Introduction
Epidemiological investigations have shown that
esophageal cancer is one of the most common malignant tumors of the
digestive tract in the world (1–3). Its
incidence and mortality rates rank 7th and 6th among all malignant
tumors worldwide according to global cancer statistics in 2018
(4). In recent years, neoadjuvant
therapy (5,6), minimally invasive surgery (7,8) and modern
precision radiotherapy (9) for the
treatments of esophageal cancer have improved survival times;
however, the overall efficacy is still not high, with a 5-year
survival rate of 18% based on cases diagnosed between 2005 and 2011
in North America (10). Natural
products are important resources for anticancer drugs (11,12), such
as gypenoside L and matrine. Studies have demonstrated that
gypenoside L inhibited the proliferation of esophageal cancer cells
and enhanced the sensitivity of them to chemotherapy (13), and matrine increased the inhibition
rate of cell proliferation by inducing cell autophagy (14). Therefore, there is an urgent
requirement to develop natural medicine with high efficiency and
minimal side effects to improve the treatment of esophageal cancer
and improve population health.
The perennial shrub, Lithocarpus polystachyus
Rehd, commonly known as ‘sweet tea’ in Chinese folk, is often used
as a source of sweets and traditional oriental medicine (15). Previous studies have shown that the
aqueous extract from the leaf of sweet tea inhibited breast cancer
proliferation and improved blood sugar status (16). Phlorizin (phloretin 2′-O-glucoside),
the main component of sweet tea, is an important member of the
dihydrochalcone family. Previous studies confirmed that phlorizin
was the main phenolic glucoside in the Malus species (17) and was one of the sodium-linked glucose
transporter inhibitors (18). A
series of bioactive functions of phlorizin have been discovered,
such as lowering blood sugar levels and improving memory, and
having anti-oxidative and anti-cancer properties (19–22).
The aim of the present study was to investigate the
therapeutic effects of phlorizin on esophageal cancer using
transcriptome sequencing and functional experiments, to provide
experimental evidence to develop phlorizin-based anticancer foods
or drugs.
Materials and methods
Phlorizin extraction
Sweet tea leaves were collected from woodland in
Bama county in Guangxi, China. The fresh sweet tea leaves were
dried, sieved and immersed in an ethanol solution. The mixture was
then extracted using ultrasonic extraction (40 kHz for 20 min at
room temperature) and vacuum filtration, concentrated by rotary
evaporation to remove the ethanol, then freeze-dried to obtain a
crude product. Subsequently, 5 g crude extract was added to water
and dissolved in a treated macroporous resin column for 30 min. The
column was washed with deionized water to remove impurities, then
the phlorizin was washed with 75% ethanol. The solution was eluted
to produce the eluate. The concentrated eluate was removed from the
ethanol and placed in a zero-degree environment to recrystallize.
The crystallized product was further filtered to obtain a dry
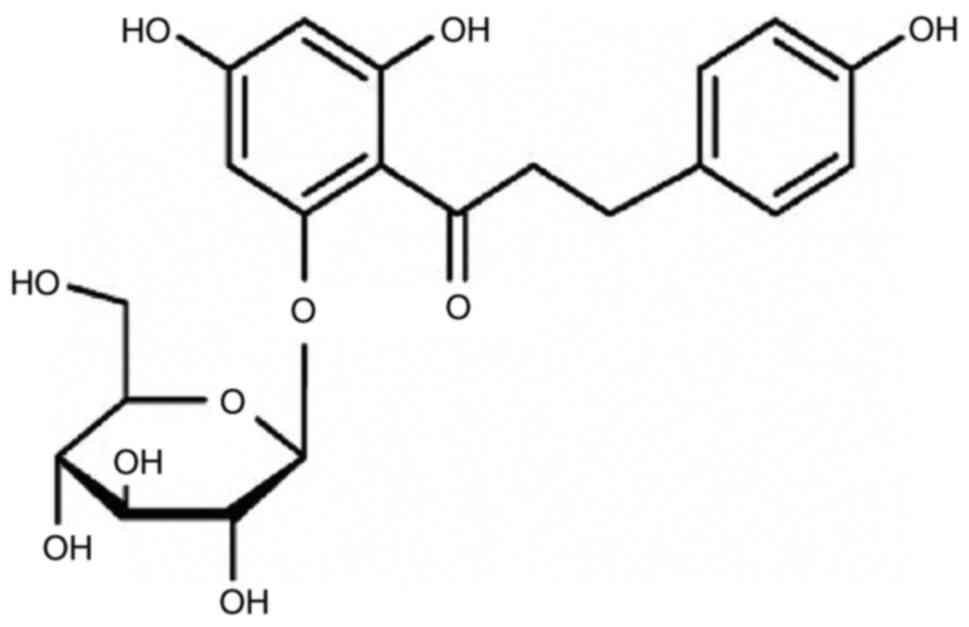
powder, which is a dry saponin monomer of phlorizin (Fig. 1).
