Human aspartate/asparagine β-hydroxylase (AspH) is a
highly conserved enzyme that is widely expressed in proliferating
placenta trophoblastic cells and is almost undetectable in normal
adult tissues (1). AspH is an ~86
kDa type II transmembrane protein located on the luminal side of
the endoplasmic reticulum (ER) that hydroxylates β-carbons of
specific aspartyl and asparaginyl residues in consensus sequences
of epidermal growth factor-like domains (EGFDs) of target proteins
in the presence of ferrous iron (2–6). In
contrast to the canonical EGFD disulfide pattern, AspH catalyzes
noncanonical EGFD substrates (Cys 1–2, 3–4, 5–6) (7). AspH, which is located at position
q12.1 of human chromosome 8, is a member of the α-ketoglutarate
(also known as 2-oxoglutarate, 2-OG)-dependent dioxygenase family
of prolyl and lysyl hydroxylases, which serve a vital role in
collagen biosynthesis (8–10). Via alternative splicing and exon
sharing, the gene encodes four functionally distinct proteins:
AspH, humbug, junctin and junctate (3,10).
Humbug serves a role in calcium homeostasis and belongs to the
N-terminal fragment that completely lacks the catalytic activity of
AspH (3,11). In contrast, the COOH-terminal region
of AspH contains the hydroxylase catalytic domain, which includes
dibasic glycine and His2 motifs that are essential for catalytic
activity (3). The 26-kDa
calsequestrin binding protein junctin and transcript junctate are
involved in regulating intracellular transient calcium release from
the sarcoplasmic reticulum in cardiac and skeletal muscle (3,10,12,13).
The 2-OG-dependent dioxygenase AspH hydroxylates
aspartate and asparagine residues in certain EGFDs of its
substrates, in particular Notch homologues or Notch ligand
homologues (4,6,18). The
Notch signaling cascade is a highly conserved pathway that affects
cell differentiation, proliferation and apoptosis by mediating
cell-cell communication, which is essential for human growth and
development (24,25). Mammals have four Notch receptors
(Notch1-4) and two ligands [Delta-like and Jagged (JAG)] (26). Both the Notch ligands and the
extracellular domain (ECD) of Notch receptors contain tandem
EGF-like repeats (27–29). Under the condition of β-hydroxylase
activity, AspH binding ligands and receptors in a ligand-dependent
manner enhances the stability and interaction between Notch
receptors and ligands, leading to conformational changes in Notch
(26,30,31).
This process makes Notch more sensitive to continuous cleavage by a
disintegrin and metalloproteinase (ADAM; S2 cleavage) and by the
multiprotein γ-secretase complex (S3 cleavage) (26). On the other hand, AspH promotes the
cleavage of the γ-secretase complex by directly interacting with
ADAM10/17, releasing the Notch intracellular domain, which enters
the nucleus and recruits coactivator proteins from the
mastermind-like 1 (MAML1) family, forming a Notch transcription
activation complex with recombination signal binding protein Jκ
(RBPJ), also known as CSL [CBF1-Su(H)-LAG1] (26,29).
Subsequently, downstream Notch-responsive genes are activated,
including hairy and enhancer of split-1 (HES1), hairy-related
transcription factor-1 (HEY1), CD44, epithelial cell adhesion
molecule, c-Myc, MMP2/9, cyclin D3 and proliferating cell nuclear
antigen (Fig. 1) (29,32).
It has been demonstrated that the Notch signaling pathway serves a
role in regulating exosomes, which are transferred from mesenchymal
cells to tumors to promote metastasis (33,34).
The activation of the AspH-Notch axis induces MMP/ADAM-mediated
exosomal synthesis and release, and the latter markedly enhances
breast cancer cell extracellular matrix (ECM)
degradation/remodeling, infiltration and metastasis (both in
vitro and in vivo) (32). In addition, the structural and
functional abnormalities of tumor blood vessels, combined with
diffusion deterioration, lead to decreased oxygen levels in regions
within solid tumors and induce the expression of stress response
proteins, such as hypoxia-inducible factor-1α (HIF-1α) (35). Chen et al (36) demonstrated that HIF-1α activates
Notch signaling by synergizing with the Notch coactivator MAML1 and
subsequently increases both HES1 and HEY1 expression levels under
hypoxia. In addition, as the upstream target gene of AspH, HIF-1α
enters the nucleus and controls AspH expression at the
transcriptional level (37).
Upregulated AspH expression stimulates the translocation of Notch
to the nucleus by binding to Notch ligands and receptors,
consequently governing downstream target genes that mediate cell
adhesion, including E-cadherin and tenascin C (30,36–38).
This novel molecular mechanism for HIF-1α-AspH-Notch signaling may
serve an important role in cancer invasion and metastasis (Fig. 1).
Several studies have indicated that the MAPK and
PI3K signaling pathways are the most general events in various
types of human cancer (39,40). The abnormal activation of these
proteins affects numerous biological processes, including cell
proliferation, differentiation, growth, survival, motility and
metabolism (39–41). It has been demonstrated that insulin
and insulin-like growth factor (IGF-1) stimulate the intrinsic
tyrosine kinase activity of the IGF-1 receptor, subsequently
activating the PI3K and MAPK signaling pathways and causing the
expression of downstream target substrates, including AKT and ERK
(42–44). de la Monte et al (45) reported that insulin and IGF-1 induce
the phosphorylation and activation of the PI3K and MAPK cascades,
which stimulate AspH expression and enhance cell motility in
hepatocellular carcinoma. Furthermore, GSK3β, which is downstream
of both the PI3K and MAPK signaling pathways, is phosphorylated
(inhibition) at Ser9 by its upstream kinases AKT and p38 (46). However, high levels of AspH lead to
decreased GSK3β phosphorylation, which delays tumor cell senescence
and promotes tumor progression by interfering with the
communication between GSK3β and upstream kinases (Fig. 2A) (47).
