Introduction
Non-coding RNA (ncRNA) refers to RNAs that lack
protein coding ability, which are ubiquitously expressed in human
cells. Findings from two large-scale genome projects, FANTOM
(1) and ENCODE (2), revealed that 80% of the human genome
is transcriptionally active, whereas only 2% of the human genome
encodes proteins. ncRNAs with a length of >200 nucleotides are
known as long ncRNAs (lncRNAs) and participate in diverse
biological processes (3).
According to a previous study, the number of identified lncRNAs in
humans is >60,000 (4).
lncRNAs were previously considered as non-functional
‘junk’ generated during the process of transcription; however,
numerous studies published more recently have reported that lncRNAs
fulfill important roles in biological processes. Studies published
to date, however, have been rather preliminary and an understanding
of the functions of lncRNAs in the cell, including in processes of
reproduction, evolution, cognition and disease, remain in the
infancy stages (5); therefore,
only a limited number of lncRNAs have been annotated (6). For these reasons, great potential and
value lies in performing research on lncRNAs and they have
consequently become a ‘hotspot’ area in biological research.
Several studies have explored the diverse and complex roles of
lncRNAs in various biological processes. lncRNAs interact with
biological molecules, such as mRNAs, DNA, proteins and microRNAs
(miRNAs), thereby modulating epigenetic, transcriptional,
post-transcriptional, translational and post-translational events
of gene expression (7,8). Wang and Chang (9) summarized and classified the
interactions that may occur between lncRNAs and these molecules
into four archetypes, namely signal, decoy, guide and scaffold, and
these archetypes may co-exist or overlap with each other.
At the molecular level, interactions between RNA and
protein are important and common, as they fulfill key roles in
cellular processes (10–14). RNA-binding proteins (RBPs) are a
class of proteins that bind RNA through one or more RNA-binding
domains (RBDs), which determines the fate or function of RNA. RBPs
are involved in virtually all aspects of RNA metabolism through the
formation of dynamic functional ribonucleoprotein (RNP) complexes
with RNA (14,15). A regulatory role for RBPs with
respect to RNA has been widely reported in previous studies on mRNA
and miRNA (16–19). However, more recent studies on
lncRNAs have reported that numerous lncRNAs are implicated in the
recruitment, organization, activation or inhibition of RBPs,
indicating that RNA is also able to regulate RBPs (15,20,21).
These results indicate that the regulatory interactions that occur
between RBPs and RNA are bidirectional, particularly in the case of
lncRNAs.
Numerous studies have examined the functions of
RBPs, and therefore, knowledge on their scope and range of
biological roles is constantly expanding. Previous studies reported
that RBPs possess certain canonical RBDs, such as the RNA
recognition motif, the KM domain and zinc finger motif, all of
which are specifically recognized and bound by RNAs (10,15).
This group of RBPs, which are referred to as ‘classical’ RBPs, has
been extensively studied. However, proteomics studies have reported
on the identification of certain non-classical RBPs that lack these
canonical RBD domains; these non-canonical domains do not affect
the binding of the RBPs to RNA (15,22,23).
The mechanism of binding of non-classical RBPs to RNAs may involve
multiple factors, including the molecular spatial structure,
intracellular localization and expression level. On the other hand,
DNA-binding proteins (DBPs), which are proteins that bind to DNA,
have mainly been studied independently of RBPs owing to their
different structural features. For instance, transcription factors
are well established as a class of typical DBPs that recognize
specific DNA sequences to regulate gene expression. However,
emerging evidence has revealed that certain lncRNAs that are
located in the nucleus are able to competitively bind to
transcription factors through sites similar to DNA-binding motifs,
thus preventing them from binding to their target DNA (24–26).
This class of proteins with the dual function of binding both RNA
and DNA are referred to as DNA- and RNA-binding proteins (DRBPs).
The distinction between the concepts of DBP and RBP has become
gradually blurred over time (20),
and this blurring has mainly occurred where lncRNAs are involved.
Therefore, the current review aimed to mainly explore ‘generalized
RBPs’, which comprise all types of proteins or protein complexes
that directly bind to and interact with RNA, including classical
and non-classical RBPs, as well as certain unique RBPs or RBP
complexes, such as transcription factors, protein kinases and
chromatin-modified complexes.
Various studies have reported that lncRNAs are
closely associated with cancer. lncRNAs are abnormally expressed in
the majority of cancer types and have been indicated to exert
regulatory roles in various cancer phenotypes through different
molecular mechanisms (27–30). In addition, previously published
studies have indicated that interactions between lncRNAs and RBPs
provide the main mechanism through which lncRNAs exert their
function (31–33). Other studies have indicated that
interactions between lncRNAs and RBPs are involved in the
occurrence and development of various types of disease, including
cancer (34–38). Therefore, interactions between
lncRNAs and RBPs have been suggested to fulfill key roles both in
carcinogenesis and in the progression of cancer. The next chapter
summarizes the common interactions that have been identified
between lncRNAs and RBPs in cancer from the perspectives of
molecular structure, expression level, subcellular localization and
interactome. The topics covered in the text of the present review
are summarized in Fig. 1 and
Table I.
 | Table I.Interaction between lncRNAs and RBPs
in cancer. |
Table I.
Interaction between lncRNAs and RBPs
in cancer.
| A, lncRNAs regulate
post-translational modification of RBPs |
|---|
|
|---|
| lncRNA | Interacting
RBP | Interaction
mechanisms | Resulting effects
on cancer | (Refs.) |
|---|
| LET | NF-90 | Downregulates NF90
protein abundance via the ubiquitin-proteasome pathway | Represses the
invasion of HCC | (41) |
| OCC-1 | HuR | Enhances binding of
ubiquitin E3 ligase β-TrCP1 to HuR | Suppresses cell
growth in CRC | (42) |
| HOTAIR | RUNX3 | Enhances binding of
E3 ligase Mex3b to RUNX3 | Enhances the
invasion of GC | (43) |
| TINCR | ACLY | Protects ACLY from
ubiquitination | Promotes
proliferation, metastasis and cisplatin resistance of NPC | (44) |
| LINRIS | IGF2BP2 | Blocks IGF2BP2
ubiquitin site K139 and downregulates its ubiquitination level | Promotes the
aerobic glycolysis in CRC | (45) |
| NKILA | NF-κB/IκB
complex | Inhibits
phosphorylation by masking the phosphorylation sites of IκB | Suppresses the
metastasis of BRC | (46) |
| HULC | PKM2 | Promotes
phosphorylation of PKM2 and inhibits its tetramer formation | Promotes aerobic
glycolysis of HCC cells | (47) |
| APAL | PLK1 | Promotes
phosphorylation and activation of PLK1 | Maintains the
survival of BRC, NSCLC cells | (48) |
| ANCR | EZH2 | Promotes
phosphorylation of EZH2 and facilitates its ubiquitination | Inhibits the
invasion and metastasis of BRC | (51) |
| RMST | FUS | Promotes
SUMOylation of FUS and inhibits its ubiquitination | Suppresses GBM cell
mitophagy | (54) |
| pSTAR | hnRNP K | Inhibits
deSUMOylation of hnRNP K and promotes formation of p53-hnRNP K
complex | Inhibits HCC cell
growth through inducing cell cycle arrest | (55) |
|
| B, lncRNAs
regulate intracellular localization of RBPs |
|
| lncRNA | Interacting
RBP | Interaction
mechanisms | Resulting
effects on cancer | (Refs.) |
|
| BLACAT2 | WDR5 | Recruits WDR5 to
the promoter region of VEGF-C gene | Promotes
lymphangiogenesis and lymphatic metastasis in BLC | (56) |
| LNMAT1 | hnRNP L | Recruits hnRNP L to
the promoter region of CCL2 | Promotes lymphatic
metastasis of BLC | (57) |
| HOXA11-AS | WDR5 | Recruits WDR5 to
the promoter region and increases expression of β-catenin | Promotes cell cycle
progression and metastasis in GC | (58) |
| HOXA11-AS | EZH2 | Recruits EZH2 to
the promoter region and inhibits the transcriptional level of
P21 | Promotes cell cycle
progression and metastasis in GC | (58) |
| AC020978 | PKM2 | Promote PKM2 to
translocate from nucleus to cytoplasm | Promotes
proliferation and glycolytic metabolism of NSCLC | (59) |
| XIST | SMAD2 | Inhibits transport
of SMAD2 into the nucleus | Promotes cell
growth and DDP chemoresistance in NSCLC | (60) |
| GAS5 | YAP | Blocks
translocation of YAP from the cytoplasm into nucleus | Inhibits
proliferation, invasion and metastasis in CRC | (61) |
| OLA1P2 (HGNC:
45277) | STAT3 | Inhibits formation
of phosphorylated STAT3 and restricts its transport into
nucleus | Inhibits
proliferation, invasion and metastasis in CRC | (62) |
| EPB41L4A-AS1 | HDAC2 | Restricts release
of HDAC2 from nucleolus to nucleoplasm | Inhibits glycolysis
and glutaminolysis in CC and HCC cells | (63) |
|
| C, lncRNAs
affect interaction network of RBPs |
|
| lncRNA | Interacting
RBP | Interaction
mechanisms | Resulting
effects on cancer | (Refs.) |
|
| FGF13-AS1 | IGF2BPs | Inhibits
stabilizing role of IGF2BPs on c-Myc mRNA | Inhibits glycolysis