Determination of the phlorizin content
of the dry powder
The purity of the dry powder (a dry saponin monomer
of phlorizin) was determined using a Waters ACQUITY ultra
performance liquid chromatography H-Class system (Waters
Corporation), equipped with an ACQUITY UPLC BEH C18 (1.7
µm; 2.1×50 mm; Waters Corporation) analytical column coupled with a
column filter and the column temperature was kept at 30°C. The
mobile phase consisted of water (A) and acetonitrile (B) in a
maintained ratio (73:27), with a flow rate of 0.2 ml/min. The
injection volume of the sample was 10 µl and the detection
wavelength was set at 285 nm. A total of 10.00 mg phlorizin
standard (Beijing Century Aoke Biotechnology Co., Ltd.) was
weighed, diluted with 10 ml methanol and completely dissolved to
obtain the internal standard. The purity of the dry saponin monomer
of phlorizin was at least 95%.
Cell culture and treatments
The human KYSE450 and KYSE30 cell lines were kindly
gifted from Dr Y. Shimada from the First Department of Surgery,
Hyogo College of Medicine (Hyogo, Japan). Both the cell lines were
then cultivated in RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.),
100 µg/ml streptomycin and 100 U/ml penicillin (Beijing Solarbio
Science and Technology Co., Ltd.) at 37°C in a humidified incubator
with 5% CO2. The cells were seeded in cell culture
plates overnight in complete medium, then treated with various
concentrations (0, 0.05, 0.10, 0.20, 0.40, 0.80 and 1.60 mM) of
phlorizin dissolved in dimethyl sulfoxide (DMSO; final
concentration <0.1%; Sigma-Aldrich; Merck KGaA).
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was utilized to detect the viability of
the cells. A total of 5×103 cells were seeded into each
well of a 96-well plate, then incubated with phlorizin at different
concentrations. At 24, 48 or 72 h, 10% CCK-8 solution was added to
the cells and incubated for another 2 h. The optical density was
measured at 450 nm using a microplate reader (Tecan Group). The
proliferation of the cells, treated with phlorizin, was normalized
to the control cells. The 50% inhibitory concentration
(IC50) in the two cell lines was calculated using SPSS
v23.0 (IBM Corporation).
Colony forming assay
The cells (3×105 cells/well) were seeded
in 6-well plates to 80% confluency. After treated with phlorizin at
0.20 or 0.80 mM for 24 h, the cells (2×103 cells/3 ml)
were digested with 0.25% trypsin (Thermo Fisher Scientific, Inc.)
and seeded into a 30 mm culture dish for 14 days. Cells without the
treatment of phlorizin served as a control. After fixing with 4%
paraformaldehyde, for 15 min at room temperature, followed by 0.1%
crystal violet staining for 30 min at room temperature, washing
with PBS and air-drying, the colonies were detected using an IX71
inverted fluorescent microscope (Olympus Corporation), at ×40
magnification.
Wound healing assay
Wound healing assay was used to determine cell
migration. The esophageal cancer cells (KYSE450 and KYSE30) were
seeded (3×105 cells/well) in a 6-well plate and cultured
at 37°C in a humidified incubator with 5% CO2. When the
cell monolayer was 100% confluent, the cells were scraped with a
sterile 200 µl pipette tip, then washed 3 times with PBS to remove
the cell debris. Subsequently, the cells were treated with
phlorizin (0.00, 0.20 and 0.80 mM) for 0, 24, 48 and 72 h. Images
of the wound gap were captured using an IX71 fluorescent microscope
(magnification, ×40; Olympus Corporation) at 0, 24, 48 and 72 h
after scratching. The distance between the edges of the wound was
then measured using ImageJ software (v1.42q; National Institutes of
Health).
Transwell assay
Transwell chambers (8-µm) coated with or without
Matrigel (incubated at 37°C for 5 h) (Corning, Inc.) were used to
determine the cell migratory and invasive abilities of the cells.
In brief, the esophageal cancer cells (3×105 cells/well)
were seeded in 6-well plates overnight and treated with phlorizin
(0.00, 0.20 and 0.80 mM) for 24 h. Subsequently, the cells
(1.5×105 cells/200 µl) were added to the upper chamber
with serum-free RPMI-1640 medium and incubated at 37°C in a
humidified incubator with 5% CO2 for 24 h. The
non-migratory and non-invasive cells were then washed away with a
cotton swab. The migrated or invaded cells in the lower chamber,
which were incubated with complete medium, were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet, both for 30
min at room temperature. An inverted fluorescent microscope
(Olympus Corporation) was used to count the invasive or migratory
cells at ×200 magnification.
RNA sequencing (RNA-Seq)
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the KYSE450
cells with or without 0.5 mM phlorizin for 48 h, in accordance with
manufacturer's instructions. A NanoPhotometer spectrophotometer
(Implen GmbH) was used to measure RNA purity. RNA integrity was
assessed using the RNA Nano 6000 Assay kit and the Bioanalyzer 2100
system (Agilent Technologies, Inc.). RNA-Seq libraries were
constructed using the NEB Next Ultra RNA Library Prep kit for
Illumina (cat. no. E7420L; New England Biolabs, Inc.). The
concentration of the RNA library was measured using a
Qubit® RNA Assay kit and a Qubit® 2.0
Flurometer (Thermo Fisher Scientific, Inc.) then the RNA was
diluted to 1 ng/µl. Poly-T oligo-attached magnetic beads were used
to enrich the RNA with a polyA tail for cDNA synthesis.
