Introduction
Breast cancer is the most commonly diagnosed cancer
and the leading cause of cancer-associated death among women in
most countries (1). In both
developed and developing countries, the incidence rates of breast
cancer far exceed those for other cancer types in women. Based on
the 2008 and 2010 data, one in eight women in the USA has the risk
of being diagnosed with breast cancer in her lifetime, compared
with the lifetime risk of one in eleven in the 1970s (2). In women, the incidence of breast
cancer is considerably higher in comparison to other cancer types.
The incidence rate of breast cancer has increased over the last few
decades in most countries (3). For
example, the incidence rates have increased rapidly in Asia,
Africa, and South America, where they had been relatively low in
the past. The primary risk factors for breast cancer cannot be
modified easily, as these factors are associated with prolonged,
endogenous hormonal exposures (1).
Hence, advanced therapeutic studies on breast cancer are urgently
required.
Recent developments in breast cancer chemotherapy
have significantly improved patient survival rates; however, the
recurrence of breast cancer remains a major problem. Systemic
treatment of breast cancer with available therapies is not curative
(4). Triple-negative breast cancer
(TNBC) is highly heterogeneous, comprising approximately 10–20% of
all breast cancer cases, and frequently occurs in women younger
than 50 years of age (5). The
prognosis of TNBC is the poorest among all types of breast cancer,
due to limited treatment options. Recently, novel therapies like
nanotherapeutics have been developed to improve the efficacy and
decrease the toxicity of antitumor drugs (6). However, the heterogeneity and
complexities of tumors hinder the widespread clinical application
of nanomedicine. None of the nanotherapeutics used clinically is
tumor specific, and targeted therapies for TNBC are still at their
early stage (7).
In order to address the aforementioned challenges,
new drugs for breast cancer therapy are urgently required. Natural
products with a wide range of physiological activities can be used
for treating specific diseases. Natural products have contributed
significantly to the discovery and development of new drugs,
especially those for cancer therapy (8,9).
Alismatis Rhizoma, a popular traditional Chinese medicine derived
from the dried rhizome of Alisma orientale (Sam.) Juz, is
recorded in Shen Nong's Herbal Classic and has been used for
removing dampness and promoting urination for thousands of years
(10). Recently, a wide range of
pharmacological activities has been reported for Alismatis Rhizoma,
including removal of dampness and elimination of edema, promotion
of water metabolism, and anti-hyperlipidemia, anti-complementary,
antioxidant, and anti-cancer properties (11). Phytochemical investigations have
revealed that protostane triterpenes are the principal constituents
of Alismatis Rhizoma, which are considered to be responsible for
its various efficacies (12).
However, the mechanisms via which its compounds act on breast
cancer remain unclear.
In the present study, the effects of four main
protostane triterpenes of Alismatis Rhizoma, including alisol A,
alisol A 24-acetate, alisol B, and alisol B 23-acetate, were
investigated in breast cancer cells. Alisol A showed significant
anticancer effects in MDA-MB-231 breast cancer cells, which is a
TNBC breast cancer cell line. The mechanisms of action of alisol A
on this cell line were also investigated. The results will provide
a comprehensive understanding regarding the anticancer effects of
alisol A in human breast cancer cells.
Materials and methods
Ethics
The use of human specimens in the present study was
approved by the Peking University Third Hospital Medical Science
Research Ethic Committee (approval no. IRB00006761-M2019343).
Informed consent was signed by all the patients.
Chemicals
Four protostane triterpenes, namely, alisol A,
alisol A 24-acetate, alisol B, and alisol B 23-acetate, were
purified from Alismatis Rhizoma by repeated chromatography on
silica gel, reversed-phase C18, Sephadex LH-20, and
semi-preparative RP-C18 high performance liquid chromatography
(HPLC), and their structures were characterized based on
comprehensive nuclear magnetic resonance, mass spectroscopy, and
ultraviolet (UV) spectral analysis. The purity of the four
compounds was greater than 98%, as assessed after normalization of
the HPLC-UV peaks observed at 210 nm (Fig. S1).
Cell culture and treatment
Human breast cancer MDA-MB-231 cells, from American
Type Culture Collection, were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% (v/v) fetal bovine serum (FBS) (HyClone;
Cytvia), 100 U/ml penicillin and streptomycin. MDA-MB-231 cells
were cultured at 37°C in a humidified incubator in the presence of
5% (v/v) CO2. After the cells reached 80% confluence,
cells were detached using trypsin (HyClone; Cytvia), counted, and
plated at the necessary density for treatment. Primary human TNBC
breast cancer cells were isolated from tumor specimens, lesion was
1×1×0.2 cm from a 56-year-old patient diagnosed with breast
invasive ductal carcinoma in July 2019, and cultured in DMEM/F12
supplemented with 5% (v/v) FBS, 0.4 µg/ml hydrocortisone
(Sigma-Aldrich; Merck KGaA), 1X Insulin-Transferrin-Selenium
(Sigma-Aldrich; Merck KGaA), 10 ng/ml epidermal growth factor (EGF)
(Invitrogen; Thermo Fisher Scientific, Inc.), 25 µg/ml adenine
(Sigma-Aldrich; Merck KGaA), 10 ng/ml cholera toxin (Sigma-Aldrich;
Merck KGaA), and 10 µmol/l Y-27632 (MedChemExpress). Alisol A,
alisol A 24-acetate, alisol B, or alisol B 23-acetate were used to
treat cells for 24 h.
