Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide with a high annual incidence and a 5-year survival
rate of <20% regardless of its stage at diagnosis (1,2). However,
when it metastasizes, the 5-year survival rate is less than 5%
(3). Non-small cell lung cancer
(NSCLC) is the most common type of lung cancer, representing ~80%
of all cases (4,5). The two most prevalent NSCLC subtypes are
lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC),
which constitute 35 and 25% of all cases, respectively (5). There have been notable advances in our
understanding of lung cancer and its underlying mechanisms of
action, which have been clearly elucidated and exemplified
(6). There have also been marked
improvements recently in the treatments available for lung cancer.
The current treatment protocol consists of a platinum doublet,
tyrosine kinase inhibitors for epithelial growth factor receptor
(EGFR), echinoderm microtubule-associated protein-like 4-anaplastic
lymphoma kinase (EML4-ALK) fusion protein and immune checkpoint
inhibitors (3). However, the
prognosis for lung cancer remains poor and a more detailed
mechanism and treatment options are needed. In addition to the
malignant cells, the surrounding microenvironment is also critical
for tumorigenesis (7,8). Amine oxidases refer to a class of
enzymes that catalyze the deamination of amine groups to produce
aldehydes, ammonia and hydrogen peroxide (9). There are a variety of amine oxidases
consisting of four classes of monoamine oxidases (MAOs), including
MAO-A and MAO-B, polyamine oxidases, lysyl oxidases, and
copper-containing amine oxidases (CAOs) (10). CAOs have been revealed to participate
in the regulation of a variety of pathological and physiological
processes, such as cell proliferation, differentiation, glucose
uptake and immune regulation (11).
Changes in CAO activity are correlated with a variety of human
diseases, including diabetes mellitus, Alzheimer's disease, and
inflammatory disorders (12,13). The four complete genes for CAOs are
amine oxidase, copper containing (AOC)1-4. AOC1 consists of
a homodimeric glycoprotein with an apparent molecular mass of 186
kDa; it is secreted as a diamine oxidase to generate hydrogen
peroxide (14,15). AOC1 is strongly expressed in the
kidneys, placenta, intestine and lungs (14). Little is known about the molecular
mechanisms regulating AOC1 gene expression. The AOC2
gene encodes retina-specific amine oxidase (16), which was originally identified in
ganglion cells. Its functions remain unclear but it may play a role
in hereditary retinal diseases (16).
The AOC4 gene encodes a soluble plasma amine oxidase in cows
as bovine AOC4 (17) but not in
humans, mice or rats. The AOC3 gene encodes vascular
adhesion protein-1, which is primarily expressed on the endothelial
cell surface but also in smooth muscle cells and adipocytes
(18). In addition to its amine
oxidase activity, AOC3 functions as a non-classical
inflammation-inducible endothelial molecule which is linked to
leukocyte-subtype specific rolling under physiological shear
(18). It has been revealed that the
enzymatic activity of AOC3 is functionally important, and leukocyte
recruitment is impaired if its activity is abolished (10).
The surrounding microenvironment of cancer
contributes to its promotion and progression (19). Due to its unique tumor
microenvironment (TME), cancer promotes and strengthens its own
progression as a result of its interactions. The cells inside the
TME include cancer-associated fibroblasts, endothelial cells and
immune cells (20), which form the
tumor immune microenvironment (TIME). The functions and densities
of different tumor-infiltrating immune cells in the TIME are
closely associated with prognosis and prediction of the treatment
response (7). Therefore, there is an
urgent need for improved understanding of immune dysfunction inside
the TIME and the mechanisms by which the tumor modifies its
environment to remove the functional immunity of the body. The
present study aimed to verify the role of AOC3 in lung cancer
progression and the relevant anticancer immunity.
Materials and methods
Cell lines and reagents
Murine Lewis lung carcinoma (LLC) cell line and
human umbilical vein endothelial cells (HUVECs) were purchased from
American Type Culture Collection (ATCC). Human lung cancer CL1-5
cells were kindly provided by Dr Pan-Chyr Yang of National Taiwan
University (Taipei City, Taiwan) and were cultured in RPMI-1640
medium (Lonza Group, Ltd.) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (Thermo
Fisher Scientific, Inc.; Waltham, MA, USA) at 37°C. Recombinant
human and mouse AOC3 were obtained from R&D Systems, Inc.
Knockdown of AOC3 in CL1-5 cells was performed using either
pLKO_005 plasmid as a control or AOC3-shRNA plasmid (14 µg shRNA
plasmid for 5×105 cells in a 6-well plate) obtained from
the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan).
The plasmid was transfected into cells using Lipofectamine 2000™
Thermo Fisher Scientific, Inc.) for 2 days, and the stable clone of
AOC3-knockdown cells were established by puromycin selection (5
µg/ml). All cells were authenticated by short tandem repeat
(Promega Corporation) and examined for mycoplasma contamination
using a MycoAlert™ mycoplasma detection kit (Lonza Group, Ltd.)
according to the manufacturer's protocol every three months.
Next generation sequencing (NGS) and
bioinformatics analysis
All of the participants selected from January 2018
to December 2019, provided written informed consent prior to
inclusion in the present study. The patients who agreed and
received surgical intervention were enrolled in this study. The
adjacent non-tumor lung and tumor tissues of ten patients (7 from
LUAD and 3 from LUSC) were obtained from the Division of Thoracic
Surgery and Division of Pulmonary and Critical Care Medicine,
Kaohsiung Medical University Hospital (Kaohsiung, Taiwan). The
protocol of the present study was reviewed and approved (approval
no. KMUH-IRB-20130054 and KMUH-IRB-G(II)-20180021) by the
Institutional Review Board of Kaohsiung Medical University
Hospital. The deep RNA-seq was carried out at a biotechnology
company (Welgene, Inc.) using the Solexa platform. RNA and small
RNA library construction was carried out using a sample preparation
kit (Illumina, Inc.) following the protocol of the TruSeq RNA or
Small RNA Sample Preparation Guide.
The expression of AOCs in lung cancer and normal
specimens (cancer vs. normal) were extracted from the
Oncomine® database (http://www.oncomine.org; Compendia Biosciences)
(21) and The Cancer Genome Atlas
(TCGA) cohort of UALCAN (http://ualcan.path.uab.edu/analysis.html) (22). The 16 cohorts from
Oncomine® database included Su et al (23), Okayama et al (24), Landi et al (25), Beer et al (26), Stearman et al (27), Selamat et al (28), Garber et al (adenocarcinoma and
squamous) (29), Hou et al
(adenocarcinoma, squamous and large cell) (30), Wachi et al (squamous) (31), Bhattacharjee et al
(adenocarcinoma, squamous, carcinoid and small cell) (32). Criteria in the analysis were fold
change >2 and P-value <10−4, which was calculated
using the Oncomine® database through two-sided Student's
t-test. The data of AOC mRNA, copy number and overall
survival in TCGA database, and AOC protein were analyzed by UALCAN
website (http://ualcan.path.uab.edu/analysis-prot.html)
(22). The immunohistochemical
staining for AOC3 in lung cancer and normal lung tissue samples
were acquired from The Human Protein Atlas (33). The association between gene expression
and clinical outcome of lung cancer patients was evaluated by
publicly available data using Kaplan-Meier (K-M) plotter and
log-rank testing (https://kmplot.com/analysis/) (34), UALCAN and PROGgeneV2 (35). The post-transcriptional regulation was
predicted using miRWalk (version 3.0) (36), miRanda (37) and miRDB (38) with restriction of >95%
confidence.