Subsequently, cDNA was ligated to a cBot Cluster Generation System
using TruSeq PE Cluster Kit v3-cBot-HS (cat. no. PE-401-3001;
Illumia, Inc.). The libraries were then sequenced on an Illumina
Hiseq 4000 platform (Illumina, Inc.) to generate 150 bp paired-end
reads. RNA-Seq and data collection was performed by Beijing Biomics
Biotech Co., Ltd.
Data preprocessing
Raw data in the FASTQ format were firstly processed
using in-house perl scripts (v5.32.1; Perl Foundation). Briefly,
clean data was obtained by removing reads containing adapter,
ploy-N and low-quality reads from the raw data. Clean and trimmed
FASTQ reads were aligned to the human hg19 genome using TopHat
(v2.0.12) (23). Cufflinks (v2.1.1)
(24) was used to assemble the mapped
reads of each sample. Fragments per kilobase of transcript per
million mapped reads was used to determine the transcription
abundance of each gene.
Biological function and pathway
enrichment analyses
The edgeR package (v3.26.8) from Bioconductor in R
language (http://www.bioconductor.org/) was used to identify
differentially expressed genes (DEGs) with the fold change of >2
and the false discovery rate of <0.01. Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analyses were performed to identify the potential enriched
biological functions of the DEGs. KEGG Orthology Based Annotation
System v2.0 software (http://kobas.cbi.pku.edu.cn) was used to performed
statistical enrichment analysis of the DEGs in the GO and KEGG
pathways. Q-value <0.05 was set as the cut-off for selecting the
significantly enriched GO terms and KEGG pathways.
Hoechst and annexin V staining
Hoechst33342 staining was used to observe the
nuclear morphology of the apoptotic cells. The esophageal cancer
cells (KYSE450 and KYSE30) were seeded (3×105
cells/well) in a 6-well plate and cultured at 37°C in a humidified
incubator with 5% CO2 overnight prior to phlorizin
treatment (0.00, 0.20 and 0.80 mM). After 48 h, the cells were
washed with PBS and fixed with 4% paraformaldehyde for 15 min at
room temperature, followed by staining with 3 µg/ml Hoechst33342
solution (Beijing Solarbio Science and Technology Co., Ltd.) for 10
min at room temperature in the dark. The stained cells were then
washed with PBS and observed using an IX71 fluorescent microscope
at ×400 magnification (Olympus Corporation).
Annexin V staining was used to detect apoptotic
cells using live cells. The KYSE450 and KYSE30 cells were seeded
(3.0×105 cells/well) in 6-well plates and cultured at
37°C in a humidified incubator with 5% CO2 overnight.
Subsequently, the cells were treated with 0.1% DMSO or phlorizin
(0.20 or 0.80 mM) for 48 h. After washing with PBS, the cells were
labeled with an Annexin V-FITC/PI apoptosis detection kit (BD
Biosciences) and were visualized using an IX71 fluorescent
microscope (magnification, ×100; Olympus Corporation).
Flow cytometry analysis
Flow cytometry analysis was also used for a cellular
apoptosis assay. Following 48 h-treatment of phlorizin, the cell
pellets were harvested and resuspended in 500 µl binding buffer
supplemented with 5 µl Annexin V-FITC and 5 µl PI (BD Biosciences)
for 20 min in dark, then detected using a flow cytometer (CytoFlex;
Beckman Coulter, Inc.). The percentage of apoptotic cells was
analyzed using FlowJo software (v10.0.7; Tree Star, Inc.).
Western blot analysis
The KYSE450 and KYSE30 esophageal cancer cells were
treated with phlorizin (0.00, 0.20 and 0.80 mM) for 48 h, then
lysed using RIPA buffer (Thermo Fisher Scientific, Inc.). The
supernatant containing the total protein, was collected by
centrifugation at 14,000 × g at 4°C for 17 min, then the
concentration of the total protein was detected using a BCA kit
(Beijing Solarbio Science and Technology Co., Ltd). Either a 10 or
12% SDS-PAGE (80 µg protein loaded per lane) was used to separate
the different proteins. Subsequently, the proteins were transferred
to nitrocellulose membranes. After blocking for 50 min with 5%
skimmed milk in TBS with 0.05% Tween-20 at room temperature, the
membranes were incubated with the primary antibody overnight at
4°C, then washed and incubated with HRP-conjugated secondary
antibodies for 30 min at 37°C. The target proteins were then
visualized using an ECL kit (GE Healthcare) and an E-Gel imager
(Universal Hood II; Bio-Rad Laboratories, Inc.), then analyzed
using ImageJ software (v1.42q; National Institutes of Health).
