Introduction
The incidence rates of kidney cancer have been
increasing worldwide, which has only received small amounts of
academic attention. For instance, nephroblastoma (also known as
Wilms' tumor), which is a type of kidney cancer, accounts for ~7%
of all pediatric malignancies and 90% of all pediatric renal tumors
(1). The most pervasive and
effective treatment for kidney cancer is nephrectomy combined with
chemotherapy (2). Although 90% of
pediatric patients with kidney cancer survive following systematic
treatment (3), conventional
treatment has proven to be ineffective in a subset of those with
high-risk kidney cancer (4). The
majority of such patients relapse several years after nephrectomy
(5,6). Therefore, it is necessary to
elucidate advanced therapeutic strategies to improve the prognosis
of patients with high-risk kidney cancer through an improved
understanding of the molecular pathogenesis of the disease.
Circular RNAs (circRNAs) are a novel type of
endogenous non-coding RNA (ncRNA) that are formed by covalently
closed loops. circRNAs are single-stranded ncRNA transcripts that
are present in cells. They bind to microRNAs (miRNAs or miRs) or
other molecules through various molecular mechanisms (7). circRNAs abundantly exist in the
eukaryotic transcriptome and consist of the precursor mRNA reverse
splicing sequences of exons or introns without upstream heads or
downstream tails (8). circRNAs
have a circular structure and are resistant to RNA exonuclease,
which makes them stable when compared with linear mRNA transcripts
(9). In the intracellular
microenvironment, circRNAs regulate the expression of important
oncogenes through various integrated molecular mechanisms,
including miRNA binding, protein interaction and novel small
molecular peptide encoding (10).
Innovative circRNA-sequencing technology and bioinformatic analysis
have resulted in the increased study and characterization of
circRNAs (11). circRNAs are
regulators of various diseases, including diabetes, neurological
diseases, immune diseases, heart failure and cancer (12). Among them, the circRNA for
miRNA-7/circular RNA ciRS-7 [cerebellar degeneration related
protein 1 antisense (CDR1as)] is the most well-studied circRNA.
CDR1as is an oncogene that promotes the growth, migration,
chemotherapeutic resistance and immunodeficiency of various types
of tumors through sponging miR-7 (13–18).
circRNA-002178 may act as a competing endogenous (ce)RNA to
upregulate the expression of programmed death-ligand 1 and
programmed cell death protein 1 in lung adenocarcinoma, which
mediates the immune escape of the tumor (19). Circ0006916 is regulated by
trinucleotide repeat-containing 6A and has been determined to be a
tumor promoter in lung cancer cells (20). circRNA polo-like kinase-1 and
hsa_circ_0002453/circRAD18 are considered to be tumor-promoting
circRNAs, as they reduce apoptosis and accelerate proliferation in
triple-negative breast cancer (21–23).
However, research on the potential molecular mechanisms and roles
of circRNAs in kidney cancer remains insufficient.
In the present study, a frequently downregulated
novel circRNA (hsa_circ_0100312, named circKL) in kidney cancer was
identified by analyzing circRNA microarray profiling data of a
previous study by our group. A series of experiments and
bioinformatic analysis were performed to examine the functions and
mechanisms of circKL in kidney cancer. The present study
demonstrated the pivotal role of the circKL-miR-182-5p-F-box/WD
repeat-containing protein 7 (FBXW7) axis in kidney cancer growth
and metastasis though the mechanism of ceRNAs. Thus, circKL may
have the potential to be a novel therapeutic target and biomarker
for kidney cancer.
Materials and methods
Clinical samples, data and ethics
approval
Kidney cancer (mainly referring to nephroblastoma)
and corresponding non-cancerous kidney tissues were collected from
10 patients at Shenzhen Children's Hospital (Shenzhen, China)
between December 2019 and June 2020. The distance between the tumor
and the matched normal adjacent tissue was >2 cm and it was
histologically confirmed to be non-cancerous. The inclusion
criteria were as follows: i) Diagnosed with kidney cancer; ii) had
not received other adjuvant treatments including chemotherapy and
radiotherapy prior to surgery; and iii) agreed to participate in
the study. The exclusion criteria were as follows: i) Failed to
cooperate with researchers; and ii) diagnosed with other diseases.
These patients included 6 male and 4 female patients with an age
range of 7–50 months (average age, 29.04±14.13 months). The age
below 18 years was the most frequently used cutoff point for kidney
cancer in China (24). The present
study was approved by the Ethics Committee of Shenzhen Children's
Hospital (Shenzhen, China). Written informed consent was obtained
from the parents or legal guardians of the patients with kidney
cancer prior to study enrollment. Animal experiments were performed
in accordance with the guiding principles of the Institutional
Animal Care and Use Committee of Shenzhen Children's Hospital
(Shenzhen, China). Detailed information regarding the demographic
and clinicopathological characteristics is provided in Table SI.
Cell culture
A total of three kidney cancer cell lines were used
in the present study: The kidney Ewing sarcoma cell line SKNEP1,
the kidney rhabdoid tumor cell line G401 and the kidney
nephroblastoma cell line HANB. A cell line originally derived from
human embryonic kidney cells, 293T, was also used. All cell lines
were purchased from the Type Culture Collection of the Chinese
Academy of Sciences and were cultured in RPMI-1640 and McCoy's 5A
medium (HyClone; Cytiva) supplemented with 10% FBS (HyClone;
Cytiva) at 37°C with 5% CO2. The authenticity of all
cell lines was verified by DNA fingerprinting (short tandem repeat
profiling).
Vector construction and
transfection
The full-length sequence of circKL (Geneseed Biotech
Co., Ltd.) was cloned into a pLCDH vector (BioVector NTCC, Inc.),
which was subsequently cotransfected with two assistant vectors
pMD2.G (cat. no. 12259; BioVector NTCC, Inc.) and psPAX2 (cat. no.
12260; BioVector NTCC, Inc.) into 293T cells to produce a
lentivirus. The control group was treated with the lentiviral
vector. Each cell line was then transfected with the circKL
overexpression lentivirus. Cells were subsequently selected
following exposure to puromycin for 7 days, after which the results
were validated by reverse transcription-quantitative (RT-q)PCR
analysis.