Compared with surgery, radiation and chemotherapy,
immunotherapy has provided important benefits to patients with
melanoma (48). The purpose of
cancer immunotherapy is to promote tumor-specific T-cell responses.
In the presence of major histocompatibility and CD28
co-stimulation, the T-cell receptor interacts with antigens to
activate T cells, which migrate to tumors, upregulate the
expression levels of immune checkpoints, such as cytotoxic T
lymphocyte antigen 4 (CTLA-4) and programmed cell death 1, and
produce cytokines such as IFN-γ, which leads to the expression of
programmed cell death ligand 1 (PD-L1) on tumor cells (48). CTLA-4 and PD-L1 are negative
regulators that inhibit T-cell activation and induce tumor cell
immune escape (49,50). Therefore, numerous efforts have been
devoted to the development of inhibitors targeting immune
checkpoints, including ipilimumab and nivolumab; these antibodies
promote antitumor CD8+ cytotoxic T lymphocyte (CTL)
responses in patients with melanoma (51). In addition, CD4+ T cells
promote both the effector and the memory functions of CTLs and
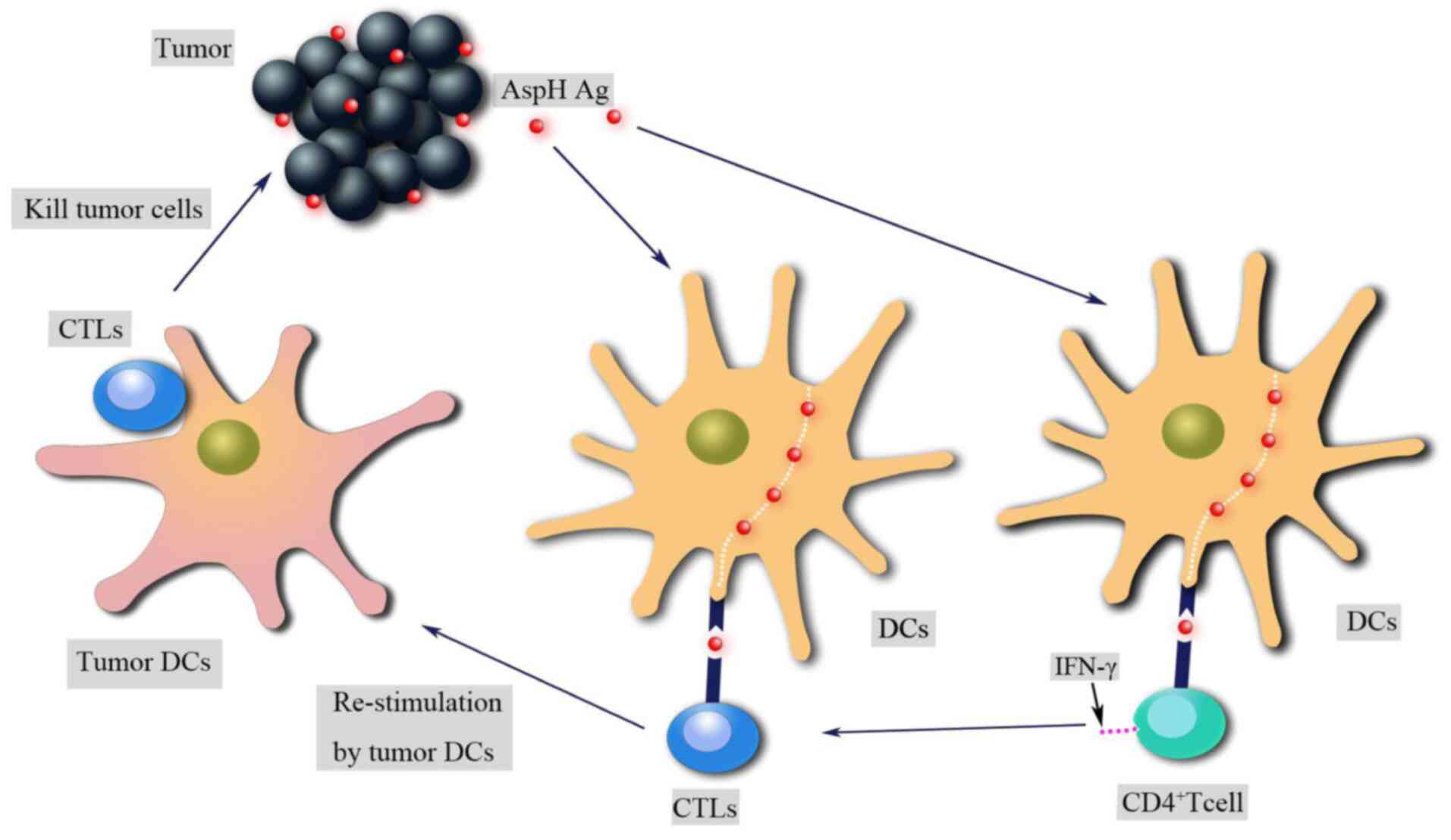
enhance their antitumor responses (51). The AspH protein is exposed to the
extracellular environment of tumor cells and can be recognized and
attacked by the host immune system (52). AspH contains both HLA class I- and
class II-limited epitopes, which stimulate AspH antigen-specific
CD4+ and CD8+ T-cell responses in human and
animal models to elicit antitumor effects (Fig. 3) (52). λ phage nanoparticles expressing
human AspH-derived proteins and AspH protein-loaded dendritic cells
(DCs) migrate from the blood to lymph nodes to activate antigen
specific CD4+ and CD8+ T cells; subsequently,
T helper (Th)1 and Th2 immune responses are induced to promote
lymphocytic infiltration and widespread necrosis in tumors
(52,53).
