Introduction
Lung cancer is the most common malignancy and one of
the main causes of cancer-related mortality worldwide (1). It is estimated that 160,000 lung
cancer-related deaths occurred in the United States in 2014,
accounting for 20% of all cancer-related deaths (2). Non-small cell lung cancer (NSCLC)
constitutes 80–85% of all lung cancer cases, with squamous cell
carcinoma and adenocarcinoma as the two major subtypes (3). Though various treatment strategies
including chemotherapy, pneumonectomy, radiation or combined
modalities have been made available (4), the 5-year survival rate of patients
with NSCLC remains unsatisfactory (5) and its molecular mechanism requires
further investigation (6). Thus, it
is essential to find new targets and therapies for NSCLC.
Natural products from medicinal plants have been
studied widely to find important anticancer agents in the last
decades (7). Hispidulin
(4′,5,7-trihydroxy-6-methoxyflavone;
C16H12O6; molecular weight, 300.3
g/mol) (Fig. 1A), a phenolic
flavonoid isolated mainly from S. involucrata, was
traditionally used in oriental medicine (8). Accumulated evidence has demonstrated
that hispidulin has various effects, such as pro-oxidant,
neuroprotective, anti-inflammatory, antiepileptic, antithrombotic,
and antiosteoporotic activities (9–13).
Furthermore, a number of in vivo and in vitro studies have
shown that hispidulin has antitumor effects on diverse
hematological and solid malignancies (14–17).
The anticancer activities of hispidulin in acute myeloid leukemia,
colorectal cancer, renal cell carcinoma, gallbladder cancer and
hepatocellular carcinoma have also been confirmed (16–18). A
previous study showed that hispidulin exerts proapoptotic and
antiproliferative effects on HepG2 cells through reactive oxygen
species (ROS)-induced endoplasmic reticulum stress (ER stress)
(19). However, whether ER stress
and apoptosis are related to the anticancer effect of hispidulin in
NSCLC remains unclear.
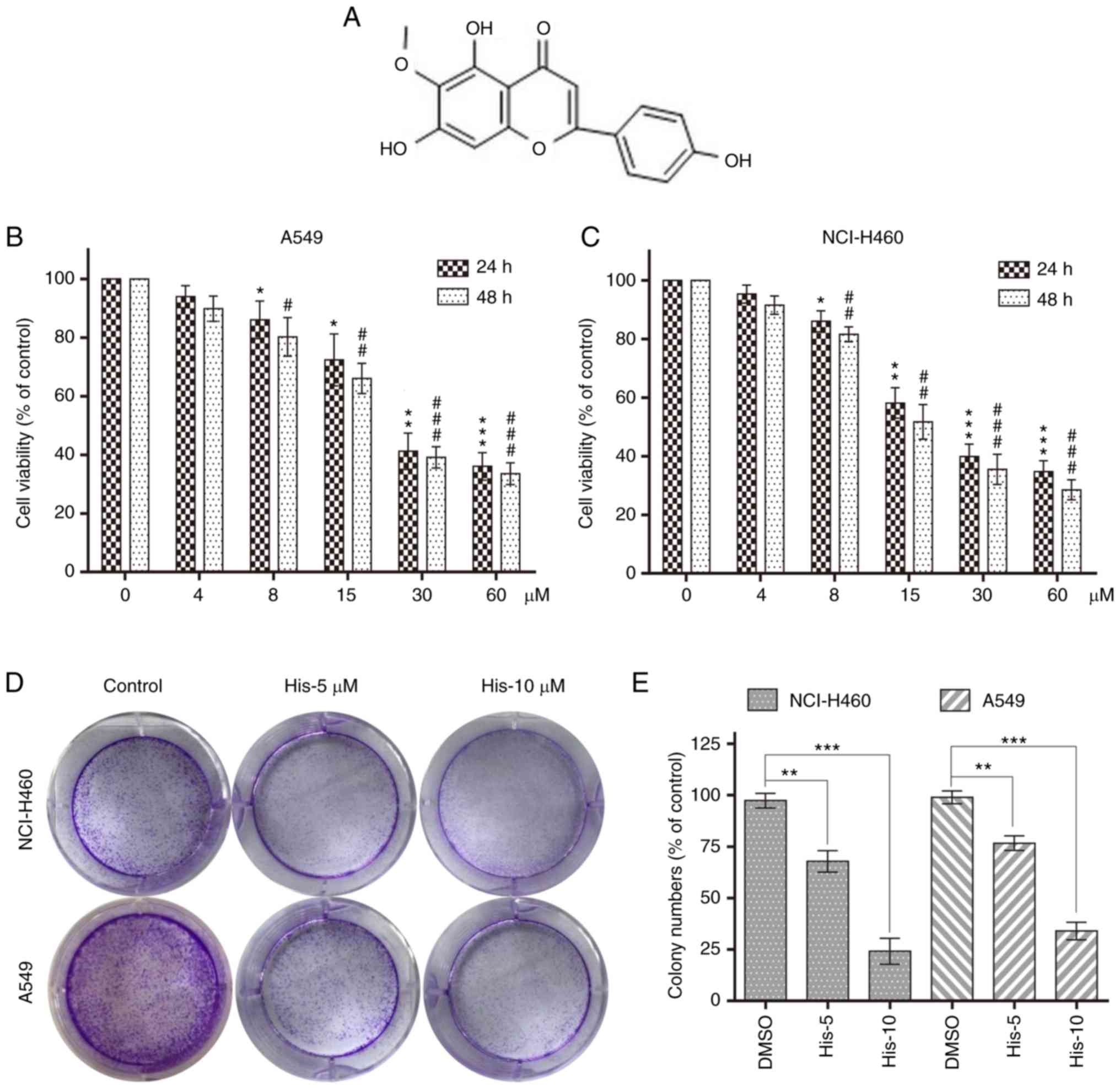 | Figure 1.Hispidulin inhibits cell viability in
human NSCLC cells. (A) Chemical structure of hispidulin. Cell
viability was analyzed using MTT assay in (B) A549 and (C) NCI-H460
cells treated with His (4, 8, 15, 30 and 60 µM) for 24 or 48 h.
*P<0.05, **P<0.01, ***P<0.001 compared to DMSO control at
24 h. #P<0.05, ##P<0.01,
###P<0.001 compared to DMSO control at 48 h. (D)
Effect of different His concentrations (5 and 10 µM) on human NSCLC
colony formation. NCI-H460 and A549 cells were incubated with His
for 12 h and allowed to grow for 8–11 days. Colonies were stained
by crystal violet dye. (E) The colony formation ability of each
group was shown in bar chart. **P<0.01, ***P<0.001 as
indicated. All images shown are representative of three independent
experiments with similar results. Data are shown as mean ± SEM
(n=3). His, hispidulin; NSCLC, non-small-cell lung cancer; His-5, 5
µM hispidulin; His-10, 10 µM hispidulin. |
The present study aimed to investigate the
anticancer effects of hispidulin in NSCLC cells. Hispidulin induced
NSCLC cell apoptosis in a time- and dose-dependent manner.