Measurement of AOC3
All of the participants provided written informed
consent prior to inclusion in the present study. The sera of 40
healthy donors and 40 lung cancer patients (healthy donors: Age
range, 40–80 years old; M/F 31%/69%; lung cancer donors: Age range
30–90 years old; M/F 46/54%) were collected from the Division of
Thoracic Surgery and Division of Pulmonary and Critical Care
Medicine, Kaohsiung Medical University Hospital (Kaohsiung, Taiwan)
from January 2018 to December 2019. The patients who agreed with
written informed consent were enrolled in this study before
starting definite treatment. These samples were assessed using
Quantikine Human VAP-1 Immunoassay (R&D Systems, Inc.). The
protocol of the present study was reviewed and approved (approval
no. KMUH-IRB-20130054) by the Institutional Review Board of
Kaohsiung Medical University Hospital.
Reverse transcription-quantitative
(RT-q) PCR
Total RNAs were extracted from CL 1–5 lung cancer
cells with TRIzol reagent (Thermo Fisher Scientific, Inc.) and
reverse transcribed into cDNA using an oligo (dT) primer and
reverse transcriptase (PrimeScript RT Reagent Kit; Takara Bio,
Inc.) following the manufacturer's protocols. The reaction
conditions were as follows: Priming for 5 min at 25°C, reverse
transcription for 20 min at 46°C, and final inactivation of reverse
transcriptase for 1 min at 95°C (40). The expression levels of specific genes
were determined by a StepOne-Plus PCR instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.), using real-time
analysis with SYBR-Green (Thermo Fisher Scientific, Inc.). The
following primers were used: AOC1 forward,
5′-AOC1_H_F2GTGATGGAGGCCAAGATGCA-3′ and reverse,
5′-AOC1_H_R2TCTGCAGTGTCTGGAAGCTG-3′; AOC2 forward,
5′-AOC2_H_F2GCCTTCCACTTCAAGCTGGA-3′ and reverse,
5′-AOC2_H_R2GCTCTCAGGTCCTCCTTTCC-3′; AOC3 forward,
5′-AOC3_H_F2GTGGGGCCATAGAAATACGA-3′ and reverse,
5′-AOC3_H_R2CAGACCCAGTTCTCCAGTCC-3′; and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) forward, 5′-TTCACCACCATGGAGAAGGC-3′ and
reverse, 5′-GGCATGGACTGTGGTCATGA-3′. The RT-qPCR was performed at
95°C for 20 sec, followed by 40 cycles at 95°C for 5 sec and 60°C
for 35 sec (39). Relative expression
levels of the cellular mRNA were normalized to GAPDH. The relative
standard method (2−ΔΔCq) was used to calculate relative
RNA expression (40).
Cell proliferation and
5-bromo-2-deoxyuridine (BrdU) incorporation
Cells (3×103 cells/well) were seeded in a
96 well plate, and then cultured for 48 or 72 h. Cell proliferation
was determined by cell proliferation reagent WST-1 proliferation
assay kit (Takara Bio, Inc.) after 2-h incubation and measured at a
450-nm wavelength according to the manufacturer's instructions.
Cells were labelled with BrdU (10 µM) at day 2 after seeding
followed by fixation. In the BrdU incorporation assay, cells were
fixed at room temperature for 30 min with 200 µl/well of the Fixing
Solution (included in the kit undermentioned) and incubated at room
temperature for 30 min. Integrated BrdU was assessed by ELISA-based
method according to the manufacturer's protocol (BrdU Cell
Proliferation Assay Kit; cat. no. 2750; EMD Millipore).
Wound healing analysis
CL 1–5 cells were seeded into a 12 well-pate at 90%
confluence and cultured in 1% of FBS-containing medium for
exogenous AOC3 (control, 10, 20 and 50 ng/ml) and 10% of
FBS-containing medium (since cell proliferation was not affected by
AOC3 knockdown, in order to mimic the physiologic conditions, 10%
of FBS-containing medium was used and cells were not serum starved)
for AOC3-shRNA knockdown at 37°C as previously described by Shao
et al (41), and the cell
migration was evaluated by measuring the migration of cells into
the acellular region formed by a sterile yellow tip. The wound
closure was observed after 8 h. The wound healing assay was closely
observed via a Nikon inverted microscope (Nikon Corporation).
CD4+ T-cell isolation
Peripheral blood mononuclear cells (PBMCs) of
healthy donors (eight healthy donors: Age range, 35–45 years old;
male only) were obtained from the Division of Pulmonary and
Critical Care Medicine, Kaohsiung Medical University Hospital
(Kaohsiung, Taiwan) from January 2020 to December 2020. The
protocol of the present study was reviewed and approved (approval
no. KMUH-IRB-20130054) by the Institutional Review Board of
Kaohsiung Medical University Hospital and the donors provided
written informed consent. PBMCs were isolated using 7.5 ml
Ficoll-Hypaque gradient reagent (EMD Millipore) in 1 ml blood
mixing with 5 ml PBS, and human CD4+ T cells were
isolated form PBMC using CD4+ T-cell Isolation Kit (MACS
MicroBeads; Miltenyi Biotec GmbH) according to the manufacturer's
instructions.
Cell adhesion and transendothelial
migration
For transendothelial migration, HUVECs
(5×104) were seeded onto inserts with polyester
membranes of 3-µm pore size (EMD Millipore) and cultured at 37°C
for 48 h to form a 100% confluent monolayer. CL1-5
(1×105) or AOC3-knockdown CL1-5 (1×105) cells
were seeded in the bottom of a 24-well plate containing RPMI-1640
culture medium. PKH26-labeled (EMD Millipore) CD4+
T-cells were seeded onto HUVEC-coated inserts, which were placed in
the wells of the 24-well plate and then incubated for 24 h at 37°C.
The migratory cells were visualized in four randomly selected
fields using a Nikon fluorescence microscope (Nikon
Corporation).
Western blot analysis
Total proteins from primary tissues and cell lines
were extracted using RIPA lysis buffer (Thermo Fisher Scientific,
Inc.). An equal amount of total protein (2 µg) was quantitated by
bicinchoninic acid (BCA) analysis and separated by SDS-PAGE (6-8%).
After transferring, the PVDF membranes containing bound proteins
were blocked at room temperature for 2 h using 5% milk containing
TBST buffer (0.02% Tween-20) and then incubated overnight at 4°C
with primary antibodies against a specific target protein. After
incubation with HRP-coupled secondary antibodies (1:5,000;
anti-mouse, 7076; anti-rabbit, 7074; Cell Signaling Technology) at
room temperature for 1 h, the protein bands were visualized using
ECL (EMD Millipore) and detected using a FluorChem HD2 System
(ProteinSimple). The following primary antibodies were used:
E-cadherin (1:500; cat. no. 610182) N-cadherin (1:500; cat. no.
610921) and vimentin (1:500; cat. no. 550513; all from BD
Biosciences), Slug (1:500; product no. 9585S; Cell Signaling
Technology, Inc.), and GAPDH (1:5,000; cat. no. MAB374; EMD
Millipore). The quantitation of the results of the western blotting
was performed using AlphaImager software (Version 6.0.0;
ProteinSimple).
miRNA mimics transfection
CL1-5 cells were transfected with microRNA
(miR)-3691-5p (AGUGGAUGAUGGAGACUCGGUAC; at a concentration of 100
nM; GE Healthcare Dharmacon, Inc.) or scrambled control (negative
control 1; UCACAACCUCCUAGAAAGAGUAGA; at a concentration of 100 nM;
GE Healthcare Dharmacon, Inc.) by using Dharmafect reagent 4 (GE
Healthcare Dharmacon, Inc.) according to the manufacturer's
instructions. The transfection efficacy was monitored by
transfecting siGLO fluorescent oligonucleotides (catalog ID:
D-001630-02-05; GE Healthcare Dharmacon, Inc.) concurrently after
24 h of transfection at 37°C according to the manufacturer's
protocol. The expression of AOC1-3, cell migration and
CD4+ T-cell migration as well as adhesion were assayed
after a 48-h transfection.
