Introduction
Adrenocortical carcinoma (ACC) is rare endocrine
neoplasia with an annual incidence rate of 0.7-2 cases/million
individuals (1). It is an
aggressive cancer type and lacks efficacious therapies. Early
diagnosis, complete surgical resection and adjuvant therapy with
mitotane are recommended as the curative treatment for ACC
(2). Immunotherapy is also
feasible and rational in the management of ACC; however, an
increasing body of evidence suggests that the clinical efficacy of
immunotherapy is modest in patients with ACC (3–5).
Productive CD8+ T-cell infiltrates induce antitumor
immune responses to promote tumor clearance. TP53 germline
mutations and Wnt/β-catenin activation have important roles in ACC
tumorigenesis, which result in impaired CD8+ function
and immunoresistance (6).
Therefore, novel therapeutic strategies are required to overcome
immunoresistance in ACC.
IFNγ is an essential cytokine for antitumor immunity
and may inhibit tumor growth by inducing cell cycle arrest and
apoptosis (7). CD8+ T
cells exhibit cytolytic activities involving perforin or the Fas
mechanism to achieve tumor clearance (8). CD8+ T cells are the major
source of IFNγ production (9,10).
Previous studies have indicated that the IFNγ produced by
CD8+ T cells appears to improve antitumor responses by
enhancing tumor sensitivity to ferroptosis (11,12).
Ferroptosis is an iron-dependent cell death that
differs from traditional apoptosis, necrosis and autophagy with
regard to both the cellular morphological changes and the and gene
expression profiles (13). This
relatively recently discovered form of regulated cell death is
characterized by lipid peroxidation and accumulation of reactive
oxygen species (ROS) (14).
Acyl-CoA synthase long chain family member 4 (ACSL4) participates
in the synthesis of oxidized cellular membrane phospholipids
capable of controlling the sensitivity of cells to ferroptosis
(15).
Solute carrier family 7 member 11 (SLC7A11) is an
important negative regulator of ferroptosis and encodes the light
chain subunit of system Xc-. System Xc- is an amino acid antiporter
located in the plasma membrane, which allows for cystine uptake and
glutamate release (16). Once
cystine enters cells, it is reduced to cysteine, which in turn is
used in the biosynthesis of glutathione (GSH). GSH is the major
intracellular antioxidant and cofactor of GSH peroxidase 4 (GPX4),
which prevents cells from experiencing oxidative stress and
therefore undergoing ferroptosis (17). Erastin is able to inhibit system
Xc-activity and subsequently cause GSH depletion, resulting in
increased ferroptosis. In addition to killing tumor cells, several
studies have demonstrated that erastin enhances the sensitivity of
cancers to chemotherapy and radiotherapy (18–20).
Previous studies have also indicated that IFNγ facilitates
erastin-induced ferroptosis, which contributes to anticancer
therapy in various types of cancer (11,12).
However, it remains elusive whether the regulatory effect of IFNγ
on ferroptosis has a positive role in the therapy of ACC.
In the present study, it was demonstrated that IFNγ
promoted erastin-induced ferroptotic cell death. In addition, IFNγ
enhanced erastin-induced ferroptosis, as evidenced by the
accumulation of iron, elevation of lipid peroxidation and increase
in mitochondrial damage. Further analysis indicated that IFNγ
enhanced ferroptosis by suppressing the expression of SLC7A11,
which was achieved through the activation of the JAK/STAT pathway
in NCI-H295R cells. Taken together, these results suggest that
targeting SLC7A11 predisposes ACC cells to ferroptosis and this may
provide a novel strategy to improve the efficacy of immunotherapy
in ACC.
Materials and methods
Identification of prognostic
ferroptosis-related differentially expressed genes (DEGs)
The RNA sequencing data of 128 normal samples and 79
ACC samples were downloaded from the UCSC Xena platform (https://xena.ucsc.edu/). In addition, the clinical
information of 79 patients with ACC was obtained from The Cancer
Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). A total of 60
ferroptosis-related genes (FRGs) were acquired from previous
research (21). All DEGs were
analyzed using R (version 4.10) (22). For DEGs, a false discovery rate of
<0.05 was used as a cutoff criterion and was processed using the
limma R package (23). Univariate
Cox analysis was performed using the survival R package, with the
aim of evaluating the interaction between FRGs and overall survival
(OS). All genes with P<0.001 were considered prognostic genes.
The Venn R package was used to draw Venn diagrams and identify the
intersection genes between the DEGs and prognostic genes. Next,
GEPIA2 (http://gepia2.cancer-pku.cn/#index) was used to
perform survival analysis of the intersection genes (24). Overall survival was estimated by
Kaplan-Meier analysis, and the P-values were determined by log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Cell culture and drugs
The human ACC cell line NCI-H295R was purchased from
Procell Life Science & Technology Co., Ltd. Cells were
incubated in DMEM/F12 media with added 6.25 µg/ml insulin, 6.25
µg/ml transferrin, 6.25 ng/ml selenium, 5.35 µg/ml linoleic acid,
10% FBS and 1% penicillin/streptomycin (all from Procell Life
Science & Technology Co., Ltd.). Cells were maintained in a
humidified incubator supplied with 5% CO2 at 37°C.
Recombinant human IFNγ (cat. no. HY-P7025A), erastin (cat. no.