and stemness of BRC | (64) |
| LINC01093 | IGF2BP1 | Facilitates GLI1
mRNA degradation by blocking the binding of IGF2BP1 | Inhibits
proliferation and metastasis of HCC | (65) |
| FILNC1 | AUF1 | Downregulates c-Myc
expression by inhibiting interaction of AUF1 with c-Myc mRNA | Represses energy
metabolism and inhibits RC development | (66) |
| MALAT1 | SFPQ | Releases PTBP2 from
the SFPQ/PTBP2 complex | Promotes tumor
growth and metastasis in CRC | (67) |
| P53RRA | G3BP1 | Displaces p53 from
the G3BP1 complex | Promotes
ferroptosis and apoptosis of LC cells | (68) |
| MYCLo-1 | HuR | Inhibits binding of
HuR to CDKN1A | Promotes the
proliferation of CRC cells | (69) |
| MYCLo-2 | hnRNP K | Inhibits binding of
hnRNP K to CDKN2B | Promotes the
proliferation of CRC cells | (69) |
| LUCAT1 | NCL | Inhibits binding of
NCL to G4 sequence in the MYC promoter | Promotes the
proliferation of CRC cells | (70) |
| THOR | IGF2BP | region Stabilizes
downstream target mRNAs of IGF2BP | Facilitates
proliferation in LC and melanoma cells | (71) |
| AFAP1-AS1 | AUF1 | Promotes binding of
AUF1 to HER-2 mRNA under exosome mediation | Promotes
trastuzumab resistance in BRC | (72) |
| HOXA11-AS | STAU1 | Promotes binding of
STAU1 to KLF2 mRNA and accelerates degradation of KLF2 mRNA | Promotes cell cycle
progression and metastasis in GC | (58) |
| RP11 | hnRNPA2B1 | Promotes binding of
hnRNPA2B1 to Siah1 and Fbxo45 mRNAs and accelerates their
degradation | Promotes migration,
invasion, EMT and liver metastasis in CRC | (73) |
| SChLAP1 | hnRNP L | Stabilizes ACTN4 by
promoting interaction between hnRNP L and ACTN4 | Promotes growth of
GBM cells | (74) |
| ZFAS1 | NOP58 | Activates NOP58 to
promote recruitment of SNORD12C and SNORD78 | Promotes
proliferation and inhibits apoptosis in CRC | (75) |
| PiHL | GRWD1 | Promotes binding of
GRWD1 to RPL11 and isolates RPL11 from MDM2 | Maintains cell
proliferation and induces 5-FU chemoresistance in CRC | (76) |
| LINC0051 | EZH2 | Enhances the
enrichment of EZH2 on IL-24 promoter and silences IL-24
expression | Promotes
proliferation and inhibits apoptosis of CRC cells | (78) |
| CYTOR | NCL, Sam68 | Scaffolds the
trimer NCL/CYTOR/Sam68 | Promotes
proliferation, migration, invasion, EMT and metastasis in CRC | (79) |
| HITTERS | MRE11-RAD50-NBS1
protein complex | Facilitates
formation of MRN protein complex as an RNA scaffold | Promotes the
invasion and lung metastasis of OSCC | (80) |
| HOTAIR | HBXIP, LSD1 | Forms an
RNA/protein complex to recruit LSD1 to the promoters of c-Myc
target genes | Promotes the
proliferation of BRC cells | (81) |
| LBCS | hnRNP K, EZH2 | Forms a complex
mediating H3K27me3 in the SOX2 promoter region | Inhibits
self-renewal and chemoresistance of BLC stem cells | (82) |
|
| D, RBPs regulate
lncRNA expression at post-transcriptional level |
|
| lncRNA | Interacting
RBP | Interaction
mechanisms | Resulting
effects on cancer | (Refs.) |
|
| NEAT1 | SRSF1 | Maintains the
stability of NEAT1 | Facilitates GBM
cell proliferation and cell cycle progression | (87) |
| NEAT1_1 | HuR | Maintains the
stability of NEAT1_1 | Promotes OC cell
proliferation and invasion | (88) |
| LincRNA-P21 | HuR | Accelerates
lincRNA-p21 degradation by recruiting let-7/RISC | Facilitates JunB
and β-catenin translation and increases the levels of these
proteins in HeLa cells | (92) |
| HOTAIR | HuR | Accelerates HOTAIR
degradation via let7/Ago2 pathway | Inhibits the
ubiquitination of Ataxin-1 and Snurportin-1 and decelerates their
degradation in HeLa cells | (93) |
| NEAT1, TUG1 | PABPN1 | Accelerates NEAT1
and TUG1 degradation via RNA exosome complexes | Promotes the
degradation of PABPN1-sensitive lncRNAs in HeLa cells | (96) |
| HULC | IGF2BP1 | Accelerates HULC
degradation by recruiting CCR4-NOT complex | Reduces the
expression of HULC in HCC cells | (98) |
|
| E, RBPs regulate
lncRNAs localization and transport |
|
| lncRNA | Interacting
RBP | Interaction
mechanisms | Resulting
effects on cancer | (Refs.) |
|
| MALAT1 | HuR/MTCH2 | Facilitates MALAT1
to shuttle into mitochondria | Promotes the
proliferation, migration and invasion of HCC | (101) |
| RMRP | HuR | Facilitates RMRP
nuclear export | Enhances oxygen
consumption rates and mitochondrial DNA replication priming in HeLa
cells | (105) |
| RMRP | GRSF1 | Facilitates RMRP
accumulation in mitochondria | Enhances oxygen
consumption rates and mitochondrial DNA replication priming in HeLa
cells | (105) |
| MALAT1 | SNRNP70 | Facilitates nuclear
and genome-wide localization of MALAT1 | Maintains the
localization of both nascent and polyadenylated lncRNA transcripts
to chromatin in HeLa cells | (106) |
| LNMAT2 | hnRNPA2B1 | Facilitates LNMAT2
loading into exosomes and secreting out of cell | Promotes lymphatic
metastasis in BLC | (109) |
|
| F, RBPs mediate
lncRNA function |
|
| lncRNA | Interacting
RBP | Interaction
mechanisms | Resulting
effects on cancer | (Refs.) |
|
| MALAT1 | SRSF1 | Mediates the
interaction between MALAT1 and mutant p53 or ID4 | Promotes
angiogenesis through repression of VEGFA165b in BRC | (110) |
| PURPL | HuR | Mediates the
interaction between PURPL and MYBBP1A | Promotes tumor
growth in CRC | (111) |
Regulation of RBPs by lncRNAs in cancer
Regulation of post-translational
modification
Interactions between lncRNAs and RBPs alter the
structure of RBPs and the most well-studied mechanism to date in
the investigation of this phenomenon has been post-translational
modification of RBP. Protein ubiquitination is widely involved in
all life activities of cells and has an important role in protein
degradation. A total of two major protein degradation pathways,
i.e., the ubiquitin-proteasome pathway and the autophagy-lysosome
pathway, are implicated in protein ubiquitination (39,40).
Previous studies have demonstrated that cancer-associated lncRNAs
are able to change the ubiquitination status of a protein after it
binds to RBP (41–43). A study on hepatocellular carcinoma
(HCC) reported that the binding of lncRNA-Low Expression in Tumor
(lncRNA-LET) promoted the ubiquitination and degradation of protein
nuclear factor 90 (NF-90) (41).
RNA immunoprecipitation (RIP) experiments using antibodies raised
against important E3 ligases (such as pirh2, wwp2 and skp2) in
liver cancer in previous studies were performed in this study;
however, lncRNA-LET was not detected. These results indicated that
lncRNA-LET may have combined with other unknown E3 ligases or may
have changed the conformation of NF-90, resulting in exposure of
ubiquitination sites, thereby increasing the ubiquitination level
of NF-90. Another study reported that lncRNA overexpressed in colon
carcinoma-1 (OCC-1) binds to classical RBP human antigen R (HuR) in
colorectal cancer (CRC). The study revealed that OCC-1 is able to
upregulate ubiquitination of HuR and decrease its expression level
through promoting the binding of HuR to the E3 ubiquitin ligase
β-Trcp1, thus inhibiting the stabilization of HuR on its target
mRNAs (42). Xue et al
(43) explored the specific
binding of HOX antisense intergenic RNA (HOTAIR) to Runt-related
transcription factor 3 (RUNX3) protein through bioinformatic
analysis combined with pull-down, RIP and truncation experiments.
Mechanistic studies demonstrated that HOTAIR promoted the binding
of RUNX3 to the E3 ligase Mex3b and accelerated degradation of
RUNX3 through the ubiquitin-proteasome pathway, thereby improving
the invasive ability of gastric cancer cells. These results
suggested that lncRNAs are able to mediate and promote interactions
between RBPs and E3 ligase, increasing the protein ubiquitination
level and downregulating the expression of key regulatory proteins
in cancer.
Conversely, other interactions that have been
identified between lncRNAs and RBPs have been indicated to lead to
inhibition of the ubiquitination level of certain cancer-associated
proteins. For instance, lncRNA terminal differentiation-induced
non-coding RNA (TINCR), which is highly expressed in nasopharyngeal
carcinoma (NPC), was indicated to reduce the ubiquitination level
after binding to ATP citrate lyase (ACLY) protein (44). Silencing TINCR led to an increase
in the ubiquitination level of ACLY. Of note, the proteasome
inhibitor MG132 was observed to reverse this effect, implying that
the stabilizing effect of TINCR on ACLY is achieved through
inhibiting the ubiquitin-proteasome pathway, and an elevated
expression of ACLY promoted both the progression and the
chemotherapeutic resistance of NPC (44). In a separate study by Wang et
al (45), long intergenic
ncRNA for insulin-like growth factor 2 mRNA-binding protein 2
(IGF2BP2) Stability (LINRIS) was indicated to be highly expressed
in CRC and to be associated with poor prognosis. They reported that
the protein IGF2BP2 is able to stabilize its downstream target,
c-Myc mRNA, which is the core regulator of aerobic glycolysis in
CRC. LINRIS interacts with IGF2BP2, thereby leading to a decrease
in its ubiquitination level. However, this function of LINRIS was
not indicated to be mediated via the proteasome pathway, but
through the autophagy-lysosomal pathway, which led to a higher
expression level of IGF2BP2, promoting the aerobic glycolysis of
CRC cells. Of note, the intrinsic molecular mechanism involved the
binding of LINRIS to block the IGF2BP2 ubiquitin site, Lys139.
