Tumor immunotherapy is a crucial treatment approach
following surgery, radiotherapy and chemotherapy. Currently,
immunotherapy is considered to be the most common treatment method
for tumors (1). Although tumor
cells cause a strong immune response, cancer persists, which may be
attributed to the fact that the immune response against tumor cells
is insufficient to prevent the development of cancer or that the
transient immune response triggers specific immune tolerance
mechanisms in tumor cells (2). A
healthy immune system maintains immune balance using co-suppressing
receptors and their ligands, known as immune checkpoint blockers
(ICBs). Therefore, tumor cells can escape immune attacks by
breaking the balance of the immune checkpoints (3). In recent years, the successful
application of combined signal receptor-targeted immune therapy has
attracted widespread attention (4,5).
Multiple clinical studies have demonstrated that monoclonal
antibodies (mAb) targeting the inhibitory receptors cytotoxic
T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell
death-1 (PD-1) exhibit favorable therapeutic effects in a variety
of human malignancies, including melanoma, non-small cell lung
cancer, renal cell carcinoma, bladder cancer and Hodgkin lymphoma
(6–8). However, despite the promising results
achieved by ICBs, there is still a considerable number of patients
who cannot benefit from the currently available therapies (9). To further exploit the advantages of
immune checkpoint inhibitor therapy, it is necessary to explore
novel immune checkpoints as therapeutic targets for patients with
cancer (10).
In recent years, nectin receptors and nectin-like
molecule (NECL) proteins have received extensive attention as
targets for cancer immunotherapy (11). NECLs are more extensively expressed
than nectins and exert more abundant functions (12). NECL-1 and NECL-4 mediate the
interaction between axons and participate in Schwann cell
differentiation and myelination (12,13).
NECL-2 functions as a tumor suppressor and immune surveillance
modulator (14). NECL-5 serves a
crucial role among NECLs due to its unique expression profile as
described below. NECL-5 is also known as CD155. CD155 also serves
as a poliovirus receptor (PVR), which promotes the migration and
proliferation of tumor cells (14,15).
CD155 is a member of the immunoglobulin superfamily, characterized
by the presence of an immunoglobulin domain V and C1-like and C2
domains in the extracellular region (16). CD155 has four splicing isoforms,
defined as α, β, γ and δ. The α and δ isoforms contain a
transmembrane domain, while the α isoform contains a longer
C-terminal domain and an immunoreceptor tyrosine-based inhibitory
motif (ITIM) necessary for the intrinsic biology of tumor cells
(17). However, the β and γ
subtypes lack transmembrane domains, and their biological functions
have not been elucidated (17).
CD155 can recruit protein-tyrosine phosphatase SHP-2 via the ITIM
domain to initiate signal transduction (18,19),
cell adhesion (20), motility
(18,21), proliferation and survival (22). CD155 is also involved in tumor
immune response. When CD155 is upregulated in different types of
tumor cells, the activating receptor CD226 and the inhibitory
receptors T cell immunoreceptor with Ig and ITIM domains (TIGIT)
and CD96, which are expressed on the cell surface of T and natural
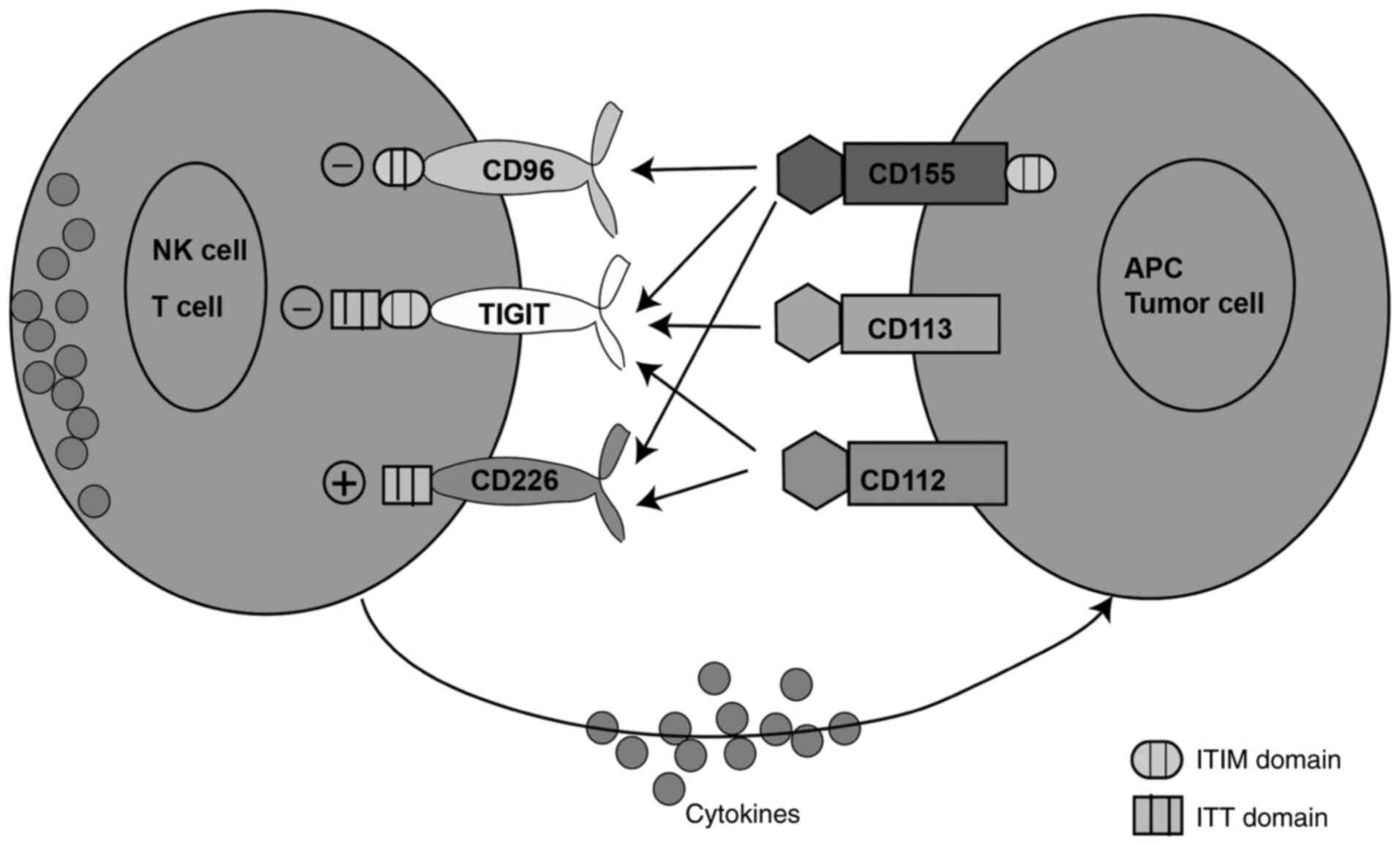
killer (NK) cells, recognize and bind to tumor cells (23). CD155 exhibits the highest binding
capacity with TIGIT, followed medium binding capacity with CD96 and
the lowest binding capacity with CD226 (Fig. 1) (24,25).