HCC is the primary hepatic malignancy, with the
highest incidence (~75%) among liver cancer worldwide in 2019
(54). Although therapeutic efforts
have improved over the last few decades, the mortality rate of HCC
has increased by 2.8 and 3.4% per year in men and women,
respectively (55). Therefore,
there is an urgent need for new treatment methods and a deeper
understanding of HCC. The relevance of AspH modification in HCC has
been extensively studied, and several studies have revealed that
AspH is highly expressed in HCC and is associated with cell
proliferation, invasion and malignant transformation (1,11,31,56–58).
AspH binding to GSK3β inhibits its phosphorylation and
inactivation, and blocks the interactions with the upstream kinases
AKT and p38 (47). Inhibition of
AspH enzymatic activity promotes HCC cell senescence and therefore
delays tumor progression by increasing the phosphorylation of GSK3β
and p16 expression (Fig. 2B)
(47). Additionally, a previous
study has revealed that AspH promotes cell proliferation by
upregulating cyclin D1 and c-Myc expression (59). MicroRNA (miR)-200a, an upstream
target gene of AspH that is rarely detected in liver tumor tissues
and cell lines, suppresses cyclin D1 and c-Myc expression by
downregulating AspH expression (59). Another study has revealed that AspH
expression can be upregulated by insulin and IGF-1 in HCC (45). Insulin and IGF-1 stimulated AspH
expression by increasing the phosphorylation of MAPK, ERK and AKT
to enhance cell motility and invasiveness (45). Malignant phenotypes, such as tumor
cell proliferation, migration, invasion and metastasis of HCC, are
partially due to the activation of insulin and IGF-1, which
increases AspH expression and subsequently activates the Notch
signaling cascade (23,30,31).
In addition, in HCC cells treated with an SMI (MO-I-1100) of
β-hydroxylase, the activation of Notch signaling was inhibited, and
the abilities of cell migration, invasion and metastasis were
decreased compared with in untreated counterparts (23). Decreased copy number and dysfunction
of mitochondrial DNA (mtDNA) are associated with the malignant
phenotypes of HCC (60). AspH
upregulation can destroy the integrity of mtDNA by blocking histone
H2A member X-mitochondrial transcription factor A signaling,
resulting in abnormal mitochondrial membrane potential, decreased
ATP generation and increased reactive oxygen species; however,
these effects can be reversed using small interfering RNAs against
AspH (60). AspH is distributed on
the surface of tumor cells, which makes it a target for
immunotherapy. AspH-loaded DCs inoculated into HCC tumor-bearing
mice can significantly suppress tumor growth, prolong survival and
delay recurrence following surgical resection (52). Furthermore, both in healthy donors
and patients with HCC, compared with α-fetoprotein-loaded DCs,
AspH-loaded DCs can stimulate the activation of antigen-specific
CD4+ T cells and CD8+ CTLs, which are
important to initiate antitumor immune responses (61–63).
CC accounted for 10–25% of primary liver tumors
globally in 2011, with a poor prognosis due to a lack of early
diagnosis and effective treatment (64,65).
It has been demonstrated that AspH is highly expressed in CC, while
AspH upregulation is not observed in normal tissues, non-neoplastic
epithelial cells and stromal cells (1). Clinicopathologically, AspH
upregulation promotes CC invasion, metastasis and poor prognosis
(66). Northern blotting suggests
that AspH expression is upregulated in CC to promote intrahepatic
spread and metastasis, since the AspH protein enhances the
sarcomatous change and epithelial-mesenchymal transition (EMT) of
CC (67). Additionally, the
activation of the Notch signaling pathway was detected in CC;
furthermore, enhanced Notch signaling and upregulation of
downstream target genes (such as HEY1 and HES1) were observed when
wild-type (wt)-AspH was transfected into HEK293 cells (68). As a cycle regulatory protein, cyclin
D1 upregulation is closely associated with the progression and
prognosis of CC (69). Knocking
down AspH significantly downregulated cyclin D1 expression;
however, overexpression of Notch partially rescued cyclin D1
levels, suggesting that AspH promotes CC cell proliferation through
Notch-mediated cyclin D1 expression (68). In addition, in in vitro
experiments, AspH-loaded DCs recruited CD3+ lymphocytes
in tumor tissues to inhibit intrahepatic CC development and
metastasis (70). In a CC model, a
large portion of BDEneu-C24 cells expressed the AspH protein,
causing a concentrated collagen matrix reaction during tumor
formation; however, CD3+ T cells can penetrate the
matrix barrier and reduce or delay the growth of CC (70). Recently, it has been reported that
AspH promotes the growth and progression of CC by regulating the
phosphorylation (and therefore inactivation) of RB1 (71). As a cancer suppressor gene, RB1
serves a vital role in cell cycle progression from
G0/G1 to S phase and cell senescence
(72,73). AspH upregulation increases the
protein-protein interaction between RB1 and cell cycle-associated
proteins, which in turn results in enhanced phosphorylation of RB1
(71). In addition, this
interaction can be suppressed by inhibitors of hydroxylase activity
(71).
PC was the third leading cause of cancer-associated
mortality in the USA in 2019, with the lowest 5-year relative
survival rate (9%) among all other types of cancer (74). The β-hydroxylase activity of AspH
was proven to boost the malignant phenotypes of PC cells, such as
cell migration, 2D and 3D invasion, EMT, ECM
degradation/remodeling, stemness, microsphere formation and
metastasis; these phenotypes were specifically suppressed using an
SMI (MO-I-1182) (75).