Mouse studies
All mice procedures were approved by and conducted
in accordance with the Institutional Animal Care and Use Committee
at Kaohsiung Medical University (IACUC Approval No. 107104;
Kaohsiung, Taiwan). C57BL/6 mice (12 males in total; weight, 18±2
g; 5 weeks old) were obtained from the Taiwan National Laboratory
Animal Center (Taipei City, Taiwan). The mice were housed in a
specific pathogen-free environment with the room temperature being
maintained at ~20°C, the humidity at ~45% and a 12-h light/dark
cycle. Each mouse had free access to food and water. The mice were
subjected to implantation of LLC cells (1×106 cells) via
tail vein and tumor growth in the lungs was allowed for 7 days.
Mice were treated with PBS or recombinant mouse (rm) AOC3 twice (10
µg/mouse; on days 7 and 14) by intra-tracheal route. At the end of
the experiment, the mice were euthanized by CO2
asphyxiation during which the CO2 gas flow rate
displaced 10 to 30% of the cage volume per minute. CD4+
T cells of the lungs of mice were isolated by mouse CD4+
T cell isolation kit (MACS MicroBeads; Miltenyi Biotec GmbH)
according to the manufacturer's instructions and counted after 21
days of LLC implantation. Lung tissue was collected and minced and
incubated in RPMI-1640 medium with collagenase type 1 (400 U/ml;
Worthington Biochemical Corporation) at 37°C for 1 h. The digested
tissues were filtered through a 70-µm cell strainer and washed with
RPMI-1640 medium. CD4+ T cells of the lung filtered
solution were isolated by mouse CD4 isolation kit and counted after
21 days of LLC implantation.
Statistical analyses
Each experiment was repeated at least three times.
Data are expressed as the mean ± standard deviation (SD) using
GraphPad Prism version 7.04 (GraphPad Software, Inc.). Two
treatment groups were compared by unpaired Student's t-test.
Multiple group comparisons were performed by two-way analysis of
variance with Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
AOC3 mRNA expression is reduced in
lung cancer
The controversial roles of AOCs have been reported
in various cancer types (41–44), therefore their effect in lung cancer
was investigated. Tumor tissue and adjacent normal tissue specimens
from 10 lung cancer patients (7 LUAD and 3 LUSC) were analyzed via
NGS (Table I). The expression of
AOC2 (7 out of 10) and AOC3 (8 out of 10) was lower
in most of the tumor tissue of patients compared with their normal
tissue, however lower AOC1 in tumor tissue was observed in
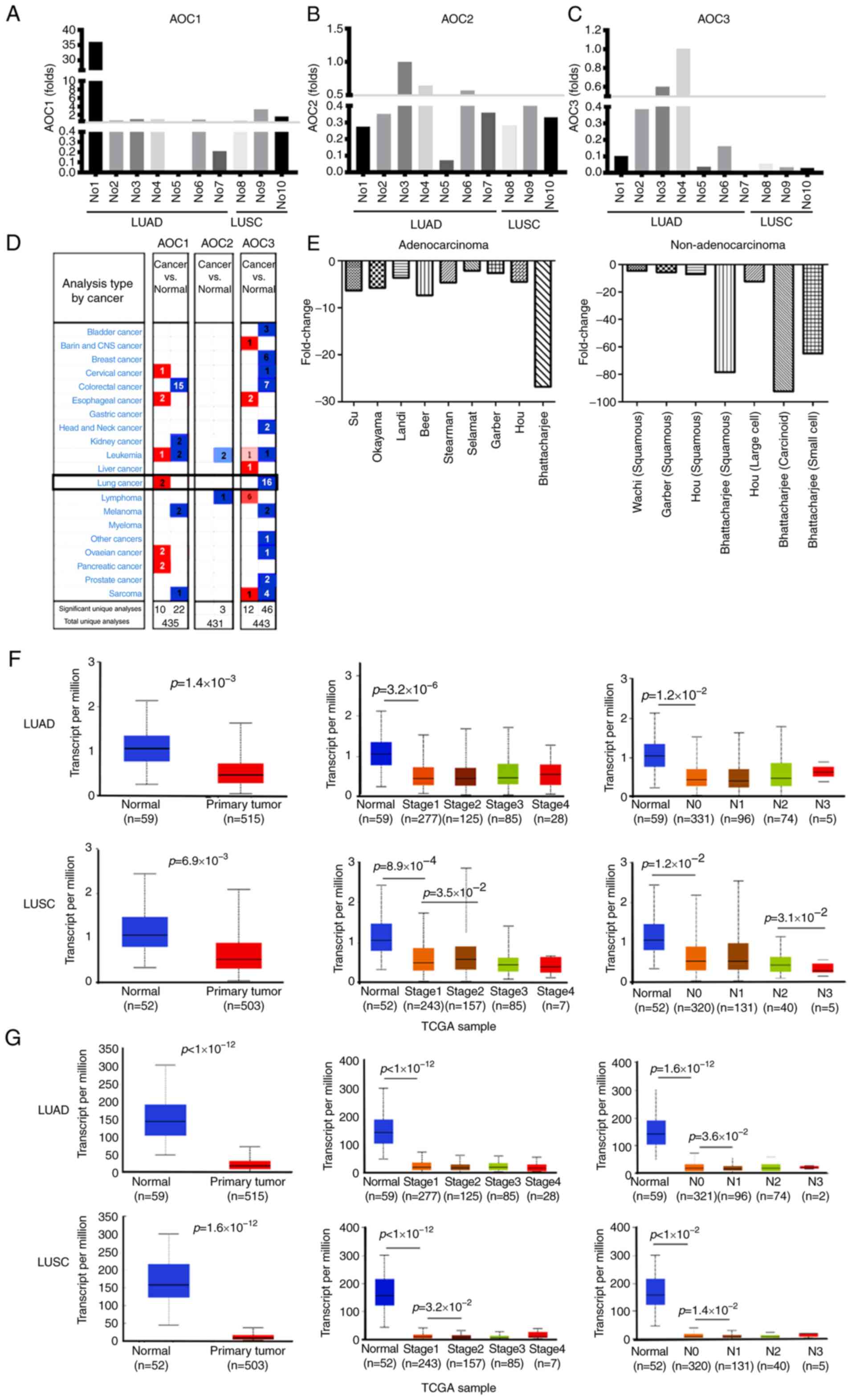
only 2 out of 10 patients with lung cancer (Fig. 1A-C). Using Oncomine®
datasets, it revealed that AOC3, but not AOC1 or
AOC2, was expressed at lower levels in tumor tissue compared
with normal tissue in 16 lung cancer cohorts (Fig. 1D). Further analysis of these 16
cohorts revealed that the expression of AOC3 mRNA was lower
in the tumor tissue for both the LUAD and non-adenocarcinoma
patients (Fig. 1E). The expression of
AOC2 and AOC3 in LUAD and LUSC was also retrieved
from TCGA cohorts. Overall AOC2 (Fig. 1F) and AOC3 (Fig. 1G) expression was significantly lower
in the tumor tissue compared with the adjacent normal tissue, even
though this trend was not observed for all stages. Moreover, the
expression of AOC3 was significantly lower in the N1 group
(with lymph node metastasis) compared with the N0 group (without
lymph node metastasis), implying that AOC3 may contribute to cancer
metastasis (Fig. 1F and G).