Molecular mechanism studies have shown that apoptosis was regulated
by ER stress activation induced by ROS. The results showed that the
pro-apoptotic effect of hispidulin was associated with ROS-induced
cell apoptosis through the activation of ER stress in NSCLC cells,
which provided theoretical basis for the future research and
development of clinical tumor-targeted drugs.
Materials and methods
General reagents
Hispidulin (Shanghai Aladdin Bio-Chem Technology
Co., Ltd.) was dissolved in 0.1% DMSO and 0.1% DMSO alone was used
as the vehicle control. Tauroursodeoxycholic acid (TUDCA; Merck
KGaA) was dissolved in saline. ROS probe
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was obtained
from Beyotime Institute of Biotechnology and glutathione (GSH) was
purchased from Sigma-Aldrich; Merck KGaA.
Cell culture and treatment
Human NSCLC cell lines NCI-H460 and A549 (Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences) were
cultured in RPMI-1640 media (Thermo Fisher Scientific, Inc.) with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were grown at 37°C and
5% CO2 (v/v) in a humidified cell incubator.
Determination of cell viability
Cell viability was determined by methyl thiazolyl
tetrazolium (MTT). DMSO was used to dissolve the formazan product.
The optical density of viable NCI-H460 and A549 cells was measured
using a spectrophotometer at 450 nm (Tecan Group, Ltd.) following
treatment with different concentrations of hispidulin (0, 4, 8, 15,
30 and 60 µM) for 24 or 48 h. Cell viability assays were performed
in triplicate.
Colony formation assay
NCI-H460 and A549 cells (1,000 cells/well) were
cultured in a six-well plate. The cultures were maintained for 12 h
upon 5 and 10 µM hispidulin treatment, and cells were grown for
8–11 days with fresh medium (RPMI-1640 with 10% FBS) at 37°C in a
humidified 5% CO2 atmosphere. The cells were
subsequently fixed with 4% paraformaldehyde for 15 min at room
temperature, stained with crystal violet for 30 min, and
photographed using a digital camera (Nikon DXM-1200; Nikon
Corporation).
Flow cytometry analysis
FITC Annexin V/PI apoptosis kit (BD Pharmingen; BD
Biosciences) was used to determine cell apoptosis. Cells were
pretreated with 2.5 mM TUDCA prior to treatment with 30 µM
hispidulin for 24 h. The NCI-H460 and A549 cells were collected,
washed, and resuspended in 195 µl Annexin V-FITC-binding buffer.
Following this, cells were incubated with Annexin V-FITC and PI (5
µl each) for 15 min at 4°C. The cells were analyzed by Accuri C6
plus flow cytometry (BD Biosciences). The flow cytometry data were
quantified using Flow Jo 7.6.1 software (Tree Star, Ashland,
Inc.).
Hoechst 33342 staining
Cell nuclear staining was performed using a Hoechst
33342 Staining kit (Beyotime Institute of Biotechnology). The
NCI-H460 and A549 cells were incubated in Hoechst 33342 (2 µg/ml)
for 30 min at 37°C 24 h post hispidulin treatment. Then, the
stained cells were examined by using a fluorescence microscope.
Measurement of ROS generation
NCI-H460 cells were seeded in six-well plates and
incubated for 24 h. After pretreatment with 5 mM GSH for 1 h and
treatment with the 15 and 30 µM hispidulin for 3 h, the cells were
incubated with 10 µM DCFH-DA for 20 min at 37°C in the dark. The
cells were subsequently washed twice with PBS and the levels of
intracellular ROS were analyzed immediately using a fluorescence
microscope (Nikon Corporation). The fluorescence intensity
measurements were performed using ImageJ (National Institutes of
Health).
Western blotting
NCI-H460 cells were cultured with hispidulin (15 or
30 µM) for 4 or 24 h and collected. Cells were pretreated with 2.5
mM TUDCA prior to treatment with 30 µM hispidulin for 24 h.
Proteins were isolated from the hispidulin-treated or control
DMSO-treated cells and the protein concentrations were determined
using the bicinchoninic acid protein assay kit (cat. no. P0010;
Beyotime Institute of Biotechnology). Total proteins (~50 µg/lane)
were subjected to 10% SDS-PAGE and transferred to a PVDF membrane
which was blocked with 5% (w/v) low-fat milk for 1 h at room
temperature. The membrane was subsequently incubated with the
following primary antibodies, cleaved-poly [ADP-ribose] polymerase
(PARP; cat. no. sc-56196; 1:200), caspase3 (cat. no. sc-56053;
1:1,000) and GAPDH (cat. no. sc-32233; 1:1,000) purchased from
Santa Cruz Biotechnology, Inc. and ATF4 (cat. no. 11815; 1:1,000),
phospho (p)-eukaryotic translation initiation factor 2 subunit α
(EIF2α; cat. no. 3398; Ser51; 1:1,000), EIF2α (cat. no. 5324;
1:1,000), C/EBP-homologous protein (CHOP; cat. no. 2895; 1:1,000),
cleaved-caspase3 (cat. no. 9664; 1:1,000) purchased from Cell
Signaling Technology, Inc. overnight at 4°C. Horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. nos. sc-2317 and
sc-2318; 1:2,000) purchased from Santa Cruz Biotechnology Inc. were
incubated with membrane for 1.5 h at room temperature. The blot
signal was detected using a chemiluminescent substrate (KPL, Inc.).
BandScan software (Glyko Biomedical, Ltd.) was used for
densitometry. All assays were performed in triplicate.
NCI-H460 ×enograft tumor growth in
nude mice
A total of 18 male athymic BALB/c mice (8 weeks old;
18–19 g) were housed at a constant room temperature with a 12/12-h
light/dark cycle and fed a standard rodent diet under standard
pathogen-free conditions. All animal experiments were approved by
the Institutional Animal Care and Use Committee of the Second
Affiliated Hospital of Kunming Medical University. NCI-H460 cells
(1×106) were subcutaneously injected into the left
dorsal flanks of the BALB/c mice. A total of 15 days after cell
injection, mice were divided randomly into three groups (6
mice/group): Vehicle group (0.9% sodium chloride with 1% DMSO), low
dose group (20 mg/kg hispidulin) and high dose group (40 mg/kg
hispidulin) once every 2 days for 20 days. Mice body weight and
tumor volumes were measured every 3 days for 21 days. All mice were
euthanized with CO2 and serum samples were aliquoted
into sterile tubes, centrifuged at 1,100 × g for 10 min at room
temperature for subsequent analysis. The activities of aspartate
transaminase (AST) and alanine transaminase (ALT) were analyzed by
using a VetScan analyzer (Abaxis, Inc.). Assay kits of AST and ALT
were purchased from Abcam (cat. nos. ab105134 and ab105135). The
harvested heart, liver and kidney tissues of mice were used for
hematoxylin and eosin (H&E) staining. Immunohistochemical (IHC)
staining were performed on NCI-H460 cell-derived xenograft tumors
as previously described (20). The
following primary antibodies were used for IHC: Proliferation
marker protein Ki-67 (1:50; cat. no sc-7846; Santa Cruz
Biotechnology, Inc.), cleaved-caspase 3 (1:100; cat. no. 9664; Cell
Signaling Technology, Inc.) and p-EIF2α (1:1,000; cat. no. 3398;
Cell Signaling Technology, Inc). HRP-conjugated secondary
antibodies (cat. nos. sc-2317 and sc-2031) were purchased from
Santa Cruz Biotechnology, Inc. Caspase-3 activity in tumor lysates
was determined using a caspase-3 activity kit (Beyotime Institute
of Biotechnology) according to the kit instructions.