HY-15763) and fludarabine (cat. no. HY-B0069) were purchased from
MedChemExpress. Erastin and fludarabine were dissolved in DMSO and
then diluted in complete culture medium to the desired
concentration, with a final DMSO concentration of <0.1%.
Cell viability assay
NCI-H295R cells were seeded into a 96-well plate at
a density of 1×104 cells/well in 100 µl culture medium.
After incubation for 24 h, cells were pretreated with or without
IFNγ (10 ng/ml) for 24 h, followed by treatment with erastin (10 or
20 µM) for 24, 48 or 72 h. Cell viability was detected using a Cell
Counting Kit-8 (CCK-8) assay (Epizyme Biotech). A total of 10 µl
CCK-8 solution was added to each well, followed. By further
incubation for 4 h at 37°C. The optical density value was measured
at 450 nm using a plate reader (Tecan infinite F200PRO; Tecan
Group, Ltd.) and the percentage of viable cells compared with the
control cells was calculated.
Ethynyldioxyuridine (EdU)
proliferation assay
An EdU staining assay was performed using an EdU
staining kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Cells were stained with 20 µM EdU solution
for 4 h and then fixed with fixative solution provided in the
staining kit. The cell nuclei were stained with DAPI. The images
were acquired using a fluorescence microscope (Eclipse 80i; Nikon
Corporation) and stained cells were analyzed using ImageJ software
(version 1.53; National Institutes of Health).
Cell death assay
Cell death was detected using a
Calcein-acetoxymethyl ester (AM)/propidium iodide (PI) LIVE/DEAD
double staining kit (cat. no. CA1630; Beijing Solarbio Science
& Technology Co., Ltd.). Cells were harvested and washed using
the included Assay Buffer three times. The harvested cells were
resuspended in Assay Buffer and subsequently stained with 2 µM
calcein-AM (for live cells) and 4.5 µM PI (for dead cells) at 37°C
for 30 min in the dark. Stained cells were imaged using a
fluorescence microscope (Eclipse 80i; Nikon Corporation) and
analyzed using ImageJ (version 1.53; National Institutes of
Health).
Western blot analysis
After treatment, cells were lysed with RIPA lysis
buffer supplemented with a protease and phosphatase inhibitor
cocktail (Epizyme Biotech). The concentration of the protein
samples was quantified using a BCA protein assay kit (Takara Bio,
Inc.), total protein (20 µg/lane) was resolved by SDS-PAGE on 10%
gels and transferred to PVDF membranes (MilliporeSigma). Membranes
were blocked with protein free rapid blocking buffer (Epizyme
Biotech) at room temperature for 15 min. Subsequently, the PVDF
membrane was incubated with primary antibodies against β-actin
(1:1,000 dilution; cat. no. ab8226; Abcam), SLC7A11 (1:1,000
dilution; cat.ARG57998; Arigobio), phosphorylated signal transducer
and activator of transcription 1 (p-STAT1; 1:1,000 dilution; cat.
no. ab109461; Abcam), STAT1 (1:10,000 dilution; cat. no. ab109320;
Abcam) p-STAT3 (1:1,000 dilution; cat. no. ab267373; Abcam), STAT3
(1:1,000 dilution; cat. no. ab68153; Abcam) or IFN regulatory
factor 1 (IRF1; 1:1,000 dilution; cat. no. ab243895; Abcam) at 4°C
overnight, followed by horseradish peroxidase (HRP)-labeled
secondary antibody (1:1,000 dilution; cat. no. LF101 or LF102;
Epizyme Biotech). Protein signals were visualized using
Chemiluminescent HRP substrate (MilliporeSigma) and analyzed using
ImageJ (version 1.53; National Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from cells using Total RNA
isolation reagent (Biosharp) and then reverse-transcribed into cDNA
using a Takara RT kit (Takara Bio, Inc.). The mixture was incubated
at 37°C for 15 min, followed by 85°C for 5 sec and then cooled at
4°C. qPCR was performed using TB Green® Premix Ex Taq™
(Takara Bio, Inc.) in a final volume of 20 µl in an ABI 7500
reactor (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
qPCR conditions consisted of an initial denaturation step for 30
sec at 95°C, followed by 40 cycles of 3 sec denaturation at 95°C
and annealing/elongation for 30 sec at 60°C. The samples were
quantified using the 2−ΔΔCq method and β-actin was used
as the internal control (25). The
PCR primer sequences (Sangon Biotech, Co., Ltd.) are listed in
Table I.
 | Table I.Primer sequences of genes for
quantitative PCR. |
Table I.