Additionally, it has been revealed that in a patient-derived
xenograft (PDX) murine model with spontaneous pulmonary metastasis
of human pancreatic ductal adenocarcinoma (PDAC), AspH promotes
primary tumor development and pulmonary metastasis; these harmful
effects can also be blocked using an SMI (MO-I-1182) (76). On the other hand, the proto-oncogene
SRC can be activated by AspH through direct interaction with
ADAM12/15 (75). Furthermore, the
highly expressed AspH-SRC axis is a marker of poor prognosis in PC
due to angiogenesis, invadopodia formation and metastasis (75,77).
AspH can promote PC growth by activating Notch signaling cascades
(29,78). Mechanistically, the ECD of Notch
receptors contains 36 consecutive EGF-like repeats for the
β-hydroxylation of aspartate/asparagine (27,29).
AspH directly stimulates Notch to upregulate downstream responsive
target genes, including HES1 and HEY1 (29). In AspH-overexpressing PDAC cell
lines, a human monoclonal antibody against AspH (SNS-622-DM1)
exerts significant antitumor effects by facilitating tumor cell
G2/M phase accumulation and increasing cellular cleaved
caspase 3 expression (79).
Additionally, SNS-622-DM1 can inhibit tumor growth and pulmonary
metastasis in a PDX murine model (79).
CRC is the fourth most deadly cancer, with ~900,000
deaths annually worldwide in 2019 (80). A bioinformatics analysis revealed
that the mRNA and protein levels of AspH are upregulated in CRC
compared with in normal tissues due to gene copy number variations
and promoter demethylation (81).
AspH accumulates at the invasive tumor margin, which may be
associated with cell invasion and infiltration (81). It has been recently reported that
Notch signaling recruits TGFβ-dependent neutrophils to drive CRC
metastasis; this pathway has an important role in the tumor
microenvironment and predicts a poor survival in patients with CRC
(82). Notably, knocking down AspH
or using specific SMIs (MO-I-1144) decreases Notch expression in
CRC, inhibiting tumor development and metastasis (81).
Studies have revealed the presence of AspH gene
amplification in invasive/advanced ductal carcinoma and AspH
silencing in normal adult breast tissues (32,83).
AspH upregulation activates the Notch signaling pathway, increases
the synthesis/release of pro-oncogenic exosomes and subsequently
enhances EMT, 2D and 3D invasion, stemness, angiogenesis and
metastases in breast cancer; these malignant phenotypes are
reversed using an SMI (MO-I-1182) (32). AspH stimulates the Notch cascade by
directly interacting with Notch receptors, ligands (JAGs) or
ADAM10/17 modulators (32). The
AspH-Notch axis is essential for the progression and prognosis of
breast cancer (32). In mouse
models, high levels of AspH induced more aggressive tumors,
characterized by rapid growth and extensive metastases (32). Notably, phage vaccination markedly
decreased pulmonary metastasis and enhanced survival in the 4T1
breast cancer model (with AspH overexpression) (53). On the other hand, in estrogen
receptor-positive breast cancer cells, the activation of MAPK and
PI3K cascades upregulates AspH mRNA expression when tamoxifen
sensitivity is decreased (84).
Furthermore, upregulated AspH expression decreases the
progression-free survival of patients with luminal B breast cancer
who received adjuvant endocrine therapy (84). Therefore, endocrine sensitivity of
endocrine-resistant breast cancer with high AspH expression may be
restored by blocking the MAPK and PI3K signaling pathways (84).
GBM was the most common primary malignant brain
tumor among adults worldwide in 2016 (85). Via analyzing whole genome
alternative splicing events in 498 GBM cases, it was revealed that
AspH expression is upregulated in GBM and is associated with the
onset and progression of cancer (86). A previous study has demonstrated
that protein levels of AspH and of the proliferation-associated
protein Ki-67 are upregulated in more aggressive GBM cases compared
with well differentiated cases (87). Furthermore, AspH knockdown or SMI
(MO-I-1100, MO-I-400, MO-I-500 and MO-I-1151) treatment targeting
hydroxylase activity decreases the viability and directional
motility of GBM cells (87).
Moreover, shorter progression-free survival and overall survival
are associated with AspH upregulation and HIF-1α expression in
patients with GBM, analyzed using immunohistochemistry (87). The Cancer Genome Atlas gene database
revealed that AspH and HIF-1α were significantly upregulated in the
mesenchymal subtype of GBM (87).
This demonstrates that both AspH and HIF-1α may be involved in
mesenchymal transformation and may subsequently induce aggressive
and invasive phenotypes (87).
Similarly to the aforementioned types of cancer,
modulation of AspH function serves a critical role in endometrial
cancer (EC), neuroblastoma, non-small cell lung carcinoma (NSCLC)
and gastric cancer (88–92). Compared with normal cell lines, AspH
expression was upregulated in EC cell lines, while miR-135a
expression was downregulated (88).