Total RNA extraction and RT-qPCR
According to the manufacturer's protocol, total RNA
was extracted from kidney cancer tissues or cultured cell lines
using the TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), after which cDNA was synthesized using the
PrimeScript® RT Master Mix (Takara Bio, Inc.). NE-PER
Nuclear and Cytoplasmic Extraction Reagents (cat. no. 78833; Thermo
Scientific) were utilized to isolate the nuclear and cytoplasmic
portions of cellular RNA. Subsequently, qPCR was performed using
the SYBR® Premix Ex Taq™ II kit (Code:
DRR081; Takara Bio, Inc.) and an ABI 7900 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The thermocycling conditions were as
follows: 95°C for 1 min, followed by 40 cycles of 95°C for 10 sec
and 60°C for 30 sec according to the manufacturer's protocol. The
primers used for qPCR were as follows: circKL forward,
5′-ATGGAATCGATGACGGGCTG-3′ and reverse, 5′-GCTTAGGGCAATGGACACCT-3′;
linear KL forward, 5′-GTGCGTCCATCTGGGATACG-3′ and reverse,
5′-TGTCGCGGAAGACGTTGTT-3′; circ0056949 forward,
5′-GCATCTTTGACTGCCCCAATG-3′ and reverse,
5′-ATCCACACAGTCGTTGCGTT-3′; circ0056860 forward,
5′-ACGTGTTATTGATCTTCGCTGT-3′ and reverse,
5′-TTCAGCATCATTTGTCAATGGC-3′; circ0056861 forward,
5′-TCTTCGCTGTAACCCAAGAACA-3′ and reverse,
5′-GGAGACAGGGTTTTCGATGA-3′; circ0039504 forward,
5′-AGCTATGGCTGGAACTTCACC-3′ and reverse,
5′-ACATTCCGAAGAAGGTGCCAT-3′; miR-182-5p forward,
5′-ATCACTTTTGGCAATGGTAGAACT-3′ and reverse,
5′-TATGGTTTTGACGACTGTGTGAT-3′; GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. The 2−ΔΔCq method was used to
determine the fold change of expression (25).
Western blot analysis
Total protein was isolated from kidney tissues and
cells using radioimmunoprecipitation (RIP) assay lysis buffer
(Nanjing KeyGen Biotech Co., Ltd.), which was then added to PMSF to
prevent degradation. Equal quantities of protein (20 µg) were
resolved by 10% SDS-PAGE, separated and transferred to PVDF
membranes (EMD Millipore) for 2 h at 300 mA. After blocking the
membranes with 5% skimmed milk (Nestle) for 2 h at room
temperature, the membrane was then incubated with the following
antibodies overnight at 4°C: Anti-FBXW7 (1:1,000 dilution; cat. no.
ab109617; Abcam) and anti-GAPDH (1:1,000 dilution; cat. no. ab8245;
Abcam). Samples were then incubated with
horseradish-peroxidase-conjugated secondary antibody (1:2,000
dilution; cat. no. ab288151; Abcam) at room temperature for 1 h.
GAPDH was used as an internal control. Band densitometry analysis
was performed using ImageJ software (version 1.8.0.112; National
Institutes of Health).
RNase R digestion assay
After 3 µg of total RNA was extracted from SKNEP1
kidney cancer cells, samples were treated with Ribonuclease R
(RNase R) (5 U/µg; cat. no. R0301; Geneseed, Inc.) or control
solution for 20 min at 37°C. Purification was then performed using
an RNeasy MinElute Cleanup Kit (cat. no. 74204; Qiagen GmbH) and
the RNAs were quantified by RT-qPCR analysis.
Actinomycin D assay
SKNEP1 kidney cancer cells were exposed to 3 µg/ml
actinomycin D (cat. no. SBR00013; MilliporeSigma) to degrade the
linear mRNA transcript for 0, 8, 16 or 24 h. SKNEP1 cells were
subsequently harvested, after which the stability of circKL and
linear KL mRNA was analyzed by RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
G401 and SKNEP1 kidney cancer cells were digested
and resuspended. Empty vector-transfected and circKL overexpression
vector-transfected cancer cells (each, 5,000 cells/well) were
seeded into a 96-well plate and incubated for 48 h at 37°C.
Subsequently, 10 µl CCK-8 solution (cat. no. C0037; Beyotime
Institute of Biotechnology) was added, followed by incubation at
37°C for 1 h prior to optical density measurement at 450 nm using a
microtiter plate reader (Epoch 2; BioTek Instruments, Inc.).
Colony-formation assay
To assess the colony formation ability of the cells
1×103 cells were seeded in six-well plates and incubated
at 37°C for 2 weeks. When macroscopic colonies (>50 cells) were
evidently observed, the cells were fixed with 100% methanol for 15
min at room temperature and stained with 0.5% crystal violet for 10
min at room temperature. The colonies were counted using an
inverted light microscope (magnification, ×100; Carl Zeiss AG). The
numbers of colonies were then counted and measured using ImageJ
software (version 1.8.0.112; National Institutes of Health). The
colony formation efficiency was calculated as the number of
colonies/plated cells ×100%.
Transwell assay
A total of 3×104 cells in serum-free
RPMI-1640 medium (HyClone; Cytiva) were resuspended and added to
the upper chamber of Transwell plates (8 µm pore size; Cell
Biolabs, Inc.). The upper chamber of Transwell plates were not
coated with Matrigel® for migration assays. Furthermore,
600 µl medium containing 10% FBS was added to the lower chamber.
After incubation for 24 h at 37°C, cells on the upper side of the
filter were removed using a cotton swab. The cells which had
migrated to the lower surface of the membrane were fixed using 4%
paraformaldehyde and stained using 0.5% crystal violet at 37°C for
15 min. Cells were then counted in five different fields
(magnification, ×200) under a light microscope (Carl Zeiss AG).
Wound-healing assay
G401 and SKNEP1 kidney cancer cells
(4×105) were seeded in 6-well plates and transfected
with vector or circKL. Subsequently, after a 70–80% confluent
culture was reached, a linear wound was scratched with a sterile
200-µl pipette tip. Cells were washed 3 times with PBS and cultured
with serum-free medium, after which cell wounds were imaged using
an inverted microscope (Carl Zeiss AG) at 0 and 24 h. The degree of
wound healing was expressed as the change in width between the two
time-points. Representative images were obtained using a light
microscope (magnification, ×100; Carl Zeiss AG) and analyzed using
ImageJ software (version 1.8.0.112; National Institutes of
Health).