β-actin was used as the control. The following primary antibodies
were used: Anti-P62/SQSTM1 (1:10,000 dilution; cat. no. ab109012;
Abcam), anti-phosphorylated(p)-JAK2 (1:5,000 dilution; cat. no.
ab32101; Abcam), anti-p-STAT3 (1:5,000 dilution; cat. no. ab68153;
Abcam), anti-JAK2 (1:5,000 dilution; cat. no. ab108596; Abcam),
anti-STAT3 (1:5,000 dilution; cat. no. ab76315; Abcam),
anti-β-actin (1:2,000 dilution; cat. no. 60008-1-lg; Proteintech
Group, Inc.) and anti-LC3B (1:2,000 dilution; cat. no. 2775; Cell
Signaling Technology, Inc.). The following secondary antibodies
were used: Goat anti-mouse IgG (1:5,000 dilution; cat. no. S0002;
Affinity Biosciences Co., Ltd), goat anti-rabbit IgG (1:5,000; cat.
no. ZB-2301; OriGene Technologies, Inc.).
Monodansylcadaverine (MDC)
staining
Cell autophagy was investigated using a MDC assay
kit (Beijing Solarbio Science and Technology Co., Ltd). The
esophageal cancer cells were harvested and adjusted to a density of
1×106 cells/ml. The cell suspension was stained with MDC
stain for 20 min at room temperature and resuspended in collection
buffer according to the manufacturer's instructions. The cell
suspension was dropped onto a slide, then covered with the cover
slip. Cell autophagy was determined using an IX71 inverted
fluorescent microscope (magnification, ×100; Olympus
Corporation).
Statistical analysis
SPSS v23.0 (IBM Corporation) and GraphPad Prism v7.0
(GraphPad Software, Inc.) were used for data analysis. The data was
presented as the mean ± SEM. To analyze the differences among
groups, either an unpaired Student's t-test or one-way ANOVA was
used with Dunnett's post hoc test. P<0.05 (two-sided) was
considered to indicate a statistically significant difference.
Results
Phlorizin inhibits cell viability in
human esophageal cancer cells
A CCK-8 assay was used to determine the effect of
phlorizin on esophageal cancer cell viability. The results showed
that phlorizin inhibited the proliferation of esophageal cancer
cells (KYSE450 and KYSE30) in a time- and dose-dependent manner
(Fig. 2A and B). This inhibitory
effect was also confirmed by colony formation experiments (Fig. 2C-F).
Phlorizin modulates esophageal cancer
migration and invasion in vitro
To further validate the effect of phlorizin on tumor
migration and invasion, the aforementioned cells were treated with
phlorizin, at different concentrations and observed at different
time points. Wound healing assay showed a marked decrease in the
edge closure speed of the wound in phlorizin-treated cells,
indicating the inhibition of phlorizin on the migration of
esophageal cells (Fig. 3A-D). The
results from a Transwell assay further supported this result
(Fig. 3E and F). To determine the
cell invasive ability, a Matrigel assay was used. As illustrated in
Fig. 3G and H, the number of invaded
cancer cells in the lower chamber was significantly reduced by the
addition of phlorizin. Taken together, these results indicated that
phlorizin had a significant effect on the migration and invasion of
esophageal cancer cells.
 | Figure 3.Cell migratory and invasive abilities
of phlorizin-treated esophageal cancer cells. (A) Wound healing
assay was performed following treatment of the KYSE450 cells with
phlorizin and images were captured at 0, 24, 48 and 72 h later, and
the results were (B) statistically analyzed. Magnification, ×40.
(C) Wound healing assay was performed following treatment of the
KYSE30 cells with phlorizin and images were captured at 0, 24, 48
and 72 h later, and the results were (D) statistically analyzed.
Magnification, ×40. (E) Cell migration was determined using a
Transwell assay following treatment of the KYSE450 and KYSE30 cells
with phlorizin and the results were (F) statistically analyzed.
Magnification, ×200. (G) Cell invasion was evaluated using a
Matrigel assay following treatment of the KYSE450 and KYSE30 cells
with phlorizin and the results were (H) statistically analyzed.
Magnification, ×200. The results are from 3 independent experiments
and normalized to the control. *P<0.05, **P<0.01,
***P<0.001 vs. control. |
DEG identification and functional
annotation
To identify the genes regulated by phlorizin in
esophageal cancer cells, DEGs were identified using RNA-Seq. There
were 749 upregulated genes and 1,405 downregulated genes in the
KYSE450 cells treated with phlorizin compared with that in the
control (Fig. 4A and B). Furthermore,
the autophagy marker gene, P62/SQSTM1 had high expression levels,
which was of interest (Fig. 4B). In
addition, GO was used to analyze the biological functions of the
DEGs, including three aspects of biology: Biological processes,
cellular components, and molecular function. The top 20 GO terms of
the DEGs were primarily involved in biological processes and
molecular functions (Fig. 5A). KEGG
pathway analysis showed that the DEGs were significantly enriched
in ‘TNF signaling pathway’ (hsa04668), ‘NF-κB signaling pathway’
(hsa04064) and ‘Pathways in cancer’ (hsa05200) (Fig. 5B). According to the number of DEGs,
the top 5 KEGG pathways included ‘pathways in cancer’ (hsa05200),
‘MAPK signaling pathway’ (hsa04010), ‘HTLV-1 infection’ (hsa05166),
‘Cytokine-cytokine receptor interaction’ (hsa04060) and ‘PI3K/AKT
signaling pathway’ (hsa04151) (Fig.