Therefore, there is accumulating evidence that the physical binding
of lncRNAs may lead to inhibition of protein ubiquitination via the
shielding of ubiquitination sites, thereby maintaining the
stability of key proteins in cancer.
Another common mode of protein modification to be
considered in terms of interactions that occur between lncRNAs and
RBPs is phosphorylation. lncRNAs are able to inhibit
phosphorylation of their binding partner RBP through a mechanism
similar to that employed in ubiquitination inhibition. For
instance, the lncRNA NF-κB interacting lncRNA binds to NF-κB/IκB
complex and inhibits the phosphorylation of IκB via IκB kinase by
‘masking’ the phosphorylation sites of IκB, thereby reducing
degradation of IκB and maintaining the inhibition of IκB on NF-κB,
ultimately suppressing breast cancer (BRC) metastasis (46). Several studies have demonstrated
how the facilitating effect of lncRNAs on RBP phosphorylation is a
common occurrence. Pyruvate kinase M2 (PKM2), an isoenzyme of
pyruvate kinase, is a key enzyme in glycolysis. PKM2 promotes tumor
growth by regulating the expression of genes involved in cell
proliferation, migration and apoptosis. The lncRNA highly
upregulated in liver cancer (HULC) was indicated to directly bind
to PKM2 and promote its phosphorylation, thereby inhibiting the
formation of the tetramer conformation (which is its activated
state), ultimately downregulating its activity (47). As another example, polo-like kinase
1 (PLK1) is a key regulator of the cell cycle and DNA damage
repair. Aurora A/PLK1-associated lncRNA binds to PLK1 and promotes
the phosphorylation and activation of PLK1, thereby inhibiting the
apoptosis of tumor cells (48).
The lncRNA-induced phosphorylation of RBP may cooperate with
ubiquitination to promote protein degradation (49,50).
For instance, enhancer of Zeste homolog 2 (EZH2) is a component of
polycomb suppress complex 2, which exerts important roles in the
occurrence and metastasis of BRC, among other cancer types.
Cyclin-dependent kinase 1 (CDK1) is able to induce the
phosphorylation of EZH2 at the Thr345 and Thr487 phosphorylation
sites, thereby promoting degradation of EZH2 through the
ubiquitin-proteasome pathway. Furthermore, the lncRNA
anti-differentiation ncRNA directly binds to EZH2. The complex that
arises promotes further interaction between CDK1 and EZH2, leading
to a heightened increase in the level of phosphorylation of EZH2 at
the Thr345 and Thr487 sites (51).
Small ubiquitin-like modifier (SUMO) is a
ubiquitin-like protein that is able to modify target proteins
through a process known as SUMOylation, which operates via a
mechanism similar to that of ubiquitination. However, SUMOylation
is different from ubiquitination, in that it does not promote
degradation of its target protein. SUMOylation fulfills an
important role in maintaining chromosomal integrity and regulating
cell proliferation. Previous studies have reported that SUMOylation
exerts a key role in cancer progression (52,53),
and lncRNAs are involved in the regulation of cancer through
modulating SUMOylation of their binding partner RBPs.
Rhabdomyosarcoma 2 associated transcript (RMST) is a highly
expressed lncRNA in glioblastoma (GBM), and RNA pull-down and RIP
experiments have indicated that it directly binds to fused in
sarcoma (FUS) protein. The interaction between RMST and FUS
promotes SUMOylation of FUS at the Lys333 site, thereby inhibiting
ubiquitination and upregulating the expression of FUS, which
ultimately leads to inhibition of the autophagy of GBM cells
mediated via downstream targets (54). Similar findings were reported by
Qin et al (55) in HCC
cells, i.e., that binding of the lncRNA p53-stabilizing and
activating RNA inhibited the deSUMOylation of RBP heterogeneous
nuclear RNP K (hnRNP K), thereby promoting the formation of the
p53-hnRNP K complex. Increased binding of hnRNP K to p53 inhibited
murine double minute 2 protein (MDM2)-dependent p53 ubiquitination
and degradation, thereby increasing p53 stability and ultimately
leading to inhibition of the proliferation of HCC cells.
Considering all of the above together, these studies
have indicated that interactions between lncRNAs and their partner
RBPs in different types of cancer modulate post-translational
modifications of RBPs either by shielding modification sites or
through linking modification enzymes. Changes in RBP structure
following the modification led to changes in the expression level
of the given RBP and this is influenced by the synergistic
mechanism of ubiquitination and other protein modifications.
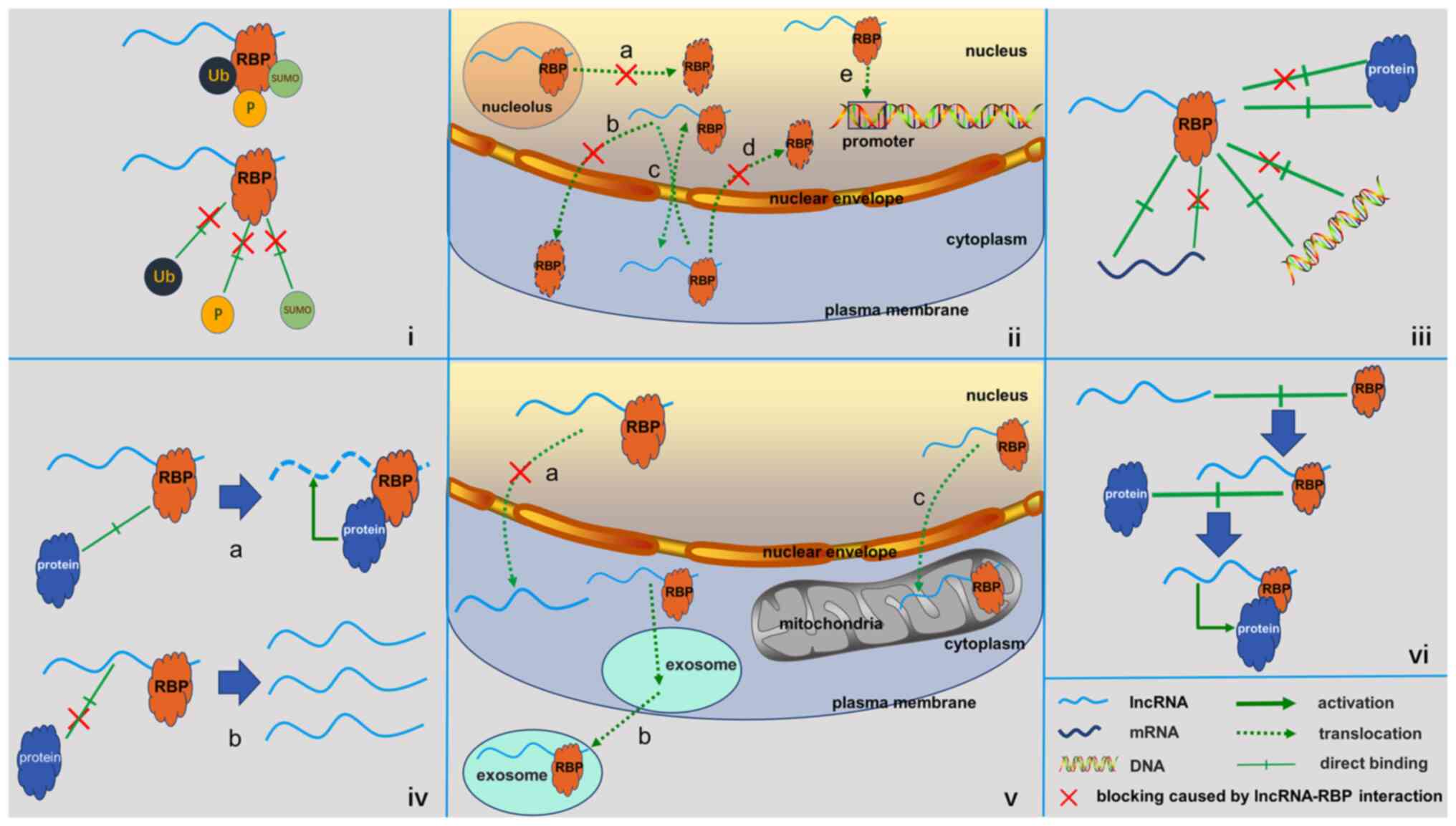
Regulation of intracellular
localization
Protein function is closely associated with the
localization of the protein of concern in the cells, and the
binding of a lncRNA may lead to a change in the intracellular
distribution of RBPs. A common regulatory mechanism of gene
expression in cancer involves the localization of transcription
factors or transcriptional co-regulators by nuclear-localized
lncRNAs precisely to the promoter region of target genes. Bladder
cancer (BLC)-associated transcript 2 is an lncRNA that is highly
expressed in BLC and which recruits WD repeat-containing protein 5
(WDR5) to the promoter region through their direct interaction,
resulting in H3K4 trimethylation of the vascular endothelial growth
factor C (VEGF-C) gene. These changes promote both VEGF-C
expression and lymphangiogenesis and lymphatic metastasis of BLC
(56). In addition, lymph node
metastasis-associated transcript 1 (LNMAT1) has been indicated to
bind to hnRNP L, recruiting it to the promoter region of chemokine
C-C motif ligand 2 (CCL2), which causes an increase in the
occupation rate of hnRNP L and H3K4 trimethylation of the promoter
region of CCL2, thereby promoting lymphatic metastasis of BLC
(57). In gastric cancer, the
antisense (AS) lncRNA-HOXA11-AS recruits WDR5 to the promoter
region and increases the expression of β-catenin via binding to
WDR5. Furthermore, HOXA11-AS has been demonstrated to recruit EZH2
to the promoter region of P21, where it causes an inhibition of the
transcription of P21 (58).