Primer sequences of genes for
quantitative PCR.
| Gene | Primer sequence
(5′-3′) |
|---|
| SLC7A11 |
|
|
Forward |
GGTCCATTACCAGCTTTTGTACG |
|
Reverse |
AATGTAGCGTCCAAATGCCAG |
| GPX4 |
|
|
Forward |
GAGGCAAGACCGAAGTAAACTAC |
|
Reverse |
CCGAACTGGTTACACGGGAA |
| ACSL4 |
|
|
Forward |
CATCCCTGGAGCAGATACTCT |
|
Reverse |
TCACTTAGGATTTCCCTGGTCC |
| β-actin |
|
|
Forward |
GACCTGTACGCCAACACAGT |
|
Reverse |
CTCAGGAGGAGCAATGATCT |
Immunofluorescence analysis
After treatment, the cells were washed with PBS
three times, fixed in 4% formaldehyde for 24 h and blocked with 5%
goat serum (cat. no. C0265; Beyotime Institute of Biotechnology)
for 60 min. Next, the cells were incubated with the anti-SLC7A11
primary antibody (1:1,000 dilution; cat. ARG57998; Arigobio) at 4°C
overnight. The following day, the cells were washed with PBS and
incubated with the secondary antibody (donkey anti-rabbit IgG
H&L; 1:1,000 dilution; cat. no. ab150075; Abcam) for 2 h at
room temperature, and the nuclei were stained with DAPI. Finally,
the fluorescently labeled cells were visualized using fluorescence
microscopy (Eclipse 80i; Nikon Corporation).
Lipid peroxidation detection using
BODIPY-C11
Lipid peroxidation was detected using a C11 BODIPY
581/591 probe (ABclonal Biotech Co., Ltd.). Cells were incubated
with 50 µM working solution for 1 h at 37°C and gently washed with
PBS three times. Finally, cells were imaged using a fluorescence
microscope (Eclipse 80i; Nikon Corporation).
Measurement of intracellular iron
levels
For assaying of intracellular iron levels, Phen
Green SK (PGSK), a fluorescent probe (Cayman Chemical Company), was
used. Cells were incubated with 50 µM PGSK at 37°C for 20 min and
then the fluorescence images were acquired. Finally, the intensity
of green fluorescence was measured using ImageJ (version 1.53;
National Institutes of Health).
Transmission electron microscopy
After treatment, cells were harvested and fixed in
2.5% glutaraldehyde at 4°C for 12 h. The samples were rinsed,
dehydrated in a gradient series of alcohol solutions, embedded and
sectioned (70–90 nm). The sections were stained using uranyl
acetate/lead citrate for 10 min prior to observation. Morphological
changes in the mitochondria were imaged using a transmission
electron microscope (H-7650; Hitachi, Ltd.). The mitochondrial area
and density were quantitatively analyzed by ImageJ software
(version 1.53; National Institutes of Health).
Detection of intracellular ROS
Intracellular ROS was measured using an ROS assay
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. In brief, after treatment, cells were
incubated with 10 µM dichloro-dihydro-fluorescein diacetate probe
at 37°C for 20 min. The fluorescent cells were observed using a
fluorescence microscope and the intracellular ROS levels were
detected using a flow cytometer (CytoFlex S; Beckman Coulter,
Inc.).
Detection of mitochondrial membrane
potential (MMP)
The JC-1 fluorescent probe (MMP assay kit with JC-1;
no. C2006; Beyotime Institute of Biotechnology) was used to
evaluate the MMP of cells according to the manufacturer's protocol.
When the MMP is high, JC-1 accumulates in the matrix of the
mitochondria to form a polymer (J-aggregates), which fluoresces
red; when the MMP is low, JC-1 does not aggregate in the
mitochondrial matrix but is present as a monomer and fluoresces
green. Thus, it is convenient to detect the changes in MMP based on
the change in fluorescence color. The relative ratio of red to
green fluorescence may be used to measure mitochondrial
depolarization. NCI-H295R cells were seeded into 6-well plates at a
density of 2×105 cells/well. Cells were pretreated with
or without IFN-γ (10 ng/ml) for 24 h, followed by treatment with
erastin (20 µM) for 48 h. Cells were washed with PBS and stained
with 1 ml of the JC-1 staining solution per ml of culture medium
added to each well of the plate, with incubation at 37°C for 20
min. The supernatant was then discarded and the cells were washed
two times with JC-1 staining buffer. The fluorescence images were
then obtained using a fluorescence microscope (Eclipse 80i; Nikon
Corporation) in 6 random fields under a magnification of ×400.
ImageJ software (version 1.53; National Institutes of Health) was
used to measure the fluorescence intensity.
Overexpression of SLC7A11 and
suppression of STAT1
pCMV-SLC7A11-ZsGreen-PURO and pcDNA3.1-ZsGreen-PURO
plasmids were purchased from Hanbio Biotechnology Co., Ltd. The
plasmid was transfected into NCI-H295R cells to overexpress
SLC7A11, while the mock plasmid was used as the negative control.
Cells were seeded in a six-well plate (2×105 cells/well)
and grown to 60–70% confluence at 37°C with 5% CO2 prior
to transfection. Transfection was performed using
Lipofectamine® 3000 reagent (Invitrogen; Themo Fisher
Scientific, Inc.) and Ultra-MEM Reduced Serum Medium (BioAgrio) for
6 h at 37°C with 5% CO2. The medium was then replaced
with supplemented culture medium for a further 18 h. Subsequently,
cells were treated with IFNγ (10 ng/ml) for 24 h, followed by
erastin (20 µM) for 48 h. The efficacy of overexpression was
analyzed by RT-qPCR and western blot analysis.
Fludarabine was used to inhibit the expression of
STAT1. Cells were seeded in a six-well plate (2×105
cells/well) at 37°C with 5% CO2 overnight; after 24 h
pre-treatment with 2 µM fludarabine at 37°C, NCI-H295R cells were
treated with IFNγ (10 ng/ml) for 24 h, followed by erastin (20 µM)
for 48 h.