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Group | Number | Sex | Age | Pathological
diagnosis | Stage | T | N | M |
|---|
| I | 01 | M | 70 | Adenocarcinoma
grade 3 | 2B | 3 | 0 | 0 |
|
| 02 | M | 66 | Adenocarcinoma
grade 3 | 4B | 2a | 0 | 1c |
|
| 03 | F | 51 | Adenocarcinoma
grade 3 | 1B | 2a | 0 | 0 |
|
| 04 | M | 53 | Adenocarcinoma
grade 3 | 3A | 3 | 2 | 0 |
|
| 05 | F | 60 | Adenocarcinoma
grade 2 | 1A | 1b | 0 | 0 |
|
| 06 | M | 67 | Adenocarcinoma
grade 1 | 1A | 1a | 0 | 0 |
|
| 07 | M | 60 | Adenocarcinoma
grade 3 | 4A | 4 | 1 | 1a |
| II | 08 | M | 84 | Squamous cell
carcinoma grade 2 | 2B | 3 | 0 | 0 |
|
| 09 | F | 65 | Squamous cell
carcinoma grade 2 | 3A | 4 | 0 | b |
|
| 10 | M | 69 | Squamous cell
carcinoma grade 2 | 2B | 2b | 1 | 0 |
AOC3 protein expression is inversely
associated with lung cancer grade
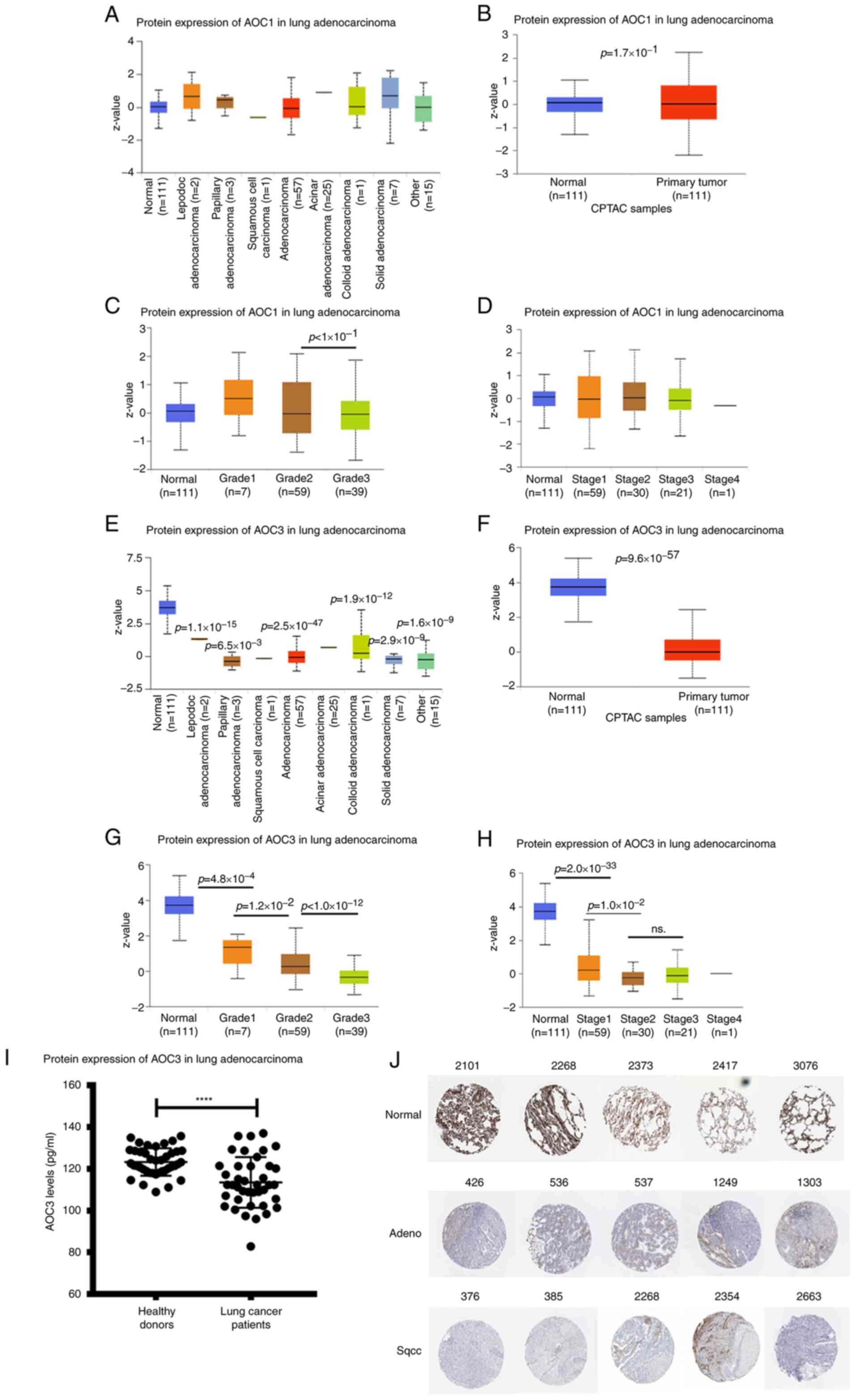
AOC protein expression was extracted from the
National Cancer Institute Clinical Proteomic Tumor Analysis
Consortium (CPTAC). AOC1 protein expression did not vary between
the tumor and normal tissue for the different types of LUAD, grades
or stages (Fig. 2A-D). However, AOC3
protein expression was lower in the tumor tissue compared with the
normal tissue in every cell type (Fig. 2E
and F). Moreover, AOC3 expression was negatively associated
with the grades and stages (early and late) of LUAD (Fig. 2G and H). The soluble form of AOC3 has
been detected in other cancer types such as colorectal cancer
(41). To evaluate the role of
soluble AOC3 in lung cancer, serum from lung cancer patients was
collected. Soluble AOC3 in the sera from lung cancer patients was
lower than in healthy donors (Fig.
2I). In addition, the public datasets for the expression of
AOC3 in lung cancer were utilized. Compared with normal tissue,
both LUAD and LUSC expressed lower levels of AOC3 from The Human
Protein Atlas (Fig. 2J). The
combination of these results and the mRNA expression results
indicated that AOC3 could be a promising tumor suppressor in lung
cancer.
Lower expression of AOC3 confers a
poorer survival time
Since the tumor tissue of lung cancers expressed
lower levels of AOC3, its prognostic vaule in patients was
evaluated by survival analysis. There are several public websites
that evaluate survival analysis, including the K-M plotter, UALCAN
and PROGgeneV2. According to the K-M plotter, low AOC1
expression did not confer a poorer survival time, and it actually
conferred a longer survival time in LUAD but not in LUSC patients
(Fig. 3A; upper panel). AOC2
revealed the same pattern in both types of lung cancer (Fig. 3A; middle panel). However, analysis of
AOC3 expression revealed that the lower the AOC3
expression was, the shorter the survival time was in LUAD patients
but not in LUSC patients (Fig. 3A,
lower panel). Moreover, the clinical implication of AOC3
expression as detemined by survival rates was validated by cohorts
extracted from the UALCAN and PROGgeneV2 websites; low expression
of AOC3 conferred a shorter surivival time but this was not
observed for AOC1 (ABP1) or AOC2 (Fig. 3B and C). These results confirmed that
AOC3 was strongly associated with clinical outcomes in lung cancer
patients (Fig. 3A and B, lower panel;
Fig. 3C).