Statistical analysis
Data are presented as the mean ± SEM from three
separate experiments. Statistical comparisons between cells were
performed by using one-way ANOVA and Dunnett's t test when
comparing more than two groups of data with control group, two-way
ANOVA, non-parametric Kruskal-Wallis test, followed by Dunn's post
hoc test when comparing multiple independent groups and one-way
repeated measures ANOVA followed by Dunnett's post hoc test when
comparing tumor volume and animal body weight. Experimental data
were analyzed by using GraphPad Prism software (version 8.0;
GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Hispidulin effectively suppresses the
cell survival in human NSCLC cells
To explore the effect of hispidulin treatment on
cell viability, human NSCLC cells (NCI-H460 and A549) were treated
with various concentrations of hispidulin (0, 4, 8, 15, 30 and 60
µM/l) for 24 and 48 h. The MTT assay results showed that hispidulin
markedly decreased the viability of A549 and NCI-H460 cell lines in
a time- and concentration-dependent manner (Fig. 1B and C). Additionally, 5 and 10 µM
hispidulin was used to treat NCI-H460 and A549 cells for 12 h
followed by a culture in a new medium for 8–11 days. Colony
formation results showed that the colony formation ability was
inhibited following treatment with 5 and 10 µM hispidulin compared
with the control group (Fig. 1D and
E). These results revealed that hispidulin inhibited the
viability of NCI-H460 and A549 cells in a time and dose-dependent
manner.
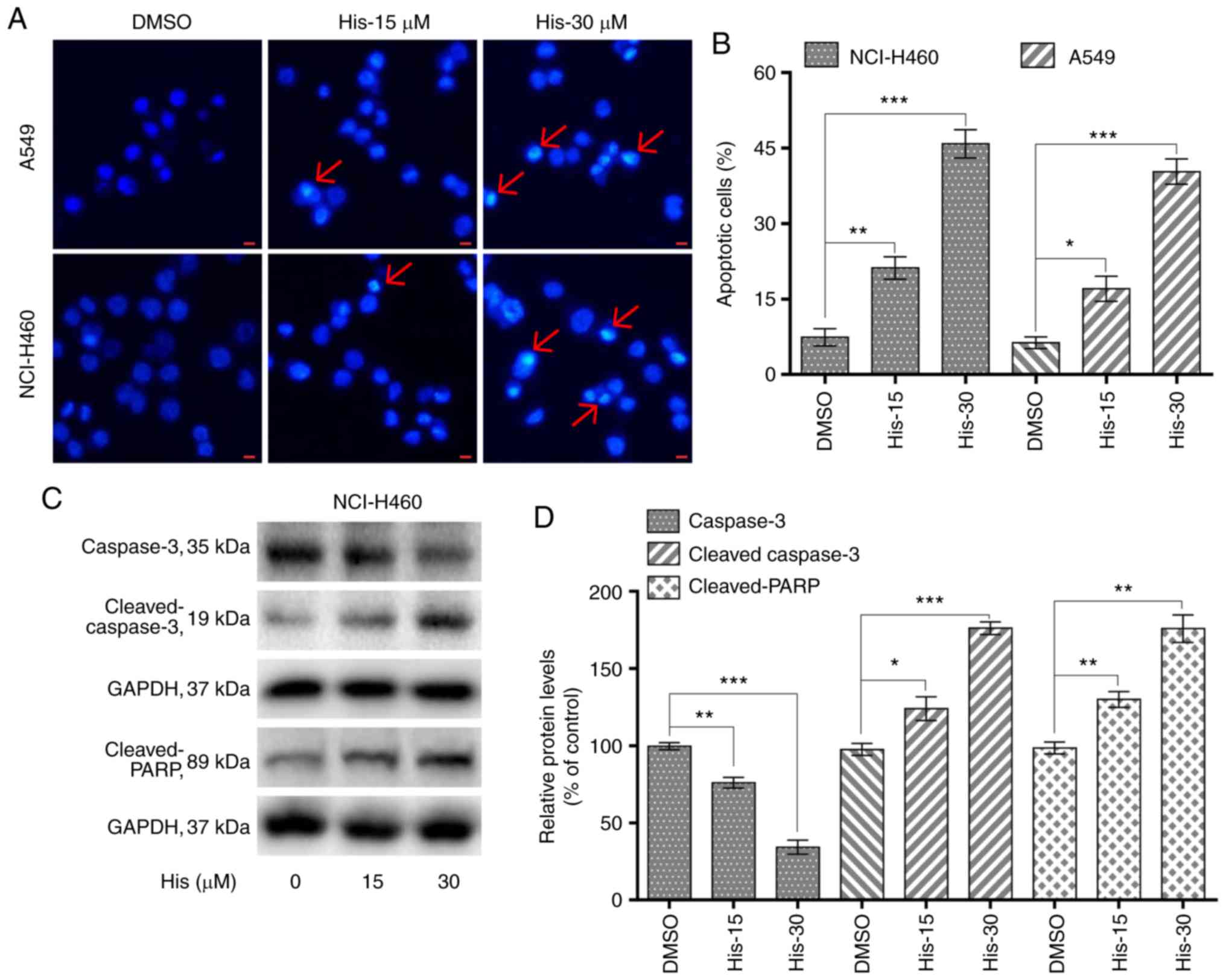
Hispidulin induces apoptosis in human
NSCLC cells in a dose-dependent manner
To explore whether hispidulin induced apoptosis in
NCI-H460 and A549 cells, flow cytometric analysis and Hoechst 33342
staining was performed. Hoechst staining results showed that 15 and
30 µM hispidulin treatment for 24 h lead to cell apoptosis in a
dose-dependent manner in both cell lines (Fig. 2A). Flow cytometry results indicated
that treatment with hispidulin (15 and 30 µM) for 24 h lead to a
significant increase in the percentage of apoptotic cells (Annexin
V-FITC+/PI− and Annexin
V-FITC+/PI+ rates). The proapoptotic effect
induced by hispidulin occurred in a concentration-dependent manner
(Figs. 2B and S1). Additionally, NCI-H460 cells were
treated with 15 and 30 µM hispidulin for 24 h and western blotting
results showed that the expression levels of caspase 3 were
markedly downregulated after exposure to the increasing
concentrations of hispidulin compared with the DMSO group. However,
the expression levels of proteolytic cleaved forms of caspase-3 as
well as cleaved PARP were found to be upregulated (Fig. 2C and D), which revealed that
hispidulin treatment could induce caspase-3 activation and
apoptosis in NCI-H460 cells.