Statistical analysis
Values are expressed as the mean ± standard
deviation of three or more independent experiments. Student's
t-test was used to analyze the differences between two groups.
Comparisons among multiple groups were performed using a one-way
ANOVA followed by a Tukey's post-hoc test. Statistical analyses
were performed using the NCSS statistical software version 21.0.3
(NCSS, LLC). P<0.05 was considered to indicate a statistically
significant difference.
Results
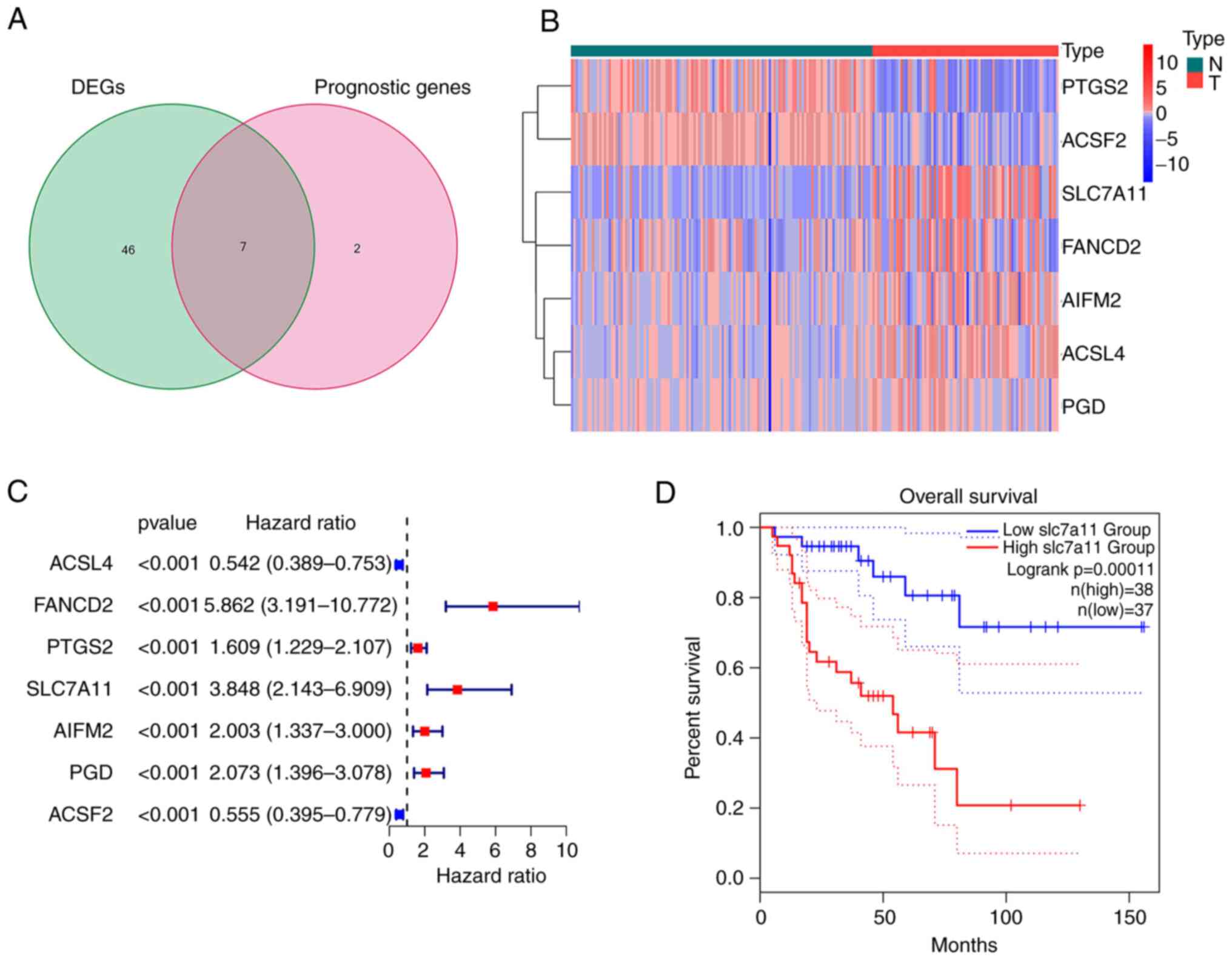
Extraction of FRGs in ACC
The majority of the FRGs (53/60, 88.33%) were
differentially expressed between the ACC and normal adrenal gland
tissues. Of these, 7 were identified as prognostic factors based on
the univariate Cox regression analysis (P<0.001; Fig. 1A). These intersection genes were
further evaluated using a heatmap and univariate Cox regression
analysis. The heatmap presents the expression levels of FRGs
between normal and tumor tissues; downregulated genes are indicated
with blue squares and upregulated genes with red squares (Fig. 1B). Analysis of the relationship
between FRGs and OS was visualized using a forest plot (Fig. 1C). The results suggested that
SLC7A11 was upregulated in patients with ACC and its high
expression was related to poor survival. Kaplan-Meier curves
suggested that low expression of SLC7A11 in patients with ACC was
associated with a favorable prognosis (Fig. 1D). The results of the Kaplan-Meier
curve analysis for the other intersection genes [Fanconi anemia
group D2 protein (FANCD2); apoptosis inducing factor mitochondria
associated 2 (AIFM2); phosphogluconate dehydrogenase (PGD); ACSL4;
acyl-CoA synthetase family member 2 (ACSF2);
prostaglandin-endoperoxide synthase 2 (PTGS2)] are provided in
Fig. S1; these findings
demonstrated that the expression of other intersection genes was
associated with the prognosis of patients with ACC (P<0.05).