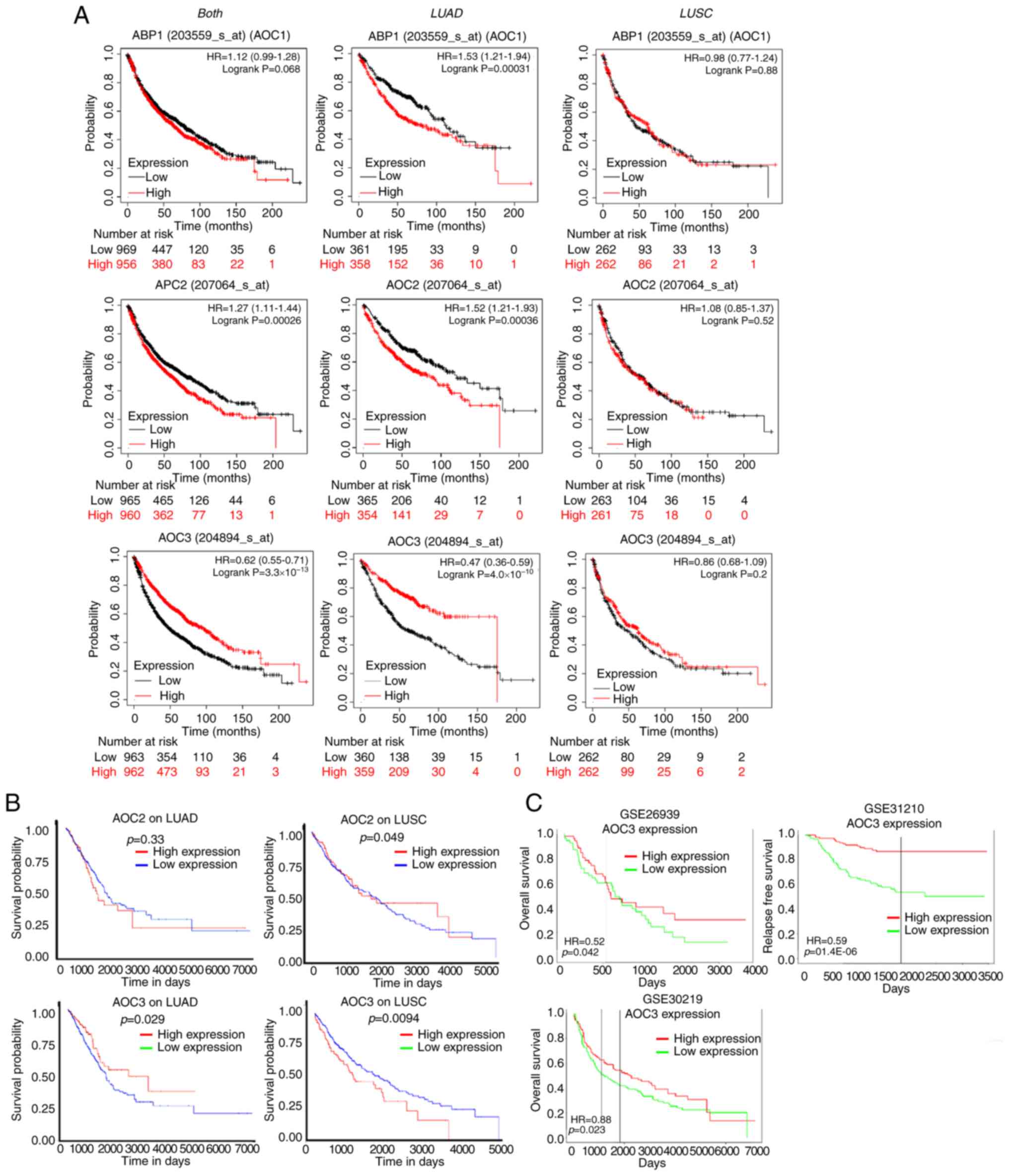 | Figure 3.Survival analysis of AOCs in lung
cancer. The survival time for lung cancer patients from cohorts
with different levels of AOC1, 2 and 3 expression
were further analyzed. In the Kaplan-Meier plotter, survival time
was analyzed for ‘both, LUAD and LUSC’, ‘LUAD’ and ‘LUSC’ (from
left to right). (A; upper panel) For AOC1, the
high-expression group was associated with a shorter survival time
compared with the low-expression group. (A; middle panel) For
AOC2, the high-expression group was not associated with a
longer survival time compared with the low-expression group. (A;
lower panel) However, for AOC3, the low-expression group was
associated with a shorter survival time in lung adenocarcinoma. (B)
In addition, UALCAN cohorts also indicated that low expression of
AOC3 but not AOC2 was associated with a shorter
survival time compared with high expression. (C) The low expression
of AOC3 also conferred shorter survival time in different
lung adenocarcinoma (GSE26919 and GSE31210) and lung cancer
(GSE30219) cohorts in the PROGgeneV2. AOC, amine oxidase, copper
containing 3; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma. |
Mechanism regulating the expression of
AOC3
Since AOC3 was revealed to be critical in the
prognosis of lung cancer patients, the dysregulation of AOC3
required investigation. Genetic modifications, as DNA copy number
variation, DNA methylation, and post-transcriptional regulation by
miRNAs were utilized. As determined by the TCGA cohort, variation
in the DNA copy number of AOC3 was not correlated with the
expression of AOC3 mRNA (R=−0.121; Fig. 4A). In addition, DNA methylation of
AOC3 was not significantly associated with the expression of
AOC3 mRNA (R=−0.034; Fig. 4B).
Concerning post-transcriptional regulation, the miRNAs that
epigenetically regulate AOC3 mRNA were predicted using
miRWalk version 3.0 with miRanda and miRDB restrictions of >95%
confidence. There was a total of 27 miRNAs listed as potential
regulators of the AOC3 (Fig.
4C). This list of miRNAs was validated using the TCGA cohort
and the ones with the highest probability were miR-3190 and
miR-3691 since both of them had significantly increased expression
in the tumor tissue compared with the normal tissue (Fig. 4D and E). Both of the predicted and
highly probable miRNAs were verified in 10 lung cancer patients.
The most likely miRNA to contribute to the regulation of
AOC3 mRNA was miR-3691-5p because there was an undetectable
read number in most specimens for miR-3190 in our samples (Fig. 4F and G).
 | Figure 4.Regulation of AOC3 mRNA
expression. The expression of AOC3 mRNA was regulated by
genetic modifications. The TCGA cohort was used to investigate
these modifications. (A) The DNA copy number of AOC3 was not
correlated with AOC3 mRNA expression. (B) Moreover,
epigenetic regulation, such as AOC3 DNA methylation was not
associated to AOC3 mRNA expression. (C) MiRNA regulation of
AOC3 mRNA was predicted using miRWalk 3.0, and the 27
possible candidate miRNAs are listed. The present study validated
the possibility of each miRNA using the TCGA cohort. The most
likely candidate miRNAs included (D) miR-3190 and (E) miR-3691.
Furthermore, the expression levels of indicated miRs were
associated with the stages respectively. (F and G) These 2 miRNAs
were further verified by data from 10 patients (including 7 lung
adenocarcinoma and 3 lung squamous cell carcinoma). AOC, amine
oxidase, copper containing 3; TCGA, The Cancer Genome Atlas; miR,
microRNA; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma. |
Low AOC3 expression mediates
epithelial-mesenchymal transition (EMT) in lung cancer
Low levels of AOC3 expression conferred poor
clinical outcomes in lung cancer patients. Therefore, the present
study set out to verify the mechanisms by which AOC3 mediated lung
cancer progression. AOC3 expression was knocked down in the LUAD
cell line (CL1-5) via the shRNA method with >50% efficiency
(Fig. 5A). The cells were then
studied to evaluate the effect of AOC3 knockdown on proliferation.
Neither the WST-1 nor the BrdU assay indicated that AOC3 affected
cell proliferation in lung cancer (Fig.
5B and C, respectively). Cell migration as evaluated via wound
healing analysis, revealed enhanced healing (increased migration
ability) after AOC3 knockdown (Fig.
