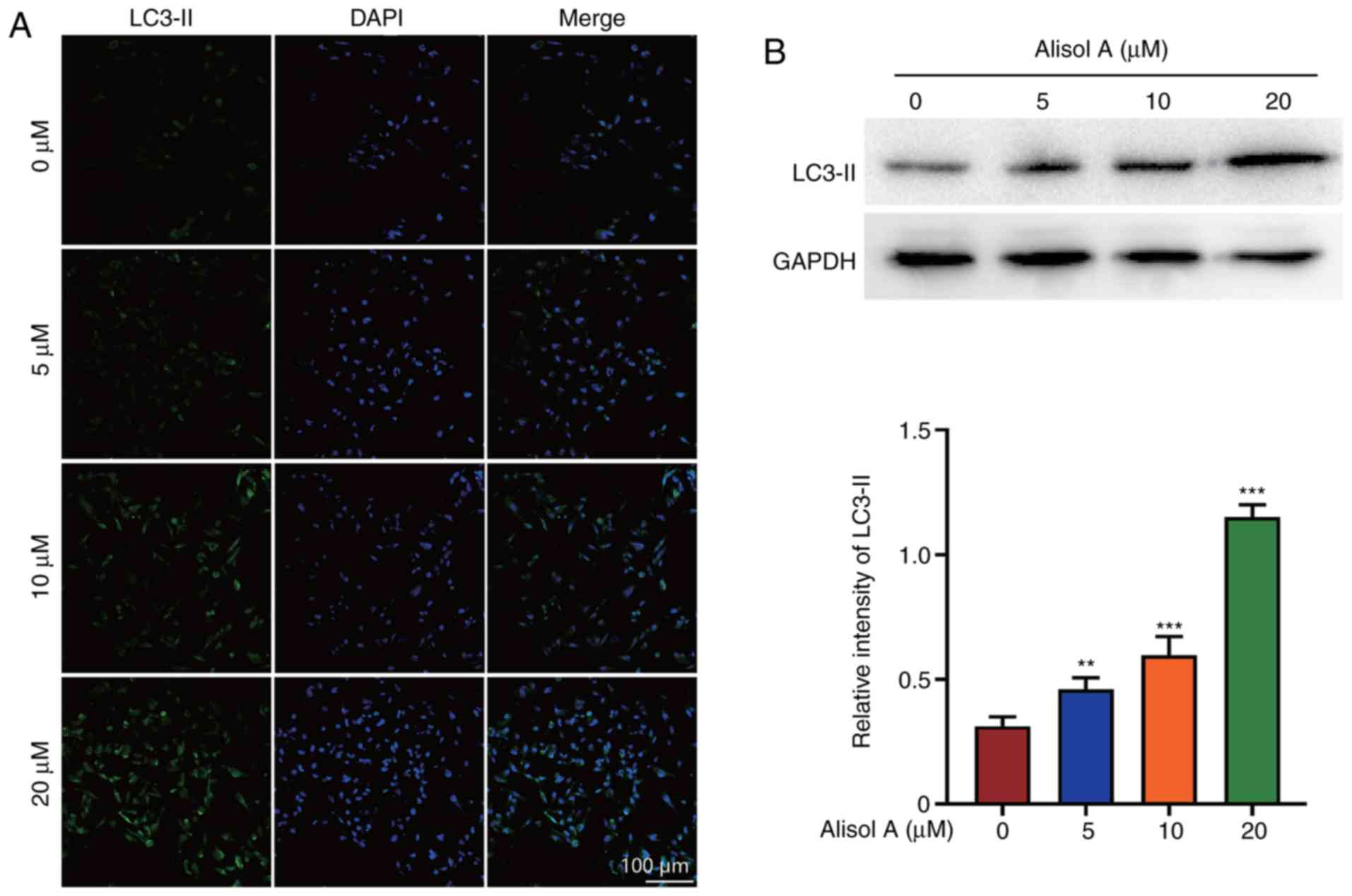
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chae SY, Ahn SH, Kim SB, Han S, Lee SH, Oh
SJ, Lee SJ, Kim HJ, Ko BS, Lee JW, et al: Diagnostic accuracy and
safety of 16alpha-[(18)F]fluoro-17β-oestradiol PET-CT for the
assessment of oestrogen receptor status in recurrent or metastatic
lesions in patients with breast cancer: A prospective cohort study.