Real-time cell viability assay
A real-time cell proliferation assay was conducted
using the ACEA RT-CES microelectronic cell sensor system (ACEA
Bioscience, Inc.) to measure the numbers of living cells. This
system works by measuring electrical impedance of sensor electrodes
integrated on the bottom of microtiter E-plates as previously
described (13). Briefly, after
treatment with 5, 10 or 20 µM compounds for 24 h, 5×103
breast cancer cells per well, including MDA-MB-231 cells and
primary human TNBC cells, were seeded in E-Plate 96 at 37°C in a
humidified incubator in the presence of 5% (v/v) CO2 and
allowed to attach for 12 h. A unitless parameter termed the cell
index was derived and used to represent the cell numbers, based on
the measured relative changes in electrical impedance that occurred
in the presence and absence of the cells in the wells. The cell
index was normalized to the baseline reading at time point 0,
following attachment. Cellular impedance was measured periodically
every 5 min. The electronic sensors provided a continuous and
quantitative measurement of the cell index (which depends on the
number of attached cells and the shape of the cells) in each well.
Cell proliferation measured using the cell index was monitored for
72 h. For primary human TNBC breast cancer cells, 10 or 20 µM
alisol A was used to confirm the effects of alisol A on breast
cancer cells.
Colony-formation assay
Colony-formation assay was performed to determine
the inhibitory effect of alisol A on MDA-MB-231 cells. Briefly,
following treatment with 10 µM Alisma orientale compounds or
5, 10 or 20 µM alisol A treatment for 24 h, cells were detached
using 0.25% trypsin, resuspended, plated at the density of
1×103 cells per 10 cm dish (Corning Inc.), and incubated
for 24 h. This was followed by the addition of alisol A. After 10
days, the cells were fixed with 4% methanol-free formalin
(Sigma-Aldrich; Merck KGaA) for 10 min and permeabilized with pure
methanol (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature. Then, the cells were stained with 0.5% crystal violet
(Sigma-Aldrich; Merck KGaA) for 10 min and washed with Dulbecco's
phosphate buffered saline (DPBS; Welgene) slowly at room
temperature until the crystal violet in the background was washed.
The number of colonies was counted using ImageJ 1.8.0 (NIH).
Cell apoptosis assay
MDA-MB-231 cells (1×105 cells) were
seeded in 6-well plates. After treatment with 5, 10 or 20 µM alisol
A for 24 h, an Annexin V-FITC/propidium iodide (PI) double-staining
Apoptosis Detection kit (Becton, Dickinson and Company) was used to
label the cells, according to the manufacturer's instructions.
MDA-MB-231 cells treated with dimethyl sulfoxide (DMSO, 0.1% (v/v))
were used as negative control. Cells were washed twice with cold
PBS, and then resuspended in 200 µl Annexin V binding buffer at
room temperature. After the cells were stained with 10 µl
FITC-labeled Annexin V and 5 µl PI at room temperature for 15 min
in the dark, the samples were immediately analyzed using flow
cytometry (Becton Dickinson FACS Calibur; Becton, Dickinson and
Company).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
To determine the effects of alisol A on DNA
fragmentation in MDA-MB-231 cells, a DeadEnd™ fluorometric terminal
deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling
(TUNEL) system (Promega Corporation) was used. The TUNEL assay was
processed according to the manufacturer's instructions. Cell nuclei
was stained with 4′6-diamidino-2-phenylindole (DAPI) at room
temperature for 10 min.
Cell cycle analysis
The MDA-MB-231 cells (1×105 cells) were
seeded in 6-well plates. After treatment with 5, 10 or 20 µM alisol
A for 24 h, the cells were harvested and washed twice with cold
PBS. MDA-MB-231 cells treated with DMSO of 0.1% (v/v) were used as
negative control. The cells were suspended in 0.5 ml 70% (v/v)
ethanol and chilled at −20°C for 24 h. After extensive washing with
PBS, the cells were resuspended in PBS containing 10 µg/ml
7-amino-actinomycin D (7AAD) (BD Biosciences) and 0.1 mg/ml RNase A
and incubated at 37°C for 30 min. The cells were subsequently
resuspended in PBS and analyzed using flow cytometry (Becton
Dickinson FACS Calibur). The results were analyzed using ModFit LT
3.2 Software (Verity Software House, Inc.).
Measurement of intracellular ROS
Intracellular ROS levels were measured using a
cell-permeable fluorogenic probe as described previously (13). Briefly, after treatment with alisol
A, cells were washed with PBS and the ROS levels were monitored
using a 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA)
molecular probe (Beyotime Institute of Biotechnology). The DCF
fluorescence distribution in the cells was observed under a
fluorescence microscope (Olympus Corporation) at ×200
magnification.