5D). In addition, AOC3 knockdown enhanced the mesenchymal
characteristics as N-cadherin, vimentin and Slug were increased
(Fig. 5E). The rmAOC3 protein
(rhAOC3) was added to confirm the observed changes in proliferation
and migration. The proliferation did not change even at a high dose
(50 ng/ml) of rhAOC3 as evaluated by either WST-1 or BrdU assays
(Fig. 5F and G, respectively). Cell
migration was reduced after the addition of rhAOC3 in a
dose-dependent manner as revealed in the wound-healing assay
(Fig. 5H). The mesenchymal
characteristics transitioned to epithelial features as E-cadherin
was increased and N-cadherin, vimentin and Slug were decreased in a
dose-dependent manner (Fig. 5I). The
aforementioned results indicated that reduced AOC3 expression
played a role in lung cancer progression by increasing cell
migration and EMT but not proliferation.
 | Figure 5.AOC3 mediates EMT in lung cancer. The
present study investigated the mechanisms by which low AOC3
expression conferred a poor lung cancer prognosis. (A) AOC3 shRNA
was used to knockdown AOC3 expression in CL1-5 cells. The knockdown
efficiency was >50%. Cell proliferation was not altered as
determined by (B) WST-1 and (C) BrdU incorporation assays. (D)
Migration potential as determined via a wound-healing assay was
enhanced after AOC3 knockdown. (E) The EMT phenomenon shifted
towards the mesenchymal characteristics as indicated by increased
N-cadherin, vimentin and Slug and decreased epithelial
characteristics as indicated by E-cadherin. Conversely, using
rhAOC3, the present study confirmed that AOC3 did not reduce
cellular proliferation as revealed by (F) WST-1 and (G) BrdU
incorporation assays. (H) The wound healing assay also revealed a
reduction in migration potential in a dose-dependent manner after
the addition of rhAOC3. (I) Concurrently, the EMT characteristics
were reversed to epithelial characteristics as E-cadherin was
increased and N-cadherin, vimentin and Slug were decreased. All
experiments were performed independently at least three times.
*P<0.05. AOC, amine oxidase, copper containing 3; EMT,
epithelial-mesenchymal transition; sh-, short hairpin; BrdU,
5-Bromo-2-deoxyuridine; rh-, recombinant human. |
Lung cancers with low levels of AOC3
fail to recruit CD4+ T cells to the tumor in vitro and
in vivo
CD4+ T-cell infiltration is a critical
factor for determining the TIME against cancer (26). The role of AOC3 in the recruitment of
CD4+ T cells remains unclear in lung cancer. To validate
the role of AOC3 in mediating the TIME in lung cancer, in
vitro and in vivo studies were performed.
CD4+ T-cell migration and attachment to lung cancer
cells were evaluated. As determined by a cell adhesion assay,
CD4+ T-cell attachment to lung cancer cells (CL1-5) was
decreased after AOC3 knockdown (Fig.
6A). Before CD4+ T cells arrive at tumor sites, they
must traverse the endothelia. A transendothelial migration assay
was utilized to reveal the transit of CD4+ T cells
through the vascular endothelia. When AOC3 was silenced in cancer
cells, CD4+ T-cell migration was reduced, (Fig. 6B) indicating that the lower the AOC3
expression, the fewer CD4+ T cells were recruited.
Conversely, when rhAOC3 was added, more CD4+ T cells
attached to the lung cancer cells (Fig.
6C). The addition of rhAOC3 increased CD4+ T-cell
migration through the vascular endothelial cells (Fig. 6D). These results indicated that AOC3
increased the recruitment of CD4+ T cells to lung cancer
sites. AOC3 facilitation of CD4+ T-cell recruitment was
validated using an animal model. The in vivo study
investigated the number of CD4+ T cells in the lungs of
mice with tumors and revealed that the number of CD4+ T
cells was increased after rmAOC3 was instilled two times (10
µg/mouse) (Fig. 6E). These results
indicated that AOC3 promoted CD4+ T-cell recruitment
into the TIME.
 | Figure 6.AOC3 attracts CD4+ T cells
to lung cancer sites. The present study attempted to clarify the
role of AOC3 in the recruitment of CD4+ T cells in lung
tumors. The in vitro study used ‘cell adhesion’ of
CD4+ T cells on lung cancer cells. After AOC3 knockdown,
PKH26-stained CD4+ T cells were added to the
fully-recovered lung cancer cells (CL1-5). (A) When compared with
the control, the AOC3-knockdown lung cancer cells were less likely
to be attached to by CD4+ T cells. (B) Moreover, the
transendothelial migration assay revealed that less CD4+
T cells were attracted to AOC3-knockdown lung cancer cells compared
with the control group. However, rhAOC3 increased CD4+
T-cell (C) attachment and (D) migration. Furthermore, the in
vivo study utilized a mouse model to verify the in vitro
findings. Lewis lung carcinoma cells were injected into mice via
their tail vein. Concurrently, rmAOC3 (10 µg/mouse) was instilled
into the trachea of mice twice, 7 days apart (on day 7 and day 14)
(intratracheal instillation). After 14 days (day 21), the mice were
sacrificed and the lungs were minced. The CD4+ T cells
were then isolated. (E) The CD4+ T cells in the mice
instilled with rmAOC3 were increased compared with the controls.
All experiments were performed independently at least three times.
*P<0.05. AOC, amine oxidase, copper containing 3; rm,
recombinant mouse; LLC, Lewis lung carcinoma. |
miR-3691-5 regulates EMT and cancer
migration via epigenetic downregulation of AOC3
To further verify the possible regulatory role of
miR-3691-5p in AOC3 expression, miR-3691 mimics were
transfected to CL1-5 cells and then their biological functions were
assessed. The transfection efficacy of miRNA mimics was monitored
by Dharmacon™ siGLO™ transfection indicators and the results
revealed that >90% efficacy was achieved (Fig. 7A). miR-3691-5p downregulated
expression of AOC3 but not of AOC1 and AOC2
(Fig. 7B). The transfected lung
cancer cells were then adopted for wound healing analysis and
revealed enhanced healing process in a dose-dependent manner
(increased migration ability) (Fig.
7C). Moreover, with miR-3691-5p transfection, AOC3 protein
expression was decreased whereas the expression of the mesenchymal
markers N-cadherin, vimentin and Slug were increased (Fig. 7D). These results indicated that
miR-3691-5p affected cell migration and EMT through AOC3.
Furthermore, to evaluate the miR-3691-5p-AOC3 axis in mediating the
TIME in lung cancer, in vitro studies were performed.
CD4+ T-cell migration and adhesion to lung cancer cells
were evaluated. As determined by a cell adhesion assay,
CD4+ T-cell attachment to lung cancer cells (CL1-5) was
decreased after miR-3691-5p transfection in a dose-dependent manner
(Fig. 7E). A transendothelial
migration assay revealed that migration of CD4+ T cells
through endothelia was reduced in a dose-dependent manner with
miR-3691-5p transfection (Fig. 7F).
These results indicated that miR-3691-5p attenuated the recruitment
of CD4+ T cells. Collectively, it was indicated that
miR-3691-5p regulated AOC3 expression to perform its
biological functions.
Discussion
The present study attempted to identify a novel
factor affecting the different aspects of lung cancer pathogenesis.
Through analysis of lung cancer patients via a high-throughput NGS
tool, and the utilization of genomic data from different cohorts,
the present study determined that AOC3 contributed to lung cancer
pathogenesis. Different findings at both transcriptional and
translational levels revealed that low levels of AOC3 were a
critical factor contributing to cancer development. Low-level AOC3
facilitated mesenchymal transformation and decreased
CD4+ T-cell recruitment to lung cancer tumors. It was
also revealed that AOC3 expression was under miR3691-5p epigenetic
regulation. The strong negative association between AOC3 and the
survival rate in patients indicated that it is a key factor
involved in lung cancer, and that AOC3 could be a valuable target
for drug development (Fig. 8). AOCs
have different effects in different types of cancer (14,42–44,45,46).