IFNγ sensitizes NCI-H295R cells to
ferroptotic cell death
NCI-H295R cells were treated with 10 ng/ml IFNγ for
24 h, followed by treatment with different concentrations of
erastin for 24, 48 and 72 h. To analyze the viability of NCI-H295R
cells, a CCK-8 assay was performed. The results indicated that
erastin exerted a dose- and time-dependent inhibitory effect on the
viability of NCI-H295R cells compared with the control group.
Pretreatment of IFNγ further enhanced the inhibitory effect of
erastin on NCI-H295R cell viability (Fig. 2A). The EdU assay suggested that the
percentage of EdU-positive cells was decreased by erastin treatment
and this was attenuated by IFNγ pretreatment (Fig. 2B). Live/Dead assay staining further
confirmed the cytotoxic effect of the above-mentioned treatment.
Green fluorescence represents live cells and red fluorescence
represents dead cells. The combination of IFNγ and erastin resulted
in a higher cell death ratio compared with cells treated with
erastin alone (Fig. 2C). All of
these results indicated that IFNγ sensitized NCI-H295R cells to
erastin-induced cell death.
IFNγ enhances erastin-induced
ferroptosis
To determine whether IFNγ enhanced the sensitivity
of NCI-H295R cells to erastin, the levels of lipid peroxidation and
intracellular iron were assessed. The C11-BODIPY581/591
fluorescence probe is a lipid peroxidation sensor, capable of
accessing the cell membrane. Once oxidized, the fluorescent
properties shift from a non-oxidative form (red fluorescence) to an
oxidative form (green fluorescence). The results suggested that
IFNγ pretreatment accelerated the process of decay of red
fluorescence to green fluorescence (Fig. 3A and B). The intracellular iron
content may quench PGSK fluorescence. The fluorescence intensity of
the PGSK probe was decreased in the IFNγ + erastin group,
indicative of an accumulation of iron (Fig. 3C and D). Both ACSL4 and GPX4 are
important indicators of ferroptosis. The RT-qPCR assay indicated
increased mRNA levels of ACSL4 and decreased mRNA levels of GPX4 in
the IFNγ + erastin group compared with the control group (Fig. 3E and F).
Mitochondrial damage caused by
erastin-induced ferroptosis is enhanced by IFNγ
The mitochondria are the primary sites of ROS
production, and ROS has important roles in ferroptosis. Therefore,
the impact of IFNγ on mitochondrial damage in erastin-treated cells
was further analyzed. MMP assays indicated an increased green/red
fluorescence ratio and loss of MMP in the erastin-treated cells
after pretreatment with IFNγ (Fig. 4A
and B). Furthermore, fluorescence microscopy and flow
cytometric analysis suggested that IFNγ enhanced intracellular ROS
generation in the NCI-H295R cells followed by treatment with
erastin (Fig. 4C-F). In addition,
the morphology of the mitochondria in the NCI-H295R cells was
observed using transmission electron microscopy. Upon exposure to
IFNγ and erastin, the mitochondria became smaller, the membrane
density increased and the area of ruptured membrane also increased
(Fig. 4G-I).
IFNγ represses SLC7A11 expression
through the JAK/STAT pathway
SLC7A11 has a critical role in ferroptosis. The
results of the western blot analysis suggested that the levels of
SLC7A11 expression were reduced in erastin-treated NCI-H295R cells.
Pretreatment with IFNγ further enhanced the inhibitory effect of
erastin on SLC7A11 expression in NCI-H295R cells (Fig. 5A and B). Similar results were also
confirmed by RT-qPCR and immunofluorescence analysis (Fig. 5C-E). IFNγ was able to activate the
JAK/STAT signaling pathway and mediate immune function and
proliferation of cells. It has been demonstrated that STAT3 reduces
the expression of SLC7A11 through binding to the promoter of
SLC7A11 (26). To further explore
the molecular mechanism of the effect of IFNγ to repress SLC7A11
expression, the levels of pSTAT1, pSTAT3 and IRF1 were determined.
The results indicated that erastin increased the expression of
pSTAT1, pSTAT3 and IRF1, which were further increased by IFNγ in
NCI-H295R cells (Fig. 5F-I),
suggesting that IFNγ may repress SLC7A11 expression through
JAK/STAT pathway.
SLC7A11 overexpression or STAT1
inhibition ameliorates IFNγ-enhanced erastin-induced
ferroptosis
Next, it was determined whether IFN-γ was able to
promote ferroptosis through repressing SLC7A11 via the JAK/STAT
pathway. A plasmid was used to overexpress SLC7A11 and fludarabine
was used to inhibit STAT1. The expression levels of SLC7A11 at the
mRNA and protein levels were significantly higher in cells
transfected with the plasmid compared with the empty vector control
(Fig. 6A and B). Cell death was
increased in cells transfected with the mock plasmid (Fig. 6C and D). The level of lipid
peroxidation and iron accumulation was reduced in
SLC7A11-overexpressing NCI-H295R cells and fludarabine-treated
NCI-H295R cells (Fig. 6E-H).