Immunofluorescence staining and
confocal microscopy
Immunofluorescence was performed as previously
described (14). After treatment
with 5, 10 or 20 µM alisol A for 24 h, the cells were washed with
PBS, and then fixed using 4% paraformaldehyde in PBS (pH 7.4) at
room temperature for 30 min. This was followed by permeabilizing
with 0.5% Triton X-100 on ice for 30 min, followed by blocking for
1 h in 1% bovine serum albumin solution at room temperature. The
cells were then incubated overnight with primary antibodies against
APE1 (cat. no. sc-17774; 1:100; Santa Cruz Biotechnology, Inc.),
γH2AX (cat. no. 9718; 1:400; Cell Signaling Technology, Inc.), and
LC3-II (cat. no. 2775; 1:200; Cell Signaling Technology, Inc.) at
4°C. After washing three times with PBS containing 0.1% Tween-20
and 0.01% Triton X-100 for 5 min each, the cells were incubated
with an appropriate FITC-conjugated secondary IgG including Alexa
Fluor 594 anti-rabbit IgG (cat. no. A21207; 1:700; Invitrogen;
Thermo Fisher Scientific, Inc.) and Alexa Fluor 488 anti-mouse IgG
(cat. no. A11029; 1:700; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature in the dark. After the nuclei
were stained with DAPI at room temperature for 5 min, the cells
were washed several times. Finally, observation was performed under
a confocal laser scanning microscope (CLSM 710; Zeiss AG) at ×400
magnification.
Western blotting
Proteins associated with cell cycle, apoptosis, and
autophagy were detected by western blot analysis. Cells treated
with alisol A or negative control cells were harvested
individually. For protein extraction, cells were suspended in Cell
lysis buffer for Western (cat. no. P0013; Beyotime Institute of
Biotechnology) containing a protease inhibitor mixture and shaken
on ice for 30 min. The cell lysate was centrifuged at 15,000 × g at
4°C for 10 min, and the supernatant was collected. The total
protein concentration was measured using the Bradford method or the
bicinchoninic acid (BCA) protein assay kit. Proteins (40 µg) were
separated on 12% (w/v) SDS-PAGE gels and electrophoretically
transferred onto polyvinylidene difluoride membranes (EMD
Millipore). The membranes were blocked in 5% (w/v) fat-free milk in
Tris-buffered saline-0.5% (v/v) Tween-20 at room temperature for 1
h and incubated overnight at 4°C with antibodies against caspase-3
(1:1,000; cat. no. 9662S; Cell Signaling Technology, Inc.),
caspase-9 (1:1,000; cat. no. 9502S; Cell Signaling Technology,
Inc.), Bcl-2 (1:1,000; cat. no. 15071S; Cell Signaling Technology,
Inc.), p-p38 (1:100; cat. no. sc-166182; Santa Cruz Biotechnology,
Inc.), cyclin A (1:100; cat. no. sc-271682; Santa Cruz
Biotechnology, Inc.), cyclin D1 (1:500; cat. no. K0062-3; MBL
International Co.), LC3-II (1:1,000; cat. no. 2775; Cell Signaling
Technology, Inc.) or GAPDH (1:200; cat. no. sc-47724; Santa Cruz
Biotechnology, Inc.). After three washes with PBS supplemented with
0.1% (v/v) Tween-20 (PBST) for 15 min, the membranes were incubated
with goat anti-rabbit IRDye 680RD (1:5,000; cat. no. 926-68071;
LI-COR Biosciences) or goat anti-mouse IRDye 800CW (1:5,000; cat.
no. 926-32210; LI-COR Biosciences) for 1 h at room temperature.
Proteins were identified by scanning the membranes using the
Odyssey Imager (LI-COR Biosciences). ImageJ 1.8.0 (National
Institutes of Health) was used to quantify the protein bands.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) of three independent experiments. The significance of
differences were analyzed using one-way analysis of variance
(ANOVA) and post-hoc Tukey's test. The half maximal inhibitory
concentration (IC50) of alisol A was calculated using
Probit regression. All statistical analyses were performed using
SPSS 23.0 software (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of Alismatis Rhizoma compounds
on breast cancer cells viability
TNBC MDA-MB-231 cells were used to evaluate the
cytotoxicity of Alismatis Rhizoma compounds on human breast cancer.