High levels of AOCs can act as oncogenes and confer worse clinical
outcomes, such as AOC1 in gastric cancer (14) and AOC3 in human glioma (45). On the other hand, low levels of AOCs
are associated with worse clinical outcomes, such as AOC3 in
colorectal (42,47) and gastric cancer (46). Moreover, decreased AOC3 levels are
correlated with lymph node and hepatic metastasis in colorectal
cancer (47). The present study is
the first one, to the best of our knowledge, which revealed that
low-level AOC3 is the critical amine oxidase in lung cancer
pathogenesis but not AOC1 or AOC2. Furthermore, miR-3691-5p
regulation on AOC3 and its biological functions were defined.
miR-3691-5p has been demonstrated to enhance migration and invasion
in hepatocellular carcinoma (48).
Low expression of AOC3 conferred poor clinical outcomes and lymph
node metastasis, supporting the theory that AOC3 acts as a tumor
suppressor in lung cancer. Biological function analyses revealed
that AOC3 did not affect cell proliferation but that it did
influence cell migration. The process of EMT is essential for the
enhancement of cell migration (49).
AOC3 knockdown enhanced mesenchymal characteristics as revealed by
an increase in N-cadherin, vimentin and Slug and attenuated
epithelial characteristics as revealed by a decrease in E-cadherin.
Exogenous rhAOC3 reversed these mesenchymal patterns. This finding
revealed that AOC3 is involved in the maintenance of epithelial
characteristics to decrease the metastasis ability of lung cancer.
For the first time, the present study has revealed the pathogenic
roles of AOC3 in malignant cells and AOC3 per se providing a
useful biomarker and prognostic factor in lung cancer patients for
clinical diagnosis and treatment.
AOC3 contributes to both innate and acquired
immunity (50). Endothelial AOC3
mediates the adhesion of tumor infiltration lymphocytes,
lymphokine-activated killer cells and natural killer cells
(51) in inflammatory tissue
(52) and tumor tissue. An absence of
AOC3 leads to a marked reduction in antigen-specific
CD4+ recruitment into the airway bronchial lymph nodes
(50). In the present study,
knockdown of AOC3 in lung cancer cells caused a reduction in
CD4+ T-cell extravasation through the endothelial layer
and attachment to cancer cells. On the other hand, exogenous rhAOC3
increased transendothelial migration and enhanced CD4+
T-cell attachment onto lung cancer cells. Furthermore, rmAOC3
facilitated CD4+ T-cell recruitment to preexisting lung
tumor in a mouse model. These results indicated that AOC3 could
modulate the TIME in lung cancer cells, and it may be possible to
potentiate its effectiveness by immunotherapy. However, before
reaching a definite conclusion, there are some limitations in the
present study. Firstly, CD4+ T cells were utilized as a
recruiting immune cell to the lung. However, there are more immune
cells such as dendritic cells and macrophages and consequently,
further investigation may be necessary. Secondly, data from
immunohistochemical staining for membrane-bound AOC3 in tumor
tissues, which would limit the role of AOC3 in lung cancer, are
lacking.
Collectively, the results of the present study
confirmed the axis of miR-3691-5p-AOC3 as having a critical role in
lung cancer via inhibiting EMT and migration and a determining
factor for the recruitment of CD4+ T cells to restore
anticancer immunity in the TME. AOC3 expression in lung cancer
specimens may provide valuable information for patient prognosis
and could have valuable applications when determining a therapeutic
strategy in immunotherapy/chemotherapy.
Acknowledgements
The authors would like to thank the CPTAC of UALCAN
and the Human Protein Atlas who generated the data used in this
publication. The authors would also like to thank the Center for
Research Resources and Development in Kaohsiung Medical University
for the assistance in Bioinformatics.
Funding
The present study was supported by the Ministry of
Science and Technology (grant nos. 110-2314-B-037-124-MY3,
109-2314-B-037-091 and 108-2320-B-037-024-MY3), the Kaohsiung
Medical University (grant no. KMU-DK108008), the Kaohsiung Medical
University Hospital (grant nos. KMUH108-8R15, KMUH108-8R16 and
MUH106-6T06) and the Kaohsiung Municipal Ta-Tung Hospital (grant
nos. KMTTH-103-019 and KMTTH-105-051).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are not publicly available due to ongoing study in
our laboratory but are available from the corresponding author on
reasonable request.
Authors' contributions
CYC, YMT and YLH conceptualized the present study.
SFJ, PHT and YCH provided the technical support, performed the
experiments and acquired the data. CYC provided the software
management and analyzed the data. YYC, JYH, WAC and IWC validated
the results. KLW, YMT and YLH performed the formal analysis. YLH
pursued the investigation and provided the resources. YMT, KLW and
YLH performed data curation and interpreted the data. YMT and KLW
wrote original draft. YYC and YLH wrote, reviewed and edited the
final manuscript. IWC performed visualization of the imaging data.
YLH supervised the study, was the project administrator and
acquired the funding. YMT and YLH critically revised the manuscript
for important intellectual content. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The protocol of the present study was approved
(approval no. KMUH-IRB-20130054 and and KMUH-IRB-G(II)-20180021) by
the Institutional Review Board of Kaohsiung Medical University
Hospital (Kaohsiung, Taiwan) and written informed consents were
acquired from all enrolled patients. All mice procedures were
approved by the Institutional Animal Care and Use Committee at
Kaohsiung Medical University (Kaohsiung, Taiwan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EML4-ALK
|
echinoderm microtubule-associated
protein-like 4-anaplastic lymphoma kinase
|
|
AOC3
|
amine oxidase, copper containing
3
|
|
CAO
|
copper-containing amine oxidase
|
|
EGFR
|
epithelial growth factor receptor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
MAO
|
monoamine oxidase
|
|
NGS
|
next generation sequencings
|
|
TIME
|
tumor immune microenvironment
|
|
TME
|
tumor microenvironment
|
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arbour KC and Riely GJ: Systemic therapy
for locally advanced and metastatic non-small cell lung cancer: A
review. JAMA. 322:764–774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Lung Screening Trial Research
Team, . Lung Cancer Incidence and mortality with extended follow-up
in the National lung screening trial. J Thorac Oncol. 14:1732–1742.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elmore LW, Greer SF, Daniels EC, Saxe CC,
Melner MH, Krawiec GM, Cance WG and Phelps WC: Blueprint for cancer
research: Critical gaps and opportunities. CA Cancer J Clin.
71:107–139. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duan Q, Zhang H, Zheng J and Zhang L:
Turning cold into hot: Firing up the tumor microenvironment. Trends
Cancer. 6:605–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vakal S, Jalkanen S, Dahlstrom KM and
Salminen TA: Human copper-containing amine oxidases in drug design
and development. Molecules. 25:12932020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mondovì B and Finazzi Agrò A: Structure
and function of amine oxidase. Adv Exp Med Biol. 148:141–153. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buffoni F and Ignesti G: The
Copper-containing amine oxidases: Biochemical aspects and
functional role. Mol Genet Metab. 71:559–564. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boomsma F, Bhaggoe UM, van der Houwen AM
and van den Meiracker AH: Plasma semicarbazide-sensitive amine
oxidase in human (patho)physiology. Biochim Biophys Acta.
1647:48–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salmi M and Jalkanen S: Vascular adhesion
protein-1: A cell surface amine oxidase in translation. Antioxid
Redox Signal. 30:314–332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu F, Xu Y, Xiong JH, Zhang JH, Wu J, Luo
J and Xiong JP: AOC1 Contributes to tumor progression by promoting
the AKT and EMT pathways in gastric cancer. Cancer Manag Res.