Cell Counting Kit-8 and wound-healing assays revealed that cell
proliferation and migration were decreased by miR-135a
overexpression. Conversely, high levels of AspH led to increased
cell proliferation and migration, and miR-135a overexpression
decreased the luciferase activity of EC cells transfected with
wt-AspH 3′-untranslated region (UTR) but not mutant-AspH 3′-UTR
(88). AspH upregulation restored
the inhibitory effects of miR-135a on EC cells (88). These observations suggest that
miR-135a affects EC growth and invasion by regulating AspH levels
(88). AspH expression was
significantly increased in neuroblastoma cells compared with in
CNS-derived primitive neuroectodermal tumor cells. Mechanistically,
insulin and IGF-1 increased directional motility by inducing AspH
expression (89). However,
treatment with AKT, ERK or cyclin-dependent kinase 5 (CDK-5)
inhibitors significantly decreased insulin- and IGF-1-stimulated
AspH mRNA expression and motility (89). These results suggest that ERK, AKT
and CDK-5 signaling may mediate insulin and IGF-1 regulation of
AspH at the level of transcription (89). In addition, high expression levels
of AspH significantly enhanced neuroblastoma Sy5y cell motility,
while the inhibition of AspH by antisense oligodeoxynucleotides
decreased the motility of Sy5y cells and enhanced the expression
levels of p21/Waf1 and p16, indicating that AspH is involved in
tumor invasion and metastasis (90). In NSCLC, FB50 immunohistochemical
staining revealed a marked increase in AspH expression,
particularly in squamous cell carcinoma (91). High levels of AspH immunoreactivity
are associated with poor survival and prognosis in patients with
NSCLC, and AspH upregulation may increase the potential for tumor
invasiveness and metastatic spread due to alterations in cell shape
and adhesion (91). Finally, as a
truncated isoform of AspH, humbug expression has been reported to
be upregulated in several gastric cancer cell lines, especially in
highly aggressive cells (92). High
expression levels of humbug increased the anchorage-independent
cell proliferation capability according to a colony formation
assay; additionally, Transwell migration assays revealed that
overexpression of humbug can promote cell migration and invasion
compared with control vector-transfected cells (92). Therefore, humbug may be a molecule
that affects the development and progression of gastric cancer
(92).
An increasing number of studies have revealed that
AspH expression is upregulated in several types of human tumor. Its
hydroxylase activity serves an essential role in promoting
malignant tumor phenotypes, including growth, proliferation,
invasion and metastasis. The present review discussed multiple key
signaling pathways and mechanisms underlying the function of AspH
in cancer. Notably, AspH activates Notch and PI3K-dependent
signaling pathways, delays tumor cell senescence, destroys the
integrity of mitochondria and subsequently leads to tumor
development and a poor prognosis (Table
I). Therefore, different specific and selective SMIs targeting
hydroxylase activity have been designed and have revealed promising
results in vitro and in vivo. Additionally, the
versatile function of AspH in the immune system has been
investigated over the last decade. Phage vaccination and DCs fused
to the AspH protein yield substantial antitumor effects in animal
models. These studies indicate that AspH may become a novel
prognostic marker and an immunotarget for antitumor agents.
Although there has been some progress with respect to the role of
AspH in tumor development, further investigations are required to
improve the efficacy of cancer treatment and provide additional
benefits to clinical patients.
Not applicable.
The present review was supported by a grant from the
National Natural Science Foundation of China (grant no.
81272681).
Not applicable.
HZ and WZ designed the study. WZ and XW wrote the
manuscript. JH prepared the figures. BB reviewed and edited the
manuscript. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lavaissiere L, Jia S, Nishiyama M, De La
Monte S, Stern AM, Wands JR and Friedman PA: Overexpression of
human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular
carcinoma and cholangiocarcinoma. J Clin Invest. 98:1313–1323.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korioth F, Gieffers C and Frey J: Cloning
and characterization of the human gene encoding aspartyl
beta-hydroxylase. Gene. 150:395–399. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinchuk JE, Henderson NL, Burn TC, Huber
R, Ho SP, Link J, O'Neil KT, Focht RJ, Scully MS, Hollis JM, et al:
Aspartyl beta-hydroxylase (Asph) and an evolutionarily conserved
isoform of Asph missing the catalytic domain share exons with
junctin. J Biol Chem. 275:39543–39554. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Q, VanDusen WJ, Petroski CJ, Garsky
VM, Stern AM and Friedman PA: Bovine liver aspartyl
beta-hydroxylase: Purification and characterization. J Biol Chem.
266:14004–14010. 1991.PubMed/NCBI
|
|
5
|
McGinnis K, Ku GM, VanDusen WJ, Fu J,
Garsky V, Stern AM and Friedman PA: Site-directed mutagenesis of
residues in a conserved region of bovine aspartyl (asparaginyl)
beta-hydroxylase: Evidence that histidine 675 has a role in binding
Fe2+. Biochemistry. 35:3957–3962. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stenflo J, Holme E, Lindstedt S,
Chandramouli N, Huang LH, Tam JP and Merrifield RB: Hydroxylation
of aspartic acid in domains homologous to the epidermal growth
factor precursor is catalyzed by a 2-oxoglutarate-dependent
dioxygenase. Proc Natl Acad Sci USA. 86:444–447. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pfeffer I, Brewitz L, Krojer T, Jensen SA,
Kochan GT, Kershaw NJ, Hewitson KS, McNeill LA, Kramer H, Münzel M,
et al: Aspartate/asparagine-β-hydroxylase crystal structures reveal
an unexpected epidermal growth factor-like domain substrate
disulfide pattern. Nat Commun. 10:49102019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gronke RS, VanDusen WJ, Garsky VM, Jacobs
JW, Sardana MK, Stern AM and Friedman PA: Aspartyl
beta-hydroxylase: In vitro hydroxylation of a synthetic peptide
based on the structure of the first growth factor-like domain of
human factor IX. Proc Natl Acad Sci USA. 86:3609–3613. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia S, VanDusen WJ, Diehl RE, Kohl NE,
Dixon RA, Elliston KO, Stern AM and Friedman PA: cDNA cloning and
expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J
Biol Chem. 267:14322–14327. 1992.PubMed/NCBI
|
|
10
|
Treves S, Feriotto G, Moccagatta L,
Gambari R and Zorzato F: Molecular cloning, expression, functional
characterization, chromosomal localization, and gene structure of
junctate, a novel integral calcium binding protein of
sarco(endo)plasmic reticulum membrane. J Biol Chem.
275:39555–39568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruix J and Llovet JM: Prognostic
prediction and treatment strategy in hepatocellular carcinoma.