Real-time cell proliferation assay and colony-formation assay were
performed to evaluate the cytotoxicity of the four major protostane
triterpenes of Alismatis Rhizoma. As shown in Fig. 1A and B, treatment with 10 µM alisol
A, alisol A 24-acetate, alisol B, and alisol B 23-acetate for 24 h
inhibited cell proliferation. Among them, alisol A was the most
effective compound, which was investigated further in this study.
The results of the real-time cell proliferation assay and
colony-formation assay showed that the viability of MDA-MB-231
cells decreased dose-dependently after treatment with alisol A
(Fig. 1C and D. Primary human TNBC
cells were further used to confirm the effects of alisol A on
breast cancer cells. As shown in Fig.
S2, after treatment with 10 or 20 µM alisol A, the cell
viability of primary human breast cancer cells decreased
dose-dependently. Following treatment with 30 µM alisol A, cell
proliferation at 80 h was inhibited to the baseline level, which is
indicative of cell death. As a result, 20 µM was the highest
concentration used in subsequent experiments. The IC50
of alisol A was 8.112 µM.
Alisol A induces apoptosis in breast
cancer cells
To evaluate whether alisol A can induce cell
apoptosis in MDA-MB-231 cells, annexin V/PI staining assay and
TUNEL assay were used. After treatment with 5, 10 or 20 µM alisol
A, the percentages of apoptosis-positive cells were 24.97±0.80,
31.81±0.36, or 33.87±0.65%, respectively, compared with 9.07±0.51%
in the negative control group (Fig.
2A). The results (Fig. 2A and
B) indicated that compared with the negative control, alisol A
induced significant apoptosis in MDA-MB-231 cells 24 h
post-treatment; the cell apoptotic effect was dose-dependent of
alisol A. Alisol A (20 µM) was used to confirm the involvement of
caspase activation in apoptosis, and activation of caspase-3 and
caspase-9 was detected (Fig. 2C).
Apoptosis-associated proteins, phosphorylated (p)- p38 was
increased, and Bcl-2 was downregulated, which was consistent with
the enhancement of apoptosis.
Alisol A induces G1 phase
cell cycle arrest in breast cancer cells
Cell cycle analysis showed that alisol A effectively
induced G1 phase cell cycle arrest in human breast
cancer MDA-MB-231 cells. After 24 h exposure to 5 µM of alisol A,
the fraction of cells in the G1 phase increased from 26.67±1.45 to
38.67±0.88%. When treated with 10 or 20 µM alisol A, the fraction
of cells in the G1 phase increased to 40.33±0.88 and
42.01±1.15%, respectively (Fig.
3A). This indicated that 5, 10 or 20 µM alisol A can induce
G1 phase cell cycle arrest. To further investigate
alisol A-mediated G1 phase arrest, the level of some
associated proteins was detected using western blotting. Consistent
with the results of the cell cycle analysis, the levels of cyclin A
and cyclin D1 were decreased after treatment with alisol A for 24 h
(Fig. 3B).
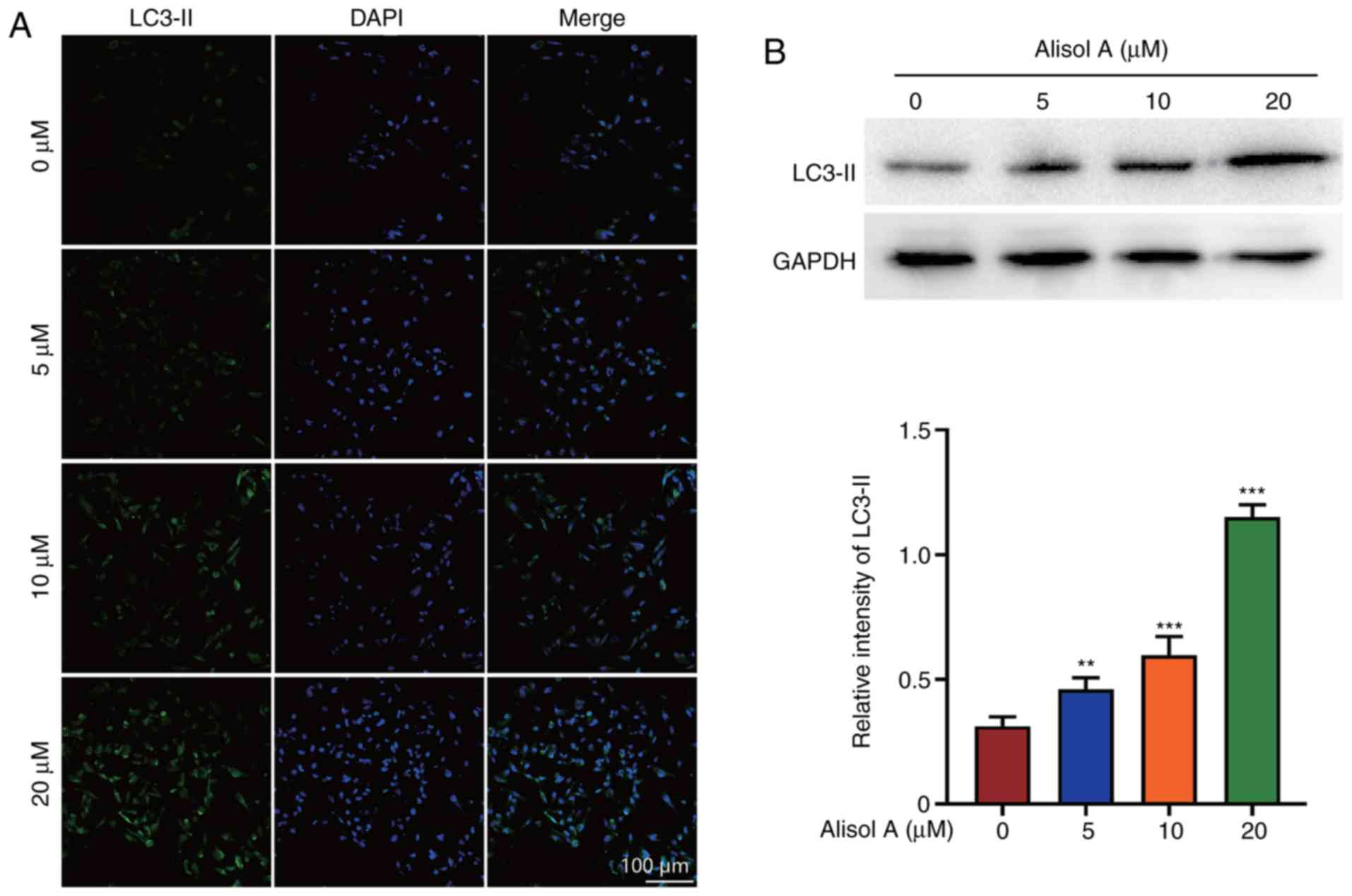
Alisol A induces autophagy in breast
cancer cells
Recent reports have shown that autophagy can
stimulate apoptosis (15). LC3-II
expression was determined to evaluate the effect of alisol A on the
induction of autophagy. LC3-II level increased significantly after
