Eur J Cancer. 50:2831–2837. 2014. View Article : Google Scholar
|
|
64
|
Sehdev A, Shih YC, Vekhter B, Bissonnette
MB, Olopade OI and Polite BN: Metformin for primary colorectal
cancer prevention in patients with diabetes: A case-control study
in a US population. Cancer. 121:1071–1078. 2015. View Article : Google Scholar
|
|
65
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56. 2018.
View Article : Google Scholar
|
|
66
|
Tsai HH, Lai HY, Chen YC, Li CF, Huang HS,
Liu HS, Tsai YS and Wang JM: Metformin promotes apoptosis in
hepatocellular carcinoma through the CEBPD-induced autophagy
pathway. Oncotarget. 8:13832–13845. 2017. View Article : Google Scholar :
|
|
67
|
Modi S, Stopeck AT, Gordon MS, Mendelson
D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, et al:
Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is
safe and active in trastuzumab-refractory HER-2 overexpressing
breast cancer: A phase I dose-escalation study. J Clin Oncol.
25:5410–5417. 2007. View Article : Google Scholar
|
|
68
|
Ma L, Yang D, Li Z, Zhang X and Pu L:
Co-delivery of paclitaxel and tanespimycin in lipid nanoparticles
enhanced anti-gastric-tumor effect in vitro and in vivo. Artif
Cells Nanomed Biotechnol. 46:904–911. 2018. View Article : Google Scholar
|
|
69
|
Ghadban T, Dibbern JL, Reeh M, Miro JT,
Tsui TY, Wellner U, Izbicki JR, Güngör C and Vashist YK: HSP90 is a
promising target in gemcitabine and 5-fluorouracil resistant
pancreatic cancer. Apoptosis. 22:369–380. 2017. View Article : Google Scholar
|
|
70
|
Hong DS, Bowles DW, Falchook GS,
Messersmith WA, George GC, O'Bryant CL, Vo AC, Klucher K, Herbst
RS, Eckhardt SG, et al: A multicenter phase I trial of PX-866, an
oral irreversible phosphatidylinositol 3-kinase inhibitor, in
patients with advanced solid tumors. Clin Cancer Res. 18:4173–4182.
2012. View Article : Google Scholar
|
|
71
|
Boér K: Fulvestrant in advanced breast
cancer: Evidence to date and place in therapy. Ther Adv Med Oncol.
9:465–479. 2017. View Article : Google Scholar :
|
|
72
|
Nathan MR and Schmid P: A review of
fulvestrant in breast cancer. Oncol Ther. 5:17–29. 2017. View Article : Google Scholar :
|
|
73
|
Fan XM, Tu SP, Lam SK, Wang WP, Wu J, Wong
WM, Yuen MF, Lin MC, Kung HF and Wong BC:
Five-lipoxygenase-activating protein inhibitor MK-886 induces
apoptosis in gastric cancer through upregulation of p27kip1 and
bax. J Gastroenterol Hepatol. 19:31–37. 2004. View Article : Google Scholar
|
|
74
|
Huang JK, Huang CC, Lu T, Chang HT, Lin
KL, Tsai JY, Liao WC, Chien JM and Jan CR: Effect of MK-886 on Ca2+
level and viability in PC3 human prostate cancer cells. Basic Clin
Pharmacol Toxicol. 104:441–447. 2009. View Article : Google Scholar
|
|
75
|
Rioux N and Castonguay A: Inhibitors of
lipoxygenase: A new class of cancer chemopreventive agents.
Carcinogenesis. 19:1393–1400. 1998. View Article : Google Scholar
|