Dual-luciferase reporter assay
G401 or SKNEP1 kidney cancer cells were seeded into
96-well-plates (5×103 cells/well). miR-182 mimics
(5′-UUUGGCAAUGGUAGAACUCACACU-3′) and negative control mimics
(miR-NC) (5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were obtained from
Guangzhou RiboBio Co., Ltd. The complementary DNA fragment of G401
or SKNEP1 kidney cancer cells containing the wild-type (WT) or
mutant type (MUT) sequence from the targeted 3′-untranslated region
(UTR) of FBXW7 were subcloned downstream of the luciferase gene
using the psiCHEK-2 vector (cat. no. C8021; Promega Corporation).
Sequences of primers used to amplify the targeted 3′-UTR of FBXW7
mRNA were as follows: Forward, 5′-CCACTGACAGCTAGACACCTA-3′ and
reverse, 5′-GAACCCAGGACAACTTGCCA-3′. The plasmid with the MUT
sequence from the 3′-UTR of FBXW7 mRNA was generated using a
Site-Directed Mutagenesis Kit (Shanghai Yeasen Biotechnology Co.,
Ltd.). With this kit, the binding sites predicted by TargetScan
(http://www.targetscan.org) of miR-182-5p
and circKL in the 3′-UTR of FBXW7 mRNA were mutated. For
transfection, cells were seeded into 24-well plates and cultured
overnight. miR-182 mimics (10 µl) or miR-NC (10 µl) and luciferase
reporter plasmid containing the WT or MUT 3′-UTR of FBXW7 (5 µg)
were transfected into cells using Lipofectamine® 3000
(Thermo Fisher Scientific, Inc.) reagent 48 h prior to performing
dual-luciferase reporter assays. Firefly and Renilla
luciferase activities were examined by employing a Dual-Luciferase
Reporter Assay system (Promega Corporation) in accordance with the
manufacturer's protocol. Renilla luciferase activity was
used as a normalization control.
RIP
RIP assays for argonaute RISC catalytic component 2
(AGO2) protein were performed using an anti-AGO2 antibody (EMD
Millipore) according to the manufacturer's protocols. The relative
expression levels of circKL, FBXW7 and miR-182-5p were assessed
after RNA purification. For the MS2-based (Escherichia coli
Bacteriophage MS2-based) immunoprecipitation assays, MS2 binding
site Renilla luciferase (MS2bs-Rluc), MS2bs-circKL and
MS2bs-circKL-mutant type (mut) plasmids were constructed using a
pcDNA3.1 vector (cat. no. V79020; Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequently, 5 µg MS2bs-Rluc, MS2bs-circKL or
MS2bs-circKL-mt was transfected into G401 and SKNEP1 cells using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) prior to performing immunoprecipitation. Normal
Mouse immunoglobulin G (IgG) (dilution, 1:150; cat. no. 17–700; EMD
Millipore) served as the control control. The degree of miR-182-5p
enrichment was measured by RT-qPCR after purification of RNA
complexes. For the in vitro assays, three replicates were
performed for each experiment.
Mouse xenograft study
SKNEP1 kidney cancer cells (2×107) that
stably overexpressed circKL, or control vectors for the control
group, were subcutaneously injected into randomly allocated BALB/c
nude mice (five mice per circKL group and vector group; body
weight, 20–25 g; age, 4 weeks). A total of 10 BALB/c nude mice were
purchased from Shanghai Laboratory Animal Research Center. All mice
were housed under specific pathogen-free conditions at 26°C and 20%
humidity, with a 12-h light/dark cycle and ad libitum access
to food and water. The tumors of the mice were measured with
Vernier calipers every 4 days and their volumes calculated
according to the following equation: 0.5 × width2 ×
length. After 28 days, the nude mice were euthanized by
intraperitoneal injection of 80–100 µl pentobarbital sodium (100
mg/kg). The tumors were then extracted and weighed.
A lung metastasis assay was also performed. SKNEP1
cells (5×105) were injected through the tail veins of
nude mice (four mice per circKL group and vector group). After 8
weeks, lung tissues were excised while mice were anesthetized with
sodium pentobarbital (100 mg/kg). Tumors were subsequently
paraffin-embedded and cut into 4-µm sections. The samples were
dewaxed in xylene and rehydrated with an ethanol gradient. The
sections were stained with hematoxylin (cat. no. ab245880; Abcam)
for 5 min at room temperature and then with eosin (cat. no.
ab245880; Abcam) for 2 min at room temperature. The number of
macroscopically visible lung metastatic nodules was quantified and
validated by a light microscope (magnification, ×40, ×100 and ×200;
Carl Zeiss AG).
Immunohistochemical staining
Tumor xenografts were fixed in 4% neutral formalin
at room temperature for 24 h. Histology sections (4 µm-thick) were
prepared, deparaffinized using xylene and hydrated using a graded
series of alcohols. The slides were incubated with Ki-67 antibodies
(1:300 dilution; cat. no. 9449; Cell Signaling Technology, Inc.)
overnight at 4°C. Subsequently, horseradish-peroxidase-conjugated
secondary antibodies (ready-to-use antibody 50–120 µl; cat. no.
8125; Cell Signaling Technology, Inc.) were applied and samples
were incubated for 45 min at room temperature. The resultant signal
was visualized using 3,3′-diaminobenzidine color reagent staining
at room temperature for 3 min, after which the slides were
counterstained with hematoxylin at room temperature for 5 min and
dehydrated in ethanol and xylene. Finally, the staining was
quantified and image acquisition was performed utilizing a light
microscope (magnification, ×200; Carl Zeiss AG).
Statistical analysis
All statistical analyses were performed using SPSS
23.0 software (SPSS, Inc.). Values are expressed as the mean ±
standard deviation of at least three independent experiments.