treatment with alisol A. Further, the increase in LC3-II level was
dose-dependent. In the negative control group, LC3-II was not
detected. In the 20 µM alisol A treatment group, almost all cells
showed LC3-II positivity (Fig. 4A).
Western blot analysis showed that the level of LC3-II increased
when treated with alisol. LC3-II expression was up-regulated
three-fold when treated with 20 µM alisol A (Fig. 4B).
Alisol A induced ROS and DNA damage in
breast cancer cells
DNA damage can promote cell autophagy and induce
cell apoptosis via activation of the caspase pathway (16). Intracellular ROS level was assayed
to obtain insights into the events underlying the mechanism of
action of alisol A in breast cancer cells. Cells were treated with
5, 10, or 20 µM alisol A, and ROS levels were measured after
treatment with the DCF-DA molecular probe. As shown in Fig. 5A, the ROS levels in the MDA-MB-231
cells were significantly higher in alisol A-treated cells than in
the negative control cells (P<0.01), and the effect was
dose-dependent. To investigate whether ROS induced oxidative DNA
damage in alisol A-treated cells, apurinic/apyrimidinic
endonuclease 1 (APE1), a surrogate marker of DNA single-strand
breaks, and phosphorylated H2AX (γH2AX), a surrogate marker of DNA
double-strand breaks, were assessed using immunofluorescence
microscopy. As shown in Fig. 5B,
the fractions of APE1- and γH2AX-positive cells increased
significantly.
Discussion
More than 2 million cases of breast cancer were
diagnosed in 2018, and more than 626,000 people succumbed to breast
cancer, establishing breast cancer as the second most common cancer
and the third most common cause of cancer-associated deaths
worldwide (1). Furthermore, TNBC is
characterized by the lack of targeted therapeutic receptors, due to
which treatment options are limited. Therefore, the identification
of new therapeutic agents for breast cancer is urgently required.
Natural products can be potentially used for cancer therapies due
to their significant effectiveness and low toxicity (17–19).
Many anticancer drugs, such as paclitaxel and vincristine, are
natural products of herbal origin, which play important roles in
current chemotherapy (20).
Alisols, triterpenes belonging to the protostane
family, are known as the major bioactive ingredients of Alismatis
Rhizoma (21). Reports indicate
that alisol derivatives possess many types of biological
activities. However, the mechanisms via which these compounds act
on breast cancer remains unclear. In the present study, the
cytotoxicity of four major alisols of Alismatis Rhizoma, including
alisol A, alisol A 24-acetate, alisol B, and alisol B 23-acetate
was first screened. The data showed that alisol A had significant
antitumor activity against breast cancer MDA-MB-231 cells, a TNBC
cell line. To comprehensively explore the changes in alisol
A-treated cells, cell apoptosis, cell cycle, and autophagy were
measured. ROS levels and DNA damage were measured to identify
whether these cellular changes were associated with oxidative DNA
damage, which is a critical inducer of cell death, and can be
utilized for selective cancer therapy (22).