The increased expression of CD155 in tumors may be
caused by different mechanisms. Firstly, the chemotherapy approach
used at present to treat patients with multiple myeloma (MM)
damages the DNA of tumor cells and inhibits the expression of DNA
polymerase, thereby affecting the replication capacity of highly
proliferating malignant cells. However, standard-dose chemotherapy
usually causes a powerful immunosuppressive effect (35,36).
Ataxia telangiectasia mutated (ATM) is a serine-threonine kinase
activated when cells are exposed to DNA double-strand breaks. ATM
is involved in cell cycle regulation, apoptosis and DNA repair
(37). Similar to ATM, Ataxia
telangiectasia and Rad3-related protein (ATR) serves a vital role
in cell cycle signal transduction and DNA damage response (38). In MM cells treated with doxorubicin
and melphalan, CD155 was upregulated at both the protein and mRNA
levels, depending on the activation status of the DNA damage
sensors, ATM and ATR (36).
Secondly, it has been suggested that the Ras
oncogene mediates the upregulation of CD155 via the
Raf/MEK/ERK/activator protein-1 signaling pathway (39). Therefore, a MEK inhibitor can block
the Ras-mediated activation of the CD155 promoter. Additionally, it
has been demonstrated that fibroblast growth factor also
upregulates the expression of CD155 via the same signaling pathway
(39).
Thirdly, sonic hedgehog (Shh), a vital morphogen, is
co-expressed in the same locations with CD155 during development.
The Shh signaling pathway serves an important role in cell
differentiation and proliferation (40). Abnormal activation of Shh-Gli has
been associated with different types of human cancer (40,41). A
previous study indicated that treatment of human NTERA-2 cells with
purified Shh protein upregulated the mRNA expression of CD155
(42).
Fourthly, immune cells expressing CD155 have been
detected in primary tumors and tumor-infiltrating leukocytes of the
draining lymph nodes; however, it seems to be mainly limited to
myeloid cells (43). Stimulation of
murine bone marrow-derived macrophages and B lymphocytes with
lipopolysaccharide (LPS) has been indicated to upregulate the
NF-κB-dependent CD155 expression in vitro (44–46).
Furthermore, Toll-like receptor agonists, including LPS, have been
reported to increase the expression of CD155 in dendritic cells
(DCs) (47).
High expression of CD155 is associated with the
invasive and migratory abilities of tumor cells. Focal adhesions
are composed of integrins and related cytoplasmic plaque proteins,
including talin, vinculin, α-actin, tenascin and paxillin and
numerous protein kinases. Integrins interact with the extracellular
matrix (ECM) (48). In
vitro, ECM proteins bind to CD155, suggesting that CD155 may
mediate cell-matrix interactions (20). Stimulation of CD155α with its ligand
has been indicated to promote the Src kinase-mediated
phosphorylation of ITIM, focal adhesion kinase (FAK) and paxillin,
ultimately inhibiting cell adhesion and enhancing cell
proliferation. The activation of CD155α also enhanced the
platelet-derived growth factor (PDGF)-mediated cell migration.
Taken together, these findings indicated that CD155 could regulate
cell adhesion and movement (Fig.
2A) (18). CD155, PDGF receptor
and integrin αvβ3 synergistically participate in the formation of a
ternary complex on the leading edge of a cell (49–51).
Ternary complexes serve a vital role in the dynamics of the leading
edge. It has been demonstrated that this ternary complex promotes
the activation of Src and DNA-binding protein RAP1, which in turn
activates Rac and inhibits RhoA, thereby resulting in improved cell
movement (16). In addition, a
previous study has revealed that CD155 could regulate cell
proliferation via enhancing the activation of the Ras/Raf/MEK/ERK
signaling pathway (22). Protein
sprouty homolog 2 (SPRY2) is a negative regulator of the growth
factor-induced cell proliferation (52,53).
SPRY2 is phosphorylated by the Src kinase following growth factor
signaling, thereby inhibiting the activation of growth
factor-induced Ras signals (52).
CD155 prolongs the activation of the proliferation-associated
signals induced by the inhibition of SPRY2, which is a negative
regulator of the Ras/Raf/MAPK signaling pathway involved in the
regulation of organogenesis, differentiation, cell migration and
proliferation (54). As illustrated
in Fig. 2B, SPRY2 can be released
from CD155 and phosphorylated by Src to inhibit the Ras pathway and
cell proliferation (16).