Multigroup comparisons were performed using one-way ANOVA followed
by Tukey's post hoc test. Comparisons between tumor and adjacent
normal tissues were performed using a paired Student's t-test. A
paired t-test was also used to compare the expression of circKL
between two matched groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
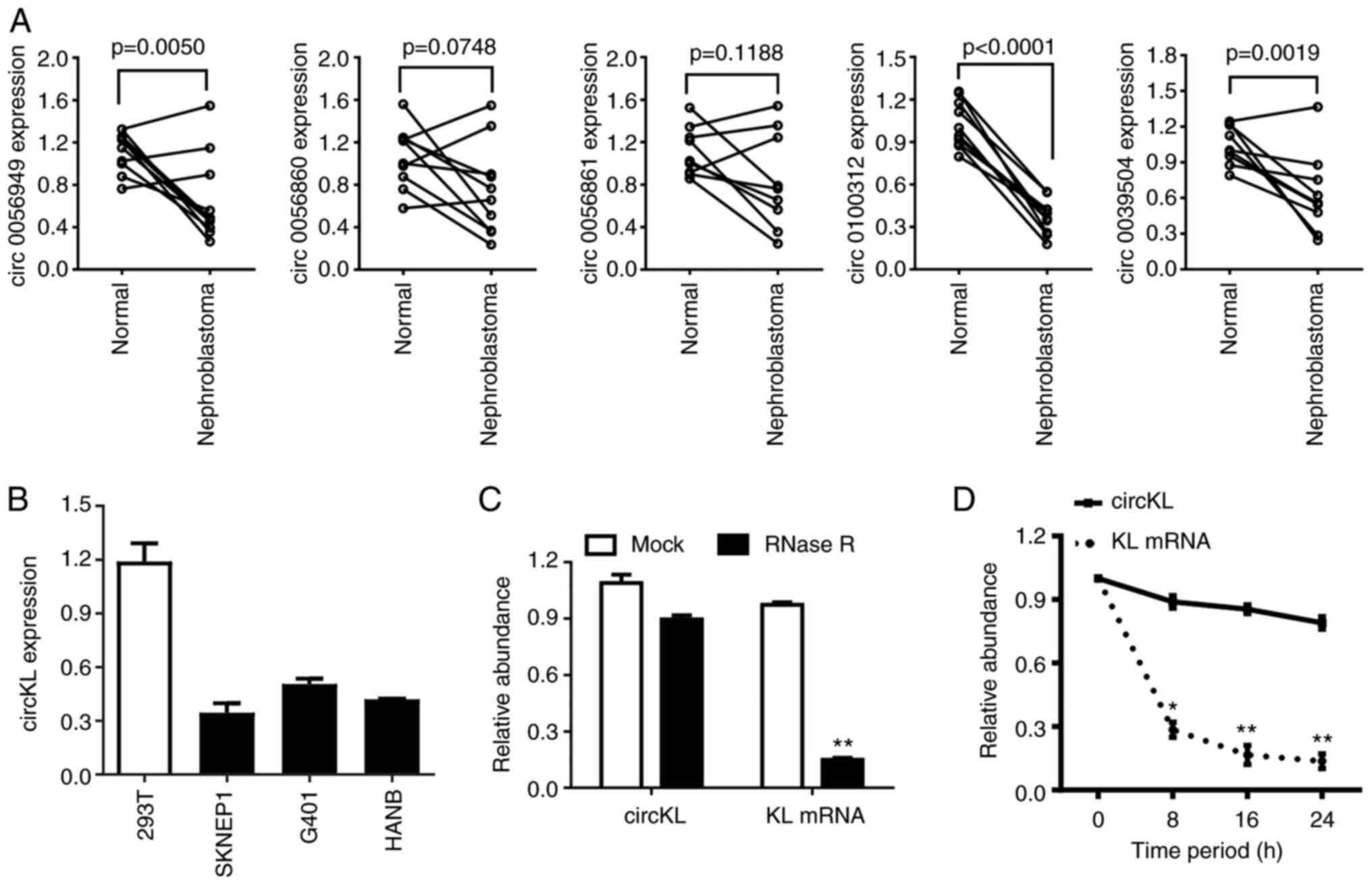
circKL has circular features and is
downregulated in kidney cancer
Following a previous analysis of high-throughput
microarray sequencing data by our group (26), RT-qPCR was performed in the present
study to verify the expression level of the top five downregulated
circRNAs in 10 pairs of kidney cancer samples and adjacent normal
kidney samples (Fig. 1A). The
results confirmed that circKL was significantly downregulated in
the tumor parts of all tumor-normal tissue pairs. Furthermore, the
expression levels of circKL were downregulated in kidney cancer
cell lines compared with those in normal kidney 293T cells
(Fig. 1B). The circular structure
and stability of circKL were further examined by performing RNase R
and actinomycin D assays. The RNase R assay results revealed that,
in contrast to linear KL mRNA, circKL was resistant to RNA
exonuclease (P<0.01; Fig. 1C).
Furthermore, actinomycin D assays confirmed that circKL had a
significantly longer half-life than linear KL mRNA (P<0.01;
Fig. 1D).
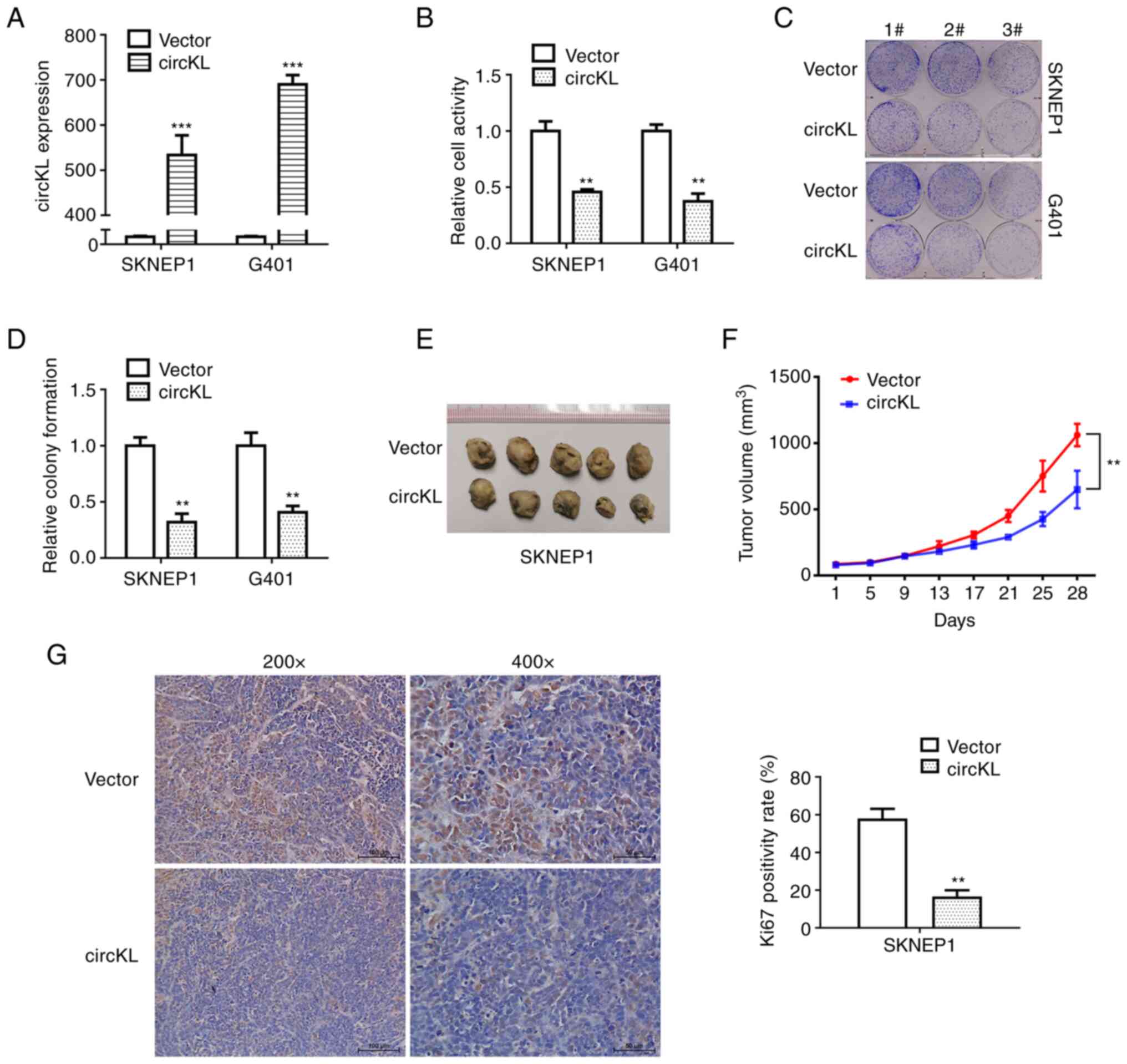
circKL overexpression inhibits the
proliferation of kidney cancer cells
A vector that continuously expressed circKL to
exogenously introduce circKL was constructed in the present study
to explore the potential role of circKL in kidney cancer
progression. G401 and SKNEP1 cells were transfected using a