5C). With respect to the KEGG classification, most of the DEGs
were involved in environmental information possessing and human
diseases (Fig. 5C).
Phlorizin induces the apoptosis of
esophageal cancer cells
As the KEGG pathway analysis showed that the DEGs
were significantly enriched in apoptosis-related pathways,
apoptosis analysis was performed. Hoechst33342 staining showed that
the KYSE30 and KYSE450 cells treated with phlorizin had notable
apoptosis characteristics, such as chromatin condensation and
nuclear fragmentation (Fig. 6A and
B). Consistently, Annexin V/PI staining showed that phlorizin
increased the number of apoptotic cells (Fig. 6C and D). In addition, flow cytometry
analysis showed that there were more early and total apoptotic
cells in esophageal cancer cells treated with phlorizin compared
with that in the control (Fig. 6E).
Notably, the high concentration of phlorizin (0.80 mM) resulted in
more apoptotic cells compared with that in cells treated with 0.20
mM phlorizin.
Phlorizin inhibits autophagy of
esophageal cancer cells
As aforementioned, phlorizin increased the mRNA
expression level of P62/SQSTM1 (Fig.
4B) and the DEGs were significantly enriched in the ‘MAPK
signaling pathway’ (Fig. 5C) in
esophageal cancer cells. It is well-known that the MAPK signaling
pathway (25,26) and P62/SQSTM1 (27–29) play
an important role in autophagy. Therefore, the effect of phlorizin
on autophagy in the esophageal cancer cells was investigated.
The cells treated with increasing concentration of
phlorizin showed an increased protein expression level of
P62/SQSTM1 and decreased protein expression level of LC3II
(Fig. 7A-D). As the critical
indicators of autophagic flux, the changes in the protein
expression level of P62/SQSTM1 and LC3II suggested that phlorizin
could be involved in cell autophagy.
In addition, MDC is a selective fluorescent marker
for autophagic vacuoles. The MDC staining results are presented in
Fig. 7E-G and showed that compared
with that in the control group, the cells treated with phlorizin
showed an increased number of autophagic cells in a dose-dependent
manner.
Phlorizin inhibits the expression of
the proteins in the JAK/STAT signaling pathway in esophageal cancer
cells
Bioinformatics analysis showed that phlorizin was
significantly enriched in the JAK2/STAT3 pathway in the KYSE450
cells (Fig. 5C). The JAK2/STAT3
signaling pathway has a regulatory effect on esophageal cancer
growth, metastasis and apoptosis (30–32). To
determine whether phlorizin was involved in JAK2 and STAT3
activation, the expression levels of the proteins involved in the
JAK2-STAT3 signaling pathway were analyzed using western blot
analysis. The results demonstrated that phlorizin not only
inhibited the phosphorylation of JAK2 and STAT3, but also the
expression of total JAK2 and STAT3 protein. Notably, phlorizin
exhibited greater JAK2/STAT3 signal antagonism at high
concentrations (Fig. 8A-F). These
findings indicated that phlorizin may have a positive therapeutic
effect on esophageal cancer by antagonizing JAK2/STAT3 signal
transduction.
Discussion
In recent years, numerous available natural
plant-derived agents, with low toxicity, have been identified to be
effective in treating cancer by suppressing the proliferation of
cells (33,34). Cancer cells exist in a complex
environment; therefore, the underlying mechanisms of how these
natural plant-derived agents are involved in pro-apoptosis,
anti-autophagy and anti-proliferation of cancer cells is still
unknown.
Phlorizin is the main component of the Chinese
Traditional Medicine ‘sweet tea’, which can be used to prevent and
treat esophageal cancer by drinking it daily. According to the
results of the present study, by suppressing esophageal cancer cell
proliferation, migration and invasion, phlorizin might serve as an
effective esophageal cancer cell inhibitor in vitro. The
concentration of phlorizin used in the present study was in-line
with previous studies, in other types of cancer (35,36). In
the present study, more efforts were made to determine the
signaling pathway that phlorizin could affect. GO and KEGG pathway
analyses showed that the DEGs were enriched in apoptosis and
autophagy-related pathways. The detection of apoptosis and
autophagy, using different methods, further verified this
prediction. Autophagy and cell apoptosis, which represent type II
and I programmed cell death respectively, have been associated with
tumor genesis and progression (37–40). The
present study showed that phlorizin induced apoptosis and
antagonized autophagy of the esophageal cancer cells. LC3 and
P62/SQSTM1, the pivotal proteins of autophagy, participate in the
formation and clearance of the autophagosome (41–44).
Physiologically, soluble LC3-I is present in the cytoplasm. When
autophagy occurs, LC3-I can be converted to LC3-II by processing
modifications. Most of the LC3-II proteins are distributed on the
autophagosome membrane and a few are distributed on the membrane of
the pre-autophagosome. LC3-II is an autophagy marker molecule,
which reflects the autophagy activity of cells (41,45–47).