Hepatology. 35:519–524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong CS, Kwon SJ and Kim DH: Multiple
functions of junctin and junctate, two distinct isoforms of
aspartyl beta-hydroxylase. Biochem Biophys Res Commun. 362:1–4.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones LR, Zhang L, Sanborn K, Jorgensen AO
and Kelley J: Purification, primary structure, and immunological
characterization of the 26-kDa calsequestrin binding protein
(junctin) from cardiac junctional sarcoplasmic reticulum. J Biol
Chem. 270:30787–30796. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siggs OM, Souzeau E and Craig JE: Loss of
ciliary zonule protein hydroxylation and lens stability as a
predicted consequence of biallelic ASPH variation. Ophthalmic
Genet. 40:12–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abarca Barriga HH, Caballero N, Trubnykova
M, Castro-Mujica MDC, La Serna-Infantes JE, Vásquez F and Hennekam
RC: A novel ASPH variant extends the phenotype of Shawaf-Traboulsi
syndrome. Am J Med Genet Part A. 176:2494–2500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kulkarni N, Lloyd IC, Ashworth J, Biswas
S, Black GCM and Clayton-Smith J; NIHR BioResource Consortium, :
Traboulsi syndrome due to ASPH mutation: An under-recognised cause
of ectopia lentis. Clin Dysmorphol. 28:184–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel N, Khan AO, Mansour A, Mohamed JY,
Al-Assiri A, Haddad R, Jia X, Xiong Y, Mégarbané A, Traboulsi EI
and Alkuraya FS: Mutations in ASPH cause facial dysmorphism, lens
dislocation, anterior-segment abnormalities, and spontaneous
filtering blebs, or Traboulsi syndrome. Am J Hum Genet. 94:755–759.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dinchuk JE, Focht RJ, Kelley JA, Henderson
NL, Zolotarjova NI, Wynn R, Neff NT, Link J, Huber RM, Burn TC, et
al: Absence of post-translational aspartyl beta-hydroxylation of
epidermal growth factor domains in mice leads to developmental
defects and an increased incidence of intestinal neoplasia. J Biol
Chem. 277:12970–12977. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gundogan F, Elwood G, Greco D, Rubin LP,
Pinar H, Carlson RI, Wands JR and de la Monte SM: Role of
aspartyl-(asparaginyl) beta-hydroxylase in placental implantation:
Relevance to early pregnancy loss. Hum Pathol. 38:50–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Song K, Xue T, Xue XP, Huyan T,
Wang W and Wang H: The distribution and expression profiles of
human aspartyl/asparaginyl beta-hydroxylase in tumor cell lines and
human tissues. Oncol Rep. 24:1257–1264. 2010.PubMed/NCBI
|
|
21
|
Ince N, de La Monte SM and Wands JR:
Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is
associated with malignant transformation. Cancer Res. 60:1261–1266.
2000.PubMed/NCBI
|
|
22
|
Zou Q, Hou Y, Wang H, Wang K, Xing X, Xia
Y, Wan X, Li J, Jiao B, Liu J, et al: Hydroxylase activity of ASPH
promotes hepatocellular carcinoma metastasis through
epithelial-to-mesenchymal transition pathway. EBioMedicine.
31:287–298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aihara A, Huang CK, Olsen MJ, Lin Q, Chung
W, Tang Q, Dong X and Wands JR: A cell-surface β-hydroxylase is a
biomarker and therapeutic target for hepatocellular carcinoma.
Hepatology. 60:1302–1313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Avila JL and Kissil JL: Notch signaling in
pancreatic cancer: Oncogene or tumor suppressor? Trends Mol Med.
19:320–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Zang C, Liu XS and Aster JC: The
role of notch receptors in transcriptional regulation. J Cell
Physiol. 230:982–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wharton KA, Johansen KM, Xu T and
Artavanis-Tsakonas S: Nucleotide sequence from the neurogenic locus
Notch implies a gene product that shares homology with proteins
containing EGF-like repeats. Cell. 43:567–581. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Penton AL, Leonard LD and Spinner NB:
Notch signaling in human development and disease. Semin Cell Dev
Biol. 23:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong X, Lin Q, Aihara A, Li Y, Huang CK,
Chung W, Tang Q, Chen X, Carlson R, Nadolny C, et al: Aspartate
β-hydroxylase expression promotes a malignant pancreatic cellular
phenotype. Oncotarget. 6:1231–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cantarini MC, de La Monte SM, Pang M, Tong
M, D'Errico A, Trevisani F and Wands JR: Aspartyl-asparagyl beta
hydroxylase over-expression in human hepatoma is linked to
activation of insulin-like growth factor and Notch signaling
mechanisms. Hepatology. 44:446–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung W, Kim M, de la Monte S, Longato L,
Carlson R, Slagle BL, Dong X and Wands JR: Activation of signal
transduction pathways during hepatic oncogenesis. Cancer Lett.
370:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Q, Chen X, Meng F, Ogawa K, Li M, Song
R, Zhang S, Zhang Z, Kong X, Xu Q, et al: ASPH-notch axis guided
exosomal delivery of prometastatic secretome renders breast cancer
multi-organ metastasis. Mol Cancer. 18:1562019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaupel P, Mayer A and Höckel M: Tumor
hypoxia and malignant progression. Methods Enzymol. 381:335–354.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lawton M, Tong M, Gundogan F, Wands JR and
de La Monte SM: Aspartyl-(asparaginyl) beta-hydroxylase,
hypoxia-inducible factor-alpha and Notch cross-talk in regulating
neuronal motility. Oxid Med Cell Longev. 3:347–356. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sivasankaran B, Degen M, Ghaffari A, Hegi
ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M,
Mergenthaler S, et al: Tenascin-C is a novel RBPJkappa-induced
target gene for Notch signaling in gliomas. Cancer Res. 69:458–465.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wagner EF and Nebreda ÁR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giorgetti S, Ballotti R, Kowalski-Chauvel
A, Tartare S and Van Obberghen E: The insulin and insulin-like
growth factor-I receptor substrate IRS-1 associates with and
activates phosphatidylinositol 3-kinase in vitro. J Biol Chem.