lentivirus that stably expressed circKL, after which the efficacy
of the overexpression vector was verified (P<0.001; Fig. 2A). CCK-8 and colony formation
assays revealed that overexpression of circKL significantly
suppressed the proliferation and colony-formation ability of G401
and SKNEP1 cell lines in vitro (P<0.01; Fig. 2B-D). Further examination of the
anti-tumor function of circKL was performed in mouse xenograft
assays. Tumor volume curves revealed that overexpression of circKL
inhibited tumor growth. Similarly, in subcutaneous tumors, the
maximum tumor diameter and mean volume in the circKL group (0.984
cm and 650.467 mm3, respectively) were significantly
smaller than those in the vector group (1.249 cm and 1061.87
mm3, respectively) after 28 days (P<0.01; Fig. 2E and F). In addition, Ki67 protein
expression in the murine xenograft tumors of the two groups was
analyzed by immunohistochemistry. The results demonstrated that
Ki67 expression was markedly decreased in the tumor tissues of the
circKL overexpression vector group (Fig. 2G).
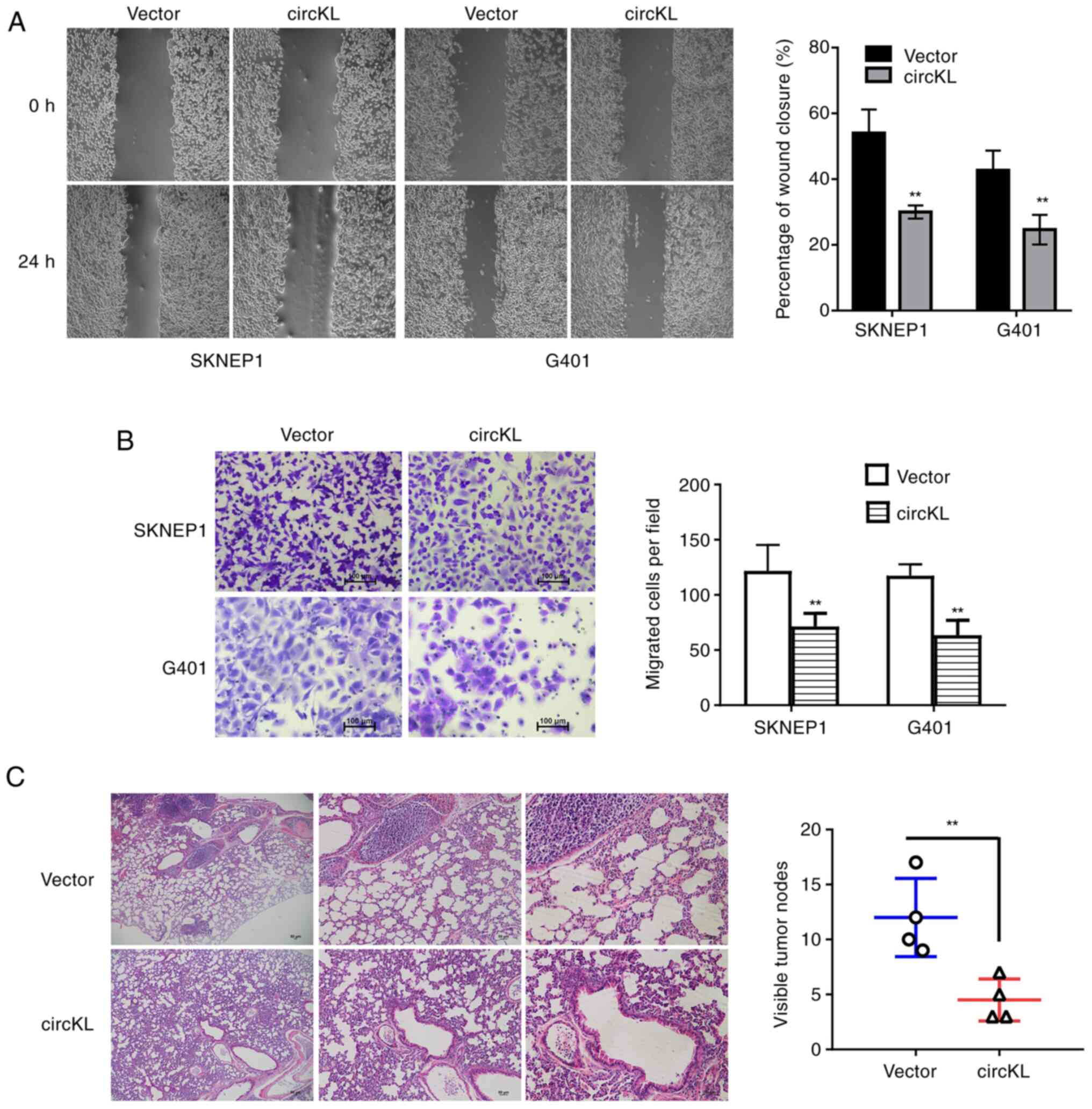
circKL overexpression inhibits
metastasis of kidney cancer cells
Migration and invasion assays were performed to
investigate whether circKL was able to suppress the metastatic
capacity of kidney cancer cells. The results revealed that
upregulation of circKL significantly inhibited the percentage of
wound closure in G401 and SKNEP1 cells (P<0.01; Fig. 3A). The results of the Transwell
assay demonstrated that circKL overexpression reduced the migration
of G401 and SKNEP1 cells (P<0.01; Fig. 3B). In concordance with the in
vitro experimental results, circKL overexpression inhibited the
metastasis of SKNEP1 cells in murine xenograft models in
vivo (P<0.01; Fig. 3C).