Additionally, CD155 promotes tumor growth. A
previous study has demonstrated that CD155 enhanced the activation
of the serum-induced Ras/Raf/MEK/ERK signaling pathway, upregulated
cyclin D2 and E, downregulated p27Kip1 and finally inhibited the
G0/G1 cell cycle arrest of NIH3T3 cells
(Fig. 2C) (22). Furthermore, knockdown of CD155
reduced the size and weight of tumors in colon cancer models and
attenuated the metastasis rate of tumors in several other mouse
tumor models (6,43,55).
To identify costimulatory or inhibitory molecules on
activated human T cells, Yu et al (24) conducted a genome search to recognize
genes with immunomodulatory receptor protein domains in T helper
(Th)17 cells. They identified a specific gene expressed on T and NK
cells, which encoded a protein containing variable immunoglobulin
domains and an ITIM, which was named TIGIT. It is considered that
TIGIT is an inhibitory receptor mainly expressed on NK,
CD8+ T, CD4+ T cells and Tregs (24). TIGIT consists of an extracellular
immunoglobulin variable domain, a type I transmembrane domain, a
short intracellular domain with an ITIM and an immunoglobulin tail
tyrosine (ITT)-like motif (24). It
has been indicated that TIGIT can bind to three ligands on tumor
cells, namely CD155, CD112 and CD113. CD155 is a high-affinity
ligand of TIGIT (Fig. 1) (24,56).
TIGIT acts as a receptor for PVR and induces intracellular
signaling, while it may serve as a competitive inhibitor of CD226
(24,56).
It has been reported that TIGIT is upregulated in
tumor-infiltrating lymphocytes (TILs) in acute myeloid leukemia
(AML), melanoma, multiple myeloma, non-small cell lung cancer,
gastric cancer and hepatocellular carcinoma (56–64).
It has been demonstrated that the expression of TIGIT was
upregulated in CD8+ T lymphocytes and tumor-infiltrating
Tregs and NK cells (65–67). TIGIT has been associated with poor
clinical outcomes in cancer. For example, the expression of TIGIT
in TILs of patients with melanoma and CD8+ T cells in
the peripheral blood of patients with gastric cancer was associated
with tumor metastasis and poor survival (62,68,69).
Furthermore, a strong association between the expression of TIGIT
on CD8+ T cells in the peripheral blood and the
recurrence of AML after transplantation has been revealed (58). In addition, a recent study on
endometrial cancer demonstrated that the high expression of TIGIT
on tumor-infiltrating NK cells was positively correlated with the
severity of the disease (Table I)
(70).
Currently, several mechanisms of the
CD155/TIGIT-mediated inhibition of effector T and NK cells have
been discovered. Immunoactivating receptor CD226 is a
co-stimulatory molecule expressed on NK cells, T lymphocytes,
monocytes and B cells, which competes with TIGIT for binding with
CD155 (71). TIGIT directly
interacts with CD226 on the cell surface and attenuates the ability
of CD226 to form homodimers. These results indicate that TIGIT can
reduce the activity of CD226 during the anti-tumor and anti-viral T
cell response (57). In NK cells,
TIGIT has been demonstrated to mediate inhibitory signals via ITIM
(56), while its engagement could
directly inhibit T cell activation and proliferation (Fig. 3A) (55,71,72).
CD155 is also expressed on DCs. It has been suggested that the
CD155-TIGIT interaction can regulate T cell-driven immune responses
via inducing interleukin IL-10 expression in DCs. Furthermore,
TIGIT-modified mature DCs can promote the production of IL-10 and
attenuate that of IL-12 (Fig. 3B)
(24). TIGIT is also highly
expressed in Tregs (66). Indeed,
it has been revealed that compared with TIGIT− Tregs,
the inhibitory effect of TIGIT+ Tregs was enhanced. In
addition, TIGIT+ Tregs were indicated to produce a
higher amount of the immunosuppressive cytokine IL-10 compared with
TIGIT− Tregs, which not only contributed to
immunosuppression in the tumor microenvironment but also promoted a
tolerogenic phenotype in DCs (Fig.
3C) (73). Additionally, IL-10
and fibrinogen-like protein 2 (Fgl2) secreted by TIGIT+
Treg cells could synergistically inhibit the secretion of IL-12 and
IL-23 by activated DCs, thereby inhibiting the Th1 and Th17 immune
responses (73). It has been also
demonstrated that TIGIT+ Treg cells secreted high
amounts of Fgl2, resulting in the inhibition of Th1 and Th17 cell
differentiation, but not Th2 differentiation (73–74).
Th2 cells can promote type 2 tumor-associated macrophage (TAM2)
differentiation by secreting IL-4. Therefore, it was demonstrated
that TAM2 suppressed T cell immune responses via secreting
inhibitory cytokines, such as IL-10 and TGF-β, and depleted
nutrients in the microenvironment via secreting arginase 1 (Arg1),
indoleamine 2 and 3-dioxygenase, further weakening T cell function
(Fig. 3D) (75).