P62/SQSTM1 is a ubiquitin-binding protein, which is expressed in
numerous types of tissue and participates in a variety of signal
transduction processes, as well as autophagy. P62/SQSTM1 is
degraded by autophagy lysosome and is negatively associated with
autophagy activity (44). To further
verify the results, MDC-specific autophagy staining was performed
and the results showed the inhibitory effect of phlorizin on
autophagy. When cell energy is in crisis, autophagy can provide an
alternative source of nutrition to the cells and prolong cell life.
Autophagy can maintain energy homeostasis, which is crucial for the
survival of mice during the early period of neonatal starvation
(48). Autophagy is also involved in
the maintenance of gene integrity by clearing the damage in
response to metabolic stress, drug treatment and radiation damage.
Therefore, inhibiting autophagy in cancer cells could improve the
sensitivity of tumor cells to radiotherapy and chemotherapy
(49).
In addition, numerous studies have shown that the
JAK/STAT signaling pathway is important for growth, metastasis and
apoptosis of esophageal cancer (50,51). STAT3
has been reported to regulate the expression of different
microRNAs, which targets autophagy-related genes. For example,
STAT3 upregulated MIR17HG via the highly conserved STAT3 binding
site in its promoter region, and family members of the MIR17HG
cluster target the autophagy-related genes, ULK1, BECN1 and BCL2L11
(52–55). The results from the current study,
suggested that phlorizin may exert an antitumor effect by
inhibiting the JAK2/STAT3signaling pathway. However, the lack of
using a JAK2/STAT3 agonist, antagonist or rescue experiments is a
limitation to the present study, and the evidence supporting the
effect of phlorizin on the JAK2/STAT3 signaling pathway is
insufficient and further verification is required. According to the
results from RNA-Seq, phlorizin might exert its effects by
affecting other signaling pathways. This possibility remains to be
verified. To further substantiate the results, in vivo
experiments will also be performed to fully clarify the molecular
mechanism of phlorizin in esophageal cancer. In addition, if a
positive control group is designed, false negatives can also be
ruled out.
In summary, the results from the present study
revealed that phlorizin inhibited cell proliferation, invasion,
migration, autophagy and activated apoptosis by antagonizing the
JAK2/STAT3 signaling pathway. The finding will provide a
theoretical basis and possibility for phlorizin as a natural food
or pharmaceutical ingredient in the treatment of esophageal
cancer.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81272613), Key Project of
Natural Science Foundation of Hebei province of China (grant no.
H2017209233), Hebei High-level Talent Cultivation Plan in
University (grant no. GCC2014050) and the Graduate Student
Innovation Fund of North China University of Science and Technology
(grant no. 2019S12).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author upon reasonable
request. Sequencing data generated for this study have been
submitted to the NCBI Sequence Read Archive (SRA; http://www.ncbi.nlm.nih.gov/sra) and the
accession number is PRJNA685263.
Authors' contributions
XZ, ZJ and ZX designed the experiments. ZJ, YX, HW,
ZW, AL and ZL performed the experiments. ZJ, ZY and ZZ performed
the analysis and interpretation of the data. ZJ and ZY drafted the
manuscript. XZ and ZZ revised the manuscript. ZJ prepared the
figures. XZ provided financial support and supervised the project.
All authors reviewed and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCK-8
|
Cell Counting Kit-8
|
|
RNA-Seq
|
RNA sequencing
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malhotra GK, Yanala U, Ravipati A, Follet
M, Vijayakumar M and Are C: Global trends in esophageal cancer. J
Surg Oncol. 115:564–579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greally M and Ilson DH: Neoadjuvant
therapy for esophageal cancer: Who, when, and what? Cancer.
124:4276–4278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenkovich TR, Subramanian M, Yan Y,
Hofstetter WL, Correa AM, Cassivi SD, Inra ML, Stiles BM, Altorki
NK, Chang AC, et al: Adjuvant therapy for node-positive esophageal
cancer after induction and surgery: A multisite study. Ann Thorac
Surg. 108:828–836. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borggreve AS, Kingma BF, Ruurda JP and van
Hillegersberg R; Dutch Upper G.I. Cancer Audit (DUCA) group, :
Safety and feasibility of minimally invasive surgical interventions
for esophageal and gastric cancer in the acute setting: A
nationwide cohort study. Surg Endosc. 35:1219–1229. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Worrell SG, Bachman KC, Sarode AL, Perry
Y, Linden PA and Towe CW: Minimally invasive esophagectomy is
associated with superior survival, lymphadenectomy and surgical
margins: Propensity matched analysis of the National Cancer
Database. Dis Esophagus. 33:doaa0172020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng W, Yang J, Ni W, Li C, Chang X, Han
W, Zhou Z, Chen D, Feng Q, Liang J, et al: Postoperative
radiotherapy in pathological T2-3N0M0 thoracic esophageal squamous
cell carcinoma: Interim report of a prospective, phase III,
randomized controlled study. Oncologist. 25:e701–e708. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cragg GM and Pezzuto JM: Natural products
as a vital source for the discovery of cancer chemotherapeutic and
chemopreventive agents. Med Princ Pract. 25 (Suppl 2):S41–S59.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanders K, Moran Z, Shi Z, Paul R and
Greenlee H: Natural products for cancer prevention: Clinical Update
2016. Semin Oncol Nurs. 32:215–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Hu X, Liao C, Xiao H, Zhu Q, Li Y,
Liu Z, Tao A, He Z, Xu C and Zheng K: Gypenoside L inhibits
proliferation of liver and esophageal cancer cells by inducing
senescence. Molecules. 24:10542019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang JH, Pi J, Jin H, Yang F and Cai JY:
Chinese herb medicine matrine induce apoptosis in human esophageal
squamous cancer KYSE-150 cells through increasing reactive oxygen
species and inhibiting mitochondrial function. Pathol Res Pract.