268:7358–7364. 1993.PubMed/NCBI
|
|
43
|
Hermanto U, Zong CS and Wang LH:
Inhibition of mitogen-activated protein kinase kinase selectively
inhibits cell proliferation in human breast cancer cells displaying
enhanced insulin-like growth factor I-mediated mitogen-activated
protein kinase activation. Cell Growth Differ. 11:655–664.
2000.PubMed/NCBI
|
|
44
|
Vuori K and Ruoslahti E: Association of
insulin receptor substrate-1 with integrins. Science.
266:1576–1578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de la Monte SM, Tamaki S, Cantarini MC,
Ince N, Wiedmann M, Carter JJ, Lahousse SA, Califano S, Maeda T,
Ueno T, et al: Aspartyl-(asparaginyl)-beta-hydroxylase regulates
hepatocellular carcinoma invasiveness. J Hepatol. 44:971–983. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ngeow KC, Friedrichsen HJ, Li L, Zeng Z,
Andrews S, Volpon L, Brunsdon H, Berridge G, Picaud S, Fischer R,
et al: BRAF/MAPK and GSK3 signaling converges to control MITF
nuclear export. Proc Natl Acad Sci USA. 115:E8668–E8677. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iwagami Y, Huang CK, Olsen MJ, Thomas JM,
Jang G, Kim M, Lin Q, Carlson RI, Wagner CE, Dong X and Wands JR:
Aspartate β-hydroxylase modulates cellular senescence through
glycogen synthase kinase 3β in hepatocellular carcinoma.
Hepatology. 63:1213–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Borst J, Ahrends T, Bąbała N, Melief CJM
and Kastenmüller W: CD4+ T cell help in cancer immunology and
immunotherapy. Nat Rev Immunol. 18:635–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tomimaru Y, Mishra S, Safran H,
Charpentier KP, Martin W, De Groot AS, Gregory SH and Wands JR:
Aspartate-β-hydroxylase induces epitope-specific T cell responses
in hepatocellular carcinoma. Vaccine. 33:1256–1266. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Iwagami Y, Casulli S, Nagaoka K, Kim M,
Carlson RI, Ogawa K, Lebowitz MS, Fuller S, Biswas B, Stewart S, et
al: Lambda phage-based vaccine induces antitumor immunity in
hepatocellular carcinoma. Heliyon. 3:e004072017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Petrick JL and McGlynn KA: The changing
epidemiology of primary liver cancer. Curr Epidemiol Reports.
6:104–111. 2019. View Article : Google Scholar
|
|
55
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual report to the nation on the status of cancer,
1975–2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tomimaru Y, Koga H, Yano H, de la Monte S,
Wands JR and Kim M: Upregulation of T-cell factor-4
isoform-responsive target genes in hepatocellular carcinoma. Liver
Int. 33:1100–1112.. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong
WM, Xia Y, Zou QF, Xi T, Shen F, et al: Overexpression of
aspartyl-(asparaginyl)-β-hydroxylase in hepatocellular carcinoma is
associated with worse surgical outcome. Hepatology. 52:164–173.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xue T, Su J, Li H and Xue X: Evaluation of
HAAH/humbug quantitative detection in the diagnosis of
hepatocellular carcinoma. Oncol Rep. 33:329–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yao WF, Liu JW and Huang DS: Mir-200a
inhibits cell proliferation and EMT by down-regulating the ASPH
expression levels and affecting ERK and PI3K/Akt pathways in human
hepatoma cells. Am J Transl Res. 10:1117–1130. 2018.PubMed/NCBI
|
|
60
|
Tang C, Hou Y, Wang H, Wang K, Xiang H,
Wan X, Xia Y, Li J, Wei W, Xu S, et al: Aspartate β-hydroxylase
disrupts mitochondrial DNA stability and function in hepatocellular
carcinoma. Oncogenesis. 6:e3622017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shimoda M, Tomimaru Y, Charpentier KP,
Safran H, Carlson RI and Wands J: Tumor progression-related
transmembrane protein aspartate-β-hydroxylase is a target for
immunotherapy of hepatocellular carcinoma. J Hepatol. 56:1129–1135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Marzo AL, Kinnear BF, Lake RA, Frelinger
JJ, Collins EJ, Robinson BW and Scott B: Tumor-specific CD4 + T
cells have a major ‘post-licensing’ role in CTL mediated anti-tumor
immunity. J Immunol. 165:6047–6055. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kennedy R and Celis E: Multiple roles for
CD4+ T cells in anti-tumor immune responses. Immunol Rev.
222:129–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Weber SM, Jarnagin WR, Klimstra D,
DeMatteo RP, Fong Y and Blumgart LH: Intrahepatic
cholangiocarcinoma: Resectability, recurrence pattern, and
outcomes. J Am Coll Surg. 193:384–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maeda T, Taguchi K, Aishima S, Shimada M,
Hintz D, Larusso N, Gores G, Tsuneyoshi M, Sugimachi K, Wands JR
and de la Monte SM: Clinicopathological correlates of aspartyl
(asparaginyl) beta-hydroxylase over-expression in
cholangiocarcinoma. Cancer Detect Prev. 28:313–318. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yoo HJ, Yun BR, Kwon JH, Ahn HS, Seol MA,
Lee MJ, Yu GR, Yu HC, Hong B, Choi K and Kim DG: Genetic and
expression alterations in association with the sarcomatous change
of cholangiocarcinoma cells. Exp Mol Med. 41:102–115. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang CK, Iwagami Y, Aihara A, Chung W, de
la Monte S, Thomas JM, Olsen M, Carlson R, Yu T, Dong X and Wands
J: Anti-tumor effects of second generation β-hydroxylase inhibitors
on cholangiocarcinoma development and progression. PLoS One.