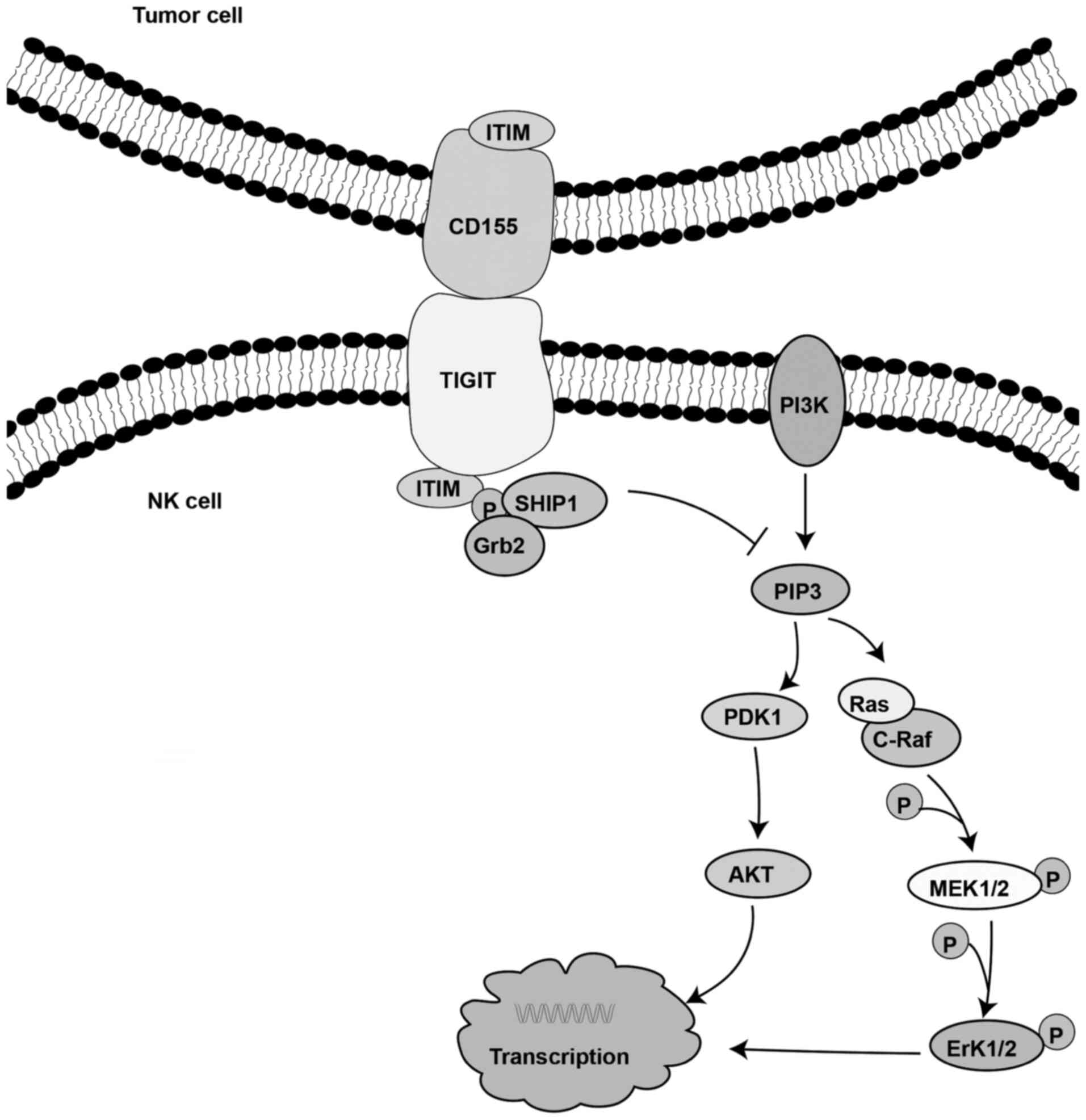
TIGIT expressed on NK cells binds with its ligand on
tumor cells to directly inhibit NK cell toxicity (56). It has been reported that the
decreased NK cell activity is mediated by the phosphorylation of
the ITT-like domain of the cytoplasmic tail of TIGIT. Following
binding of TIGIT to its ligand, the ITT-like motif is
phosphorylated at Tyr225. Subsequently, the complex binds to the
cytosolic adaptor growth factor receptor-bound protein 2 (Grb2) and
recruits SH2-containing inositol phosphatase-1 (SHIP-1), which in
turn promotes signal inhibition, thereby attenuating the cytotoxic
activity of NK cells (76). It has
also been demonstrated that TIGIT recruits SHIP-1 to prematurely
terminate the activation of AKT, ERK and MEK, thereby inhibiting NK
cell cytotoxicity (Fig. 4)
(76).
β-arrestins are common inhibitory protein. The
interaction of β-arrestins and with their partners can regulate
cell positioning, translocation and stability, while they are also
involved in the regulation of the immune response (77). Emerging evidence has suggested that
IκBα can directly interact with β-arrestin 2 to prevent
phosphorylation and degradation of IκBα (77). Furthermore, TNF receptor-associated
factor 6 (TRAF6) serves an important role in NF-κB signal
transduction (77–79). Therefore, a previous study
demonstrated that β-arrestin 2 could bind to the ITT-like motif of
phosphorylated TIGIT, recruit SHIP-1 and reduce TRAF6
autoubiquitination, thereby preventing NF-κB activation and
inhibiting IFN-γ production. However, SHIP-1 silencing could
restore IFN-γ production (Fig. 5A)
(77).
It has been reported that the CD155/TIGIT
interaction reduces the activation of the AKT/mTOR pathway,
resulting in the inhibition of metabolism and cytokine production.
In a co-culture system of the human gastric cancer cells SGC7901
with CD8+ T cells, downregulation of CD155 in SGC7901
cells increased the phosphorylation of AKT, p70S6 kinase and 4E
binding protein 1 in CD8+ T cells (63). In addition, glucose uptake, lactic
acid production and IFN-γ secretion were also increased.
Furthermore, TIGIT has been indicated to inhibit the metabolic
pathway in CD8+ T cells (63). TIGIT blockade was demonstrated to
promote the activation of the AKT/mTOR pathway, leading to
metabolism upregulation and cytokine production in CD8+
T cells. These findings indicated that CD155/TIGIT signaling in
CD8+ T cells reduced the activation of the AKT/mTOR
pathway, thereby resulting in metabolism downregulation and
suppression of cytokine production (Fig. 5B) (63).
In mouse tumor models, TIGIT deficiency
significantly attenuated the proliferation of B16F10 melanoma and
MC38 colon cancer cells, compared with wild-type controls (65). In addition, a recent study has
demonstrated that the serum levels of monoclonal immunoglobulin
proteins in TIGIT-deficient mice increased, thus reducing the tumor
burden and prolonging the survival period of mice, suggesting that
TIGIT inhibited the anti-tumor responses in multiple myeloma.