In the present study, MDA-MB-231 cells were used to
investigate the potential therapeutic effects of alisol A on TNBC.
The results showed that alisol A can significantly inhibit the
proliferation of MDA-MB-231 cells, using a real-time cell
proliferation assay that measured the cell index in real time.
While analyzing the cellular changes induced by alisol A treatment,
it was observed that the percentage of apoptotic cells increased.
Resistance to apoptosis is a major obstacle leading to chemotherapy
failure during cancer treatment (23). Drugs that can circumvent this
obstacle will be effective for cancer therapy. Moreover, the
results showed that cleaved caspase-3 and caspase-9 were activated,
which can trigger cell apoptosis. A recent study reported that
caspase-3 activation can offer insights into cancer chemotherapy
(24). Bcl-2 protein was found to
be downregulated after alisol A treatment. Bcl-2, as an
anti-apoptotic protein, could control mitochondrial outer membrane
permeabilization and has also been reported to be involved in
chemotherapy-induced cell death (25). Alisol A treatment increased p-p38
level in MDA-MB-231 cells, which plays critical roles in mediating
cellular response to stressors, and is involved in the p38-MAPK
signaling pathway, thereby linking apoptosis with ROS production
(26). Previous studies have shown
that p38 is involved in the p38-MAPK signaling pathway and its
activation is associated with cell apoptosis in different tissues
(27–31). In the present study, the expression
of the members of this signaling pathway was assessed. It was found
that following alisol A treatment, intracellular ROS and DNA damage
were induced, p38 was activated, and Bcl-2 was inhibited, which
activated the mitochondrial apoptotic caspase cascade, including
caspase-9/3.
Cell cycle arrest at the G1 phase is one
of the main triggers of apoptosis. It was found that the number of
cells at the G1 phase was increased after alisol A
treatment, indicating that the G1 cell cycle arrest was
induced by alisol A. Cyclin A and cyclin D1, the two critical
molecules involved in controlling cell cycle progression, were
downregulated by alisol A treatment. Cyclins function as regulators
of CDK to regulate mitotic events. Cyclin D1 acts to control the
G1/S transition by regulating the activity of CDK4/CDK6.
Defects in cyclin D1, which is under the complex regulation of
upstream proteins, is sufficient to induce abnormal G1/S
transition and G1 cell cycle arrest (32,33).
In addition, cyclin A interacts with CDK2 to control DNA synthesis,
and hence, defects in cyclin A lead to the G1/S arrest
(34,35). Cell cycle arrest and induction of
apoptosis are the two major causes for suppression of cell
proliferation (19).
Basal levels of autophagy could ensure the
physiological turnover of damaged organelles, while a large
accumulation of autophagic vacuoles may induce cell death.
Autophagy, which is induced in response to many types of stress,
including chemotherapeutic intervention, could ultimately lead to
apoptosis (36). Several types of
interactions between apoptosis and autophagy have been described,
indicating a complex mechanistic overlap and interaction between
the apoptotic mechanisms and autophagy-associated proteins
(37). Apoptosis may begin with
autophagy, and autophagy often culminates in apoptosis. When
autophagy is induced, LC3-I is converted to LC3-II, which is an
important autophagosome marker (38). It found that alisol A promoted the
induction of autophagy in breast cancer cells.
The induction of DNA damage is considered to be one
of the important mechanisms of action of cancer therapeutics
(39). Previously, ROS has been
reported to induce oxidative DNA damage (13). Whether alisol A enhanced the
efficacy of cancer therapy via induction of ROS and oxidative DNA
damage remains unclear. In the present study, it was shown that
alisol A increased intracellular ROS levels and induced APE1 and
γH2AX accumulation in breast cancer cells. As previously described,
APE1 is a critical regulator of the cellular response to ROS and is
well known for the DNA backbone cleavage activity during base
excision repair, while γH2AX is required for the stabilization of
various DNA damage response factors at the sites of DNA lesions
(13). DNA-damaging drugs are
commonly used in cancer therapy. Studies on the role of DNA damage
response in drug discovery are essential for identifying novel
cancer therapeutic options (39).
The findings of the present study indicate that alisol A could play
an efficient role against breast cancer via the induction of ROS
and DNA damage. Thus, alisol A could offer a new therapeutic option
for breast cancer.
In conclusion, the present study suggests that
alisol A from Alismatis Rhizoma may be used as a novel agent for
breast cancer therapy. The results are consistent with the
observations of a previous study on the effects of alisol A on
breast cancer cells (40). More
cell lines should be used to confirm the effects of alisol A, to
validate its potential of therapeutic effect. As reported
previously, different cell lines were used to show the effects of
alisol A (40). The results showed
that MDA-MB-231 cell line was the most significant cell line
(40). The present study was a
pilot study that screened the cytotoxicity of four major alisols of
Alismatis Rhizoma, including alisol A, alisol A 24-acetate, alisol
B and alisol B 23-acetate. According to the previous study
(40), MDA-MB-231 cell line was
first used to attain the primary goal. After screening, primary
human TNBC cells were used to confirm the effects of alisol A on
breast cancer cells, as shown in Fig.