circKL acts as a sponge of miR-182-5p
in kidney cancer
After different cellular proportions were detected
by qPCR, it was revealed that circKL predominantly existed in the
cytoplasm of cells (Fig. 4A). The
Circular RNA Interactome database (freely accessible at http://circinteractome.nia.nih.gov) was therefore
used to evaluate the potential interactions between circRNA and
various miRNAs (27). Among the
miRNA candidates, only miR-182-5p was predicted to bind to the
circKL sequence at four possible interaction sites (Fig. 4B). In kidney cancer cell lines,
RT-qPCR analysis revealed that miR-182-5p was significantly
upregulated (Fig. 4C).
Furthermore, the AGO2-related RIP assay confirmed the direct
interaction between circKL and miR-182-5p. In addition, it was
determined that miR-182-5p was predominantly enriched in the
MS2bs-circKL overexpression vector group (P<0.01; Fig. 4D), indicating that circKL directly
interacted with miR-182-5p and may act as a sponge for
miR-182-5p.
circKL inhibits kidney cancer
progression through the circKL-miR-182-5p-FBXW7 axis
TargetScan (http://www.targetscan.org) was used to predict the
potential targeting genes of miR-182-5p (27). Among the possible genes, FBXW7 was
identified as a putative downstream target gene of miR-182-5p
(Fig. 5A). The results of the qPCR
analysis revealed that FBXW7 was markedly downregulated in kidney
cancer cells (Fig. 5B). Whether
miR-182-5p was able to directly bind to the 3′-UTR of FBXW7 mRNA
was subsequently examined. The relative luciferase activity of G401
and SKNEP1 kidney cancer cells was significantly decreased
following the transfection of miR-182-5p and the wild-type
3′-UTR-FBXW7 plasmids. However, after co-transfection with the
mutated luciferase reporter vector, no such effect was observed
(P<0.01; Fig. 5C). The
exogenous introduction of miR-182-5p contributed to the reduction
of FBXW7 mRNA expression levels (P<0.01; Fig. 5D). In addition, AGO2-related RIP
assays revealed that circKL, miR-182-5p and FBXW7 were all highly
enriched in the anti-AGO2 G401 and SKNEP1 kidney cancer cell groups
(P<0.01; Fig. 5E). Furthermore,
the mRNA level of FBXW7 was markedly decreased following circKL
overexpression (P<0.01; Fig.
5F). After transfection with miR-182-5p mimics, FBXW7 protein
was decreased in both G401 and SKNEP1 kidney cancer cell lines
(Fig. 5G). Western blot analysis
also revealed that circKL overexpression significantly increased
FBXW7 protein levels (Fig.
5H).
 | Figure 5.circKL inhibits kidney cancer
progression through the circKL-miR-182-5p-FBXW7 axis. (A) Two
sequences from the 3′UTR of FBXW7 were predicted as a downstream
target of miR-182-5p, according to the TargetScan online website.
(B) Relative expression level of FBXW7 in kidney cancer cell lines.
(C) Luciferase reporter assay using SKNEP1 and G401 cell lines
co-transfected with miR-182-5p mimics and luciferase reporter
plasmid containing the wild/mutant-type fragment from the 3′-UTR of
FBXW7. (D) Overexpression of miR-182-5p contributed to the
reduction of FBXW7 expression in SKNEP1 and G401 cell lines, as
detected by reverse transcription-quantitative PCR analysis. (E)
Enrichment of circKL, FBXW7 and miR-182-5p on AGO2 assessed by RIP
assay. (F) Enrichment of FBXW7 to AGO2 was decreased after
overexpression of circKL. (G) Overexpression of miR-182-5p
contributed to the reduction of FBXW7 expression in SKNEP1 and G401
cell lines, as detected by western blot analysis. (H)
Overexpression of circKL increased the expression of FBXW7 in
SKNEP1 and G401 cell lines, as detected by western blot analysis.
Each assay was performed as three biological replicates.
**P<0.01 vs. miR-NC, vector. FBXW7, F-box and WD-40 domain
protein 7; wt, wild-type; mut, mutant-type; NC, negative control;
Ago2, argonaute RISC catalytic component 2; RIP, RNA
immunoprecipitation; circKL, circular RNA KL, hsa_circ_0100312;
miR, microRNA. |
Discussion
circRNAs have become a focus of ncRNA research in
recent years. Due to their high expression efficiency, structural
stability and disease specificity, scientists have been able to
utilize high-throughput sequencing technology and bioinformatics
analysis to discover and study various circRNAs (28). circRNAs are novel ncRNAs that occur
as covalently closed loops. They are widely expressed in mammalian
tissues and exhibit tissue-specific and cell-specific expression
patterns (29). Although
originally thought to be useless products of mRNA pre-splicing,
these unique ncRNAs with circular structures are currently
recognized as relatively well-established biomarkers in cancer
diagnosis (30). With the
popularization of high-throughput technology, hundreds of circRNAs
have been discovered as novel predictive biomarkers and promising
therapeutic targets for cancer therapy in recent years (31). For instance, circAGO2 was indicated
to harbor oncogenic properties through activating human antigen R
in different types of cancer (32). Furthermore, circAHNAK1 (Desmoyokin)
and circRNA of Homo sapiens G protein subunit β1 have been
identified as critical regulatory factors for competing endogenous
(ce)RNA mechanisms in triple-negative breast cancer (33,34).