Importantly, blocking TIGIT with monoclonal antibodies could
enhance the function of CD8+ T effector cells and
inhibit the development of multiple myeloma (60). Furthermore, it was demonstrated that
treatment with anti-TIGIT could significantly delay tumor growth in
a mouse model of transgenic head and neck squamous cell carcinoma
and enhance the anti-tumor immune response via activating
CD8+ T effector cells and reducing the number of Tregs.
In vitro, co-culture studies revealed that anti-TIGIT
treatment could significantly eliminate the immunosuppressive
ability of myeloid suppressor cells via downregulating Arg1 and
preventing Treg inhibition by reducing the secretion of TGF-β1
(33). In AML, TIGIT downregulation
using the small interfering RNA technology, could inhibit the
immunosuppressive effect of TIGIT+ CD8+ T
cells in the blood, thereby resulting in increased secretion of
IFN-γ and TNF-α and decreased apoptosis (58).
A clinical trial has indicated that the mismatch
repair status can predict the clinical benefit of treatment with
the anti-PD-1 mAb pembrolizumab. However, one third of patients
still did not respond to anti-PD-1 therapy (80). Preclinical studies on the effect of
TIGIT on regulating anti-tumor immune responses indicated that
TIGIT combined with current immunotherapy was a promising target.
For example, it has been suggested that a combined treatment
approach with TIGIT and PD-1 or T-cell immunoglobulin mucin
receptor 3 (TIM-3) on malignant tumors was more beneficial compared
with a single-targeted one (11,65).
Treatment of patients with TIGIT/PD-1 co-blockade also enhanced the
expansion and cytotoxicity of CD8+ T cells in gastric
cancer (63), non-Hodgkin lymphoma
(81) and glioblastoma (82) compared with a single blockade
therapy. Furthermore, the combination of anti-TIGIT therapy with
other immune checkpoint inhibitors has been also examined in mouse
models. For instance, treatment of TIGIT−/− mice with
anti-TIM-3 antibodies significantly reduced tumor growth compared
with TIGIT−/− deficiency alone (65). Blocking TIGIT could also increase
the cytokine expression (such as TNF-α, IFN-γ and IL-2) of
TIGIT+ cells; however, this was less effective than the
combined blockade of TIGIT, PD-1 and TIM-3 (83).
Immunotherapy can provide an important anti-tumor
response in patients with advanced and metastatic tumors. However,
even in sensitive types of tumors, a large proportion of patients
does not respond to these therapies. Therefore, novel immune
targets as a supplement to immunotherapy are urgently needed. TIGIT
is considered a promising target that exhibits an immunomodulatory
role in several processes involved in carcinogenesis (84). It is hypothesized that the combined
application of anti-TIGIT and anti-PD-1 will have a synergistic
effect similar to that of the combined application of anti-PD-1 and
anti-CTLA-4 antibodies in melanoma, which can increase the response
rate to therapy (85). A growing
number of preclinical studies have indicated that combining
anti-TIGIT with other immunological agents can increase the
anti-tumor response in patients (Table
II). Therefore, more favorable results in future studies based
on the application of anti-TIGIT therapy are anticipated.
Not applicable.
The study was conducted at Qilu Hospital, Shandong
University and was supported by the National Natural Science
Foundation of China (grant no. 81572559) and the Key Research
Project of Shandong Province (grant no. 2017CXGC1210).
Not applicable.
LL wrote the manuscript; XWY and YS designed and
drew the figures; SH and JHZ edited the figures; YZZ conceived and
designed the study. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Trapani J and Darcy P: Immunotherapy of
cancer. Aust Fam Physician. 46:194–199. 2017.PubMed/NCBI
|
|
2
|
Willimsky G and Blankenstein T: Sporadic
immunogenic tumours avoid destruction by inducing T-cell tolerance.
Nature. 437:141–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang G, Tai R, Wu Y, Yang S, Wang J, Yu X,
Lei L, Shan Z and Li N: The expression and immunoregulation of
immune checkpoint molecule VISTA in autoimmune diseases and
cancers. Cytokine Growth Factor Rev. 52:1–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv3242016. View Article : Google Scholar
|
|
6
|
Smyth MJ, Ngiow SF, Ribas A and Teng MWL:
Combination cancer immunotherapies tailored to the tumour
microenvironment. Nat Rev Clin Oncol. 13:143–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kruger S, Ilmer M, Kobold S, Cadilha BL,
Endres S, Ormanns S, Schuebbe G, Renz BW, D'Haese JG, Schloesser H,
et al: Advances in cancer immunotherapy 2019-latest trends. J Exp
Clin Cancer Res. 38:2682019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darvin P, Toor SM, Nair VS and Elkord E:
Immune checkpoint inhibitors: Recent progress and potential
biomarkers. Exp Mol Med. 50:1652018. View Article : Google Scholar
|
|
10
|
Sharma P and Allison JP: Immune checkpoint
targeting in cancer therapy: Toward combination strategies with
curative potential. Cell. 161:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dougall WC, Kurtulus S, Smyth MJ and
Anderson AC: TIGIT and CD96: New checkpoint receptor targets for
cancer immunotherapy. Immunol Rev. 276:112–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kakunaga S, Ikeda W, Itoh S,
Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A and Takai Y: Nectin-Like
molecule-1/TSLL1/SynCAM3: A neural tissue-specific
immunoglobulin-like cell-cell adhesion molecule localizing at
non-junctional contact sites of presynaptic nerve terminals, axons
and glia cell processes. J Cell Sci. 118:1267–1277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spiegel I, Adamsky K, Eshed Y, Milo R,
Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN and
Peles E: A central role for Necl4 (SynCAM4) in Schwann cell-axon
interaction and myelination. Nat Neurosci. 10:861–869. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuramochi M, Fukuhara H, Nobukuni T, Kanbe
T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura
T, et al: TSLC1 is a tumor-suppressor gene in human non-small-cell
lung cancer. Nat Genet. 27:427–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujito T, Ikeda W, Kakunaga S, Minami Y,
Kajita M, Sakamoto Y, Monden M and Takai Y: Inhibition of cell
movement and proliferation by cell-cell contact-induced interaction
of necl-5 with nectin-3. J Cell Biol. 171:165–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takai Y, Miyoshi J, Ikeda W and Ogita H:
Nectins and nectin-like molecules: Roles in contact inhibition of
cell movement and proliferation. Nat Rev Mol Cell Biol. 9:603–615.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koike S, Horie H, Ise I, Okitsu A, Yoshida
M, Iizuka N, Takeuchi K, Takegami T and Nomoto A: The poliovirus
receptor protein is produced both as membrane-bound and secreted
forms. EMBO J. 9:3217–3224. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oda T, Ohka S and Nomoto A: Ligand
stimulation of CD155alpha inhibits cell adhesion and enhances cell
migration in fibroblasts. Biochem Biophys Res Commun.