Collectively, these results suggest that lncRNAs are implicated in
the localization of RBPs on the promoter regions of their target
genes, where they elicit either positive or negative regulation of
transcription of the genes concerned.
The roles of lncRNAs in nucleocytoplasmic
localization of the RBPs that they bind are diverse. For instance,
lncRNA-AC020978 is upregulated in non-small cell lung cancer
(NSCLC) and its upregulation is strongly correlated with TNM
staging and the clinical prognosis of NSCLC. AC020978 is also able
to promote the translocation of PKM2 from the nucleus to the
cytoplasm through their direct interaction, thereby promoting the
activation of hypoxia-inducible factor-1α transcription during
glucose starvation and hypoxia (59). In addition, the lncRNA X
inactive-specific transcript was indicated to inhibit transport of
the TGF-β effector factor SMAD2 into the nucleus through their
direct binding, leading to transcriptional inhibition of both p53
and NLR family pyrin domain containing 3, which are key regulators
of apoptosis and pyrolysis, ultimately leading to the facilitation
of tumor growth in NSCLC and the promotion of cisplatin resistance
(60). As a further example,
dysregulation of the Hippo/Yes-associated protein (YAP) signaling
pathway promotes tumorigenesis of CRC and other cancers, with YAP
being a key factor in the Hippo signaling pathway. A previous study
reported that growth arrest-specific 5 is able to block
translocation of YAP from the cytoplasm into the nucleus through
its binding to YAP, resulting in an accumulation of YAP in the
cytoplasm, thereby promoting the ubiquitination degradation of YAP
(61). Aspirin-induced
lncRNA-OLA1P2 was reported to inhibit the formation of the
phosphorylated STAT3 homodimer by binding to Tyr705 and restricting
its entry into the nucleus, thus inhibiting metastasis of CRC
(62). A study by Liao et
al (63) reported that the
regulation mediated by lncRNAs on RBP localization occurs in a very
precise manner. lncRNA-EPB41L4A-AS1 is regulated by p53, which is
expressed at only a low level or is even deleted in a variety of
human cancer types, a phenomenon that is associated with poor
prognosis. EPB41L4A-AS1 is able to bind to histone deacetylase 2
(HDAC2) and co-localize with HDAC2 in the nucleolus. HDAC2 is
subsequently released from the nucleolus into the nucleoplasm after
silencing of EPB41L4A-AS1. In addition, an increased level of HDAC2
in the nucleoplasm enhances its binding on the promoter regions of
the Von Hippel-Lindau and voltage-dependent anion channel 1 genes,
which ultimately accelerates the processes of glycolysis and
glutamine metabolism. These findings indicate that the roles of
lncRNAs in terms of intracellular localization of RBP are not
limited to intracellular and extracellular distribution but also
involve intranuclear localization.
Taken together, it has been amply demonstrated that
the binding of lncRNAs to certain partner RBPs leads to significant
changes in their expression and function through regulating their
intracellular distribution, thus regulating the pathological
processes of RBP-associated cancers.
Effects on the RBP interaction
network
General
In addition to RNAs, RBPs are able to bind to
several other types of biological molecules, including proteins and
DNA. Studies have reported that binding of lncRNAs also affects the
interaction network of RBPs and this mode of regulation is
implicated in various human diseases, including cancer (58,64–82).
Common ways in which lncRNAs regulate RBP-interaction networks in
cancer are summarized below.
Negative regulation
The binding of lncRNAs has been indicated to
suppress interactions between RBPs and other biomolecules through a
mechanism similar to that employed by competing endogenous RNA,
which is known as ‘decoy’ or ‘competitive combination’. Competitive
binding of lncRNAs is a common mechanism in cancer that leads to
inhibition of the interactions between RBPs and their downstream
cancer-associated mRNAs, proteins, DNA and other targets.
The inhibitory effects mediated by lncRNAs on the
interactions between RBPs and mRNA frequently lead to increased
degradation of the target mRNA, resulting in decreased expression
at the post-transcriptional level. For instance, the lncRNA
fibroblast growth factor 13-AS1 binds IGF2BPs, affecting their
stabilizing role on c-Myc mRNA and reducing the expression level of
c-Myc, thereby inhibiting glycolysis and the stemness of BRC cells
(64). A new liver-specific
lncRNA, LINC01093, was reported to competitively combine with
IGF2BP1 and block its binding to glioma-associated oncogene homolog
1 (GLI1) mRNA, resulting in GLI1 mRNA degradation and leading to
the suppression of proliferation and metastasis of HCC (65). It is noteworthy that certain
stimulating factors are able to induce activation of this
mechanism. For instance, FoxO-induced long non-coding RNA 1 causes
downregulation of the expression of c-Myc protein under low-energy
conditions via modulating the interaction of
ARE/poly(U)-binding/degradation factor 1 (AUF1) with c-Myc mRNA
through their direct competitive combination. Subsequently,
c-Myc-mediated energy metabolism is inhibited following a decrease
in c-Myc protein expression, leading to apoptosis and inhibition of
the proliferation of renal cancer cells (66).
Competitive binding of lncRNAs may result in an
inability of RBPs to form activated complexes with other proteins,
producing a ‘sequestration’ effect that effectively suppresses the
function of target proteins at the post-translational level. RBP
polypyrimidine-tract-binding protein (PTBP2) has been implicated in
promoting the growth of ovarian cancer and other tumors, and SFPQ
protein, also known as PTB-associated splicing factor, is able to
bind to PTBP2 and inhibit its function. Metastasis associated with
lung adenocarcinoma transcript-1 (MALAT1) has been indicated to
competitively bind to SFPQ and to release PTBP2 from the SFPQ/PTBP2
complex, thereby increasing tumor growth and metastasis (67). Similarly, binding of lncRNA p53RRA
to Ras GTPase-activating protein-binding protein 1 (G3BP1) is able
to displace p53 from the G3BP1 complex, leading to retention of p53
in the nucleus and consequently promoting cell cycle arrest,
apoptosis and ferroptosis (68).
Furthermore, lncRNA binding is able to inhibit the
DNA-binding ability of certain DRBPs, leading to inhibition of the
expression of target genes at the transcriptional level.
Cyclin-dependent kinase inhibitor 1A (CDKN1A) and CDKN2B are key
target genes for the transcription factor MYC in mediating
tumorigenesis. Kim et al (69) reported that a group of lncRNAs
termed ‘MYCLo’ are induced by MYC, where MYCLo-1 and MYCLo-2
inhibit binding of the RBPs HuR and hnRNPK to the promoters of
CDKN1A and CDKN2B, respectively. These inhibitory effects result in
dysregulation of CDKN1A and CDKN2B expression and promote the
proliferation of CRC. In addition, G-quadruplex (G4) is a negative
regulator of transcription and a study performed on CRC by Wu et
al (70) revealed that the
lncRNA lung cancer associated transcript 1 is able to bind to
nucleolin (NCL) through its G4 formation sequence, thereby
inhibiting the binding of NCL to the G4 sequence in the MYC
promoter region. This competitive binding leads to upregulation in
the expression of MYC and further promotes the proliferation of CRC
cells.
These findings collectively indicate that the
binding of lncRNAs results in significant negative effects on the
interactions between RBPs and other biomolecules and these are
implicated in different stages of cancer progression.
Positive regulation
Activation of RBP function may require the
participation of lncRNAs. In addition to the competitive inhibition
mechanism, direct combination of lncRNAs may either guide or
activate RBPs to function with other biomolecules. This type of
positive regulation of lncRNAs occurs commonly in various types of
cancer and results in an increase in the complexity of the
RBP-interaction network.
Facilitating the interactions of RBPs with their
downstream target mRNAs may be the most common mechanism through
which lncRNAs activate RBP function by binding to RBPs without
altering their expression levels. RBPs activated by lncRNAs in turn
regulate the expression of certain cancer-associated genes by
changing the stability of mRNA after direct binding has occurred,
and they therefore participate in regulating the pathological
processes of various types of cancer. Hosono et al (71) characterized the highly conserved
oncogenic lncRNA Testis-associated highly-conserved oncogenic long
non-coding RNA (THOR), which, although mainly expressed in normal
tissue of the testis, is highly expressed in various types of
cancer. Binding of THOR to IGF2BP promotes the stabilization of a
series of related target mRNAs. Of note, the same effects of
lncRNAs may be transmitted through exosomes. Han et al
(72) reported that in BRC, lncRNA
actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1),
secreted by trastuzumab-resistant cells, becomes packed into
exosomes. AFAP1-AS1, when combined with the RBP AUF1 under exosomal
mediation, promotes the binding of AUF1 to HER-2 mRNA, thereby
activating its translation without affecting the expression level,
with a consequent increase in the expression of HER-2 protein
promoting both trastuzumab resistance and metastasis of the BRC
cells. Furthermore, interactions between lncRNAs and RBPs may lead
to a reduction in the stability of target mRNAs bound to RBPs. For
instance, in gastric cancer, lncRNA HOXA11-AS was indicated to
induce degradation of Kruppel-like factor 2 (KLF2) mRNA through
interacting with staufen-1, thereby downregulating the protein
expression of KLF2 and promoting tumor proliferation and metastasis
(58). Furthermore, the lncRNA
RP11 was demonstrated to exhibit similar activity. High expression
levels of RP11 are significantly positively correlated with the
metastasis of CRC and this has been implicated in promoting the
post-translational expression of Zinc finger E-box binding homeobox
1 (Zeb1) protein. After their direct combination, RP11 promotes
binding of hnRNPA2B1 to E3 ligase seven in absentia homolog 1 and
F-box only protein 45 mRNAs, thereby accelerating their
degradation, a process that inhibits degradation of Zeb1 through
the proteasomal pathway that is associated with the two ligases
(73).