11:e01503362016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sugimachi K, Aishima S, Taguchi K, Tanaka
S, Shimada M, Kajiyama K, Sugimachi K and Tsuneyoshi M: The role of
overexpression and gene amplification of cyclin D1 in intrahepatic
cholangiocarcinoma. J Hepatol. 35:74–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Noda T, Shimoda M, Ortiz V, Sirica AE and
Wands JR: Immunization with aspartate-β-hydroxylase-loaded
dendritic cells produces antitumor effects in a rat model of
intrahepatic cholangiocarcinoma. Hepatology. 55:86–97. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang CK, Iwagami Y, Zou J, Casulli S, Lu
S, Nagaoka K, Ji C, Ogawa K, Cao KY, Gao JS, et al: Aspartate
beta-hydroxylase promotes cholangiocarcinoma progression by
modulating RB1 phosphorylation. Cancer Lett. 429:1–10. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Narita M, Nũnez S, Heard E, Narita M, Lin
AW, Hearn SA, Spector DL, Hannon GJ and Lowe SW: Rb-mediated
heterochromatin formation and silencing of E2F target genes during
cellular senescence. Cell. 113:703–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ogawa K, Lin Q, Li L, Bai X, Chen X, Chen
H, Kong R, Wang Y, Zhu H, He F, et al: Aspartate β-hydroxylase
promotes pancreatic ductal adenocarcinoma metastasis through
activation of SRC signaling pathway. J Hematol Oncol. 12:1442019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ogawa K, Lin Q, Li L, Bai X, Chen X, Chen
H, Kong R, Wang Y, Zhu H, He F, et al: Prometastatic secretome
trafficking via exosomes initiates pancreatic cancer pulmonary
metastasis. Cancer Lett. 481:63–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jove R and Hanafusa H: Cell transformation
by the viral src oncogene. Annu Rev Cell Biol. 3:31–56. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hou G, Xu B, Bi Y, Wu C, Ru B, Sun B and
Bai X: Recent advances in research on aspartate β-hydroxylase
(ASPH) in pancreatic cancer: A brief update. Bosn J Basic Med Sci.
18:297–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nagaoka K, Bai X, Ogawa K, Dong X, Zhang
S, Zhou Y, Carlson RI, Jiang ZG, Fuller S, Lebowitz MS, et al:
Anti-tumor activity of antibody drug conjugate targeting
aspartate-β-hydroxylase in pancreatic ductal adenocarcinoma. Cancer
Lett. 449:87–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Benelli R, Costa D, Mastracci L, Grillo F,
Olsen MJ, Barboro P, Poggi A and Ferrari N:
Aspartate-β-hydroxylase: A promising target to limit the local
invasiveness of colorectal cancer. Cancers (Basel). 12:9712020.
View Article : Google Scholar
|
|
82
|
Jackstadt R, van Hooff SR, Leach JD,
Cortes-Lavaud X, Lohuis JO, Ridgway RA, Wouters VM, Roper J,
Kendall TJ, Roxburgh CS, et al: Epithelial NOTCH signaling rewires
the tumor microenvironment of colorectal cancer to drive
poor-prognosis subtypes and metastasis. Cancer Cell. 36:319–336.e7.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kadota M, Sato M, Duncan B, Ooshima A,
Yang HH, Diaz-Meyer N, Gere S, Kageyama S, Fukuoka J, Nagata T, et
al: Identification of novel gene amplifications in breast cancer
and coexistence of gene amplification with an activating mutation
of PIK3CA. Cancer Res. 69:7357–7365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shimoda M, Hori A, Wands JR, Tsunashima R,
Naoi Y, Miyake T, Tanei T, Kagara N, Shimazu K, Kim SJ and Noguchi
S: Endocrine sensitivity of estrogen receptor-positive breast
cancer is negatively correlated with aspartate-β-hydroxylase
expression. Cancer Sci. 108:2454–2461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen X, Zhao C, Guo B, Zhao Z, Wang H and
Fang Z: Systematic profiling of alternative mRNA splicing signature
for predicting glioblastoma prognosis. Front Oncol. 9:9282019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sturla LM, Tong M, Hebda N, Gao J, Thomas
JM, Olsen M and de la Monte SM: Aspartate-β-hydroxylase (ASPH): A
potential therapeutic target in human malignant gliomas. Heliyon.
2:e002032016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen X, Jin P, Tang H and Zhang L:
miR-135a acts as a tumor suppressor by targeting ASPH in
endometrial cancer. Int J Clin Exp Pathol. 12:3384–3389.
2019.PubMed/NCBI
|
|
89
|
Lahousse SA, Carter JJ, Xu XJ, Wands JR
and de la Monte SM: Differential growth factor regulation of
aspartyl-(asparaginyl)-β-hydroxylase family genes in SH-Sy5y human
neuroblastoma cells. BMC Cell Biol. 7:412006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sepe PS, Lahousse SA, Gemelli B, Chang H,
Maeda T, Wands JR and de la Monte SM: Role of the
aspartyl-asparaginyl-beta-hydroxylase gene in neuroblastoma cell
motility. Lab Invest. 82:881–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luu M, Sabo E, de la Monte SM, Greaves W,
Wang J, Tavares R, Simao L, Wands JR, Resnick MB and Wang L:
Prognostic value of aspartyl (asparaginyl)-beta-hydroxylase/humbug
expression in non-small cell lung carcinoma. Hum Pathol.
40:639–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lee JH: Overexpression of humbug promotes
malignant progression in human gastric cancer cells. Oncol Rep.
19:795–800. 2008.PubMed/NCBI
|