319:1253–1264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yusa Si, Catina TL and Campbell KS: SHP-1-
and phosphotyrosine-independent inhibitory signaling by a killer
cell Ig-like receptor cytoplasmic domain in human NK cells. J
Immunol. 168:5047–5057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lange R, Peng X, Wimmer E, Lipp M and
Bernhardt G: The poliovirus receptor CD155 mediates cell-to-matrix
contacts by specifically binding to vitronectin. Virology.
285:218–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reymond N, Imbert AM, Devilard E, Fabre S,
Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A,
et al: DNAM-1 and PVR regulate monocyte migration through
endothelial junctions. J Exp Med. 199:1331–1341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kakunaga S, Ikeda W, Shingai T, Fujito T,
Yamada A, Minami Y, Imai T and Takai Y: Enhancement of serum- and
platelet-derived growth factor-induced cell proliferation by
necl-5/tage4/poliovirus receptor/CD155 through the ras-raf-MEK-ERK
signaling. J Biol. 27:36419–36425. 2004.
|
|
23
|
Molfetta R, Zitti B, Lecce M, Milito ND,
Stabile H, Fionda C, Cippitelli M, Gismondi A, Santoni A and
Paolini R: CD155: A multi-functional molecule in tumor progression.
Int J Mol Sci. 21:9222020. View Article : Google Scholar
|
|
24
|
Yu X, Harden K, Gonzalez LC, Francesco M,
Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al: The
surface protein TIGIT suppresses T cell activation by promoting the
generation of mature immunoregulatory dendritic cells. Nat Immunol.
10:48–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deuss FA, Watson GM, Fu Z, Rossjohn J and
Berry R: Structural basis for CD96 immune receptor recognition of
nectin-like protein-5, CD155. Structure. 5:219–228. 2019.
View Article : Google Scholar
|
|
26
|
Bevelacqua V, Bevelacqua Y, Candido S,
Skarmoutsou E, Amoroso A, Guarneri C, Strazzanti A, Gangemi P,
Mazzarino MC, D'Amico F, et al: Nectin like-5 overexpression
correlates with the malignant phenotype in cutaneous melanoma.
Oncotarget. 3:882–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakai R, Maniwa Y, Tanaka Y, Nishio W,
Yoshimura M, Okita Y, Ohbayashi C, Satoh N, Ogita H, Takai Y and
Hayashi Y: Overexpression of necl-5 correlates with unfavorable
prognosis in patients with lung adenocarcinoma. Cancer Sci.
101:1326–1330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishiwada S, Sho M, Yasuda S, Shimada K,
Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N and Nakajima
Y: Clinical significance of CD155 expression in human pancreatic
cancer. Anticancer Res. 35:2287–2297. 2015.PubMed/NCBI
|
|
29
|
Smazynski J, Hamilton PT, Thornton S,
Milne K, Wouters MC, Webb JR and Nelson BH: The immune suppressive
factors CD155 and PD-L1 show contrasting expression patterns and
immune correlates in ovarian and other cancers. Gynecol Oncol.
158:167–177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pende D, Spaggiari GM, Marcenaro S,
Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I,
Zambello R, et al: Analysis of the receptor-ligand interactions in
the natural killer-mediated lysis of freshly isolated myeloid or
lymphoblastic leukemias: Evidence for the involvement of the
poliovirus receptor (CD155) and nectin-2 (CD112). Blood.
105:2066–2073. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gromeier M, Lachmann S, Rosenfeld MR,
Gutin PH and Wimmer E: Intergeneric poliovirus recombinants for the
treatment of malignant glioma. Proc Natl Acad Sci USA.
97:6803–6808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang DW, Huang M, Lin XS and Huang Q:
CD155 expression and its correlation with clinicopathologic
characteristics, angiogenesis, and prognosis in human
cholangiocarcinoma. Onco Targets Ther. 10:3817–3825. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang
LL, Wu H, Bu LL, Kulkarni AB, Zhang WF and Sun ZJ: Blockade of
TIGIT/CD155 signaling reverses t-cell exhaustion and enhances
antitumor capability in head and neck squamous cell carcinoma.
Cancer Immunol Res. 7:1700–1713. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iguchi-Manaka A, Okumura G, Kojima H, Cho
Y, Hirochika R, Bando H, Sato T, Yoshikawa H, Hara H and Shibuya A:
Increased soluble CD155 in the serum of cancer patients. PLoS One.