Similarly, lncRNA is able to bind to RBP and
facilitate its interactions with other proteins or protein
complexes. For instance, in glioblastoma, the lncRNA SWI/SNF
complex antagonist associated with prostate cancer 1 has an
important role as a binding-protein partner of hnRNP L, promoting
its interaction with α-actinin-4 (ACTN4). hnRNP L binds and
stabilizes ACTN4 by blocking the ubiquitin-proteasome pathway,
thereby activating the NF-κB signaling pathway, which accelerates
the rate of cancer progression (74). In a recent study, Wu et al
(75) reported on a novel
mechanism that linked the lncRNA zinc finger NFX1-type containing 1
antisense RNA 1 (ZFAS1) with CRC progression. ZFAS1 is able to bind
directly with nucleolar protein 58 (NOP58), the core component of
small nucleolar ribonucleoprotein complex (SNORNP); upon activation
of NOP58, this promotes the recruitment of the other proteins that
are involved in the complexes SNORD12C and SNORD78, and further
promotes the assembly of the three components to form SNORNP.
Upregulated SNORNP promotes 2′-O methylation of 28S rRNA, leading
to a high expression level of downstream target genes such as
EIF4A3 and LAMC2, resulting in inhibition of the proliferation and
invasion of CRC cells. Similarly, glutamate-rich WD
repeat-containing protein 1 (GRWD1) has been reported to bind to
p53 inhibiting lncRNA (PiHL) and ribosomal protein L11 (RPL11) in
CRC (76). Of particular interest
is that PiHL promotes binding of GRWD1 to RPL11, thereby isolating
RPL11 from MDM2, followed by enhanced p53 ubiquitination,
ultimately leading to rapid cell proliferation and chemotherapy
resistance in CRC (76).
Of note, RBPs without direct DNA-binding ability are
able to interact with DNA through the modulating effects of
lncRNAs. Co-participation of the lncRNA lincRNA-p21 and hnRNP-K is
involved in the p53 (a classical tumor suppressor gene) signaling
pathway. The lincRNA-p21-hnRNP-K complex mediates binding of
hnRNP-K to the promoter region of p53 target gene, thereby
suppressing the expression of its target genes (77). Lu et al (78) reported that the upregulation of
LINC0051 promoted the progression of CRC, accompanied by
downregulation of the expression of IL-24. LINC0051 combines with
EZH2 and their interaction activates the silencing effect of EZH2
on IL-24 expression via enhancing its enrichment on the IL-24
promoter region. It is important to note that, in all the above
cases, this RBP activation function of the lncRNA is always
accompanied by its localization to the target gene promoter region
of its partner RBP mediated by lncRNA.
In addition, lncRNAs are able to bind to multiple
RBPs at the same time as a scaffold or platform, whereby the
functions of the different RBPs are integrated, thus activating the
protein complexes and promoting their functions of regulating gene
expression. For instance, formation of the trimer NCL/CYTOR/Sam68
in CRC leads to acceleration of tumor cell
epithelial-to-mesenchymal transition (EMT) and tumor progression
via activating the NF-κB signaling pathway. Furthermore, the lncRNA
cytoskeleton regulator has been indicated to activate the
interaction between two RBPs, namely NCL and Sam68, as a scaffold
in the trimer (79). A study by Wu
et al (80) reported that
the lncRNA HERPUD1 intronic transcript of endoplasmic reticulum
(ER) stress has a role as an RNA scaffold, facilitating formation
of the MRE11-RAD50-NBS1 protein complex. Formation of this complex
leads to inhibition of apoptosis of oral squamous cell carcinoma
cells induced by ER stress, further promoting tumor growth and
invasion. In addition, lncRNAs are able to participate in chromatin
modification through scaffolding the modification complex. This
mechanism is associated with the function of c-Myc as a
transcription factor in BRC. Li et al (81) reported that an oncoprotein,
hepatitis B X-interacting protein (HBXIP), directly binds to c-Myc
as a co-activator, leading to activation of the transcription of
c-Myc target genes. Subsequent experiments that aimed to unravel
the mechanism of action reported that HBXIP and lysine-specific
demethylase 1 (LSD1) are scaffolded by the lncRNA HOTAIR to form an
RNA-protein complex, thereby activating the demethylation of H3K4
through recruiting LSD1 to the promoters of c-Myc target genes. In
a separate study (82), a novel
lncRNA named as ‘low expressed in bladder cancer stem cells’ (LBCS)
was reported to be active in bladder cancer stem cells (BCSCs).
LBCS acts as a scaffold to integrate hnRNPK and EZH2, which
subsequently form a complex that mediates the induction of H3K27
trimethylation in the SOX2 promoter region, a process that inhibits
SOX2 expression and results in suppression of self-renewal and
chemotherapeutic resistance of the BCSCs (82).
Taken together, these results suggest that lncRNAs
act either as activators or mediators to facilitate interactions
between their binding proteins and various biomolecules, thereby
expanding the interaction network of cancer-associated RBPs. This
feature may explain in part why lncRNAs are implicated in most
processes of cancer pathogenesis.
Regulation of lncRNAs by RBPs in cancer
Regulation of lncRNAs by RBPs has not been widely
explored in comparison with the regulation of mRNAs and miRNAs.
Advances in techniques for studying protein-RNA interactions,
however, have resulted in an increase in the number of studies that
explore the direct regulation of lncRNAs through binding of RBPs, a
process that is now known to be implicated in the pathogenesis of
several diseases, including cancer (15,33,83–85).
An in-depth review of this topic has been given elsewhere (86); however, in the current review, the
topic is also outlined, as it is an important aspect of lncRNA-RBP
interactions, and recent examples are provided.
Regulation of lncRNA expression at the
post-transcriptional level
Previous studies on cancer report that changes in
expression of RBP are associated with alterations of lncRNA
expression at the post-transcriptional level (86–98),
implying that RBPs have a role in regulating the expression of
lncRNAs. In addition, direct binding may be the most common mode
through which RBPs regulate the stability of lncRNAs in cancer.
RBPs are able to enhance the expression of
cancer-associated lncRNA transcripts by maintaining RNA stability
through direct binding. For instance, RBP serine/arginine rich
splicing factor 1 (SRSF1) is an oncogenic factor of glioma and a
key regulator of the cell cycle. SRSF1 directly binds to
nuclear-enriched abundant transcript 1 (NEAT1) and maintains RNA
stability, whereas NEAT1 is involved in the occurrence and
progression of glioma through regulating the cell cycle (87). Furthermore, the classical RBP HuR,
which is enriched in several different cancer types, combines with
NEAT1_1 and stabilizes it, whereas the abnormal expression of
NEAT1_1 is correlated with cell proliferation and invasion in
ovarian cancer (88). Although
several studies have reported on interactions between RBPs and
lncRNA transcripts, the molecular mechanism underlying how RBPs
enhance the stability of lncRNAs after their direct combination has
yet to be fully elucidated. However, previous studies have
suggested the most likely mechanism is that physical binding of
RBPs blocks certain specific binding sites associated with the
degradation pathway of lncRNA (88–90).
On the other hand, binding of RBPs may lead to an
acceleration of the degradation of cancer-linked lncRNAs in cancer,
thereby reducing the expression level of lncRNA transcripts and
ultimately regulating various cancer phenotypes. Several potential
mechanisms of lncRNA degradation induced by binding to RBP have
been explored. The first mechanism involves the let-7/Ago2
signaling pathway. Ago2 is the core component of RNA-induced
silencing complex (RISC), whereas miRNA let-7 is the key factor
that mediates synthesis of RISC induced by Ago2. Therefore, the
let-7-Ago2 complex is able to mediate the cleavage of RNA by RISC.
For instance, HuR protein has been indicated to be a promoter of
mRNA degradation (91) and other
studies have reported that it may also induce the degradation of
lncRNAs. For instance, previous studies have explored the effect of
HuR on lincRNA-p21 (92) and
HOTAIR (93). The findings
obtained suggested that HuR mediates the interaction between lncRNA
and the let-7-Ago2 complex through direct combination, thereby
promoting degradation of the lncRNA. The second mechanism that has
been indicated to be involved is the RNA exosome pathway. Exosomes
have a central role in RNA metabolism and various types of RNA
molecules are degraded through the RNA exosome complex (94,95).
Knockdown of poly(A)-binding protein nuclear 1 (PABPN1) in HeLa
cells affects the expression level of polyadenylated lncRNAs,
including several classic cancer-associated lncRNAs such as NEAT1
and taurine-upregulated gene 1. PABPN1 binds to these lncRNAs and
promotes their interaction with RNA-exosome complexes, thereby
promoting the degradation of lncRNAs (96). The third mechanism of action that
has been implicated involves the carbon catabolite repression
4-negative on TATA-less (CCR4-NOT)-deadenylase complex, which is a
highly conserved multifunctional protein complex implicated in RNA
decay (97). For example, the RBP
IGF2BP1 has been implicated in the degradation of HULC, a lncRNA
that is significantly upregulated in human liver cancer. Hammerle
et al (98) reported that
specific binding occurs between IGF2BP1 and HULC, and verified that
elimination of IGF2BP1 may increase the expression level of HULC
through prolonging its half-life. IGF2BP1 acts as an adapter
protein and recruits the CCR4-NOT complex by binding to CNOT1, the
scaffold of the CCR4-NOT deadenylase complex, thereby initiating
the degradation of HULC. Therefore, RBPs have been indicated to
accelerate the degradation of certain lncRNAs by binding physically
with them and either recruiting or mediating specific biomolecules
or complexes implicated in RNA degradation.