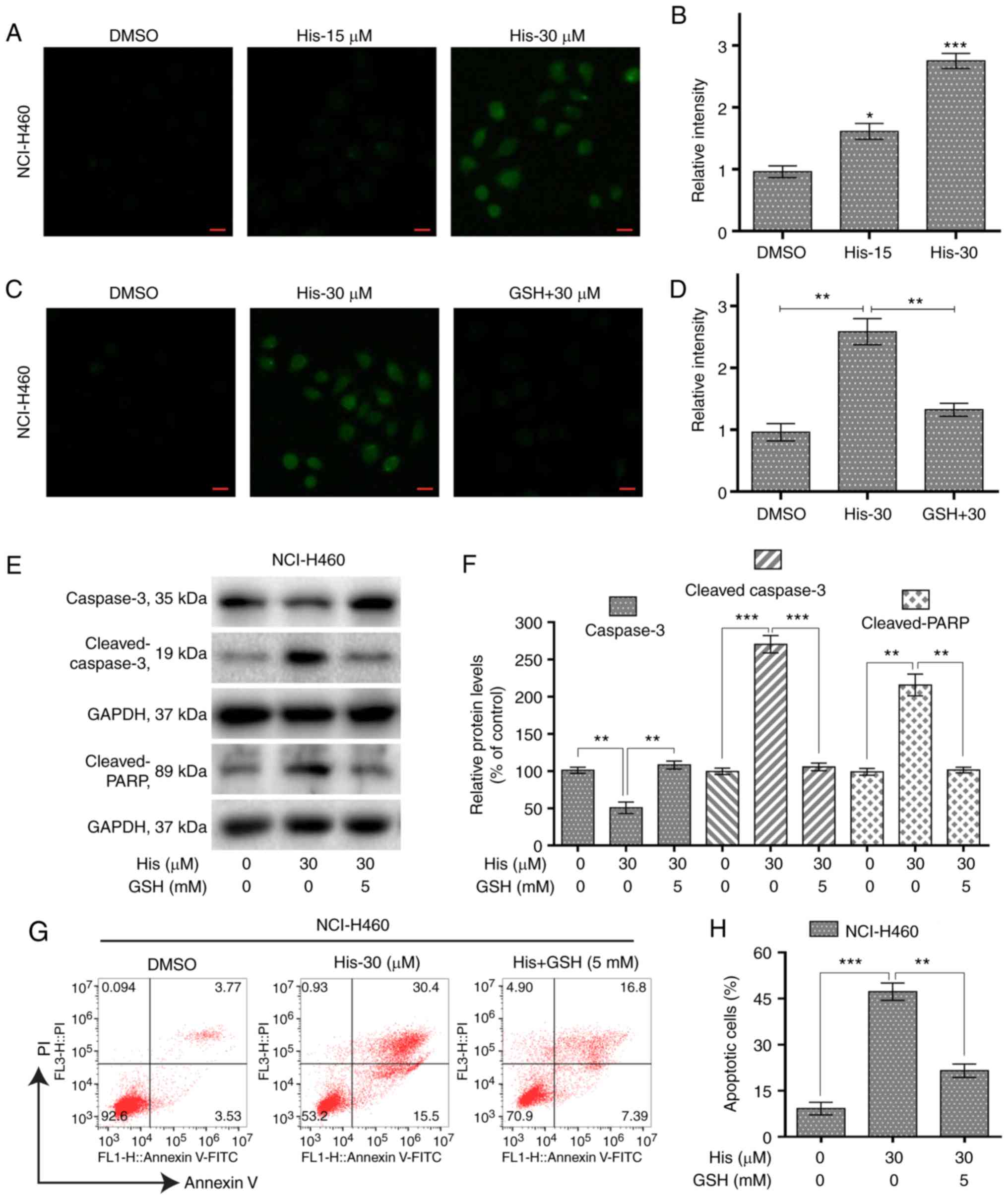
Hispidulin induces ROS-mediated
apoptosis in human NSCLC cells
To investigate the role of ROS in hispidulin-induced
apoptosis, the ROS level was measured in NCI-H460 cells treated
with various concentrations of hispidulin for 3 h. The results
showed that the ROS level was significantly increased by hispidulin
in a concentration-dependent manner (Fig. 3A and B). However, pre-treatment of
NCI-H460 cells with 5 mM ROS scavenger GSH showed a significantly
decreased level of ROS compared with the hispidulin-treated cells
(Fig. 3C and D). Further western
blotting results indicated that GSH pre-treatment attenuated
hispidulin-induced expression of apoptosis-related proteins in
NCI-H460 cells (Fig. 3E and F). The
same results were observed using the Annexin V/PI staining assay
(Fig. 3G and H). These results
demonstrated that ROS generation was significantly involved in
hispidulin-induced apoptosis.
Hispidulin causes ER stress activation
and cell apoptosis in NSCLC cells
To explore the relationship between ER stress and
cancer cell apoptosis, the expression levels of ER stress-related
proteins including p-eIF2α and cyclic AMP-dependent transcription
factor ATF4 were detected in NCI-H460 cells treated with
hispidulin. Western blot analysis results revealed that the
expression of p-eIF2α and ATF4 were increased upon 4-h hispidulin
treatment in a dose-dependent manner (Figs. 4A and B and S2). CHOP is an important factor mediating
ER stress-induced apoptosis (21).
The current study found that 10-h hispidulin treatment lead to a
significant increase in the protein expression level of CHOP
(Fig. 4A and B). Then the role of
ROS in hispidulin-induced ER stress progress was evaluated. The
expression of p-eif2α, ATF4 and CHOP was significantly decreased
upon 5 mM GSH pre-treatment for 1 h compared with hispidulin
treatment alone in NCI-H460 cells (Figs. 4C and D and S2). TUDCA, a chemical chaperone
frequently used for block the ER stress process (22), was used to investigate whether ER
stress was involved in the anticancer effect of hispidulin.
Pre-treatment with TUDCA (2.5 mM) for 1 h in NCI-H460 and A549
cells effectively reversed the increase in CHOP protein expression
levels induced by treatment with hispidulin for 10 h (Figs. 4E and F and S3A and B). Besides, Pre-treatment of
NCI-H460 or A549 cells with TUDCA significantly decreased apoptosis
rate induced by Hispidulin treatment for 24 h (Figs. 4G and S3C and D). All these findings demonstrate
that hispidulin-induced cell apoptosis may be mediated through
activation of the ER stress pathway.