214:691–699. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Li W and Liu Z: Preparative
isolation, quantification and antioxidant activity of
dihydrochalcones from Sweet Tea (Lithocarpus polystachyus Rehd.). J
Chromatogr B Analyt Technol Biomed Life Sci. 1002:372–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou SZ, Chen SX, Huang S, Jiang DX, Zhou
CJ, Chen CQ, Liang YM and Lai XP: The hypoglycemic activity of
Lithocarpus polystachyus Rehd. leaves in the experimental
hyperglycemic rats. J Ethnopharmacol. 138:142–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gosch C, Halbwirth H and Stich K:
Phloridzin: Biosynthesis, distribution and physiological relevance
in plants. Phytochemistry. 71:838–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panayotova-Heiermann M, Loo DD and Wright
EM: Kinetics of steady-state currents and charge movements
associated with the rat Na+/glucose cotransporter. J Biol Chem.
270:27099–27105. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ehrenkranz JR, Lewis NG, Kahn CR and Roth
J: Phlorizin: A review. Diabetes Metab Res Rev. 21:31–38. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Cheng J, Wang H, Wang M, Zhao J
and Wu Z: Protective effect of apple phlorizin on hydrogen
peroxide-induced cell damage in HepG2 cells. J Food Biochem.
43:e130522019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Gao Z, Wang A, Jia L, Zhang X,
Fang M, Yi K, Li Q and Hu H: Comparative oral and intravenous
pharmacokinetics of phlorizin in rats having type 2 diabetes and in
normal rats based on phase II metabolism. Food Funct. 10:1582–1594.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park J, Kwon O, Cho SY, Cho MC, Paick JS
and Kim SW: Comparison of improving effects for diabetic erectile
dysfunction according to the anti-glycemic agents: Phlorizin and
insulin. World J Mens Health. 37:210–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh S and Chan CK: Analysis of RNA-Seq
data using TopHat and cufflinks. Methods Mol Biol. 1374:339–361.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan J, Ren D, Wang J, Liu X, Zhang H, Wu M
and Yang G: Bruceine D induces lung cancer cell apoptosis and
autophagy via the ROS/MAPK signaling pathway in vitro and in vivo.
Cell Death Dis. 11:1262020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou YY, Li Y, Jiang WQ and Zhou LF:
MAPK/JNK signalling: A potential autophagy regulation pathway.
Biosci Rep. 35:e001992015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goruppi S, Jo SH, Laszlo C, Clocchiatti A,
Neel V and Dotto GP: Autophagy controls CSL/RBPJκ stability through
a p62/SQSTM1-dependent mechanism. Cell Rep. 24:3108–3114.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee M, Nam HY, Kang HB, Lee WH, Lee GH,
Sung GJ, Han MW, Cho KJ, Chang EJ, Choi KC, et al: Epigenetic
regulation of p62/SQSTM1 overcomes the radioresistance of head and
neck cancer cells via autophagy-dependent senescence induction.
Cell Death Dis. 12:2502021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu Z, Lu C, Li C, Jiao Y, Li Y and Zhang
G: Dracorhodin perchlorate induces apoptosis and G2/M cell cycle
arrest in human esophageal squamous cell carcinoma through
inhibition of the JAK2/STAT3 and AKT/FOXO3a pathways. Mol Med Rep.
20:2091–2100. 2019.PubMed/NCBI
|
|
31
|
Song M, Yoon G, Choi JS, Kim E, Liu X, Oh
HN, Chae JI, Lee MH and Shim JH: Janus kinase 2 inhibition by
Licochalcone B suppresses esophageal squamous cell carcinoma
growth. Phytother Res. 34:2032–2043. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Zhou P, Qin S, Xu D, Liu Y, Fu W,
Ruan B, Zhang L, Zhang Y, Wang X, et al: The curcumin analogs
2-pyridyl cyclohexanone induce apoptosis via inhibition of the
JAK2-STAT3 pathway in human esophageal squamous cell carcinoma
cells. Front Pharmacol. 9:8202018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R
and Darwiche N: Cell death mechanisms of plant-derived anticancer
drugs: Beyond apoptosis. Apoptosis. 20:1531–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ammoury C, Younes M, El Khoury M, Hodroj
MH, Haykal T, Nasr P, Sily M, Taleb RI, Sarkis R, Khalife R and
Rizk S: The pro-apoptotic effect of a Terpene-rich Annona cherimola
leaf extract on leukemic cell lines. BMC Complement Altern Med.