S2. The present study demonstrated that the alternative
induction of ROS and DNA damage was the mechanism underlying alisol
A-induced cellular changes, which has not been previously reported.
Further studies using animal models are required to confirm the
functions of alisol A in breast cancer therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant nos. 81672610 and 81872978), and
Major National Science and Technology Project of China (grant no.
2014ZX09304307-001-011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, MW and PW performed the experiments and wrote
the manuscript. TZ performed the western blot analysis. JY and LS
performed the experiments for protostane triterpenes purification
and assessment. ML, HW, QZ and HZ designed the study. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The use of human specimens in the present study was
approved by the Peking University Third Hospital Medical Science
Research Ethic Committee (approval no. IRB00006761-M2019343).
Informed consent was obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TNBC
|
triple-negative breast cancer
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling
|
|
PI
|
propidium iodide
|
|
APE1
|
apurinic/apyrimidinic endonuclease
1
|
|
γH2AX
|
phosphorylated H2AX
|
|
ROS
|
reactive oxygen species
|
|
DNA
|
deoxyribonucleic acid
|
|
HPLC
|
high performance liquid
chromatography
|
|
UV
|
ultraviolet
|
|
FITC
|
fluorescein isothiocyanate isomer
I
|
|
DMSO
|
dimethyl sulfoxide
|
|
7AAD
|
7-amino-actinomycin D
|
|
DCF-DA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
PBS
|
phosphate buffer saline
|
|
DAPI
|
4′6-diamidino-2-phenylindole
|
|
p-p38
|
phosphorylated p38
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chae SY, Ahn SH, Kim SB, Han S, Lee SH, Oh
SJ, Lee SJ, Kim HJ, Ko BS, Lee JW, et al: Diagnostic accuracy and
safety of 16alpha-[(18)F]fluoro-17β-oestradiol PET-CT for the
assessment of oestrogen receptor status in recurrent or metastatic
lesions in patients with breast cancer: A prospective cohort study.
Lancet Oncol. 20:546–555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venkitaraman R: Triple-negative/basal-like
breast cancer: Clinical, pathologic and molecular features. Expert
Rev Anticancer Ther. 10:199–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi J, Kantoff PW, Wooster R and Farokhzad
OC: Cancer nanomedicine: Progress, challenges and opportunities.
Nat Rev Cancer. 17:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X,
Xiao Y, Yu KD, Liu YR, Yu Y, et al: Genomic and transcriptomic
landscape of triple-negative breast cancers: Subtypes and treatment
strategies. Cancer Cell. 35:428–440.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Huang T, Li MJ, Zhang CX, Yu XC, Yin
YY, Liu C, Wang X, Feng HW, Zhang T, et al: The histone
modification reader ZCWPW1 is required for meiosis prophase I in
male but not in female mice. Sci Adv. 5:eaax11012019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao M, Shang H, Li Y, Li T, Wang M, Zheng
Y, Hou W and Liu C: An integrated approach to uncover quality
marker underlying the effects of Alisma orientale on lipid
metabolism, using chemical analysis and network pharmacology.
Phytomedicine. 45:93–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Xu W, Xu YL, Chen X, Huang M and
Lu J: Therapeutic potential of rhizoma alismatis: A review on
ethnomedicinal application, phytochemistry, pharmacology, and
toxicology. Ann N Y Acad Sci. 1401:90–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian T, Chen H and Zhao YY: Traditional
uses, phytochemistry, pharmacology, toxicology and quality control
of Alisma orientale (Sam.) Juzep: A review. J Ethnopharmacol.
158:373–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Wang P, Guo Y, Liang X, Li Y and
Ding S: Helicobacter pylori-induced DNA damage is a potential
driver for human gastric cancer AGS cells. DNA Cell Biol.
38:272–280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang X, Jin Y, Wang H, Meng X, Tan Z,
Huang T and Fan S: Transgelin 2 is required for embryo implantation
by promoting actin polymerization. FASEB J. 33:5667–5675. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasprowska-Liśkiewicz D: The cell on the
edge of life and death: Crosstalk between autophagy and apoptosis.
Postepy Hig Med Dosw (Online). 71:825–841. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Chen R, Chen L, Qiu P, Ai X, Huang
E, Huang W, Chen C, Liu C, Lin Z, et al: Effects of DDIT4 in
methamphetamine-induced autophagy and apoptosis in dopaminergic
neurons. Mol Neurobiol. 54:1642–1660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo F, Gu J, Chen L and Xu X: Systems
pharmacology strategies for anticancer drug discovery based on
natural products. Mol biosyst. 10:1912–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crrowell JA: The chemopreventive agent
development research program in the division of cancer prevention
of the US National Cancer Institute: An overview. Eur J Cancer.