Lancet Oncol. 20:546–555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venkitaraman R: Triple-negative/basal-like
breast cancer: Clinical, pathologic and molecular features. Expert
Rev Anticancer Ther. 10:199–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi J, Kantoff PW, Wooster R and Farokhzad
OC: Cancer nanomedicine: Progress, challenges and opportunities.
Nat Rev Cancer. 17:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X,
Xiao Y, Yu KD, Liu YR, Yu Y, et al: Genomic and transcriptomic
landscape of triple-negative breast cancers: Subtypes and treatment
strategies. Cancer Cell. 35:428–440.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Huang T, Li MJ, Zhang CX, Yu XC, Yin
YY, Liu C, Wang X, Feng HW, Zhang T, et al: The histone
modification reader ZCWPW1 is required for meiosis prophase I in
male but not in female mice. Sci Adv. 5:eaax11012019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao M, Shang H, Li Y, Li T, Wang M, Zheng
Y, Hou W and Liu C: An integrated approach to uncover quality
marker underlying the effects of Alisma orientale on lipid
metabolism, using chemical analysis and network pharmacology.
Phytomedicine. 45:93–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Xu W, Xu YL, Chen X, Huang M and
Lu J: Therapeutic potential of rhizoma alismatis: A review on
ethnomedicinal application, phytochemistry, pharmacology, and
toxicology. Ann N Y Acad Sci. 1401:90–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian T, Chen H and Zhao YY: Traditional
uses, phytochemistry, pharmacology, toxicology and quality control
of Alisma orientale (Sam.) Juzep: A review. J Ethnopharmacol.
158:373–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Wang P, Guo Y, Liang X, Li Y and
Ding S: Helicobacter pylori-induced DNA damage is a potential
driver for human gastric cancer AGS cells. DNA Cell Biol.
38:272–280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang X, Jin Y, Wang H, Meng X, Tan Z,
Huang T and Fan S: Transgelin 2 is required for embryo implantation
by promoting actin polymerization. FASEB J. 33:5667–5675. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasprowska-Liśkiewicz D: The cell on the
edge of life and death: Crosstalk between autophagy and apoptosis.
Postepy Hig Med Dosw (Online). 71:825–841. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Chen R, Chen L, Qiu P, Ai X, Huang
E, Huang W, Chen C, Liu C, Lin Z, et al: Effects of DDIT4 in
methamphetamine-induced autophagy and apoptosis in dopaminergic
neurons. Mol Neurobiol. 54:1642–1660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo F, Gu J, Chen L and Xu X: Systems
pharmacology strategies for anticancer drug discovery based on
natural products. Mol biosyst. 10:1912–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crrowell JA: The chemopreventive agent
development research program in the division of cancer prevention
of the US National Cancer Institute: An overview. Eur J Cancer.
41:1889–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Wu QS, Meng FC, Tang ZH, Chen X,
Lin LG, Chen P, Qiang WA, Wang YT, Zhang QW and Lu JJ:
Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest,
triggers apoptosis and inhibits migration and invasion in ovarian
cancer cells. Phytomedicine. 23:1555–1565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Fátima A, Terra BS, da Silva CM, da
Silva DL, Araujo DP, da Silva Neto L and Nascimento de Aquino RA:
From nature to market: Examples of natural products that became
drugs. Recent Pat Biotechnol. 8:76–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murata T, Imai Y, Hirata T and Miyamoto M:
Biological-active trieterpenes of Alismatis rhizoma. I. Isolation
of the alisols. Chem Pharm Bull (Tokyo). 18:1347–1353. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan
K, Cheng H, Jin K, Ni Q, Yu X and Liu C: The role of necroptosis in
cancer biology and therapy. Mol Cancer. 18:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonzalez PS, O'Prey J, Cardaci S, Barthet
VJA, Sakamaki JI, Beaumatin F, Roseweir A, Gay DM, Mackay G,
Malviya G, et al: Mannose impairs tumour growth and enhances
chemotherapy. Nature. 563:719–723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Peng W, Zheng Y, Hao H, Li S, Yao
Y, Ding Y, Zhang J, Lyu J and Zeng Q: Upregulation of UCP2
expression protects against LPS-induced oxidative stress and
apoptosis in cardiomyocytes. Oxid Med Cell Longev.