Regulation of lncRNA localization and
transport
The cellular localization of lncRNAs has an
important participatory role in their function of gene regulation.
Of note, the binding of RBPs to lncRNAs results in changes in their
cellular localization. MALAT1 is involved in the maintenance of
normal mitochondrial functions (99,100). A study wherein RIP experiments
were performed on the RBP HuR and mitochondrial carrier homolog 2
(MTCH2) reported that the two are able to interact with MALAT1,
both in isolated mitochondria and in the whole cell, suggesting
that MALAT1 is shuttled into mitochondria by physically binding to
HuR and MTCH2 complexes (101).
Furthermore, the lncRNA RMRP, the RNA component of mitochondrial
RNA processing endoribonuclease, is involved in the progression of
a variety of human tumors (102–104). A previous study reported on two
RBPs, namely HuR and G-rich RNA sequence binding protein (GRSF1),
which are implicated in translocation of RNA component of
mitochondrial RNA-processing endoribonuclease (RMRP) from the
nucleus to mitochondria. HuR binds to RMRP in the nucleus and
mediates its nuclear export, whereas GRSF1 binds to RMRP and
facilitates its accumulation in the mitochondrial matrix (105). These results suggested that the
intracellular distribution of lncRNAs may be mediated via the
synergistic action of transport- and localization-associated
RBPs.
The structural basis of lncRNA localization has yet
to be fully explored; however, the intracellular distribution of
lncRNAs may be associated with a specific domain. For instance, U1
small nuclear ribonucleoprotein (U1 snRNP) interacts extensively
with lncRNAs and recruits them to chromatin in a
transcription-dependent manner. Yin et al (106) reported that the rapid degradation
of SNRNP70, the protein component of U1 snRNP, reduces the
localization of several nascent and polyadenylated lncRNA
transcripts in chromatin and significantly disrupts nuclear and
genome-wide localization of MALAT1, which has been associated with
multiple cancers. Furthermore, this study demonstrated that the U1
recognition motif contained in these lncRNAs may be the factor
responsible for their localization. Lubelsky and Ulitsky (107) reported that SINE-derived nuclear
localization (SIRLOIN) element with its special sequence has a key
role in the nuclear accumulation of lncRNAs. HnRNPK may bind to
lncRNAs through SIRLOIN elements and promote their enrichment in,
and localization to, the nucleus. In addition, the RIDL domain is
implicated in the subcellular localization of lncRNAs (108). Collectively, these findings
indicate that these specific domains frequently mediate
interactions between RBPs and lncRNAs, thereby affecting the
localization of the lncRNAs.
Furthermore, RBPs are implicated in lncRNA transport
through exosomes. Chen et al (109) reported that LNMAT2, an exosomal
lncRNA, is able to promote lymphangiogenesis and lymph node
metastasis in BLC. Their analysis indicated that LNMAT2 is able to
directly bind to the RBP hnRNPA2B1 and was thereby loaded into
exosomes secreted by BLC cells. This finding provides a novel
research direction for investigating the interactions between RBPs
and lncRNAs, and the underlying mechanism(s) merit further
attention.
In conclusion, RBPs act as the regulators of the
subcellular distribution and transmembrane transport of lncRNAs by
binding to specific domains, thereby affecting the regulatory
effects of lncRNAs on the progression of cancer.
Mediation of lncRNA function
lncRNAs are multi-functional biomolecules that
interact with other biomolecules. Binding of RBPs may promote a
wider interactome of lncRNAs. Certain RBPs may bind to lncRNAs and
mediate the formation of complexes with other proteins. Dangelmaier
and Lal (31) named this class of
RBPs as ‘adaptor proteins’. Pruszko et al (110) performed RIP experiments using BRC
cells and fixed the cells using formaldehyde cross-linking and
ultraviolet (UV) approaches. Their results suggested that
formaldehyde cross-linked both protein-protein and protein-RNA
complexes, whereas UV was only able to cross-link proteins and
their directly binding RNA. Of note, antibodies against mutant p53
protein or ID4 protein downregulated lncRNA MALAT1 in the
formaldehyde cross-linking group, but not in the UV cross-linking
group, indicating an indirect interaction between MALAT1 and mutant
p53 or inhibitor of differentiation 4 (ID4) protein. Subsequently,
this study revealed that the RBP SRSF1 acts as an adaptor protein
that connects MALAT1 and mutant p53 or ID4. Furthermore, lncRNA p53
upregulated regulator of p53 levels (PURPL) suppressed the
expression of p53 protein in CRC through blocking the formation of
p53-MYB binding protein 1A (MYBBP1A) protein complex, which has
been implicated in maintaining p53 stability. RNA pull-down
experiments demonstrated an interaction between MYBBP1A and PURPL.
However, RIP experiments with UV cross-linking did not detect any
direct combination between the two, indicating that the interaction
between PURPL and MYBBP1A involved indirect binding. The subsequent
steps of this study suggested that HuR mediates the interaction
between PURPL and MYBBP1A as an adaptor protein (111). Taken together, these findings
indicated that adaptor proteins mediate interactions between
lncRNAs and proteins lacking RNA binding ability.
Conclusions and future perspectives
lncRNAs and RBPs, in addition to their respective
networks, serve an important role in oncogenesis and progression of
cancer. Interactions between lncRNAs and RBPs provide the most
extensive mode through which they exert their respective biological
functions and their interactions affect their respective
interaction network with other biomolecules. However, further
studies require to be performed to explore the interactions between
lncRNAs and RBPs in greater detail. Several studies have reported
on the bidirectionality of the regulatory effects between lncRNAs
and RBPs, and the polyfunctionality of generalized RBPs, thereby
delineating the intricate interactive network that exists among
various biomolecules. These studies have also provided a basis for
further research. It is anticipated that exploring the common
features and key intersections in the interaction network of
lncRNAs and RBPs will reveal the underlying mechanisms of
oncogenesis and progression of cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 82160575) and the
Outstanding Young Technological and Innovative Talent Cultivation
Project of Zunyi Municipal Science and Technology Bureau, 2021 (no.
10).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
HH and KW conceived the review. HH and LL were
involved in the collection of references. HH wrote the manuscript.
LL constructed the figure. HH, LL and KW checked and revised the
manuscript. HH was responsible for the organization, revision and
submission of this manuscript. All authors read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
FANTOM Consortium and the RIKEN PMI and
CLST (DGT), ; Forrest AR, Kawaji H, Rehli M, Baillie JK, de Hoon
MJ, Haberle V, Lassmann T, Kulakovskiy IV, Lizio M, et al: A
promoter-level mammalian expression atlas. Nature. 507:462–470.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee H, Zhang Z and Krause HM: Long
noncoding RNAs and repetitive elements: Junk or intimate
evolutionary partners? Trends Genet. 35:892–902. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uszczynska-Ratajczak B, Lagarde J,
Frankish A, Guigo R and Johnson R: Towards a complete map of the
human long non-coding RNA transcriptome. Nat Rev Genet. 19:535–548.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Wang W, Zhu W, Dong J, Cheng Y,
Yin Z and Shen F: Mechanisms and functions of long non-coding RNAs
at multiple regulatory levels. Int J Mol Sci. 20:55732019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerstberger S, Hafner M and Tuschl T: A
census of human RNA-binding proteins. Nat Rev Genet. 15:829–845.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rinn JL and Ule J: 'Oming in on
RNA-protein interactions. Genome Biol. 15:4012014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Licatalosi DD and Darnell RB: RNA
processing and its regulation: Global insights into biological
networks. Nat Rev Genet. 11:75–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jankowsky E and Harris ME: Specificity and
nonspecificity in RNA-protein interactions. Nat Rev Mol Cell Biol.
16:533–544. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarvelin AI, Noerenberg M, Davis I and
Castello A: The new (dis)order in RNA regulation. Cell Commun
Signal. 14:92016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hentze MW, Castello A, Schwarzl T and
Preiss T: A brave new world of RNA-binding proteins. Nat Rev Mol
Cell Biol. 19:327–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitchell SF and Parker R: Principles and
properties of eukaryotic mRNPs. Mol Cell. 54:547–558. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller-McNicoll M and Neugebauer KM: How
cells get the message: Dynamic assembly and function of
mRNA-protein complexes. Nat Rev Genet. 14:275–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zealy RW, Wrenn SP, Davila S, Min KW and
Yoon JH: microRNA-binding proteins: Specificity and function. Wiley
Interdiscip Rev RNA. 8:52017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hudson WH and Ortlund EA: The structure,
function and evolution of proteins that bind DNA and RNA. Nat Rev
Mol Cell Biol. 15:749–760. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cech TR and Steitz JA: The noncoding RNA
revolution-trashing old rules to forge new ones. Cell. 157:77–94.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moore S, Järvelin AI, Davis I, Bond GL and
Castello A: Expanding horizons: New roles for non-canonical
RNA-binding proteins in cancer. Curr Opin Genet Dev. 48:112–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castello A, Fischer B, Eichelbaum K, Horos
R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T,
Steinmetz LM, et al: Insights into RNA biology from an atlas of
mammalian mRNA-binding proteins. Cell. 149:1393–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rapicavoli NA, Qu K, Zhang J, Mikhail M,
Laberge RM and Chang HY: A mammalian pseudogene lncRNA at the
interface of inflammation and anti-inflammatory therapeutics.
Elife. 2:e007622013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hung T, Wang Y, Lin MF, Koegel AK, Kotake
Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al:
Extensive and coordinated transcription of noncoding RNAs within
cell-cycle promoters. Nat Genet. 43:621–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calle AS, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dangelmaier E and Lal A: Adaptor proteins
in long noncoding RNA biology. Biochim Biophys Acta Gene Regul
Mech. 1863:1943702020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim C, Kang D, Lee EK and Lee JS: Long
noncoding RNAs and RNA-binding proteins in oxidative stress,
cellular senescence, and age-related diseases. Oxid Med Cell
Longev. 2017:20623842017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferrè F, Colantoni A and Helmer-Citterich
M: Revealing protein-lncRNA interaction. Brief Bioinform.
17:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu F, Jin L, Jin Y, Nie Z and Zheng H:
Long noncoding RNAs in autoimmune diseases. J Biomed Mater Res A.
107:468–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo J, Liu Z and Gong R: Long noncoding
RNA: An emerging player in diabetes and diabetic kidney disease.
Clin Sci (Lond). 133:1321–1339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kazimierczyk M, Kasprowicz MK, Kasprzyk ME
and Wrzesinski J: Human long noncoding RNA interactome: Detection,
characterization and function. Int J Mol Sci. 21:10272020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lorenzen JM and Thum T: Long noncoding
RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol.
12:360–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji CH and Kwon YT: Crosstalk and interplay
between the ubiquitin-proteasome system and autophagy. Mol Cells.
40:441–449. 2017.PubMed/NCBI
|
|
40
|
Dikic I: Proteasomal and autophagic
degradation systems. Annu Rev Biochem. 86:193–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang F, Huo XS, Yuan SX, Zhang L, Zhou WP,
Wang F and Sun SH: Repression of the long noncoding RNA-LET by
histone deacetylase 3 contributes to hypoxia-mediated metastasis.
Mol Cell. 49:1083–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lan Y, Xiao X, He Z, Luo Y, Wu C, Li L and
Song X: Long noncoding RNA OCC-1 suppresses cell growth through
destabilizing HuR protein in colorectal cancer. Nucleic Acids Res.
46:5809–5821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue M, Chen LY, Wang WJ, Su TT, Shi LH,
Wang L, Zhang W, Si JM, Wang LJ and Chen SJ: HOTAIR induces the
ubiquitination of Runx3 by interacting with Mex3b and enhances the
invasion of gastric cancer cells. Gastric Cancer. 21:756–764. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng ZQ, Li ZX, Guan JL, Liu X, Li JY,
Chen Y, Lin L, Kou J, Lv JW, Zhang LL, et al: Long noncoding RNA
TINCR-mediated regulation of acetyl-CoA metabolism promotes
nasopharyngeal carcinoma progression and chemoresistance. Cancer
Res. 80:5174–5188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen
YX, Liu J, LuoX J, Meng Q, Pu HY, et al: lncRNA LINRIS stabilizes
IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer.
Mol Cancer. 18:1742019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-κB interacting
long noncoding RNA blocks IκB phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang C, Li Y, Yan S, Wang H, Shao X, Xiao
M, Yang B, Qin G, Kong R, Chen R and Zhang N: Interactome analysis
reveals that lncRNA HULC promotes aerobic glycolysis through LDHA
and PKM2. Nat Commun. 11:31622020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo ML, Li J, Shen L, Chu J, Guo Q, Liang
G, Wu W, Chen J, Chen R and Song E: The role of APAL/ST8SIA6-AS1
lncRNA in PLK1 activation and mitotic catastrophe of tumor cells. J
Natl Cancer Inst. 112:356–368. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu SC and Zhang Y: Cyclin-dependent kinase
1 (CDK1)-mediated phosphorylation of enhancer of zeste 2 (Ezh2)
regulates its stability. J Biol Chem. 286:28511–28519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lo PW, Shie JJ, Chen CH, Wu CY, Hsu TL and
Wong CH: O-GlcNAcylation regulates the stability and enzymatic
activity of the histone methyltransferase EZH2. Proc Natl Acad Sci
USA. 115:7302–7307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Z, Hou P, Fan D, Dong M, Ma M, Li H,
Yao R, Li Y, Wang G, Geng P, et al: The degradation of EZH2
mediated by lncRNA ANCR attenuated the invasion and metastasis of
breast cancer. Cell Death Differ. 24:59–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eifler K and Vertegaal ACO:
SUMOylation-mediated regulation of cell cycle progression and
cancer. Trends Biochem Sci. 40:779–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
He X, Riceberg J, Pulukuri SM, Grossman S,
Shinde V, Shah P, Brownell JE, Dick L, Newcomb J and Bence N:
Characterization of the loss of SUMO pathway function on cancer
cells and tumor proliferation. PLoS One. 10:e01238822015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu C, Peng Z, Li P, Fu H, Feng J, Zhang
Y, Liu T, Liu Y, Liu Q, Liu Q, et al: lncRNA RMST suppressed GBM
cell mitophagy through enhancing FUS SUMOylation. Mol Ther Nucleic
Acids. 19:1198–1208. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qin G, Tu X, Li H, Cao P, Chen X, Song J,
Han H, Li Y, Guo B, Yang L, et al: Long noncoding RNA
p53-stabilizing and activating RNA promotes p53 signaling by
inhibiting heterogeneous nuclear ribonucleoprotein K deSUMOylation
and suppresses hepatocellular carcinoma. Hepatology. 71:112–129.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He W, Zhong G, Jiang N, Wang B, Fan X,
Chen C, Chen X, Huang J and Lin T: Long noncoding RNA BLACAT2
promotes bladder cancer-associated lymphangiogenesis and lymphatic
metastasis. J Clin Invest. 128:861–875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen C, He W, Huang J, Wang B, Li H, Cai
Q, Su F, Bi J, Liu H, Zhang B, et al: LNMAT1 promotes lymphatic
metastasis of bladder cancer via CCL2 dependent macrophage
recruitment. Nat Commun. 9:38262018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu Z, Chen Z, Fan R, Jiang B, Chen X,
Chen Q, Nie F, Lu K and Sun M: Over-expressed long noncoding RNA
HOXA11-AS promotes cell cycle progression and metastasis in gastric
cancer. Mol Cancer. 16:822017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hua Q, Mi B, Xu F, Wen J, Zhao L, Liu J
and Huang G: Hypoxia-induced lncRNA-AC020978 promotes proliferation
and glycolytic metabolism of non-small cell lung cancer by
regulating PKM2/HIF-1α axis. Theranostics. 10:4762–4778. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu X, Zhou X, Chen Z, Gao C, Zhao L and
Cui Y: Silencing of lncRNA XIST inhibits non-small cell lung cancer
growth and promotes chemosensitivity to cisplatin. Aging (Albany
NY). 12:4711–4726. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou
A, Liu J, Che L and Li J: Long noncoding RNA GAS5 inhibits
progression of colorectal cancer by interacting with and triggering
YAP phosphorylation and degradation and is negatively regulated by
the m6A reader YTHDF3. Mol Cancer. 18:1432019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo H, Liu J, Ben Q, Qu Y, Li M, Wang Y,
Chen W and Zhang J: The aspirin-induced long non-coding RNA OLA1P2
blocks phosphorylated STAT3 homodimer formation. Genome Biol.
17:242016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liao M, Liao W, Xu N, Li B, Liu F, Zhang
S, Wang Y, Wang S, Zhu Y, Chen D, et al: lncRNA EPB41L4A-AS1
regulates glycolysis and glutaminolysis by mediating nucleolar
translocation of HDAC2. EBioMedicine. 41:200–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ma F, Liu X, Zhou S, Li W, Liu C, Chadwick
M and Qian C: Long non-coding RNA FGF13-AS1 inhibits glycolysis and
stemness properties of breast cancer cells through
FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 450:63–75. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
He J, Zuo Q, Hu B, Jin H, Wang C, Cheng Z,
Deng X, Yang C, Ruan H, Yu C, et al: A novel, liver-specific long
noncoding RNA LINC01093 suppresses HCC progression by interaction
with IGF2BP1 to facilitate decay of GLI1 mRNA. Cancer Lett.
450:98–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xiao ZD, Han L, Lee H, Zhuang L, Zhang Y,
Baddour J, Nagrath D, Wood CG, Gu J, Wu X, et al: Energy
stress-induced lncRNA FILNC1 represses c-Myc-mediated energy
metabolism and inhibits renal tumor development. Nat Commun.
8:7832017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han
Z, Sui H, Tang Y, Wang Y, Liu N, et al: Long non-coding RNA MALAT1
promotes tumour growth and metastasis in colorectal cancer through
binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2
complex. Br J Cancer. 111:736–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang
Y, Shi Y, Shen Y, Liu X, Lai W, et al: A G3BP1-interacting lncRNA
promotes ferroptosis and apoptosis in cancer via nuclear
sequestration of p53. Cancer Res. 78:3484–3496. 2018.PubMed/NCBI
|
|
69
|
Kim T, Jeon YJ, Cui R, Lee JH, Peng Y, Kim
SH, Tili E, Alder H and Croce CM: Role of MYC-regulated long
noncoding RNAs in cell cycle regulation and tumorigenesis. J Natl
Cancer Inst. 107:dju5052015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu R, Li L, Bai Y, Yu B, Xie C, Wu H,
Zhang Y, Huang L, Yan Y, Li X and Lin C: The long noncoding RNA
LUCAT1 promotes colorectal cancer cell proliferation by
antagonizing Nucleolin to regulate MYC expression. Cell Death Dis.