41:1889–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Wu QS, Meng FC, Tang ZH, Chen X,
Lin LG, Chen P, Qiang WA, Wang YT, Zhang QW and Lu JJ:
Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest,
triggers apoptosis and inhibits migration and invasion in ovarian
cancer cells. Phytomedicine. 23:1555–1565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Fátima A, Terra BS, da Silva CM, da
Silva DL, Araujo DP, da Silva Neto L and Nascimento de Aquino RA:
From nature to market: Examples of natural products that became
drugs. Recent Pat Biotechnol. 8:76–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murata T, Imai Y, Hirata T and Miyamoto M:
Biological-active trieterpenes of Alismatis rhizoma. I. Isolation
of the alisols. Chem Pharm Bull (Tokyo). 18:1347–1353. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan
K, Cheng H, Jin K, Ni Q, Yu X and Liu C: The role of necroptosis in
cancer biology and therapy. Mol Cancer. 18:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonzalez PS, O'Prey J, Cardaci S, Barthet
VJA, Sakamaki JI, Beaumatin F, Roseweir A, Gay DM, Mackay G,
Malviya G, et al: Mannose impairs tumour growth and enhances
chemotherapy. Nature. 563:719–723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Peng W, Zheng Y, Hao H, Li S, Yao
Y, Ding Y, Zhang J, Lyu J and Zeng Q: Upregulation of UCP2
expression protects against LPS-induced oxidative stress and
apoptosis in cardiomyocytes. Oxid Med Cell Longev.
2019:27582622019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian Z, Chang J, Jiang F, Ge D, Yang L, Li
Y, Chen H and Cao X: Excess administration of miR-340-5p
ameliorates spinal cord injury-induced neuroinflammation and
apoptosis by modulating the P38-MAPK signaling pathway. Brain Behav
Immun. 2020.(Epub ahead of print). View Article : Google Scholar
|
|
28
|
He J, Huang Z, He M, Liao J, Zhang Q, Wang
S, Xie L, Ouyang L, Koeffler HP, Yin D and Liu A: Circular RNA
MAPK4 (circ-MAPK4) inhibits cell apoptosis via MAPK signaling
pathway by sponging miR-125a-3p in gliomas. Mol Cancer. 19:172020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akter M, Jangra A, Choi SA, Choi EH and
Han I: Non-thermal atmospheric pressure bio-compatible plasma
stimulates apoptosis via p38/MAPK mechanism in U87 malignant
glioblastoma. Cancers (Basel). 12:2452020. View Article : Google Scholar
|
|
30
|
Xu Y, Yao H, Wang Q, Xu W, Liu K, Zhang J,
Zhao H and Hou G: Aquaporin-3 attenuates oxidative stress-induced
nucleus pulposus cell apoptosis through regulating the P38 MAPK
pathway. Cell Physiol Biochem. 50:1687–1697. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Masamha CP and Benbrook DM: Cyclin D1
degradation is sufficient to induce G1 cell cycle arrest despite
constitutive expression of cyclin E2 in ovarian cancer cells.
Cancer Res. 69:6565–6572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen ZH, Jing YJ, Yu JB, Jin ZS, Li Z, He
TT and Su XZ: ESRP1 induces cervical cancer cell G1-phase arrest
via regulating cyclin A2 mRNA stability. Int J Mol Sci.
20:37052019. View Article : Google Scholar
|
|
35
|
Zhang X, Liu J, Zhang P, Dai L, Wu Z, Wang
L, Cao M and Jiang J: Silibinin induces G1 arrest, apoptosis and
JNK/SAPK upregulation in SW1990 human pancreatic cancer cells.
Oncol Lett. 15:9868–9876. 2018.PubMed/NCBI
|
|
36
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Delgado ME, Dyck L, Laussmann MA and Rehm
M: Modulation of apoptosis sensitivity through the interplay with
autophagic and proteasomal degradation pathways. Cell Death Dis.
5:e10112014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu D, Wu H, Wang C, Li Y, Tian H, Siraj
S, Sehgal SA, Wang X, Wang J, Shang Y, et al: STING directly
activates autophagy to tune the innate immune response. Cell Death
Differ. 26:1735–1749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Almeida LC, Bauermeister A,
Rezende-Teixeira P, Santos EAD, Moraes LAB, Machado-Neto JA and
Costa-Lotufo LV: Pradimicin-IRD exhibits antineoplastic effects by
inducing DNA damage in colon cancer cells. Biochem Pharmacol.
168:38–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lou C, Xu X, Chen Y and Zhao H: Alisol A
suppresses proliferation, migration, and invasion in human breast
cancer MDA-MB-231 cells. Molecules. 24:36512019. View Article : Google Scholar
|