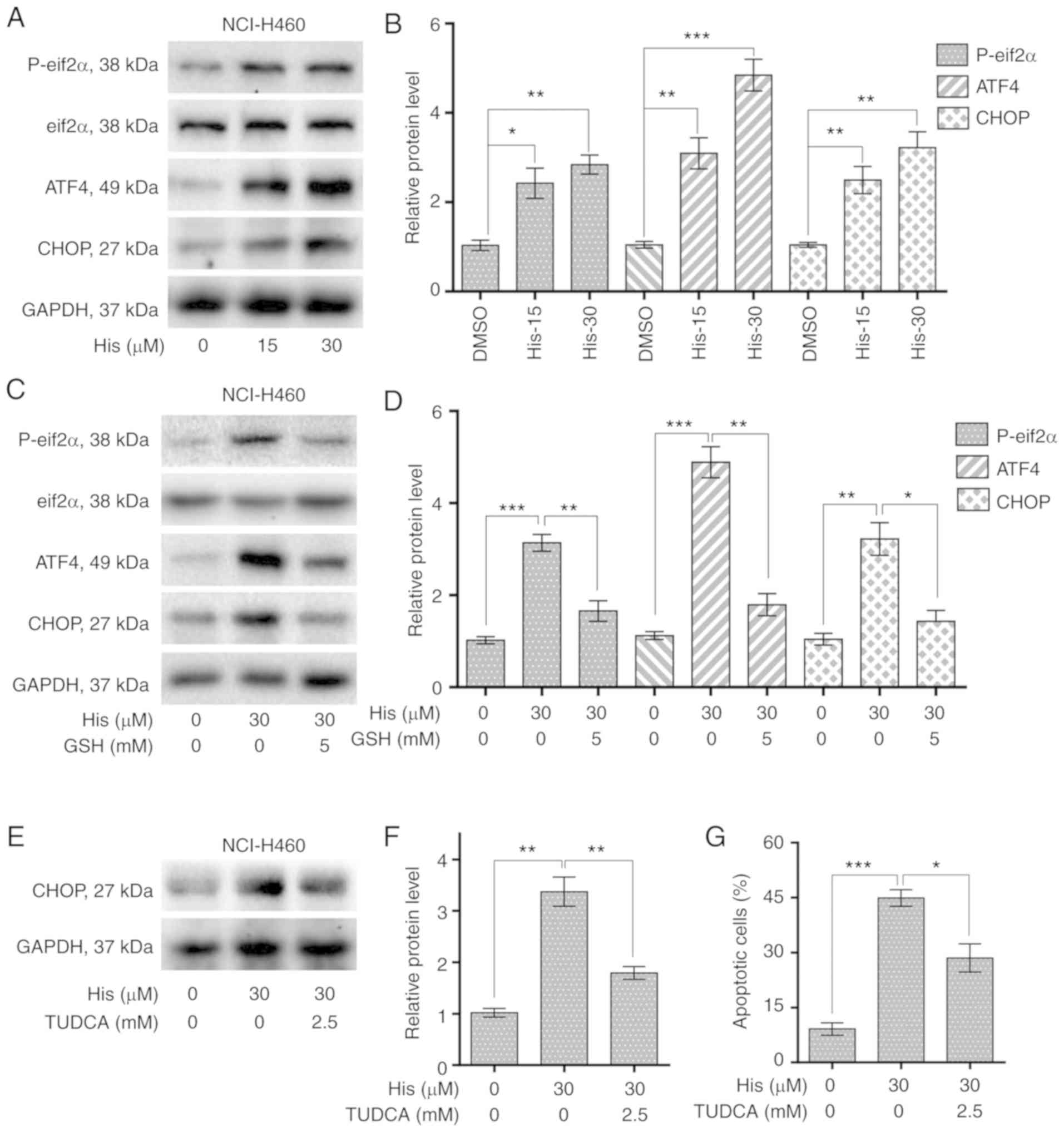 | Figure 4.Hispidulin induces apoptosis through
ROS-dependent ER stress pathway in human NSCLC cells. (A) Protein
expression of ER stress pathway-associated proteins, p-eIF2α,
eIF2α, ATF4 and CHOP in human NSCLC cells. NCI-H460 cells were
exposed to 15 and 30 µM His for 4 h for p-eIF2α, eIF2α and ATF4 or
10 h for CHOP. (B) Quantification of western blotting results
flowing treatment with His. (C) Protein expression of ERs
pathway-associated proteins, p-eIF2α, eIF2α, ATF4 and CHOP in human
NSCLC cells exposed to 30 µM His and 5 mM GSH. (D) Quantification
of western blotting results flowing treatment with His and GSH. (E)
Effect of TUDCA pretreatment on His-induced CHOP protein expression
in NCI-H460 cells. TUDCA was used at 2.5 mM for 1 h before exposure
to 30 µM His. (F) Quantification of CHOP protein expression levels.
(G) Effect of TUDCA on His-induced apoptosis in NCI-H460 cells was
determined by Annexin V/PI staining. TUDCA was used at 2.5 mM for 1
h before exposure to His. All images shown are representative of
three independent experiments with similar results. Data are
presented as the mean ± SEM, n=3. *P<0.05, **P<0.01 and
***P<0.001 as indicated. GSH, glutathione; His, hispidulin;
TUDCA, tauroursodeoxycholic acid; ATF4, cyclic AMP-dependent
transcription factor ATF4; p-, phosphorylated; eIF2α, eukaryotic
translation initiation factor 2 subunit α; CHOP, C/EBP-homologous
protein; NSCLC, non-small-cell lung cancer. |
Hispidulin suppresses NSCLC xenograft
tumor growth in nude mice
The in vivo effect of hispidulin was assessed
using an NSCLC xenograft mouse model. NCI-H460 cells were implanted
in BALB/c mice and then the mice were injected with different doses
of hispidulin (20 and 40 mg/kg) or vehicle control. Hispidulin
attenuated the xenograft tumor growth compared with vehicle group
at both doses 21 days after the first treatment (Fig. 5A). However, there were no
significant differences in the body weights of the mice in
different treatment groups (Fig.
5B). Moreover, the side effects of hispidulin were evaluated
using normal hepatocytes of tumor-bearing mice. The effects on
serum makers of liver function including ALT and AST were tested
immediately at the end of the treatment. There was no significant
difference in these indices between the vehicle group and
hispidulin-treated groups (Fig. 5C and
D). Furthermore, H&E staining showed that hispidulin
treatment did not induce marked histological changes compared with
the vehicle group (Fig. 5E). IHC
staining was used to assess the protein expression of
representative tumor progression (Ki-67), ER stress (p-eIF2α) or
apoptosis marker (cleaved caspase-3) markers in the xenograft
tumors which were harvested from the three groups. The results
revealed that Ki-67 expression was decreased in the His-treated
groups compared with the vehicle group, while the cleaved caspase-3
and p-eIF2α expression levels were increased (Fig. 5F). Furthermore, hispidulin
effectively induced cell apoptosis in vivo, as verified by
caspase-3 activity assay (Fig. 5G).
These results suggested that hispidulin induced NSCLC xenograft
tumor growth inhibition as well as apoptosis in vivo.