Certain circRNAs, including circfam114a2 (35) and circitch (36), have been determined to act as tumor
suppressors through different molecular mechanisms. However, the
potential molecular mechanisms and biological roles of circRNAs in
kidney cancer have remained largely elusive.
For the present study, high-throughput circRNA
microarray data from a previous study by our group were analyzed to
screen for the differentially expressed circRNAs in three pairs of
kidney cancer tissues (26).
circKL was identified as a significantly downregulated circRNA in
both kidney cancer cells and tissues. A circKL overexpression
plasmid was then constructed to investigate the function of circKL
in kidney cancer. circKL overexpression significantly inhibited the
proliferation and migration of kidney cancer cells in vitro
and in vivo. RIP analysis and a luciferase reporter assay
were also performed in the present study to reveal the underlying
mechanisms of the actions of circKL. The results demonstrated that
circKL inhibited the progression of kidney cancer via miR-182-5p
sponging, which upregulated FBXW7 expression.
circRNAs have been known to serve as miRNA sponges
for several years (37).
Theoretically, ceRNAs, mRNAs, long non-coding RNAs and circRNAs are
able to regulate and communicate through the competitive binding of
shared miRNAs (38). In the
present study, miR-182-5p was indicated to interact with circKL in
kidney cancer. miR-182-5p promotes the progression of
hepatocellular carcinoma by inhibiting forkhead box (FOX)O3a
expression, which is a potential predictor of early hepatocellular
carcinoma recurrence in patients who underwent curative surgery
(39). Regulated by
circRNA_0025202, miR-182-5p attenuates tamoxifen resistance by
downregulating FOXO3a expression in breast cancer (40). In addition, circRNA BCRC-3
suppresses cancer cell metastasis and proliferation through the
miR-182-5p/p27 axis in bladder cancer (41). FBXW7 encodes a member of the F-box
protein family, which is a motif characterized by ~40 amino acids
that was originally identified in the cell cycle. The F-box protein
has an important role in phosphorylation-dependent ubiquitination
and is one of the four subunits of the ubiquitin protein ligase
complex, Skp1-Cullin-F-box. FBXW7 has been proven to be an
important tumor suppressor in multiple types of cancer (42,43).
Of note, FBXW7 expression may be regulated by its circular
transcription (44). CircFBXW7
inhibits the proliferation and invasion of glioma and colorectal
cancer cells by translating a 21 kDa novel protein (FBXW7-185AA)
and sponging miRNA (44,45). In the present study, FBXW7 was
significantly upregulated following circKL overexpression in kidney
cancer cells, which was consistent with the results of previous
studies (43). These findings
identified the important roles of circRNAs in the downstream
regulation and modulation of cancer progression.
In summary, the present study elucidated the
biological role of circKL in the growth and metastasis of kidney
cancer through the miR-182-5p/FBXW7 axis. The results of the
current study are of great significance for the development of
novel treatment strategies and potential prognostic biomarkers for
patients with kidney cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Clinical Research
Project of Shenzhen Healthcare Research Project (grant no.
SZLY2018015), Shenzhen Fund for Guangdong Provincial High-level
Clinical Key Specialties (grant no. SZGSP012) and Shenzhen Key
Medical Discipline Construction Fund (grant no. SZXK034).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC performed data analyses and wrote the initial
manuscript. JC and SL designed the study and revised the
manuscript. JC, UY, LL, SC and HX performed the cell and animal
experiments. XY performed the bioinformatics analysis. MY
contributed clinical information and samples, as well as technical
support for multiple software applications. JC and SL checked and
confirmed the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of Shenzhen Children's Hospital (Shenzhen, China;
no. 201903902). The legal guardians of all participants provided
written informed consent. All experiments involving animals were
approved by the Animal Ethics Committee of Shenzhen Children's
Hospital (Shenzhen, China; no. 20200102).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Treger TD, Chowdhury T, Pritchard-Jones K
and Behjati S: The genetic changes of Wilms tumour. Nat Rev
Nephrol. 15:240–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chowdhury N and Drake CG: Kidney cancer:
An overview of current therapeutic approaches. Urol Clin North Am.
47:419–431. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anvar Z, Acurzio B, Roma J, Cerrato F and
Verde G: Origins of DNA methylation defects in Wilms tumors. Cancer
Lett. 457:119–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lange J, Peterson SM, Takashima JR,
Grigoriev Y, Ritchey ML, Shamberger RC, Beckwith JB, Perlman E,
Green DM and Breslow NE: Risk factors for end stage renal disease
in non-WT1-syndromic Wilms tumor. J Urol. 186:378–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dome JS, Graf N, Geller JI, Fernandez CV,
Mullen EA, Spreafico F, Van den Heuvel-Eibrink M and
Pritchard-Jones K: Advances in Wilms tumor treatment and biology:
Progress through international collaboration. J Clin Oncol.
33:2999–3007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clericuzio CL and Johnson C: Screening for
Wilms tumor in high-risk individuals. Hematol Oncol Clin North Am.
9:1253–1265. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goodall GJ and Wickramasinghe VO: RNA in
cancer. Nat Rev Cancer. 21:22–36. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ebbesen KK, Hansen TB and Kjems J:
Insights into circular RNA biology. RNA Biol. 14:1035–1045. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen LL: The expanding regulatory
mechanisms and cellular functions of circular RNAs. Nat Rev Mol
Cell Biol. 21:475–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao
L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, et al: The
landscape of circular RNA in cancer. Cell. 176:869–881.e13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han B, Chao J and Yao H: Circular RNA and
its mechanisms in disease: From the bench to the clinic. Pharmacol
Ther. 187:31–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su Y, Lv X, Yin W, Zhou L, Hu Y, Zhou A
and Qi F: circRNA Cdr1as functions as a competitive endogenous RNA
to promote hepatocellular carcinoma progression. Aging (Albany NY).
11:8182–8203. 2019.
|
|
14
|
Yang W, Yang X, Wang X, Gu J, Zhou D, Wang
Y, Yin B, Guo J and Zhou M: Silencing CDR1as enhances the
sensitivity of breast cancer cells to drug resistance by acting as
a miR-7 sponge to down-regulate REGγ. J Cell Mol Med. 23:4921–4932.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou Y, Zheng S, Deng X, Yang A and Xie X,
Tang H and Xie X: The role of circular RNA CDR1as/ciRS-7 in
regulating tumor microenvironment: A pan-cancer analysis.