19:3652019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Liu J, Geng S, Liu Y, Ma H, Zheng
J, Liu B and Liang G: Lipase-catalyzed synthesis mechanism of
tri-acetylated phloridzin and its antiproliferative activity
against HepG2 cancer cells. Food Chem. 277:186–194. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin X, Xing YF, Zhou Z and Yao Y:
Dihydrochalcone compounds isolated from crabapple leaves showed
anticancer effects on human cancer cell lines. Molecules.
20:21193–21203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma C, Zhu L, Wang J, He H, Chang X, Gao J,
Shumin W and Yan T: Anti-inflammatory effects of water extract of
Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced
inflammation in acute lung injury by suppressing PI3K/Akt/mTOR
signaling pathway. J Ethnopharmacol. 168:349–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deng X, Liu J, Liu L, Sun X, Huang J and
Dong J: Drp1-mediated mitochondrial fission contributes to
baicalein-induced apoptosis and autophagy in lung cancer via
activation of AMPK signaling pathway. Int J Biol Sci. 16:1403–1416.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao B, Sun Q, Yuan Y, Yin Y, Qiao J and
Jiang P: Histone deacetylase inhibitor MGCD0103 causes cell cycle
arrest, apoptosis, and autophagy in liver cancer cells. J Cancer.
11:1915–1926. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu W, Chai Y, Hu L, Wang J, Pan X, Yuan
H, Zhao Z, Song Y and Zhang Y: Polyphyllin VI induces apoptosis and
autophagy via reactive oxygen species mediated JNK and P38
activation in glioma. Onco Targets Ther. 13:2275–2288. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yorimitsu T and Klionsky DJ: Autophagy:
Molecular machinery for self-eating. Cell Death Differ. 12 (Suppl
2):S1542–S1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin X, Li S, Zhao Y, Ma X, Zhang K, He X
and Wang Z: Interaction domains of p62: A bridge between p62 and
selective autophagy. DNA Cell Biol. 32:220–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bansal M, Moharir SC and Swarup G:
Autophagy receptor optineurin promotes autophagosome formation by
potentiating LC3-II production and phagophore maturation. Commun
Integ Biol. 11:1–4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
do Nascimento-Neto LG, Cabral MG, Carneiro
RF, Silva Z, Arruda FVS, Nagano CS, Fernandes AR, Sampaio AH,
Teixeira EH and Videira PA: Halilectin-3, a lectin from the marine
sponge haliclona caerulea, induces apoptosis and autophagy in human
breast cancer MCF7 cells through caspase-9 pathway and LC3-II
protein expression. Anticancer Agents Med Chem. 18:521–528. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang N, Li L, Wang J, Cao M, Liu G, Xie
G, Yang Z and Li Y: Study of autophagy-related protein light chain
3 (LC3)-II expression levels in thyroid diseases. Biomed
Pharmacother. 69:306–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kuma A, Hatano M, Matsui M, Yamamoto A,
Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T and Mizushima N: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo JY, Xia B and White E:
Autophagy-mediated tumor promotion. Cell. 155:1216–1219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Timme S, Ihde S, Fichter CD, Waehle V,
Bogatyreva L, Atanasov K, Kohler I, Schöpflin A, Geddert H, Faller
G, et al: STAT3 expression, activity and functional consequences of
STAT3 inhibition in esophageal squamous cell carcinomas and
Barrett's adenocarcinomas. Oncogene. 33:3256–3266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu JR, Wu WJ, Liu SX, Zuo LF, Wang Y,
Yang JZ and Nan YM: Nimesulide inhibits the growth of human
esophageal carcinoma cells by inactivating the JAK2/STAT3 pathway.
Pathol Res Pract. 211:426–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brock M, Trenkmann M, Gay RE, Michel BA,
Gay S, Fischler M, Ulrich S, Speich R and Huber LC: Interleukin-6
modulates the expression of the bone morphogenic protein receptor
type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ
Res. 104:1184–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu H, Wang F, Hu S, Yin C, Li X, Zhao S,
Wang J and Yan X: MiR-20a and miR-106b negatively regulate
autophagy induced by leucine deprivation via suppression of ULK1
expression in C2C12 myoblasts. Cell Signal. 24:2179–2186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chatterjee A, Chattopadhyay D and
Chakrabarti G: miR-17-5p downregulation contributes to paclitaxel
resistance of lung cancer cells through altering beclin1
expression. PLoS One. 9:e957162014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Spaccarotella E, Pellegrino E, Ferracin M,
Ferreri C, Cuccuru G, Liu C, Iqbal J, Cantarella D, Taulli R,
Provero P, et al: STAT3-mediated activation of microRNA cluster
17~92 promotes proliferation and survival of ALK-positive
anaplastic large cell lymphoma. Haematologica. 99:116–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|