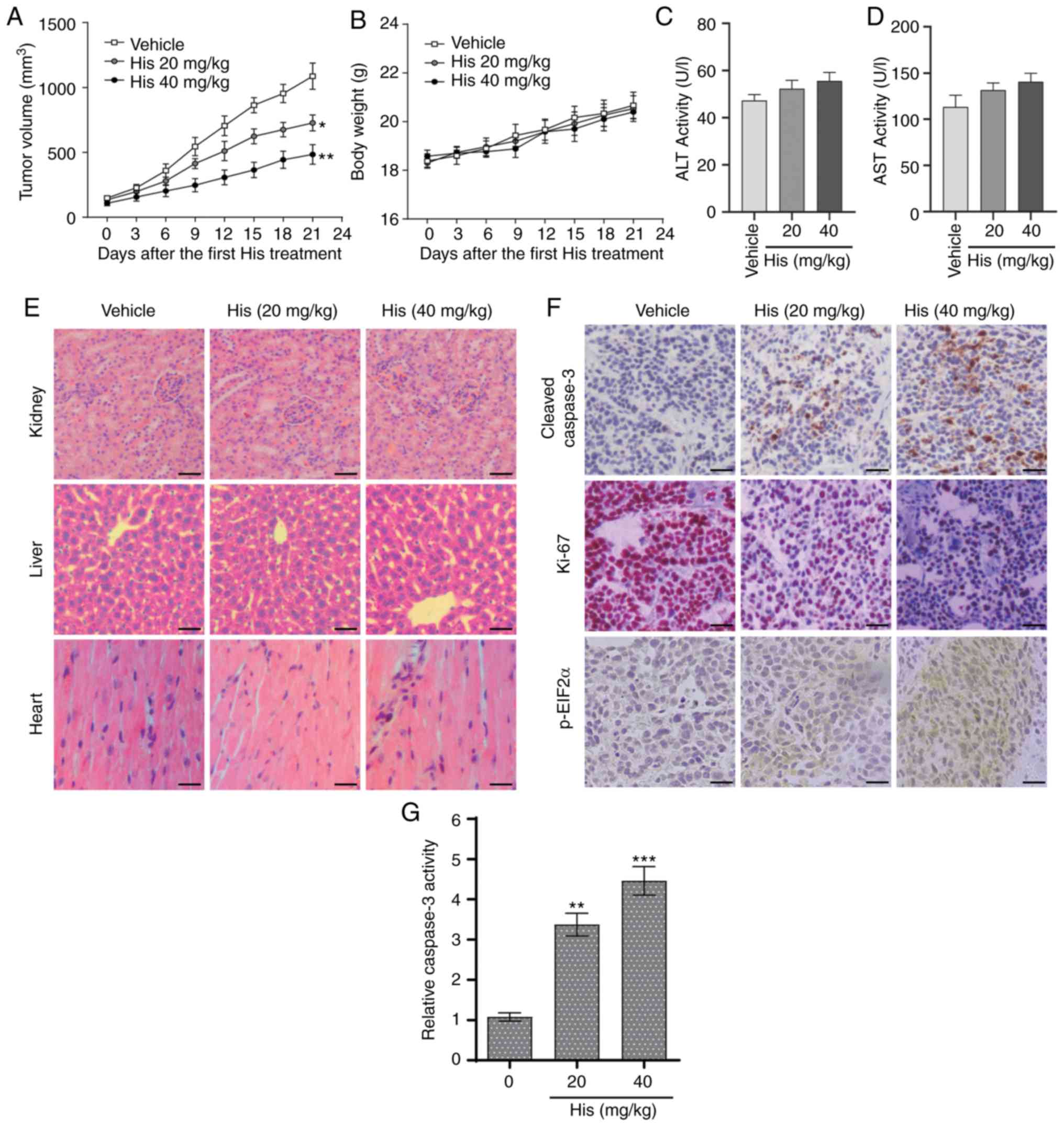 | Figure 5.Hispidulin suppresses the growth of
NSCLC xenografts in nude mice. (A) Vehicle or 20 and 40 mg/kg His
were intraperitoneally injected into tumor-bearing nude mice for 3
weeks, and tumor growth was monitored every 3 days. (B) Measurement
of the body weight every 3 days in a NSCLC xenograft mice model
after treatment with different dosages of His or vehicle. Serum
samples were collected from tumor-bearing mice and serum activities
of (C) ALT and (D) AST were detected. Values were normalized to
those from the vehicle control group. (E) Kidneys, livers and heart
tissues were sectioned at 5 µm and the slides were stained with
hematoxylin and eosin. All images were obtained at ×20
magnification. Hispidulin administration did not cause histological
abnormalities in kidneys, livers and hearts (scale bar=50 µm). (F)
Immunohistochemical staining was performed on the cryostat sections
(5-µm thick) of NSCLC xenograft tumors to detect the expression of
cleaved caspase-3, p-eIF2α and Ki-67 after treatment with
hispidulin (scale bar=50 µm). (G) At the end of the study, tumor
tissue lysates were prepared and subjected to caspase-3 activity
assay. Data are presented as the mean ± SEM, n=6. *P<0.05,
**P<0.01 and ***P<0.001 vs. the vehicle group. His,
hispidulin; ALT, alanine transaminase; AST, aspartate transaminase;
Ki-67, proliferation marker protein Ki-67; p-eIF2α, phosphorylated
eukaryotic translation initiation factor 2 subunit α; NSCLC,
non-small-cell lung cancer. |
Discussion
The current study investigated the anticancer effect
of hispidulin in human NSCLC cells. Our results revealed that
hispidulin inhibited proliferation and induced apoptosis in NSCLC
cells. In addition, hispidulin induced ROS-dependent apoptosis in
human NSCLC cells and caused ER stress activation. The
hispidulin-induced cell apoptosis effect was mediated through the
activation of the ER stress pathway.
Hispidulin is a natural bioactive flavone with
pharmacological effects. Previous studies have demonstrated that
hispidulin inhibited gastric cancer cell growth in a time- and
concentration-dependent manner by inducing G1/S phase
arrest and apoptosis (18,23,24).
Furthermore, hispidulin inhibited human pancreatic tumor growth
in vivo in xenograft model mice. Vascular endothelial growth
factor-induced cell invasion, migration, and capillary-like
structure formation were also inhibited by hispidulin (25). The effect of hispidulin on the liver
system has also been reported (15). However, the molecular mechanisms of
anticancer effect induced by hispidulin in NSCLC cells remain
unclear.
The elevated generation of ROS triggers
caspase-related pathways resulting in apoptosis (26–28).
Therefore, the current study detected the levels of ROS in NSCLC
cells treated with different concentrations of hispidulin and
pretreated with GSH, a selective ROS inhibitor. The results
suggested that ROS was markedly involved in hispidulin-induced
apoptosis.
ER stress is a regulator of different pathologies
and an important mechanism of cancer cell death due to therapeutic
drugs (29,30). In certain cases, oxidative stress
and ER stress occur simultaneously, and are closely linked to
signaling events (31,32). The proline-rich receptor-like
protein kinase (PERK) signaling is mediated through phosphorylation
of eIF2α and PERK phosphorylates multiple substrates to protect
cells from oxidative stress (33,34).