Biomolecules. 9:4292019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou Y, Zheng S, Deng X, Yang A, Kong Y,
Kohansal M, Hu X and Xie X: Diagnostic and prognostic value of
circular RNA CDR1as/ciRS-7 for solid tumours: A systematic review
and meta-analysis. J Cell Mol Med. 24:9507–9517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang C, Zeng X, Shan R, Wen W, Li J, Tan
J, Li L and Wan R: The emerging picture of the roles of
circRNA-CDR1as in cancer. Front Cell Dev Biol. 8:5904782020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Zhao X, Wang Y, Ren F, Sun D, Yan
Y, Kong X, Bu J, Liu M and Xu S: circRNA-002178 act as a ceRNA to
promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis.
11:322020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai X, Zhang N, Cheng Y, Yang T, Chen Y,
Liu Z, Wang Z, Yang C and Jiang Y: RNA-binding protein
trinucleotide repeat-containing 6A regulates the formation of
circular RNA circ0006916, with important functions in lung cancer
cells. Carcinogenesis. 39:981–992. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin G, Wang S, Zhang X and Wang D:
Circular RNA circPLK1 promotes breast cancer cell proliferation,
migration and invasion by regulating miR-4500/IGF1 axis. Cancer
Cell Int. 20:5932020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong Y, Yang L, Wei W, Lyu N, Zou Y, Gao
G, Ou X, Xie X and Tang H: CircPLK1 sponges miR-296-5p to
facilitate triple-negative breast cancer progression. Epigenomics.
11:1163–1176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou Y, Zheng S, Xiao W and Xie X, Yang A,
Gao G, Xiong Z, Xue Z, Tang H and Xie X: circRAD18 sponges
miR-208a/3164 to promote triple-negative breast cancer progression
through regulating IGF1 and FGF2 expression. Carcinogenesis.
40:1469–1479. 2019.PubMed/NCBI
|
|
24
|
Huang Y, Zhang W, Song H and Sun N: A
nomogram for prediction of distant metastasis in children with
Wilms tumor: A study based on SEER database. J Pediatr Urol.
16:473.e1–473.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chi J, Liu S, Wu Z, Shi Y, Shi C, Zhang T,
Xiong B, Zeng Y and Dong X: circNSUN2 promotes the malignant
biological behavior of colorectal cancer cells via the
miR-181a-5p/ROCK2 axis. Oncol Rep. 46:1422021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao J, Huang Z, Ou S, Wen F, Yang G, Miao
Q, Zhang H, Wang Y, He X, Shan Y, et al: circ0093740 promotes tumor
growth and metastasis by sponging miR-136/145 and upregulating
DNMT3A in Wilms tumor. Front Oncol. 11:6473522021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong Y, He D, Peng Z, Peng W, Shi W, Wang
J, Li B, Zhang C and Duan C: Circular RNAs in cancer: An emerging
key player. J Hematol Oncol. 10:22017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bolha L, Ravnik-Glavač M and Glavač D:
Circular RNAs: Biogenesis, function, and a role as possible cancer
biomarkers. Int J Genomic. 2017:62183532017.PubMed/NCBI
|
|
31
|
Li S and Han L: Circular RNAs as promising
biomarkers in cancer: Detection, function, and beyond. Genome Med.
11:152019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Yang F, Fang E, Xiao W, Mei H, Li
H, Li D, Song H, Wang J, Hong M, et al: Circular RNA circAGO2
drives cancer progression through facilitating HuR-repressed
functions of AGO2-miRNA complexes. Cell Death Differ. 26:1346–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao W, Zheng S, Zou Y, Yang A and Xie X,
Tang H and Xie X: CircAHNAK1 inhibits proliferation and metastasis
of triple-negative breast cancer by modulating miR-421 and RASA1.
Aging (Albany NY). 11:12043–12056. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu P, Zou Y, Li X, Yang A, Ye F, Zhang J,
Wei W and Kong Y: circGNB1 facilitates triple-negative breast
cancer progression by regulating miR-141-5p-IGF1R axis. Front
Genet. 11:1932020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Lu Q, Liu J, Xie S, Feng B, Zhu W,
Liu M, Liu Y, Zhou X, Sun W, et al: Circular RNA FAM114A2
suppresses progression of bladder cancer via regulating ∆NP63 by
sponging miR-762. Cell Death Dis. 11:472020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Ge YZ, Xu L and Jia R: Circular RNA
ITCH: A novel tumor suppressor in multiple cancers. Life Sci.
254:1171762020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verduci L, Strano S, Yarden Y and Blandino
G: The circRNA-microRNA code: Emerging implications for cancer
diagnosis and treatment. Mol Oncol. 13:669–680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao MQ, You AB, Zhu XD, Zhang W, Zhang YY,
Zhang SZ, Zhang KW, Cai H, Shi WK, Li XL, et al: miR-182-5p
promotes hepatocellular carcinoma progression by repressing FOXO3a.
J Hematol Oncol. 11:122018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sang Y, Chen B, Song X, Li Y, Liang Y, Han
D, Zhang N, Zhang H, Liu Y, Chen T, et al: circRNA_0025202
regulates tamoxifen sensitivity and tumor progression via
regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther.
27:1638–1652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie F, Li Y, Wang M, Huang C, Tao D, Zheng
F, Zhang H, Zeng F, Xiao X and Jiang G: Circular RNA BCRC-3
suppresses bladder cancer proliferation through miR-182-5p/p27
axis. Mol Cancer. 17:1442018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yeh CH, Bellon M and Nicot C: FBXW7: A
critical tumor suppressor of human cancers. Mol Cancer. 17:1152018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yumimoto K and Nakayama KI: Recent insight
into the role of FBXW7 as a tumor suppressor. Semin Cancer Biol.
67:1–15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Y, Qiu A, Peng F, Tan X, Wang J and
Gong X: Exosomal transfer of circular RNA FBXW7 ameliorates the
chemoresistance to oxaliplatin in colorectal cancer by sponging
miR-18b-5p. Neoplasma. 68:108–118. 2021. View Article : Google Scholar : PubMed/NCBI
|