11:e01529822016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soriani A, Zingoni A, Cerboni C, Iannitto
ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C,
Petrucci MT, Guarini A, et al: ATM-ATR-Dependent up-regulation of
DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic
agents results in enhanced NK-cell susceptibility and is associated
with a senescent phenotype. Blood. 113:3503–3511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soriani A, Fionda C, Ricci B, Iannitto ML,
Cippitelli M and Santoni A: Chemotherapy-Elicited upregulation of
NKG2D and DNAM-1 ligands as a therapeutic target in multiple
myeloma. Oncoimmunology. 2:e266632014. View Article : Google Scholar
|
|
37
|
Lee JH and Paull TT: Activation and
regulation of ATM kinase activity in response to DNA double-strand
breaks. Oncogene. 26:7741–7748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vauzour D, Vafeiadou K, Rice-Evans C,
Cadenas E and Spencer JP: Inhibition of cellular proliferation by
the genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone is
mediated by DNA damage and activation of the ATR signalling
pathway. Arch Biochem Biophys. 468:159–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirota T, Irie K, Okamoto R, Ikeda W and
Takai Y: Transcriptional activation of the mouse
necl-5/tage4/PVR/CD155 gene by fibroblast growth factor or
oncogenic ras through the raf-MEK-ERK-AP-1 pathway. Oncogene.
24:2229–2235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers (Basel). 8:222016.
View Article : Google Scholar
|
|
41
|
Athar M, Li C, Kim AL, Spiegelman VS and
Bickers DR: Sonic hedgehog signaling in basal cell nevus syndrome.
Cancer Res. 74:4967–4975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Solecki DJ, Gromeier M, Mueller S,
Bernhardt G and Wimmer E: Expression of the human poliovirus
receptor/CD155 gene is activated by sonic hedgehog. J Biol Chem.
277:25697–25702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li XY, Das I, Lepletier A, Addala V, Bald
T, Stannard K, Barkauskas D, Liu J, Aguilera AR, Takeda K, et al:
CD155 loss enhances tumor suppression via combined host and
tumor-intrinsic mechanisms. J Clin Invest. 128:2613–2625. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Escalante NK, von Rossum A, Lee M and Choy
JC: CD155 on human vascular endothelial cells attenuates the
acquisition of effector functions in CD8 T cells. Arterioscler
Thromb Vasc Biol. 31:1177–1184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kamran N, Takai Y, Miyoshi J, Biswas SK,
Wong JSB and Gasser S: Toll-Like receptor ligands induce expression
of the costimulatory molecule CD155 on antigen-presenting cells.
PLoS One. 8:e544062013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pende D, Castriconi R, Romagnani P,
Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini
S, Rivera P, et al: Expression of the DNAM-1 ligands, Nectin-2
(CD112) and poliovirus receptor (CD155), on dendritic cells:
Relevance for natural killer-dendritic cell interaction. Blood.
107:2030–2036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gilfillan S, Chan CJ, Cella M, Haynes NM,
Rapaport AS, Boles KS, Andrews DM, Smyth MJ and Colonna M: DNAM-1
promotes activation of cytotoxic lymphocytes by nonprofessional
antigen-presenting cells and tumors. J Exp Med. 205:2965–2973.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gumbiner BM: Cell adhesion: The molecular
basis of tissue architecture and morphogenesis. Cell. 84:345–357.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Minami Y, Ikeda W, Kajita M, Fujito T,
Amano H, Tamaru Y, Kuramitsu K, Sakamoto Y, Monden M and Takai Y:
Necl-5/Poliovirus receptor interacts in cis with integrin
alphaVbeta3 and regulates its clustering and focal complex
formation. J Biol Chem. 282:18481–18496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Amano H, Ikeda W, Kawano S, Kajita M,
Tamaru Y, Inoue N, Minami Y, Yamada A and Takai Y: Interaction and
localization of necl-5 and PDGF receptor beta at the leading edges
of moving NIH3T3 cells: Implications for directional cell movement.
Genes Cells. 13:269–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takahashi M, Rikitake Y, Nagamatsu Y, Hara
T, Ikeda W, Hirata Ki and Takai Y: Sequential activation of rap1
and rac1 small G proteins by PDGF locally at leading edges of
NIH3T3 cells. Genes Cells. 13:549–569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Christofori G: Split personalities: The
agonistic antagonist sprouty. Nat Cell Biol. 5:377–379. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim HJ and Bar-Sagi D: Modulation of
signalling by sprouty: A developing story. Nat Rev Mol Cell Biol.
5:441–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Reich A, Sapir A and Shilo B: Sprouty is a
general inhibitor of receptor tyrosine kinase signaling.
Development. 126:4139–4147. 1999.PubMed/NCBI
|
|
55
|
Zheng Q, Wang B, Gao J, Xin N, Wang W,
Song X, Shao Y and Zhao C: CD155 knockdown promotes apoptosis via
AKT/bcl-2/bax in colon cancer cells. J Cell Mol Med. 22:131–140.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Stanietsky N, Simic H, Arapovic J, Toporik
A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al:
The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell
cytotoxicity. Proc Natl Acad Sci USA. 106:17858–17863. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T
cell effector function. Cancer Cell. 26:923–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kong Y, Zhu L, Schell TD, Zhang J, Claxton
DF, Ehmann WC, Rybka WB, George MR, Zeng H and Zheng H: T-Cell
immunoglobulin and ITIM domain (TIGIT) associates with
CD8+ T-cell exhaustion and poor clinical outcome in AML
patients. Clin Cancer Res. 22:3057–3066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chauvin JM, Pagliano O, Fourcade J, Sun Z,
Wang H, Sander C, Kirkwood JM, Chen Th, Maurer M, Korman AJ and
Zarour HM: TIGIT and PD-1 impair tumor antigen-specific
CD8+ T cells in melanoma patients. J Clin Invest.
125:2046–2058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guillerey C, Harjunpää H, Carrié N, Kassem
S, Teo T, Miles K, Krumeich S, Weulersse M, Cuisinier M and
Stannard K: TIGIT immune checkpoint blockade restores
CD8+ T-cell immunity against multiple myeloma. Blood.