IRE1α-dependent mitogen-activated protein kinase activation can
activate CHOP to contribute to ROS generation (35). Further, ROS can also regulate ER
stress-induced apoptotic responses through the ER-calcium signaling
(36). The levels of ER
stress-related proteins, such as ATF4 and p-eIF2α in
hispidulin-treated NCI-H460 cells were detected in the current
study and hispidulin treatment increased the levels of p-eIF2α and
ATF4 in a dose-dependent manner. CHOP is involved in repressing
transcription of the anti-apoptotic BCL2 protein, leading to
enhanced oxidant injury and apoptosis. Previous studies have shown
that the antitumor capacity of numerous small molecule anti-tumor
compounds that activate the ER stress pathway is significantly
reduced after silencing CHOP protein (37,38).
Based on the results of the current in vitro
study, an NCI-H460 ×enograft mouse model was used to test the in
vivo therapeutic effects of hispidulin. Previous research
revealed that injection of isoliensinine significantly suppressed
the Huh-7 ×enograft tumors (39).
In the current study, hispidulin markedly inhibited the tumor
growth at doses of 20 and 40 mg/kg. The mouse body weight was not
significantly affected by hispidulin in HCC xenograft mouse models
(15). Consistent with this
finding, the current study found that the body weight of NCI-H460
×enograft mouse models changed slightly at both doses and was not
significantly different compared with vehicle control. In a
previous study, isoliensinine induced cell apoptosis in xenograft
HCC tissues in vivo, as demonstrated by a caspase-3 activity
assay (40). Similarly, as
demonstrated in the current study, hispidulin had the capacity to
induce cell apoptosis in vivo.
In conclusion, hispidulin lead to cell apoptosis
through ROS-mediated ER stress in NSCLC cells. This study began to
elucidate the underlying mechanism of hispidulin in NSCLC, and
indicated that targeting ER stress and ROS may be an effective step
in the development of anti-NSCLC drugs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Research
of Yunnan Science and Technology Planning Project [grant no.
2017FE468 (−201)], the China Health Promotion Foundation
Anti-angiogenesis Research Project (grant no. JJKXG20170503), the
National Natural Science Foundation of China (grant no. 81960423)
and the Second Affiliated Hospital of Kunming Medical University
Program (grant no. 2019YK001).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived and designed that study. LL and WZ
acquired, analyzed and interpreted the data. TL, LJ and XL
participated in the study design, data collection and revision
process. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Second
Affiliated Hospital of Kunming Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Yang F, Sui X, Chen X, Zhang L, Wang X,
Wang S and Wang J: Sublobar resection versus lobectomy in surgical
treatment of elderly patients with early-stage non-small cell lung
cancer (STEPS): Study protocol for a randomized controlled trial.
Trials. 17:1912016. View Article : Google Scholar :
|
|
3
|
Shtivelman E, Hensing T, Simon GR, Dennis
PA, Otterson GA, Bueno R and Salgia R: Molecular pathways and
therapeutic targets in lung cancer. Oncotarget. 5:1392–1433. 2014.
View Article : Google Scholar :
|
|
4
|
Li S, Fan J, Liu J, Zhou J, Ren Y, Shen C
and Che G: Neoadjuvant therapy and risk of bronchopleural fistula
after lung cancer surgery: A systematic meta-analysis of 14 912
patients. Jpn J Clin Oncol. 46:534–546. 2016. View Article : Google Scholar
|
|
5
|
Socinski MA, Stinchcombe TE, Moore DT,
Gettinger SN, Decker RH, Petty WJ, Blackstock AW, Schwartz G,
Lankford S, Khandani A and Morris DE: Incorporating bevacizumab and
erlotinib in the combined-modality treatment of stage III
non-small-cell lung cancer: Results of a phase I/II trial. J Clin
Oncol. 30:3953–3959. 2012. View Article : Google Scholar
|
|
6
|
Wagner KW, Alam H, Dhar SS, Giri U, Li N,
Wei Y, Giri D, Cascone T, Kim JH, Ye Y, et al: KDM2A promotes lung
tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin
Invest. 123:5231–5246. 2013. View
Article : Google Scholar :
|
|
7
|
Bishayee A and Sethi G: Bioactive natural
products in cancer prevention and therapy: Progress and promise.
Sem Cancer Biol. 40:1–3. 2016. View Article : Google Scholar
|
|
8
|
Xu YJ, Zhao DX, Fu CX, Cheng LQ, Wang NF,
Han LJ and Ma FS: Determination of flavonoid compounds from
Saussurea involucrata by liquid chromatography electrospray
ionisation mass spectrometry. Nat Prod Res. 23:1689–1698. 2009.
View Article : Google Scholar
|
|
9
|
Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen
T and Xu Q: Anti-Inflammatory and immunosuppressive effect of
flavones isolated from artemisia vestita. J Ethnopharmacol.
120:1–6. 2008. View Article : Google Scholar
|
|
10
|
Zhou R, Wang Z and Ma C: Hispidulin exerts
anti-osteoporotic activity in ovariectomized mice via activating
AMPK signaling pathway. Cell Biochem Biophys. 69:311–317. 2014.
View Article : Google Scholar
|
|
11
|
Yang JM, Hung CM, Fu CN, Lee JC, Huang CH,
Yang MH, Lin CL, Kao JY and Way TD: Hispidulin sensitizes human
ovarian cancer cells to TRAIL-Induced apoptosis by AMPK activation
leading to Mcl-1 block in translation. J Agric Food Chem.
58:10020–10026. 2010. View Article : Google Scholar
|
|
12
|
Xie J, Gao H, Peng J, Han Y, Chen X, Jiang
Q and Wang C: Hispidulin prevents hypoxia-induced
epithelial-mesenchymal transition in human colon carcinoma cells.
Am J Cancer Res. 5:1047–1061. 2015.
|
|
13
|
Lee-Hilz YY, Boerboom AM, Westphal AH,
Berkel WJ, Aarts JM and Rietjens IM: Pro-Oxidant activity of
flavonoids induces EpRE-Mediated gene expression. Chem Res Toxicol.
19:1499–1505. 2006. View Article : Google Scholar
|
|
14
|
Wang YG, Liu W, He X and Fei Z: Hispidulin
enhances the anti-tumor effects of temozolomide in glioblastoma by
activating AMPK. Cell Biochem Biophys. 71:701–706. 2015. View Article : Google Scholar
|
|
15
|
Han M, Gao H, Xie J, Yuan YP, Yuan Q, Gao
MQ, Liu KL, Chen XH, Han YT and Han ZW: Hispidulin induces ER
stress-mediated apoptosis in human hepatocellular carcinoma cells
in vitro and in vivo by activating AMPK signaling pathway. Acta
Pharmacol Sin. 40:666–676. 2019. View Article : Google Scholar
|
|
16
|
Liu K, Gao H, Wang Q, Wang L, Zhang B, Han
Z, Chen X, Han M and Gao M: Hispidulin suppresses cell growth and
metastasis by targeting PIM1 through JAK2/STAT3 signaling in
colorectal cancer. Cancer Sci. 109:1369–1381. 2018. View Article : Google Scholar :
|
|
17
|
Gao MQ, Gao H, Han M, Liu KL, Peng JJ and
Han YT: Hispidulin suppresses tumor growth and metastasis in renal
cell carcinoma by modulating ceramide-sphingosine 1-phosphate
rheostat. Am J Cancer Res. 7:1501–1514. 2017.
|
|
18
|
Gao H, Gao MQ, Peng JJ, Han M, Liu KL and
Han YT: Hispidulin mediates apoptosis in human renal cell carcinoma
by inducing ceramide accumulation. Acta Pharmacol Sin.