12:1789–1798. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lopes de Carvalho L, Bligt-Linden E,
Ramaiah A, Johnson MS and Salminen TA: Evolution and functional
classification of mammalian copper amine oxidases. Mol Phylogenet
Evol. 139:1065712019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imamura Y, Kubota R, Wang Y, Asakawa S,
Kudoh J, Mashima Y, Oguchi Y and Shimizu N: Human retina-specific
amine oxidase (RAO): cDNA cloning, tissue expression, and
chromosomal mapping. Genomics. 40:277–283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonaiuto E, Lunelli M, Scarpa M, Vettor R,
Milan G and Di Paolo ML: A structure-activity study to identify
novel and efficient substrates of the human semicarbazide-sensitive
amine oxidase/VAP-1 enzyme. Biochimie. 92:858–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salmi M and Jalkanen S: A 90-kilodalton
endothelial cell molecule mediating lymphocyte binding in humans.
Science. 257:1407–1409. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stankovic B, Bjørhovde HAK, Skarshaug R,
Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold
ES, Woldbæk PR, et al: Immune cell composition in human non-small
cell lung cancer. Front Immunol. 9:31012019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oja AE, Piet B, van der Zwan D,
Blaauwgeers H, Mensink M, de Kivit S, Borst J, Nolte MA, van Lier
RAW, Stark R and Hombrink P: Functional heterogeneity of
CD4+ tumor-infiltrating lymphocytes with a resident
memory phenotype in NSCLC. Front Immunol. 9:26542018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ,
Liang SC, Lin CH, Whang-Peng J, Hsu SL, Chen CH and Huang CY:
Selection of DDX5 as a novel internal control for Q-RT-PCR from
microarray data using a block bootstrap re-sampling scheme. BMC
Genomics. 8:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Landi MT, Dracheva T, Rotunno M, Figueroa
JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, et
al: Gene expression signature of cigarette smoking and its role in
lung adenocarcinoma development and survival. PLoS One.
3:e16512008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beer DG, Kardia SL, Huang CC, Giordano TJ,
Levin AM, Misek DE, Lin L, Chen G, Gharib TG, Thomas DG, et al:
Gene-expression profiles predict survival of patients with lung
adenocarcinoma. Nat Med. 8:816–824. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stearman RS, Dwyer-Nield L, Zerbe L,
Blaine SA, Chan Z, Bunn PA Jr, Johnson GL, Hirsch FR, Merrick DT,
Franklin WA, et al: Analysis of orthologous gene expression between
human pulmonary adenocarcinoma and a carcinogen-induced murine
model. Am J Pathol. 167:1763–1775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Selamat SA, Chung BS, Girard L, Zhang W,
Zhang Y, Campan M, Siegmund KD, Koss MN, Hagen JA, Lam WL, et al:
Genome-scale analysis of DNA methylation in lung adenocarcinoma and
integration with mRNA expression. Genome Res. 22:1197–1211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garber ME, Troyanskaya OG, Schluens K,
Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen
GD, Perou CM, Whyte RI, et al: Diversity of gene expression in
adenocarcinoma of the lung. Proc Natl Acad Sci USA. 98:13784–13789.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens
JA, Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wachi S, Yoneda K and Wu R:
Interactome-transcriptome analysis reveals the high centrality of
genes differentially expressed in lung cancer tissues.
Bioinformatics. 21:4205–4208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhattacharjee A, Richards WG, Staunton J,
Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et
al: Classification of human lung carcinomas by mRNA expression
profiling reveals distinct adenocarcinoma subclasses. Proc Natl
Acad Sci USA. 98:13790–13795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gyorffy B, Surowiak P, Budczies J and
Lanczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mende DR, Letunic I, Maistrenko OM,
Schmidt TS, Milanese A, Paoli L, Hernández-Plaza A, Orakov AN,
Forslund SK, Sunagawa S, et al: proGenomes2: An improved database
for accurate and consistent habitat, taxonomic and functional
annotations of prokaryotic genomes. Nucleic Acids Res.
48:D621–D625. 2020.PubMed/NCBI
|
|
36
|
Sticht C, De La Torre C, Parveen A and
Gretz N: miRWalk: An online resource for prediction of microRNA
binding sites. PLoS One. 13:e02062392018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ke HL, Li WM, Lin HH, Hsu WC, Hsu YL,
Chang LL, Huang CN, Li CC, Chang HP, Yeh HC, et al:
Hypoxia-regulated MicroRNA-210 overexpression is associated with
tumor development and progression in upper tract urothelial
carcinoma. Int J Med Sci. 14:578–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shao L, Li H, Chen J, Song H, Zhang Y, Wu
F, Wang W, Zhang W, Wang F, Li H and Tang D: Irisin suppresses the
migration, proliferation, and invasion of lung cancer cells via
inhibition of epithelial-to-mesenchymal transition. Biochem Biophys
Res Commun. 485:598–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ward ST, Weston CJ, Shepherd EL, Hejmadi
R, Ismail T and Adams DH: Evaluation of serum and tissue levels of
VAP-1 in colorectal cancer. BMC Cancer. 16:1542016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun WY, Choi J, Cha YJ and Koo JS:
Evaluation of the expression of amine oxidase proteins in breast
cancer. Int J Mol Sci. 18:27752017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kostoro J, Chang SJ, Clark Lai YC, Wu CC,
Chai CY and Kwan AL: Overexpression of vascular adhesion protein-1
is associated with poor prognosis of astrocytomas. APMIS.
124:462–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang SJ, Tu HP, Lai YCC, Luo CW, Nejo T,
Tanaka S, Chai CY and Kwan AL: Increased vascular adhesion protein
1 (VAP-1) levels are associated with alternative M2 macrophage
activation and poor prognosis for human gliomas. Diagnostics
(Basel). 10:2562020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yasuda H, Toiyama Y, Ohi M, Mohri Y, Miki
C and Kusunoki M: Serum soluble vascular adhesion protein-1 is a
valuable prognostic marker in gastric cancer. J Surg Oncol.
103:695–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Toiyama Y, Miki C, Inoue Y, Kawamoto A and
Kusunoki M: Circulating form of human vascular adhesion protein-1
(VAP-1): Decreased serum levels in progression of colorectal cancer
and predictive marker of lymphatic and hepatic metastasis. J Surg
Oncol. 99:368–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Du W, Zhang X and Wan Z: miR-3691-5p
promotes hepatocellular carcinoma cell migration and invasion
through activating PI3K/Akt signaling by targeting PTEN. Onco
Targets Ther. 12:4897–4906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Leggett SE, Hruska AM, Guo M and Wong IY:
The epithelial-mesenchymal transition and the cytoskeleton in
bioengineered systems. Cell Commun Signal. 19:322021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dunkel J, Aguilar-Pimentel JA, Ollert M,
Fuchs H, Gailus-Durner V, de Angelis MH, Jalkanen S, Salmi M and
Veres TZ: Endothelial amine oxidase AOC3 transiently contributes to
adaptive immune responses in the airways. Eur J Immunol.
44:3232–3239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Irjala H, Salmi M, Alanen K, Grénman R and
Jalkanen S: Vascular adhesion protein 1 mediates binding of
immunotherapeutic effector cells to tumor endothelium. J Immunol.
166:6937–6943. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stolen CM, Marttila-Ichihara F, Koskinen
K, Yegutkin GG, Turja R, Bono P, Skurnik M, Hänninen A, Jalkanen S
and Salmi M: Absence of the endothelial oxidase AOC3 leads to
abnormal leukocyte traffic in vivo. Immunity. 22:105–115. 2005.
View Article : Google Scholar : PubMed/NCBI
|