11:9082020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hosono Y, Niknafs YS, Prensner JR, Iyer
MK, Dhanasekaran SM, Mehra R, Pitchiaya S, Tien J, Escara-Wilke J,
Poliakov A, et al: Oncogenic role of THOR, a conserved
cancer/testis long non-coding RNA. Cell. 171:1559–1572.e20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Han M, Gu Y, Lu P, Li J, Cao H, Li X, Qian
X, Yu C, Yang Y, Yang X, et al: Exosome-mediated lncRNA AFAP1-AS1
promotes trastuzumab resistance through binding with AUF1 and
activating ERBB2 translation. Mol Cancer. 19:262020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu Y, Yang X, Chen Z, Tian L, Jiang G,
Chen F, Li J, An P, Lu L, Luo N, et al: m6A-induced
lncRNA RP11 triggers the dissemination of colorectal cancer cells
via upregulation of Zeb1. Mol Cancer. 18:872019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ji J, Xu R, Ding K, Bao G, Zhang X, Huang
B, Wang X, Martinez A, Wang X, Li G, et al: Long noncoding RNA
SChLAP1 forms a growth-promoting complex with HNRNPL in human
glioblastoma through stabilization of ACTN4 and activation of NF-κB
signaling. Clin Cancer Res. 25:6868–6881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu H, Qin W, Lu S, Wang X, Zhang J, Sun T,
Hu X, Li Y, Chen Q, Wang Y, et al: Long noncoding RNA ZFAS1
promoting small nucleolar RNA-mediated 2′-O-methylation via NOP58
recruitment in colorectal cancer. Mol Cancer. 19:952020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Deng X, Li S, Kong F, Ruan H, Xu X, Zhang
X, Wu Z, Zhang L, Xu Y, Yuan H, et al: Long noncoding RNA PiHL
regulates p53 protein stability through GRWD1/RPL11/MDM2 axis in
colorectal cancer. Theranostics. 10:265–280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lu Y, Yu Y, Liu F, Han Y, Xue H, Sun X,
Jiang Y and Tian Z: LINC00511-dependent inhibition of IL-24
contributes to the oncogenic role of HNF4α in colorectal cancer. Am
J Physiol Gastrointest Liver Physiol. 320:338–350. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang X, Yu H, Sun W, Kong J, Zhang L, Tang
J, Wang J, Xu E, Lai M and Zhang H: The long non-coding RNA CYTOR
drives colorectal cancer progression by interacting with NCL and
Sam68. Mol Cancer. 17:1102018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu C, Chen W, Yu F, Yuan Y, Chen Y, Hurst
DR, Li Y, Li L and Liu Z: Long noncoding RNA HITTERS protects oral
squamous cell carcinoma cells from endoplasmic reticulum
stress-induced apoptosis via promoting MRE11-RAD50-NBS1 complex
formation. Adv Sci (Weinh). 7:20027472020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li Y, Wang Z, Shi H, Li H, Li L, Fang R,
Cai X, Liu B, Zhang X and Ye L: HBXIP and LSD1 scaffolded by lncRNA
hotair mediate transcriptional activation by c-Myc. Cancer Res.
76:293–304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen X, Xie R, Gu P, Huang M, Han J, Dong
W, Xie W, Wang B, He W, Zhong G, et al: Long noncoding RNA LBCS
inhibits self-renewal and chemoresistance of bladder cancer stem
cells through epigenetic silencing of SOX2. Clin Cancer Res.
25:1389–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ramanathan M, Porter DF and Khavari PA:
Methods to study RNA-protein interactions. Nat Methods. 16:225–234.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhu J, Fu H, Wu Y and Zheng X: Function of
lncRNAs and approaches to lncRNA-protein interactions. Sci China
Life Sci. 56:876–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Corley M, Burns MC and Yeo GW: How
RNA-binding proteins interact with RNA: Molecules and mechanisms.
Mol Cell. 78:9–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jonas K, Calin GA and Pichler M:
RNA-binding proteins as important regulators of long non-coding
RNAs in cancer. Int J Mol Sci. 21:29692020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhou X, Li X, Yu L, Wang R, Hua D, Shi C,
Sun C, Luo W, Rao C, Jiang Z, et al: The RNA-binding protein SRSF1
is a key cell cycle regulator via stabilizing NEAT1 in glioma. Int
J Biochem Cell Biol. 113:75–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chai Y, Liu J, Zhang Z and Liu L:
HuR-regulated lncRNA NEAT1 stability in tumorigenesis and
progression of ovarian cancer. Cancer Med. 5:1588–1598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Abdelmohsen K and Gorospe M:
Posttranscriptional regulation of cancer traits by HuR. Wiley
Interdiscip Rev RNA. 1:214–229. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hu YP, Jin YP, Wu XS, Yang Y, Li YS, Li
HF, Xiang SS, Song XL, Jiang L, Zhang YJ, et al: lncRNA-HGBC
stabilized by HuR promotes gallbladder cancer progression by
regulating miR-502-3p/SET/AKT axis. Mol Cancer. 18:1672019.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim HH, Kuwano Y, Srikantan S, Lee EK,
Martindale JL and Gorospe M: HuR recruits let-7/RISC to repress
c-Myc expression. Genes Dev. 23:1743–1748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yoon JH, Abdelmohsen K, Srikantan S, Yang
X, Martindale JL, De S, Huarte M, Zhan M, Becker KG and Gorospe M:
LincRNA-p21 suppresses target mRNA translation. Mol Cell.
47:648–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yoon JH, Abdelmohsen K, Kim J, Yang X,
Martindale JL, Tominaga-Yamanaka K, White EJ, Orjalo AV, Rinn JL,
Kreft SG, et al: Scaffold function of long non-coding RNA HOTAIR in
protein ubiquitination. Nat Commun. 4:29392013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Meola N, Domanski M, Karadoulama E, Chen
Y, Gentil C, Pultz D, Vitting-Seerup K, Lykke-Andersen S, Andersen
JS, Sandelin A and Jensen TH: Identification of a nuclear exosome
decay pathway for processed transcripts. Mol Cell. 64:520–533.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kilchert C, Wittmann S and Vasiljeva L:
The regulation and functions of the nuclear RNA exosome complex.
Nat Rev Mol Cell Biol. 17:227–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Beaulieu YB, Kleinman CL, Landry-Voyer AM,
Majewski J and Bachand F: Polyadenylation-dependent control of long
noncoding RNA expression by the poly(A)-binding protein nuclear 1.
PLoS Genet. 8:e10030782012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hagkarim NC and Grand RJ: The regulatory
properties of the Ccr4-not complex. Cells. 9:23792020. View Article : Google Scholar
|
|
98
|
Hammerle M, Gutschner T, Uckelmann H,
Ozgur S, Fiskin E, Gross M, Skawran B, Geffers R, Longerich T,
Breuhahn K, et al: Posttranscriptional destabilization of the
liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding
protein 1 (IGF2BP1). Hepatology. 58:1703–1712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Best J, Schotten C, Theysohn JM, Wetter A,
Müller S, Radünz S, Schulze M, Canbay A, Dechêne A and Gerken G:
Novel implications in the treatment of hepatocellular carcinoma.
Ann Gastroenterol. 30:23–32. 2017.PubMed/NCBI
|
|
100
|
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang
Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al: Long noncoding RNA
MALAT1 suppresses breast cancer metastasis. Nat Genet.
50:1705–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhao Y, Zhou L, Li H, Sun T, Wen X, Li X,
Meng Y, Li Y, Liu M, Liu S, et al: Nuclear-encoded lncRNA MALAT1
epigenetically controls metabolic reprogramming in HCC cells
through the mitophagy pathway. Mol Ther Nucleic Acids. 23:264–276.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Y, Luo X, Liu Y, Han G and Sun D:
Long noncoding RNA RMRP promotes proliferation and invasion via
targeting miR-1-3p in non-small-cell lung cancer. J Cell Biochem.
120:15170–15181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hongfeng Z, Andong J, Liwen S, Mingping B,
Xiaowei Y, Mingyong L and Aimin Y: lncRNA RMRP knockdown suppress
hepatocellular carcinoma biological activities via regulation
miRNA-206/TACR1. J Cell Biochem. 121:1690–1702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhou N, He Z, Tang H, Jiang B and Cheng W:
lncRNA RMRP/miR-613 axis is associated with poor prognosis and
enhances the tumorigenesis of hepatocellular carcinoma by impacting
oncogenic phenotypes. Am J Transl Res. 15:2801–2815.
2019.PubMed/NCBI
|
|
105
|
Noh JH, Kim KM, Abdelmohsen K, Yoon JH,
Panda AC, Munk R, Kim J, Curtis J, Moad CA, Wohler CM, et al: HuR
and GRSF1 modulate the nuclear export and mitochondrial
localization of the lncRNA RMRP. Genes Dev. 30:1224–1239.
2016.PubMed/NCBI
|
|
106
|
Yin Y, Lu JY, Zhang X, Shao W, Xu Y, Li P,
Hong Y, Cui L, Shan G, Tian B, et al: U1 snRNP regulates chromatin
retention of noncoding RNAs. Nature. 580:147–150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lubelsky Y and Ulitsky I: Sequences
enriched in alu repeats drive nuclear localization of long RNAs in
human cells. Nature. 555:107–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Carlevaro-Fita J, Polidori T, Das M,
Navarro C, Zoller TI and Johnson R: Ancient exapted transposable
elements promote nuclear enrichment of human long noncoding RNAs.
Genome Res. 29:208–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu
H, Zhong G, Li Y, Li J, Huang J, et al: Exosomal long noncoding RNA
LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin
Invest. 130:404–421. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Pruszko M, Milano E, Forcato M, Donzelli
S, Ganci F, Agostino SD, Panfilis SD, Fazi F, Bates DO, Bicciato S,
et al: The mutant p53-ID4 complex controls VEGFA isoforms by
recruiting lncRNA MALAT1. EMBO Rep. 18:1331–1351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li XL, Subramanian M, Jones MF, Chaudhary
R, Singh DK, Zong X, Gryder B, Sindri S, Mo M, Schetter A, et al:
Long noncoding RNA PURPL suppresses basal p53 levels and promotes
tumorigenicity in colorectal cancer. Cell Rep. 20:2408–2423. 2017.
View Article : Google Scholar : PubMed/NCBI
|