Furthermore, SLC7A11 overexpression prevented mitochondrial damage
in the cells, and STAT1 inhibition exerted a similar effect,
although it was weaker (Fig.
6I-K). These results indicated that SLC7A11 inhibited
IFNγ-enhanced erastin-induced ferroptosis, and STAT1 aggravated
this effect.
Discussion
ACC has a poor prognosis, with a 5-year survival
rate of 15% in patients with advanced-stage disease (20). Although the use of immunotherapy
for the management of malignant neoplasms has increased, the
immunotherapeutic efficacy in ACC is limited owing to the intrinsic
mechanisms of immunoresistance. Ferroptosis has an important role
in affecting the efficacy of cancer immunotherapy (27). It is well established that
ferroptosis inducers exert anti-tumor effects in various types of
cancer (28). In the present
study, the effect of IFNγ on the sensitivity of the ACC cell line
NCI-H295R to a ferroptosis inducer, erastin, was determined.
The differentially expressed FRGs between normal
adrenal glands and ACC samples were determined, and univariate Cox
regression analysis was then performed to identify prognostic genes
(ACSL4, FANCD2, PTGS2, SLC7A11, AIFM2, PGD and ACSF2). Recent
research has revealed that a combination of IFNγ derived from
immunotherapy-activated CD8+ T cells and
radiotherapy-activated ATM promote cancer ferroptosis via
synergistic repression of SLC7A11 (29). Thus, it was speculated that SLC7A11
was a novel common gene involved in both ferroptosis and cancer
immunotherapy. SLC7A11, an important subunit of cystine/glutamate
antiporter system Xc-, mediates cystine uptake and GSH
biosynthesis, resulting in the suppression of ferroptosis and
reducing oxidative stress (30).
Several studies have indicated that SLC7A11 expression is
upregulated in various types of cancer (31–33).
Weigand et al (34)
reported that SLC7A11 was upregulated in 33ACC tissues compared
with 10 normal adrenal gland samples from the Gene Expression
Omnibus dataset GSE10927. Furthermore, the present study determined
that SLC7A11 was upregulated in 79 ACC tissues compared with 128
normal adrenal gland tissues based on the TCGA dataset and GTEx
dataset. Chen et al (35)
determined SLC7A11 was correlated with OS of patients with ACC from
the TCGA dataset. Shi et al (36) reported that high expression of
SLC7A11 was significantly associated with a shortened OS in
patients with ACC according to the GEPIA2 database; thus, SLC7A11
is a candidate prognostic biomarker for ACC. The present study also
indicated that SLC7A11 is a prognosis-related ferroptosis gene in
patients with ACC and further explored relevant regulatory
mechanisms of SLC7A11 in the ferroptosis of ACC cells. The present
study suggested that targeting SLC7A11 was able to promote
ferroptosis in ACC cells through IFNγ. Therefore, SLC7A11 may be
considered a potential immunological therapeutic target in ACC.
Erastin is a classical ferroptosis inducer that
inhibits the activity of System Xc-, thereby facilitating an
increase in sensitivity to ferroptosis in tumor cells (37). Erastin has excellent prospects for
clinical use, enhancing the sensitivity of various types of human
cancer to chemotherapy or radiotherapy (18,19,38).
Weigand et al (34)
demonstrated that ACC cells were insensitive to treatment with
Erastin for 24 h. However, recent research suggested that knockdown
of SLC7A11 or IFNγ-mediated downregulation of SLC7A11 increased the
sensitivity of tumor cells to Erastin-induced ferroptosis (11). Therefore, it was hypothesized that
there exists crosstalk between IFNγ-mediated signaling and
ferroptosis in ACC. In the present study, it was indicated that
pretreatment with IFNγ prolonged the period of action of erastin to
inhibit the viability of the NCI-H295R ACC cells.
Ferroptosis is characterized by iron-dependent lipid
peroxidation and ROS production. A higher level of ROS generation
promotes cancer suppression (39,40).
The results of the present study suggested that IFNγ together with
erastin enhanced the ferroptosis-like changes, including
accumulation of intracellular iron content, elevated levels of
lipid peroxidation, increased ROS production and mitochondrial
damage. Both GPX4 and ACSL4 are essential determinants of
sensitivity to ferroptosis. ACSL4 is involved in the formation of
lipid ROS, which leads to ferroptosis, whereas GPX4, using GSH as a
substrate, protects cells from lipid peroxidation (15). The results of the present study
indicated that the expression of ACSL4 was upregulated in the IFNγ
+ erastin group, whereas the expression of GPX4 was downregulated.
To summarize, these results suggested that IFNγ enhanced
erastin-induced ferroptosis in an ACC cell line, highlighting a
potential therapeutic approach for the management of ACC.