38:1618–1631. 2017. View Article : Google Scholar :
|
|
19
|
Scoparo C, Valdameri G, Worfel P, Guterres
FA, Martinez GR, Winnischofer SM, Di Pietro A and Rocha ME: Dual
properties of hispidulin: Antiproliferative effects on HepG2 cancer
cells and selective inhibition of ABCG2 transport activity. Mol
Cell Biochem. 409:123–133. 2015. View Article : Google Scholar
|
|
20
|
Han Y, Yang X, Zhao N, Peng J, Gao H and
Qiu X: Alpinumisoflavone induces apoptosis in esophageal squamous
cell carcinoma by modulating miR-370/PIM1 signaling. Am J Cancer
Res. 6:2755–2771. 2016.
|
|
21
|
Zinszner H, Kuroda M, Wang XZ, Batchvarova
N, Ron D, Lightfoot RT, Remotti H and Stevens JL: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar :
|
|
22
|
Launay N, Ruiz M, Grau L, Ortega FJ,
Ilieva EV, Martínez JJ, Galea E, Ferrer I, Knecht E, Pujol A and
Fourcade S: Tauroursodeoxycholic bile acid arrests axonal
degeneration by inhibiting the unfolded protein response in
X-linked adrenoleukodystrophy. Acta Neuropathol. 133:283–301. 2017.
View Article : Google Scholar
|
|
23
|
Yu CY, Su KY, Lee PL, Jhan JY, Tsao PH,
Chan DC and Chen YL: Potential therapeutic role of hispidulin in
gastric cancer through induction of apoptosis via NAG-1 signaling.
Evid Based Complement Alternat Med. 2013:5183012013. View Article : Google Scholar :
|
|
24
|
Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL,
Wei CW, Kao JY and Way TD: Hispidulin potently inhibits human
glioblastoma multiforme cells through activation of AMP-activated
protein kinase (AMPK). J Agric Food Chem. 58:9511–9517. 2010.
View Article : Google Scholar
|
|
25
|
He L, Wu Y, Lin L, Wang J, Wu Y, Chen Y,
Yi Z, Liu M and Pang X: Hispidulin, a small flavonoid molecule,
suppresses the angiogenesis and growth of human pancreatic cancer
by targeting vascular endothelial growth factor receptor 2-mediated
PI3K/Akt/mTOR signaling pathway. Cancer Sci. 102:219–225. 2011.
View Article : Google Scholar
|
|
26
|
Lu Z, Zhang G, Zhang Y, Hua P, Fang M, Wu
M and Liu T: Isoalantolactone induces apoptosis through reactive
oxygen species-dependent upregulation of death receptor 5 in human
esophageal cancer cells. Toxicol Appl Pharmacol. 352:46–58. 2018.
View Article : Google Scholar
|
|
27
|
Jin C, Zhang G, Zhang Y, Hua P, Zhang X,
Song G, Sun M, Li X, Tong T and Li B: Isoalantolactone induces
intrinsic apoptosis through p53 signaling pathway in human lung
squamous carcinoma cells. PLoS One. 12:e01817312017. View Article : Google Scholar :
|
|
28
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar :
|
|
29
|
Huang P, Zhang YH, Zheng XW, Liu YJ, Zhang
H, Fang L, Zhang YW, Yang C, Islam K, Wang C and Naranmandura H:
Phenylarsine oxide (PAO) induces apoptosis in HepG2 cells via
ROS-mediated mitochondria and ER-stress dependent signaling
pathways. Metallomics. 9:1756–1764. 2017. View Article : Google Scholar
|
|
30
|
Yang J, Wei J, Wu Y, Wang Z, Guo Y, Lee P
and Li X: Metformin induces ER stress-dependent apoptosis through
miR-708-5p/NNAT pathway in prostate cancer. Oncogensis.
193:e1582015. View Article : Google Scholar
|
|
31
|
Maryam A, Mehmood T, Yan Q, Li Y, Khan M
and Ma T: Proscillaridin A promotes oxidative stress and ER stress,
inhibits STAT3 activation, and induces apoptosis in A549 lung
adenocarcinoma cells. Oxid Med Cell Longev. 11:38534092018.
|
|
32
|
Zhang L, Sang BK, Luitel K and Shay JW:
Cholesterol depletion by TASIN-1 induces apoptotic cell death
through the ER Stress/ROS/JNK signaling in colon cancer cells. Mol
Cancer Ther. 17:943–951. 2018. View Article : Google Scholar
|
|
33
|
Ron D: Translational control in the
endoplasmic reticulum stress response. J Clin Invest.
110:1383–1388. 2002. View Article : Google Scholar :
|
|
34
|
Scheuner D, Song B, Mcewen E, Liu C,
Laybutt R, Gillespie P, Saunders T, Bonner-Weir S and Kaufman RJ:
Translational control is required for the unfolded protein response
and in vivo glucose. Mol Cell. 7:1165–1176. 2001. View Article : Google Scholar
|
|
35
|
Harding HP, Zhang Y, Zeng H, Novoa I, Lu
PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al: An
integrated stress response regulates amino acid metabolism and
resistance to oxidative stress. Mol Cell. 11:619–633. 2003.
View Article : Google Scholar
|
|
36
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2294. 2007.
View Article : Google Scholar
|
|
37
|
Lee DH, Jung Jung Y, Koh D, Lim Y, Lee YH
and Shin SY: A synthetic chalcone,
2′-hydroxy-2,3,5′-trimethoxychalcone triggers unfolded protein
response-mediated apoptosis in breast cancer cells. Cancer Lett.
372:1–9. 2016. View Article : Google Scholar
|
|
38
|
Prasad S, Yadav VR, Ravindran J and
Aggarwal BB: ROS and CHOP are critical for dibenzylideneacetone to
sensitize tumor cells to TRAIL through induction of death receptors
and downregulation of cell survival proteins. Cancer Res.
71:538–549. 2011. View Article : Google Scholar
|
|
39
|
Shu G, Yue L, Zhao W, Xu C, Yang J, Wang S
and Yang X: Isoliensinine, a bioactive alkaloid derived from
embryos of nelumbo nucifera, induces hepatocellular carcinoma cell
apoptosis through suppression of NF-κB signaling. J Agric Food
Chem. 63:8793–8803. 2015. View Article : Google Scholar
|
|
40
|
Zhang W, Liu S, Liu K, Ji B, Wang Y and
Liu Y: Knockout of ADAM10 enhances sorafenib antitumor activity of
hepatocellular carcinoma in vitro and in vivo. Oncol Rep.
32:1913–1922. 2014. View Article : Google Scholar
|