2019:27582622019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian Z, Chang J, Jiang F, Ge D, Yang L, Li
Y, Chen H and Cao X: Excess administration of miR-340-5p
ameliorates spinal cord injury-induced neuroinflammation and
apoptosis by modulating the P38-MAPK signaling pathway. Brain Behav
Immun. 2020.(Epub ahead of print). View Article : Google Scholar
|
|
28
|
He J, Huang Z, He M, Liao J, Zhang Q, Wang
S, Xie L, Ouyang L, Koeffler HP, Yin D and Liu A: Circular RNA
MAPK4 (circ-MAPK4) inhibits cell apoptosis via MAPK signaling
pathway by sponging miR-125a-3p in gliomas. Mol Cancer. 19:172020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akter M, Jangra A, Choi SA, Choi EH and
Han I: Non-thermal atmospheric pressure bio-compatible plasma
stimulates apoptosis via p38/MAPK mechanism in U87 malignant
glioblastoma. Cancers (Basel). 12:2452020. View Article : Google Scholar
|
|
30
|
Xu Y, Yao H, Wang Q, Xu W, Liu K, Zhang J,
Zhao H and Hou G: Aquaporin-3 attenuates oxidative stress-induced
nucleus pulposus cell apoptosis through regulating the P38 MAPK
pathway. Cell Physiol Biochem. 50:1687–1697. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Masamha CP and Benbrook DM: Cyclin D1
degradation is sufficient to induce G1 cell cycle arrest despite
constitutive expression of cyclin E2 in ovarian cancer cells.
Cancer Res. 69:6565–6572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen ZH, Jing YJ, Yu JB, Jin ZS, Li Z, He
TT and Su XZ: ESRP1 induces cervical cancer cell G1-phase arrest
via regulating cyclin A2 mRNA stability. Int J Mol Sci.
20:37052019. View Article : Google Scholar
|
|
35
|
Zhang X, Liu J, Zhang P, Dai L, Wu Z, Wang
L, Cao M and Jiang J: Silibinin induces G1 arrest, apoptosis and
JNK/SAPK upregulation in SW1990 human pancreatic cancer cells.
Oncol Lett. 15:9868–9876. 2018.PubMed/NCBI
|
|
36
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Delgado ME, Dyck L, Laussmann MA and Rehm
M: Modulation of apoptosis sensitivity through the interplay with
autophagic and proteasomal degradation pathways. Cell Death Dis.
5:e10112014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu D, Wu H, Wang C, Li Y, Tian H, Siraj
S, Sehgal SA, Wang X, Wang J, Shang Y, et al: STING directly
activates autophagy to tune the innate immune response. Cell Death
Differ. 26:1735–1749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Almeida LC, Bauermeister A,
Rezende-Teixeira P, Santos EAD, Moraes LAB, Machado-Neto JA and
Costa-Lotufo LV: Pradimicin-IRD exhibits antineoplastic effects by
inducing DNA damage in colon cancer cells. Biochem Pharmacol.
168:38–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lou C, Xu X, Chen Y and Zhao H: Alisol A
suppresses proliferation, migration, and invasion in human breast
cancer MDA-MB-231 cells. Molecules. 24:36512019. View Article : Google Scholar
|