132:1689–1694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee WJ, Lee YJ, Choi ME, Yun KA, Won CH,
Lee MW, Choi JH and Chang SE: Expression of lymphocyte-activating
gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM
domains in cutaneous melanoma and their correlation with programmed
cell death 1 expression in tumor-infiltrating lymphocytes. J Am
Acad Dermatol. 81:219–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
O'Brien SM, Klampatsa A, Thompson JC,
Martinez MC, Hwang WT, Rao AS, Standalick JE, Kim S, Cantu E,
Litzky LA, et al: Function of human tumor-infiltrating lymphocytes
in early-stage non-small cell lung cancer. Cancer Immunol Res.
7:896–909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He W, Zhang H, Han F, Chen X, Lin R, Wang
W, Qiu H, Zhuang Z, Liao Q, Zhang W, et al: CD155T/TIGIT signaling
regulates CD8+ T-cell metabolism and promotes tumor
progression in human gastric cancer. Cancer Res. 77:6375–6388.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang C, Wang Y, Xun X, Wang S, Xiang X,
Hu S, Cheng Q, Guo J, Li Z and Zhu J: TIGIT can exert
immunosuppressive effects on CD8+ T cells by the
CD155/TIGIT signaling pathway for hepatocellular carcinoma in
vitro. J Immunother. 43:236–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kurtulus S, Sakuishi K, Ngiow SF, Joller
N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK and Anderson AC: TIGIT
predominantly regulates the immune response via regulatory T cells.
J Clin Invest. 125:4053–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fourcade J, Sun Z, Chauvin JM, Ka M, Davar
D, Pagliano O, Wang H, Saada S, Menna C, Amin R, et al: CD226
opposes TIGIT to disrupt tregs in melanoma. JCI Insight.
26:e1211572018. View Article : Google Scholar
|
|
67
|
Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu
W, Wang Z, Wu Q, Peng H, Wei H, et al: Blockade of the checkpoint
receptor TIGIT prevents NK cell exhaustion and elicits potent
anti-tumor immunity. Nat Immunol. 19:723–732. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stålhammar G, Seregard S and Grossniklaus
HE: Expression of immune checkpoint receptors Indoleamine
2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus
nonmetastatic choroidal melanoma. Cancer Med. 8:2784–2792.
2019.PubMed/NCBI
|
|
69
|
Tang W, Pan X, Han D, Rong D, Zhang M,
Yang L, Ying J, Guan H, Chen Z and Wang X: Clinical significance of
CD8+ T cell immunoreceptor with Ig and ITIM
domains+ in locally advanced gastric cancer treated with
SOX regimen after D2 gastrectomy. Oncoimmunology. 8:e15938072019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Degos C, Heinemann M, Barrou J, Boucherit
N, Lambaudie E, Savina A, Gorvel L and Olive D: Endometrial tumor
microenvironment alters human NK cell recruitment, and resident NK
cell phenotype and function. Front Immunol. 10:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang B, Zhao W, Li H, Chen Y, Tian H, Li
L, Zhang L, Gao C and Zheng J: Immunoreceptor TIGIT inhibits the
cytotoxicity of human cytokine-induced killer cells by interacting
with CD155. Cancer Immunol Immunother. 65:305–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Joller N, Hafler JP, Brynedal B, Kassam N,
Spoerl S, Levin SD, Sharpe AH and Kuchroo VK: Cutting edge: TIGIT
has T cell-intrinsic inhibitory functions. J Immunol.
186:1338–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Joller N, Lozano E, Burkett PR, Patel B,
Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, et al: Treg cells
expressing the coinhibitory molecule TIGIT selectively inhibit
proinflammatory Th1 and Th17 cell responses. Immunity. 40:569–581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lozano E, Dominguez-Villar M, Kuchroo V
and Hafler DA: The TIGIT/CD226 axis regulates human T cell
function. J Immunol. 188:3869–3875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
De Vlaeminck Y, González-Rascón A,
Goyvaerts C and Breckpot K: Cancer-Associated myeloid regulatory
cells. Front Immunol. 7:1132016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu S, Zhang H, Li M, Hu D, Li C, Ge B,
Jin B and Fan Z: Recruitment of Grb2 and SHIP1 by the ITT-like
motif of TIGIT suppresses granule polarization and cytotoxicity of
NK cells. Cell Death Differ. 20:456–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B
and Pei G: Identification of beta-arrestin2 as a G protein-coupled
receptor-stimulated regulator of NF-kappaB pathways. Mol Cell.
14:303–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun L, Deng L, Ea CK, Xia ZP and Chen ZJ:
The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation
by BCL10 and MALT1 in T lymphocytes. Mol Cell. 14:289–301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen ZJ: Ubiquitination in signaling to
and activation of IKK. Immunol Rev. 246:95–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Asaoka Y, Ijichi H and Koike K: PD-1
blockade in tumors with mismatch-repair deficiency. N Engl J Med.
373:19792015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Josefsson SE, Beiske K, Blaker YN, Førsund
MS, Holte H, Østenstad B, Kimby E, Köksal H, Wälchli S, Bai B, et
al: TIGIT and PD-1 mark intratumoral T cells with reduced effector
function in B-cell non-hodgkin lymphoma. Cancer Immunol Res.
7:355–362. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hung AL, Maxwell R, Theodros D, Belcaid Z,
Mathios D, Luksik AS, Kim E, Wu A, Xia Y, Garzon-Muvdi T, et al:
TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity
and survival in GBM. Oncoimmunology. 7:e14667692018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang X, Zhang H, Chen L, Feng Z, Gao L
and Li Q: TIGIT expression is upregulated in T cells and causes T
cell dysfunction independent of PD-1 and Tim-3 in adult B lineage
acute lymphoblastic leukemia. Cell Immunol. 344:1039582019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Harjunpaa H and Guillerey C: TIGIT as an
emerging immune checkpoint. Clin Exp Immunol. 200:108–119. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Valsecchi ME: Combined nivolumab and
ipilimumab or monotherapy in untreated melanoma. N Engl J Med.
373:12702015. View Article : Google Scholar : PubMed/NCBI
|