Pro-inflammatory cytokines, such as IFNγ, are
released from immune cells and are responsible for anti-tumor
immunity. IFNγ binds to specific receptors at the cell membrane to
trigger the phosphorylation of STAT1 and activation of IRF1. This
results in the activation of the JAK/STAT signaling pathway, which
is an essential signaling pathway involved in regulating SLC7A11
expression (11,12). Recent research confirmed that IFNγ
promoted STAT1-mediated suppression of SLC7A11 in HT-1080 cells
(11). In addition, STAT3 and
STAT5 contribute to the regulation of SLC7A11 in numerous types of
cancer, such as breast cancer (41). The results of the present study
suggested that dual treatment with IFNγ and erastin significantly
suppressed the expression of SLC7A11. Furthermore, IFNγ treatment
indirectly upregulated the expression of pSTAT1, pSTAT3 and IRF1.
In addition, SLC7A11 overexpression or STAT1 inhibition
significantly ameliorated IFNγ-enhanced erastin-induced
ferroptosis. Therefore, it may be hypothesized that IFNγ inhibits
SLC7A11 via the JAK/STAT pathway.
The present study has certain limitations. First,
ferroptosis-inhibiting drugs, such as ferrostatin-1, were not used
to explore their protective effects under IFNγ-enhanced
erastin-induced ferroptosis. Furthermore, the role of IFNγ was not
verified in vivo or in another ACC cell line. In addition, a
comparison of the expression status of SLC7A11 between normal
adrenocortical cells and NCI-H295R was not performed due to the
lack of normal adrenocortical cells. The above-mentioned factors
will be investigated in future studies.
In conclusion, it was demonstrated for the first
time that IFNγ treatment increased the sensitivity of ACC cells to
erastin-induced ferroptosis via downregulation of SLC7A11 via the
JAK/STAT pathway. Furthermore, it increased the levels of lipid
peroxidation and ROS and increased mitochondrial damage, which in
turn led to ferroptosis in ACC tumor cells. The present study
provides critical insight into ferroptosis and how it may be used
to improve the efficacy of cancer immunotherapy in ACC.
Supplementary Material
Supporting Data
Acknowledgements
The authors express their gratitude to Dr Lei Guo
(Department of Orthopaedics, Ruijin Hospital, Shanghai Jiao Tong
University School of Medicine, Shanghai, China) for providing
experimental assistance for this research.
Funding
This work was supported by the Natural Science Foundation of
Shanghai, China (grant no. 21ZR1440700).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
XY and DZ contributed to the design of the study,
performed the study and wrote the manuscript. BL contributed to the
statistical analysis of the data. WK and YC contributed to cell
culture. YZ analyzed the data and edited the manuscript. All
authors contributed to the article, and read and approved the final
version. XY and YZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Assié G, Letouzé E, Fassnacht M, Jouinot
A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K,
René-Corail F, et al: Integrated genomic characterization of
adrenocortical carcinoma. Nat Genet. 46:607–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crona J and Beuschlein F: Adrenocortical
carcinoma-towards genomics guided clinical care. Nat Rev
Endocrinol. 15:548–560. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Tourneau C, Hoimes C, Zarwan C, Wong
DJ, Bauer S, Claus R, Wermke M, Hariharan S, von Heydebreck A,
Kasturi V, et al: Avelumab in patients with previously treated
metastatic adrenocortical carcinoma: phase 1b results from the
JAVELIN solid tumor trial. J Immunother Cancer. 6:1112018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sperone P, Ferrero A, Daffara F, Priola A,
Zaggia B, Volante M, Santini D, Vincenzi B, Badalamenti G,
Intrivici C, et al: Gemcitabine plus metronomic 5-fluorouracil or
capecitabine as a second-/third-line chemotherapy in advanced
adrenocortical carcinoma: A multicenter phase II study. Endocr
Relat Cancer. 17:445–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Henning JEK, Deutschbein T, Altieri B,
Steinhauer S, Kircher S, Sbiera S, Wild V, Schlötelburg W, Kroiss
M, Perotti P, et al: Gemcitabine-based chemotherapy in
adrenocortical carcinoma: A multicenter study of efficacy and
predictive factors. J Clin Endocrinol Metab. 102:4323–4332. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cosentini D, Grisanti S, Dalla Volta A,
Laganà M, Fiorentini C, Perotti P, Sigala S and Berruti A:
Immunotherapy failure in adrenocortical cancer: Where next? Endocr
Connect. 7:E5–E8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jorgovanovic D, Song M, Wang L and Zhang
Y: Roles of IFN-γ in tumor progression and regression: A review.
Biomark Res. 8:492020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni L and Lu J: Interferon gamma in cancer
immunotherapy. Cancer Med. 7:4509–4516. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ligocki AJ, Brown JR and Niederkorn JY:
Role of interferon-γ and cytotoxic T lymphocytes in intraocular
tumor rejection. J Leukoc Biol. 99:735–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Ma P, Li J, Cui X and Song W:
Improvement of the cytotoxic T lymphocyte response against
hepatocellular carcinoma by transduction of cancer cells with an
adeno-associated virus carrying the interferon-γ gene. Mol Med Rep.
13:3197–3205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Green M, Choi JE, Gijon M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al: CD8(+) T
cells regulate tumour ferroptosis during cancer immunotherapy.
Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong R, Wang N, Han W, Bao W and Lu J:
IFNγ-mediated repression of system xc− drives
vulnerability to induced ferroptosis in hepatocellular carcinoma
cells. J Leukoc Biol. 110:301–314. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X and Li X: Abnormal iron and lipid
metabolism mediated ferroptosis in kidney diseases and its
therapeutic potential. Metabolites. 12:582022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D and Li Y: The interaction between
ferroptosis and lipid metabolism in cancer. Signal Transduct Target
Ther. 5:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang X, Chen W, Liu H, Liu N, Chen D, Tian
D and Wang J: Research progress on SLC7A11 in the regulation of
cystine/cysteine metabolism in tumors. Oncol Lett. 23:472022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wei Z, Pan K, Li J and Chen Q: The
function and mechanism of ferroptosis in cancer. Apoptosis.
25:786–798. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cobler L, Zhang H, Suri P, Park C and
Timmerman L: xCT inhibition sensitizes tumors to γ-radiation via
glutathione reduction. Oncotarget. 9:32280–32297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Li X, Liu L, Yu B, Xue Y and Liu
Y: Erastin sensitizes glioblastoma cells to temozolomide by
restraining xCT and cystathionine-γ-lyase function. Oncol Rep.
33:1465–1474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roh JL, Kim EH, Jang HJ, Park JY and Shin
D: Induction of ferroptotic cell death for overcoming cisplatin
resistance of head and neck cancer. Cancer Lett. 381:96–103. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang JY, Wang DS, Lin HC, Chen XX, Yang
H, Zheng Y and Li YH: A novel ferroptosis-related gene signature
for overall survival prediction in patients with hepatocellular
carcinoma. Int J Biol Sci. 16:2430–2441. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Team C: Team RDC.R: A Language And
Environment For Statistical Computing. R Foundation for Statistical
Computing; Vienna: 2012
|
|
23
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linher-Melville K, Haftchenary S, Gunning
P and Singh G: Signal transducer and activator of transcription 3
and 5 regulate system Xc- and redox balance in human breast cancer
cells. Mol Cell Biochem. 405:205–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun L, Linghu D and Hung M: Ferroptosis: A
promising target for cancer immunotherapy. Am J Cancer Res.
11:5856–5863. 2021.PubMed/NCBI
|
|
28
|
Xia X, Fan X, Zhao M and Zhu P: The
Relationship between ferroptosis and tumors: A novel landscape for
therapeutic approach. Curr Gene Ther. 19:117–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lang X, Green MD, Wang W, Yu J, Choi JE,
Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al: Radiotherapy and
immunotherapy promote tumoral lipid oxidation and ferroptosis via
synergistic repression of SLC7A11. Cancer Discov. 9:1673–1685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiozaki A, Iitaka D, Ichikawa D,
Nakashima S, Fujiwara H, Okamoto K, Kubota T, Komatsu S, Kosuga T,
Takeshita H, et al: xCT, component of cysteine/glutamate
transporter, as an independent prognostic factor in human
esophageal squamous cell carcinoma. J Gastroenterol. 49:853–863.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeuchi S, Wada K, Toyooka T, Shinomiya
N, Shimazaki H, Nakanishi K, Nagatani K, Otani N, Osada H, Uozumi
Y, et al: Increased xCT expression correlates with tumor invasion
and outcome in patients with glioblastomas. Neurosurgery. 72:33–41;
discussion 41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weigand I, Schreiner J, Rohrig F, Sun N,
Landwehr LS, Urlaub H, Kendl S, Kiseljak-Vassiliades K, Wierman ME,
Angeli JPF, et al: Active steroid hormone synthesis renders
adrenocortical cells highly susceptible to type II ferroptosis
induction. Cell Death Dis. 11:1922020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Yan L, Jiang F, Lu Y, Zeng N, Yang
S and Ma X: Identification of a ferroptosis-related signature
associated with prognosis and immune infiltration in adrenocortical
carcinoma. Int J Endocrinol. 2021:46543022021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi Z, Tao H, Fan Z, Song S and Bai J:
Prognostic and immunological role of key genes of ferroptosis in
pan-cancer. Front Cell Dev Biol. 9:7489252021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang R, Xu J, Zhang B, Liu J, Liang C, Hua
J, Meng Q, Yu X and Shi S: Ferroptosis, necroptosis, and pyroptosis
in anticancer immunity. J Hematol Oncol. 13:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamaguchi H, Hsu JL, Chen CT, Wang YN, Hsu
MC, Chang SS, Du Y, Ko HW, Herbst R and Hung MC:
Caspase-independent cell death is involved in the negative effect
of EGF receptor inhibitors on cisplatin in non-small cell lung
cancer cells. Clin Cancer Res. 19:845–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Belavgeni A, Bornstein SR, von
Mässenhausen A, Tonnus W, Stumpf J, Meyer C, Othmar E, Latk M,
Kanczkowski W, Kroiss M, et al: Exquisite sensitivity of
adrenocortical carcinomas to induction of ferroptosis. Proc Natl
Acad Sci USA. 116:22269–22274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu S, Li T, Liu W and Huang Y: Ferroptosis
and cancer: Complex relationship and potential application of
exosomes. Front Cell Dev Biol. 9:7337512021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Linher-Melville K and Singh G: The complex
roles of STAT3 and STAT5 in maintaining redox balance: Lessons from
STAT-mediated xCT expression in cancer cells. Mol Cell Endocrinol.
451:40–52. 2017. View Article : Google Scholar : PubMed/NCBI
|